Abstract
Introduction
The SITS-UTMOST (Safe Implementation of Thrombolysis in Upper Time window Monitoring Study) was a registry-based prospective study of intravenous alteplase used in the extended time window (3–4.5 h) in acute ischaemic stroke to evaluate the impact of the approval of the extended time window on routine clinical practice.
Patients and methods
Inclusion of at least 1000 patients treated within 3–4.5 h according to the licensed criteria and actively registered in the SITS-International Stroke Thrombolysis Registry was planned. Prospective data collection started 2 May 2012 and ended 2 November 2014. A historical cohort was identified for 2 years preceding May 2012. Clinical management and outcome were contrasted between patients treated within 3 h versus 3–4.5 h in the prospective cohort and between historical and prospective cohorts for the 3 h time window. Outcomes were functional independency (modified Rankin scale, mRS) 0–2, favourable outcome (mRS 0–1), and death at 3 months and symptomatic intracerebral haemorrhage (SICH) per SITS.
Results
4157 patients from 81 centres in 12 EU countries were entered prospectively (N = 1118 in the 3–4.5 h, N = 3039 in the 0–3 h time window) and 3454 retrospective patients in the 0–3 h time window who met the marketing approval conditions. In the prospective cohort, median arrival to treatment time was longer in the 3–4.5 h than 3 h window (79 vs. 55 min). Within the 3 h time window, treatment delays were shorter for prospective than historical patients (55 vs. 63). There was no significant difference between the 3–4.5 h versus 3 h prospective cohort with regard to percentage of reported SICH (1.6 vs. 1.7), death (11.6 vs. 11.1), functional independency (66 vs. 65) at 3 months or favourable outcome (51 vs. 50).
Discussion
Main weakness is the observational design of the study.
Conclusion
This study neither identified negative impact on treatment delay, nor on outcome, following extension of the approved time window to 4.5 h for use of alteplase in stroke.
Keywords: Stroke, thrombolysis, ischaemia, haemorrhage, time to treatment, Actilyse
Background
Intravenous (IV) thrombolysis with recombinant tissue plasminogen activator (rt-PA) is a highly effective treatment within 3 h after onset of stroke symptoms in selected patients with acute ischaemic stroke.1–6 An extended treatment window up to 4.5 h has also proven to be efficacious in the randomised ECASS III trial7 and recent meta-analysis,6 which is supported by observational SITS-International Stroke Thrombolysis Registry (ISTR) studies.8–10 Safety and efficacy of a treatment in acute ischaemic stroke may differ between the settings of RCTs and during implementation into clinical routine. Recent studies have shed some light into the importance of optimising hospital management of stroke patients in the acute phase.11–14 The European Stroke Organisation15 and the American Stroke Association16,17 Guidelines recommend to treat with IV thrombolysis in acute ischemic stroke if symptoms onset is within 4.5 h. However, there are some discrepancy between European15 and 2013 American guidelines16 such as American guidelines recommend to treat with IV thrombolysis within 4.5 h except for patients over 80 years old, those taking oral anticoagulants regardless of INR, those with a baseline NIHSS score >25, those with imaging evidence of ischemic injury involving more than one-third of the MCA territory, or those with a history of both stroke and diabetes mellitus. The recently updated American guidelines recommends treatment between 3 and 4.5 h for carefully selected patients including those over 80 years, those taking oral anticoagulants with INR<1.7 and those with a history of both stroke and diabetes mellitus.17
The SITS-UTMOST (Safe Implementation of Thrombolysis in Upper Time window Monitoring Study) was a registry-based prospective observational study carried out upon post-approval request of the Competent Authorities in European Union (EU) with Mutual Recognition Procedure (MRP)-countries of IV thrombolysis by Actilyse in the extended time window (3–4.5 h) after onset of acute ischaemic stroke symptoms. Authorities were concerned that extending the time window might lead to patients being treated more slowly with increased door to needle (DNT) times. The purpose of this study was to evaluate the impact of the approval of the extended time window up to 4.5 h on routine clinical practice treated according to the EU Summary of Product Characteristics (SmPC) criteria.
