Abstract
Background
Embolic strokes of undetermined source comprise up to 20% of ischemic strokes. The stroke recurrence rate is substantial with aspirin, widely used for secondary prevention. The New Approach riVaroxaban Inhibition of Factor Xa in a Global trial versus ASA to prevenT Embolism in Embolic Stroke of Undetermined Source international trial will compare the efficacy and safety of rivaroxaban, an oral factor Xa inhibitor, versus aspirin for secondary prevention in patients with recent embolic strokes of undetermined source.
Main hypothesis
In patients with recent embolic strokes of undetermined source, rivaroxaban 15 mg once daily will reduce the risk of recurrent stroke (both ischemic and hemorrhagic) and systemic embolism (primary efficacy outcome) compared with aspirin 100 mg once daily.
Design
Double-blind, randomized trial in patients with embolic strokes of undetermined source, defined as nonlacunar cryptogenic ischemic stroke, enrolled between seven days and six months from the qualifying stroke. The planned sample size of 7000 participants will be recruited from approximately 480 sites in 31 countries between 2014 and 2017 and followed for a mean of about two years until at least 450 primary efficacy outcome events have occurred. The primary safety outcome is major bleeding. Two substudies assess (1) the relative effect of treatments on MRI-determined covert brain infarcts and (2) the biological underpinnings of embolic strokes of undetermined source using genomic and biomarker approaches.
Summary
The New Approach riVaroxaban Inhibition of Factor Xa in a Global trial versus ASA to prevenT Embolism in Embolic Stroke of Undetermined Source trial is evaluating the benefits and risks of rivaroxaban for secondary stroke prevention in embolic strokes of undetermined source patients. Main results are anticipated in 2018.
Keywords: Stroke, cryptogenic stroke, cerebral embolism, embolic stroke of undetermined source, stroke prevention, rivaroxaban, aspirin, randomized trial
Introduction
Ischemic strokes traditionally classified as cryptogenic remain frequent despite advances in the diagnostic techniques to determine stroke etiology.1,2 Most nonlacunar cryptogenic ischemic strokes are presumed due to emboli, originating from a multitude of cardiac and arterial sources or occasionally from venous thromboembolism (i.e. via paradoxical embolism).1 Cryptogenic conventionally denotes a stroke where high-risk sources of embolism (such as atrial fibrillation) are absent, but many patients diagnosed with cryptogenic stroke are found to have one or more potential embolic sources if thoroughly evaluated, and it is often not possible to be certain of the specific origin of the suspected embolus.3,4 These observations have led to the construct of embolic strokes of undetermined source (ESUS) in order to define a cohort of patients that may respond better to anticoagulation than antiplatelet therapy for secondary stroke prevention.1 In brief, ESUS is diagnosed when a nonlacunar ischemic stroke occurs in a patient in whom subsequent investigations do not show another specifically treatable underlying stroke etiology, primarily >50% stenosis in a proximal extracranial or intracranial artery, atrial fibrillation, or other major-risk cardioembolic source. A recent prospective global registry reported that 16% of ischemic stroke patients met criteria for ESUS (19% if stroke patients who did not undergo the complete evaluation required for diagnosis were excluded).5
Supported by their efficacy for prevention of embolic stroke in atrial fibrillation patients,6,7 anticoagulants have been hypothesized to be more efficacious than antiplatelet drugs for secondary prevention following ESUS.1 The most promising anticoagulants for the prevention of embolic stroke and systemic embolism are the nonvitamin K antagonist direct oral anticoagulants (DOACs). Compared with warfarin and its congeners, DOACs carry a lower risk of intracranial hemorrhage, the most devastating complication of anticoagulation.8 The ESUS construct combined with the availability of efficacious and safe DOACs has prompted the initiation of several randomized trials aimed at reducing recurrent stroke in ESUS patients.9–11 Here, the design highlights and key protocol issues of the New Approach riVaroxaban Inhibition of Factor Xa in a Global trial versus ASA to prevenT Embolism in Embolic Stroke of Undetermined Source (NAVIGATE ESUS) trial are presented.