Methods
At least 1000 patients treated by IV thrombolysis within 3–4.5 h time window after onset of acute ischaemic stroke from EU centres actively registered in the SITS-ISTR were planned to be included in the study. The sample size of the study was not based on any formal power calculation, rather chosen pragmatically in consultation with authorities, based on numbers achievable within a 2-year period. Controls were also recorded: 0–3 h prospective and historical. SITS centres are obliged to enter consecutive patients in the registry for the part of the registry the centre intend to use such as IV thrombolysis, thrombectomy, general stroke registry. The current study is based on centres used the registry for IV thrombolysis.
Centre selection
Ninety-four centres from EU countries were considered to be included in the post-approval part of the study based on their active participation in SITS-ISTR during 2010 and 2011. The criteria used to select centres required regular treatment of acute ischaemic stroke patients with Actilyse and registered into SITS registry (≥ 1 patient/ month during January 2010 and January 2012) with sufficient quality of the data; completeness of three months outcome data > 70% and acute data >75%.
The prospective part of the registry commenced on 2 May 2012 after the majority of EU Health Authorities had approved the extended AIS treatment time window up to 4.5 h (except for Poland and Italy where the start date based on local approvals had been set to 15 July 2010 and 3 October 2013, respectively). UTMOST database was locked on 2 November 2014 when reached target for at least 1000 patients in the 3–4.5 h time window. A historical cohort for the prior 2 years was extracted from the registry from the same centres that contributed to the prospective cohort, dated 1 May 2010 to 1 May 2012 except for Poland and Italy. For Poland, retrospective cohort data extraction was from 15 July 2008 to 14 July 2010 and for Italy was 3 October 2011 to 2 October 2013.
All centres routinely providing data to the academic SITS-ISTR Registry were informed about the study through the SITS website (website: sitsinternational.org/sits-projects/sits-utmost), regardless of their actual participation. SITS-UTMOST extracted data from the existing academic registry SITS-ISTR. Centres, chosen for the purposes of SITS-UTMOST data analysis, were contacted prior to final data analysis to confirm agreement for having their data included in the study. All centres agreed to include their data in the analysis.
The SITS-ISTR is an ongoing, prospective, internet-based, academic-driven, multinational, observational monitoring register for clinical centres using thrombolysis for the treatment of acute ischemic stroke. The methodology of the SITS-ISTR, including procedures for data collection and management, patient identification and verification of source data, has been described previously.2,8,9 We collected baseline and demographic characteristics, stroke severity as measured by NIHSS score, time logistics, medication history, and imaging data on admission and 24 h after thrombolysis (preferably within 22–36 h or earlier if clinically indicated) and follow-up, 3-months outcome as measured by modified Rankin scale (mRS) score.
Ethics approval and data monitoring
The study was approved by Ethics Committee Karolinska Institutet, Stockholm. Ethics approval and patient consent for participation in the SITS-ISTR were obtained in countries that required this; other countries approved the register for anonymized audit. The SITS International Coordination Office monitored the SITS-ISTR data online and checked individual patient data monthly to identify errors or inconsistencies. The study was performed according to a protocol approved by the ethics committee. Since the study is not an RCT and did not influence treatment allocation it did not require clinical trial registration.
Outcome measurements
Primary outcome measurements were symptomatic intracerebral haemorrhage (SICH), death and independency as measured by the mRS 0–2 and favourable outcome (mRS 0–1) at 3-months.
Secondary outcome measurements were patients’ management time delays: Onset of symptoms to treatment/needle time and its components: onset of symptoms to door; door to imaging scan, imaging to needle and door to needle times (DNT).
The following definitions of SICH were used in our study:
SICH per SITS-MOST: PH2 (parenchymal haemorrhage type 2) or remote parenchymal haemorrhage type (PHr2) on imaging 22–36 h after treatment, or earlier if the scan was performed due to clinical deterioration, combined with a neurological deterioration of ≥4 NIHSS points or leading to death within 24 h;
SICH per ECASS II: any ICH on any post-treatment imaging after the start of thrombolysis and increase of ≥4 NIHSS points or leading to death, within 7 days;
SICH per NINDS: any ICH on any post-treatment imaging and any deterioration in NIHSS or death within 7 days.
All SICH events were adjudicated centrally by the SITS International Coordination Office based on submitted clinical and imaging reports; images were not available for review. All assessments of imaging studies, neurological and functional status were done according to clinical routine at centres participating in the SITS-ISTR. Training in mRS assessment was not mandated by SITS.
Statistical analysis
We contrasted baseline data and clinical outcome data by comparing between patients treated within 3 h and 3–4.5 h in the post-approval/prospective cohort. We also compared the pre-approval/ retrospective cohort to post-approval/prospective cohort for patients treated within 3 h. For categorical variables, we calculated percentage proportions by dividing the number of events by the total number of patients excluding missing or unknown cases. Pearson’s Chi square tests were used for categorical variables and Mann–Whitney U-tests were used for continuous and ordinal variables. We further compared the proportions of functional independency and death and SICH. Analyses were also made with regards to patient’s management time intervals to evaluate if an extended time window results in undue delays in treatment. We also performed multivariable logistic regression analysis after adjusting for variables that were statistically significant in the univariate analysis at 10% level. All p-values presented are at nominal 5% alpha level as statistically significant.
Results
Twelve European countries recorded data in the registry during the prospective study period: Belgium, Bulgaria, Czech Republic, Finland, Germany, Italy, Poland, Portugal, Slovenia, Spain, Sweden, United Kingdom.
Recruitment of patients
In the prospective cohort, 4157 patients and in the retrospective cohort, 3454 patients were recorded to have received IV thrombolysis according to the EU SmPC. Among the prospective cohort, 1118 patients were treated with IV thrombolysis between 3 and 4.5 h from onset of stroke.
Recruitment per country is shown in Table 1A in the Appendix.
Baseline and clinical data
Baseline and clinical characteristics of patients in full compliance with other European SmPC for the prospective and retrospective cohorts are given in Table 1. The proportion of females was higher in the upper time window (3–4.5 h) compared to ≤3 h time window (45% vs. 41%). Frequency of hyperlipidemia and atrial fibrillation was lower in the upper time window compared to ≤3 h time window. Baseline stroke severity was 2 points (median) lower in the 3–4.5 h time window period compared to ≤3 h time window and median NIHSS score was 1 point lower in the prospective 3 h cohort than retrospective 3 h. In general, the patient baseline characteristics treated within 3 h were very similar in the prospective and retrospective cohorts.
Table 1.
Baseline and clinical characteristics.
| Baseline and demographic variables | Prospective 3–4 5 h (n = 1118) | Prospective within 3 h (n = 3039) | p-valuesa | Retrospective within 3 h (n = 3454) | p-valuesb |
|---|---|---|---|---|---|
| Age (years) | 68 (58-75) | 68 (59-75) | 0.553 | 68 (59-75) | 0.948 |
| Sex: female | 498/ 1118 (44.5) | 1239/ 3039 (40.8) | 0.031 | 1428/ 3454 (41.3) | 0.658 |
| Hypertension | 699/ 1114 (62.8) | 1876/ 3034 (61.8) | 0.616 | 2134/ 3433 (62.2) | 0.806 |
| Diabetes mellitus | 217/ 1117 (19.4) | 494/ 3033 (16.3) | 0.020 | 559/ 3439 (16.3) | 0.999 |
| Hyperlipidaemia | 307/ 3433 (27.5) | 941/ 3021 (31.2) | 0.027 | 1088/ 3382 (32.2) | 0.395 |
| Smoking | 0.878 | 0.028 | |||
| current | 204/ 1090 (18.7) | 566/ 2922 (19.4) | 662/ 3306 (20.0) | ||
| previous | 128/ 1090 (11.7) | 347/ 2922 (11.9) | 461/ 3306 (13.9) | ||