Design
Overview and timelines
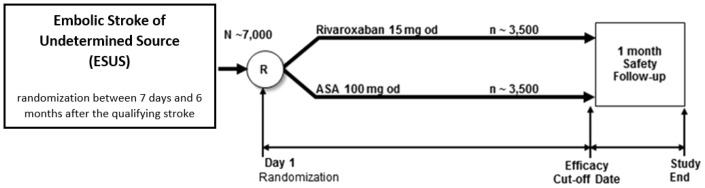
NAVIGATE ESUS (Clinicaltrials.gov.NCT02313909) is an international, double-blinded, randomized phase III trial comparing rivaroxaban 15 mg once daily (immediate-release, film-coated tablets) with aspirin (enteric-coated) 100 mg once daily, both to be taken with food, in patients with recent ESUS (Figure 1). Matching placebos will be used in this double-blinded trial. The primary hypothesis is that rivaroxaban is superior to aspirin for reducing the risk of recurrent stroke and systemic embolism (primary efficacy outcome). Seven thousand patients will be enrolled to detect a ≥30% reduction in primary efficacy outcome events with 90% power by assignment to rivaroxaban based on an estimated rate of 3.8% per year among aspirin-assigned patients.1 Participants will be randomized between seven days and six months following the qualifying ESUS at about 480 sites in 31 countries (Figure 2). Patient recruitment began in December 2014 and is anticipated to finish in 2017. Mean patient follow-up is expected to be about two years, but the study will continue until at least 450 participants have experienced a primary efficacy outcome event. The main results are anticipated to be available in 2018.
Figure 1.
NAVIGATE ESUS design overview.
Figure 2.
Countries participating in NAVIGATE ESUS.
Study population
Screening ischemic stroke patients for participation is based on five key eligibility criteria as summarized in Table 1. In short, patients ≥50 years old with nonlacunar ischemic stroke visualized by neuroimaging and without a clear etiology are included for whom most current guidelines recommend antiplatelet therapy for secondary prevention (Figure 3). After the qualifying stroke, at least 20 h of cardiac rhythm monitoring is required to exclude atrial fibrillation lasting >6 min, although investigators can choose to monitor for longer periods per local clinical practice standards. Intracranial arterial imaging is not required, but if done, the presence of >50% intracranial atherosclerotic stenosis supplying the ischemic area excludes participation. Patients with left ventricular systolic dysfunction of any severity, patent foramen ovale (PFO), and all types of aortic arch plaque are eligible if anticoagulation (or PFO closure) is not planned and potential randomization to aspirin is acceptable to local investigators. Transthoracic echocardiography is mandatory, with transesophageal echocardiography (TEE) optional and an acceptable substitute, with intracardiac thrombus detected by either technique an exclusion criterion. Patients with carotid artery atherosclerotic plaques causing ≤50% stenosis are eligible regardless of ulceration or other features. The outer time limit (i.e. six months) between qualifying stroke and randomization was chosen because the temporal pattern of recurrent stroke is not known for ESUS patients and it was deemed worthwhile to determine the absolute benefits of anticoagulation for ESUS patients who are identified after the acute phase due to diagnostic delays.
Table 1.
Inclusion criteria.
| Embolic stroke of undetermined source (ESUS)a between seven daysb and six months: |
| 1. Ischemic stroke visualized by CT or MRI that is not lacunarc |
| 2. Absence of extracranial and, if intracranial imaging performed, intracranial atherosclerosis causing >50% luminal stenosis of arteries supplying the area of ischemiad |
| 3. No atrial fibrillation by history, ECG, or after >20 h of cardiac rhythm monitoringe |
| 4. No intracardiac thrombus by echocardiography |
| 5. No other specific cause of ischemic stroke identified (e.g. high-risk cardiac sourcef usually requiring anticoagulation, cardiac tumor, arteritis, dissection, migraine/vasospasm, cerebral venous thrombosis, drug abuse) |
| Age ≥50 yearsg |
| Ability and willingness to provide written informed consent |
CT: computed tomography; ECG: electrocardiogram; MRI: magnetic resonance imaging.
Criteria for ESUS contrast with the original definition proposed by the Cryptogenic Stroke/ESUS International Working Group in three main ways (1): intracranial arterial imaging is not required, intracranial arterial occlusion does not exclude participation if diagnosed as embolic, and exclusion based on echocardiography is limited to intracardiac thrombus.
Patients with minor stroke (NIH stroke scale score ≤3) can be entered as soon as three days after onset and not before 10 days in case of hemorrhagic transformation or intravenous thrombolysis therapy and unless repeat CT or MRI performed before randomization documents the absence of new or extension of bleeding.
Lacunar defined as an infarct ≤1.5 cm in largest dimension involving any subcortical area of the cerebral hemispheres, pons, and midbrain.
Arterial imaging includes sonography (including transcranial Doppler) or CT, MR, or digital subtraction angiography of the relevant arteries.
Cardiac rhythm monitoring using automated rhythm detection strongly encouraged, but cardiac telemetry carried out in an inpatient stroke unit acceptable.
Mechanical prosthetic cardiac valve, atrial myxoma or other cardiac tumors, severe mitral stenosis, or infective endocarditis.