| Previous stroke >3 months before | 105/1116 (9.4) | 276/ 3027 (9.1) | 0.821 | 286/ 3435 (8.3) | 0.279 |
| Previous TIA | 79/ 1117 (7.1) | 183/ 3028 (6.0) | 0.256 | 218/ 3037 (7.2) | 0.084 |
| Atrial fibrillation | 146/ 1116 (13.1) | 481/ 3028 (15.9) | 0.029 | 655/ 3433 (19.1) | 0.001 |
| Congestive heart failure | 75/ 1114 (6.7) | 192/ 3023 (6.4) | 0.710 | 233/ 3439 (6.8) | 0.525 |
| Aspirin | 310/ 1110 (27.9) | 874/ 3017 (29.0) | 0.537 | 1067 / 3435 (31.1) | 0.072 |
| Dipyridamol | 14/ 1114 (1.3) | 45/ 3022 (1.5) | 0.681 | 82/ 3442 (2.4) | 0.013 |
| Clopidogrel | 86/ 1113 (7.7) | 168/ 3022 (5.6) | 0.012 | 167/ 3443 (4.9) | 0.220 |
| Other anti-platelet | 8/ 1114 (0.72) | 25/ 3021 (0.83) | 0.878 | 50/ 3442 (1.5) | 0.026 |
| Oral anti-hypertensives | 628/ 1111 (56.5) | 1742/ 3019 (57.7) | 0.521 | 1882/ 3429 (54.9) | 0.025 |
| Statin | 326/ 1113 (29.3) | 947/ 3019 (31.4) | 0.213 | 852/ 3021 (28.2) | 0.008 |
| Current infarct at baseline imaging | 167/ 1099 (15.2) | 425/ 2991 (14.2) | 0.457 | 502/ 3363 (14.9) | 0.439 |
| Weight in kg | 78 (70–90) | 79 (70–90) | 0.430 | 78 (69–89) | 0.013 |
| Dose of Actilyse (mg) | 70 (61–80) | 70 (61–80) | 0.546 | 70 (60–80) | |
| Blood glucose (mmol/L) | 6.7 (5.7–8.0) | 6.5 (5.7–7.8) | 0.281 | 6.5 (5.7–7.8) | 0.810 |
| NIHSS) score | 8 (5–14) | 10 (6–16) | <0.001 | 11 (6–17) | 0.002 |
| Systolic blood pressure (mm Hg) | 150 (135–162) | 150 (135–163) | 0.301 | 150 (135–160) | 0.159 |
| Diastolic blood pressure (mm Hg) | 80 (72–90) | 80 (73–90) | 0.219 | 80 (72–90) | 0.332 |
Comparison between 3–4.5 h and ≤3 h for the prospective cohort.
Comparison between ≤3 h prospective and ≤3 h retrospective cohorts. Data are median (IQR) for continuous and ordinal variables and n/N (%) for categorical variables.
Time logistics of patients’ management
Table 2 shows the time logistics. The management times within the hospital are somewhat longer for the 3–4.5 h time window compared to the ≤3 h time window. Median door to imaging time was 7 min and DNT was 24 min longer in the 3–4.5 h time window compared to the ≤3 h time window. Median DNT was 8 min shorter in the ≤3 h prospective cohort compared to ≤3 h retrospective cohort.
Table 2.
Time logistics according to onset to treatment time.
| Median (IQR) time logistics in minutes | Prospective 3–4 5 h (n = 1118) | Prospective within 3 h (n = 3039) | p-valuesa | Retrospective within 3 h (n = 3454) | p-valuesb |
|---|---|---|---|---|---|
| Stroke onset to door time | 137 (100–171) | 67 (50–90) | <0.001 | 65 (46–86) | <0.001 |
| Door to imaging | 29 (17–47) | 22 (13–32) | <0.001 | 23 (14–35) | 0.002 |
| Imaging to treatment | 45 (28–70) | 32 (20–48) | <0.001 | 37 (24–55) | <0.001 |
| Door to needle time | 79 (54–111) | 55 (40–75) | <0.001 | 63 (45–84) | <0.001 |
| Stroke onset to treatment time | 217 (200–240) | 129 (105–155) | <0.001 | 135 (106–157) | <0.001 |
Comparison between 3–4.5 h and ≤3 h for the prospective cohort.
Comparison between ≤3 h prospective and ≤3 h retrospective cohorts using Mann–Whitney U-test.
In Table 3, the hospital management time is presented based on when the patient arrived to the hospital (i.e. prehospital time). For the prospective cohort (≤4.5 h treatment time window), in hospital management times (door to imaging and DNT) were shorter for patients whose stroke onset to hospital arrival time was longer. When comparing patients who arrived at hospital within 60 min of symptom onset between prospective and historical control, we observed a 9 min shorter median DNT in the prospective compared to historical control and similar DNT (median 60 min) for patients who arrived hospital within 61–120 min of symptom onset.
Table 3.
Time logistics of patients within the prospective cohort depending on arrival time to hospital (OTD onset to door). Data for retrospective cohort are provided for the first 2 h of hospital arrival.
| Prospective cohort |
Retrospective cohort |
||||||
|---|---|---|---|---|---|---|---|
| Time in minutes | OTD 181–270 (n = 211) | OTD 121–180 (n = 604) | OTD 61–120 (n = 1865) | OTD 0–60 min (n = 1321) | p-valuesa | OTD 61–120 (n = 1592) | OTD 0–60 min (n = 1535) |
| Door to imaging | 18 (11–28) | 21 (13–32) | 23 (14–35) | 24 (15–35) | 0.001 | 21 (12–32) | 26 (15–39) |
| Imaging to needle time | 25 (15–33) | 34 (20–48) | 37 (22–55) | 35 (23–57) | <0.001 | 35 (23–50) | 40 (26–60) |
| Door to needle time | 43 (30–55) | 58 (40–76) | 60 (45–85) | 61 (45–90) | <0.001 | 60 (43–75) | 70 (52–93) |
| Onset to treatment | 250 (240–260) | 205 (185–226) | 150 (126–175) | 110 (90–135) | <0.001 | 145 (129–165) | 115 (95–135) |
Comparison using Kruskal–Wallis equality-of-populations rank test for all groups for the prospective cohort. Data are median (IQR). 156 patients were excluded from this analysis due to unclear OTD time.
Clinical outcome data
Table 4 shows proportion and adjusted odds ratio for the SICH and 3-month outcomes. In the prospective cohort, there was no significant difference in proportions of SICH mortality and functional outcome at 3 months between 3–4.5 h and ≤3 h time window. Multivariate analyses showed no difference in SICH and mortality between 3–4.5 h and ≤3 h time window in the prospective cohorts. There was a significantly lower odds ratio for functional independence at 3 months in the 3–4.5 h cohort compared to ≤3 h cohort.
Table 4.
SICH and 3-months outcomes.
| Outcomes | Prospective 3–4 5 h n/N (%) aOR (95% CI) | Prospective within 3 h n/N (%) aOR (95% CI) | p-valuesa | Retrospective within 3 h n/N (%) aOR (95% CI) | p-valuesb |
|---|---|---|---|---|---|
| SICH (SITS-MOST)c | 17/ 1082 (1.57) | 49/ 2953 (1.66) | 0.956 | 60/ 3391 (1.77) | 0.811 |
| Adjusted OR | 1.08 (0.61–1.90) | – | 0.787 | 0.91 (0.61–1.35) | 0.639 |
| SICH (ECASS II)d | 42/ 1074 (3.91) | 97/ 2946 (3.29) | 0.395 | 131/ 3369 (3.89) | 0.231 |
| Adjusted OR | 1.46 (0.99–2.15) | – | 0.053 | 0.87 (0.65–1.15) | 0.331 |
| SICH (NINDS)d | 59/ 1081 (5.46) | 144/ 2950 (4.88) | 0.509 | 194/ 3386 (5.7) | 0.149 |
| Adjusted OR | 1.35 (0.98–1.87) | – | 0.068 | 0.89 (0.70–1.12) | 0.326 |
| 3 months (mRS 0–1) | 399/ 782 (51.0) | 1109/ 2230 (49.7) | 0.562 | 1450/ 2951 (49.1) | 0.692 |
| Adjusted OR | 0.87 (0.72–1.05) | – | 0.159 | 0.96 (0.84–1.09) | 0.514 |
| 3 months (mRS 0–2) | 512/ 782 (65.5) | 1453/ 2230 (65.2) | 0.908 | 1878/ 2951 (63.4) | 0.272 |
| Adjusted OR | 0.81 (0.67–0.99) | – | 0.044 | 1.01 (0.88–1.15) | 0.925 |
| 3 months mortality | 93/ 801 (11.6) | 251/ 2267 (11.1) | 0.726 | 333/ 3021 (11.0) | 0.990 |
| Adjusted OR | 1.30 (0.98–1.73) | – | 0.066 | 1.10 (0.90–1.34) | 0.339 |
SICH: symptomatic intracerebral haemorrhage; mRS: modified Rankin scale; aOR: adjusted odds ratio.
For the prospective 3–4.5 h compared to prospective ≤3 h cohort. Multivariate analysis adjusted for age, sex, baseline NIHSS, history of diabetes mellitus, hyperlipidaemia and atrial fibrillation and treatment with Clopidogel at baseline.
For ≤3 h prospective cohort compared to ≤3 h retrospective cohort. Multivariate analysis adjusted for age, baseline NIHSS, history of TIA, atrial fibrillation, and smoking, aspirin, antihypertensive and statin treatment at baseline.
A local or remote parenchymal haemorrhage type 2 on the 22- to 36-h post-treatment imaging scan or earlier if clinically indicated, combined with a neurological worsening of ≥4 points between baseline and 24 h, or leading to death.
Any intracerebral haemorrhage on any post-treatment imaging scans combined with NIHSSS worsening ≥4 points between baseline and 7d, or leading to death.
Any intracerebral haemorrhage on any post-treatment imaging scans combined with any decline in neurologic status as measured by NIHSS between baseline and 7d, or leading to death.
In the ≤3 h time window, there was no significant difference in any outcome parameter between the prospective and retrospective cohort.
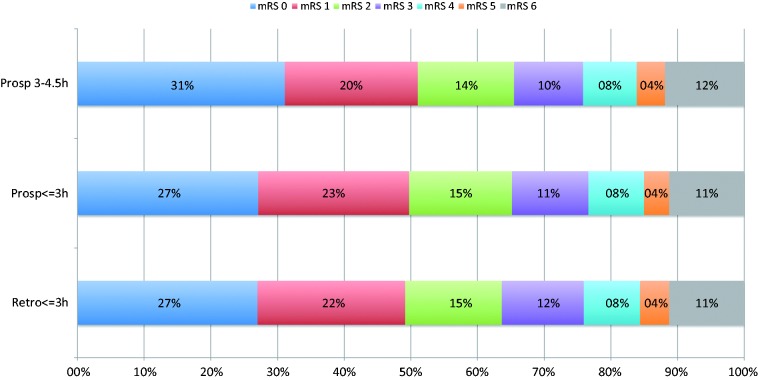
Figure 1 shows the similar distribution of mRS score at 3 months between the cohorts.
Figure 1.
Modified Rankin scale score at 3 m.
Discussion
During the 30 months of the prospective study period, the SITS-UTMOST registry achieved the expected sample size of more than 1000 patients treated within the extended time window (3–4.5 h) fulfilling all other SmPC criteria. In the prospective cohort, there were minor differences in the baseline and demographic characteristics between 3–4.5 h and ≤3 h time window which are not clinically important. The only clinically important difference was 2 points lower baseline median NIHSS score in the 3–4.5 h time window than the ≤3 h in prospective cohort, which favoured 3–4.5 h time window. This may either represent milder stroke patients seeking hospital later than severe stroke or a greater proportion of patients with milder stroke severity being treated in recent years compared to previous years. When comparing ≤3 h time window between prospective and retrospective cohorts, in general the baseline and demographic characteristics were very similar. Most of the statistically significant differences were not clinically significant other than 1 point lower median NIHSS score in the ≤3 h prospective cohort than in the ≤3 h retrospective cohort.
As we observed in previous studies,8–10 there was a longer hospital management time (7 min longer median door to imaging time and 24 min longer median DNT) in the 3–4.5 h time window compared to ≤3 h time window. Patients treated in the 3–4.5 h time window had milder strokes, which may have led to different management at the hospital. It is important to note that the median DNT in the prospective ≤3 h time window was 8 min shorter than in the corresponding ≤3 h retrospective cohort. Some patients in the 3–4.5 h cohort might not have received treatment under the original licence due to ≤3 h time window restriction. After extension of the time window beyond 3 h, centres would have more time to assess and thus greater potential to treat such patients with IV thrombolysis.
We also observed that in hospital management times (door to imaging, DNT) were longer when stroke onset to hospital arrival time was shorter (stroke onset to door time). These results may suggest that patients arriving at the limit of therapeutic time window are managed more rapidly than those arriving earlier. However, this interpretation may not be the sole explanation. It may be due to a mathematical reason since we have an upper time limit for start of treatment (4.5 h for prospective and 3 h for historical control). It is important to note that there was no negative impact on hospital management time for patients who arrived at hospital within first 2 h of symptom onset between the prospective and historical control. However, 60 min DNT is still long and hospitals should aim for DNT less than 40 min.
In the prospective cohort, we did not observe any difference in the SICH, mortality and functional outcome between the 3–4.5 h and ≤3 h time window. A similar observation was also noted for ≤3 h prospective and retrospective cohorts. These results were consistent in the multivariable analysis after adjustment for baseline imbalances, with the exception of a lower odds ratio for functional independence (mRS 0–2) at 3 months in the 3–4.5 h cohort compared to ≤3 h cohort. It is biologically plausible that later initiation of treatment will mean that the amount of core damage which is already established will be greater, and the salvageable penumbral tissue smaller, readily explaining this finding.
This study has certain limitations. Main limitation is the observational design with all its inherent weakness. Some level of missing data for 3-month follow-up of the mRS may have influenced the results.
In conclusion, this observational study uncovered no evidence of poorer safety or functional outcome from treatment with IV thrombolysis in the 3–4.5 h time window after acute ischaemic stroke. We did not observe a negative impact of the extended time window on the hospital management logistics compared to those of the historically treated ≤3 h time window patients. The extended hospital management time for patients in the 3–4.5 h cohort is suboptimal and indicates scope for service improvement. This should have high priority since repeated pooled analyses have consistently shown that earlier initiation of treatment increases the odds for better outcome.3,4,6
Acknowledgements
We thank all SITS-UTMOST investigators and their centers for their participation. We also pass on our thanks to all patients who participated in SITS-UTMOST. The current SITS registry is developed, maintained and upgraded by Zitelab, Copenhagen, Denmark, in close collaboration with SITS.
Appendix
Table 1A.
Recruitment of patients per country.
| Prospective cohort |
Retrospective cohort |
|||
|---|---|---|---|---|
| Country | Number | Percentage | Number | Percentage |
| Belgium | 59 | 1.4 | 39 | 1.1 |
| Bulgaria | 87 | 2.1 | 35 | 1.0 |
| Czech Republic | 854 | 20.5 | 456 | 13.2 |
| Finland | 38 | 0.9 | 148 | 4.3 |
| Germany | 242 | 5.8 | 318 | 9.2 |
| Italy | 595 | 14.3 | 798 | 23.1 |
| Poland | 266 | 6.4 | 131 | 3.8 |
| Portugal | 192 | 4.6 | 201 | 5.8 |
| Slovenia | 72 | 1.7 | 44 | 1.3 |
| Spain | 127 | 3.1 | 54 | 1.6 |
| Sweden | 361 | 8.7 | 247 | 7.2 |
| UK | 1264 | 30.4 | 983 | 28.5 |
| Total | 4157 | 100 | 3454 | 100 |
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: N Ahmed is a senior researcher in SITS International, which receives a grant from Boehringer Ingelheim and Ferrer for the SITS-MOST/SITS-ISTR. Karin Hermansson, Erich Bluhmki, and Thierry Danays are employees of Boehringer Ingelheim. R. Mikulik, Ana Paiva Nunes and A Kenton: has received conference hospitality from Boehringer Ingelheim. D Toni is a member of an Advisory Board (regarding dabigatran) and has received speaker honoraria from Boehringer Ingelheim. G Ford has received personal honoraria and support to attend a scientific meeting from Boehringer Ingelheim, manufacturer of alteplase. KR Lees' institution has received grant support from Genentech, unrelated to the present study. He has received fees from Boehringer Ingelheim for his role as member of DSMB boards. N Wahlgren has received expenses from Boehringer Ingelheim for his role as member of the Steering Committee in relation to the ECASS III trial with alteplase and served as a consultant to Thrombogenics as chairman of the DSMB. SITS International (chaired by N Wahlgren) received a grant from Boehringer Ingelheim and Ferrer for the SITS-MOST/SITS-ISTR. His institution has also received grant support towards administrative expenses for coordination of the ECASS III trial. N Wahlgren has also received lecture fees from Boehringer Ingelheim and Ferrer.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study protocol was drafted by SITS and developed in close collaboration between SITS, Boehringer Ingelheim and the approving Competent Authorities of the EU within the MRP (Mutual Recognition Procedure). All data collection and analysis were conducted independently by SITS. To monitor the study, interim reports were written by SITS and Boehringer Ingelheim and submitted to Competent Authorities in EU during the prospective phase. Boehringer Ingelheim representatives (KH, TD and EB) contributed to the manuscript by their comments. N Ahmed and N Wahlgren had full access to all data in this study, and final responsibility for the preparation and content of this manuscript and its submission for publication. R. Mikulik was supported by the project no. LQ1605 from the National Program of Sustainability II.
Ethical approval and informed consent
The study was approved by Ethics Committee Karolinska Institutet, Stockholm. Ethics approval and patient consent for participation in the SITS-ISTR were obtained in countries that required this; other countries approved the register for anonymized audit.
Guarantor
N Ahmed and N Wahlgren had full access to all data in this study, and final responsibility for the preparation and content of this manuscript and its submission for publication.
Contributorship
The study protocol was drafted by SITS and developed in close collaboration between SITS, Boehringer Ingelheim and the approving Competent Authorities of the EU within the MRP (Mutual Recognition Procedure). All data collection and analysis were conducted independently by SITS. To monitor the study, interim reports were written by SITS and Boehringer Ingelheim and submitted to Competent Authorities in EU during the prospective phase. N Ahmed wrote the initial draft of the manuscript. All authors including Boehringer Ingelheim representatives (KH, TD and EB) contributed to the manuscript by their comments.
References
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group: Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581–1587. [DOI] [PubMed]
- 2.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): An observational study. Lancet 2007; 369: 275–282. [DOI] [PubMed] [Google Scholar]
- 3.Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004; 363: 768–774. [DOI] [PubMed] [Google Scholar]
- 4.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010; 375: 1695–1703. [DOI] [PubMed] [Google Scholar]
- 5.IST-3 Collaborative Group. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): A randomised controlled trial. Lancet 2012; 379: 2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 8.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase 3 - 4.5 h after acute ischaemic stroke (SITS-ISTR): An observational study. Lancet 2008; 372: 1303–1309. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed N, Wahlgren N, Grond M, et al. Implementation and outcome of thrombolysis with alteplase 3-4.5 h after an acute stroke: An updated analysis from SITS-ISTR. Lancet Neurol 2010; 9: 866–874. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed N, Kellert L, Lees KR, et al. Results of intravenous thrombolysis within 4.5 to 6 hours and updated results within 3 to 4.5 hours of onset of acute ischemic stroke recorded in the Safe Implementation of Treatment in Stroke International Stroke Thrombolysis Register (SITS-ISTR): An observational study. JAMA Neurol 2013; 70: 837–844. [DOI] [PubMed] [Google Scholar]
- 11.Fonarow GC, Smith EE, Saver JL, et al. Improving door-to-needle times in acute ischemic stroke: The design and rationale for the American Heart Association/American Stroke Association’s Target: Stroke Initiative. Stroke 2011; 42: 2983–2989. [DOI] [PubMed] [Google Scholar]
- 12.Mikulik R, Kadlecova P, Czlonkowska A, et al. Factors influencing in-hospital delay in treatment with intravenous thrombolysis. Stroke 2012; 43: 1578–1583. [DOI] [PubMed] [Google Scholar]
- 13.Ford AL, Williams JA, Spencer M, et al. Reducing door-to-needle times using Toyota’s lean manufacturing principles and value stream analysis. Stroke 2012; 43: 3395–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strbian D, Michel P, Ringleb P, et al. Relationship between onset-to-door time and door-to-thrombolysis time: A pooled analysis of 10 dedicated stroke centers. Stroke; 44: 2808–2813. [DOI] [PubMed]
- 15.ESO Guidelines: eso-stroke.org/eso-stroke/education/education-guidelines.html, ESO guideline updated, 2009.
- 16.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 870–947. [DOI] [PubMed] [Google Scholar]
- 17.Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016; 47: 581–641. [DOI] [PubMed] [Google Scholar]