Patients between age 50 and 59 must have one or more additional stroke risk factors (hypertension, tobacco smoking at time of qualifying stroke, ischemic stroke or TIA prior to qualifying stroke, heart failure, or diabetes); patients age ≥18–50 years with additional risk factors were eligible prior to the protocol amendment of late 2015.
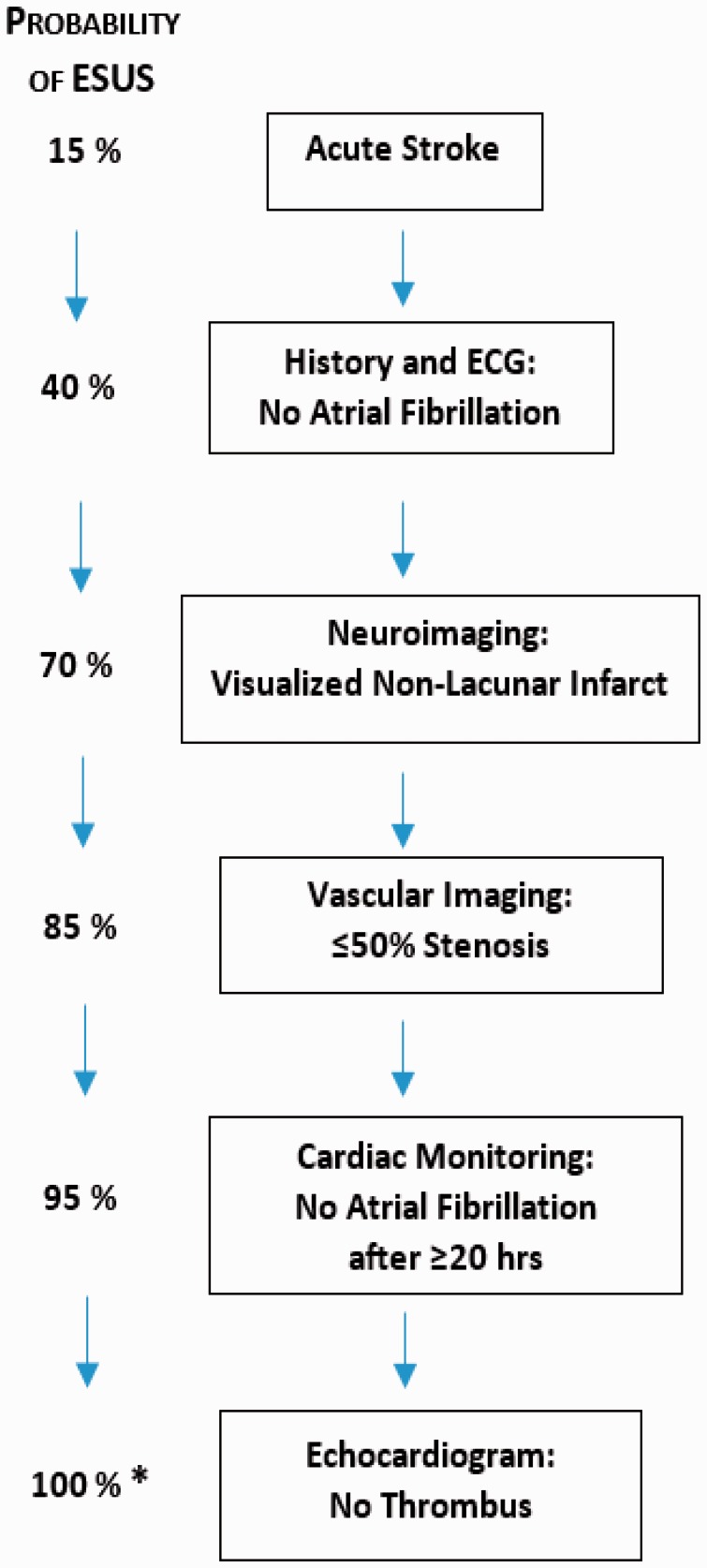
Figure 3.
Conceptual scheme for screening to identify ESUS patients. Prior to diagnostic testing, approximately 15% of strokes would be ESUS. With each additional step in screening, the probability increases toward 100%.5 *After excluding less uncommon stroke etiologies (e.g. dissections).
Exclusion criteria include severely disabling stroke (modified Rankin score ≥4 at screening), the presence or plan to insert an implantable ECG loop recorder, specific indication for chronic anticoagulation or for chronic antiplatelet therapy, ongoing regular use of conventional nonsteroidal anti-inflammatory drugs, previous nontraumatic intracranial hemorrhage (an exception is hemorrhagic transformation of ischemic stroke), and required use of strong inhibitors of both cytochrome P450 isoenzyme 3A4 (CYP3A4) and P-glycoprotein (e.g. protease inhibitors and several azole-antimycotic agents) (Table 2).
Table 2.
Key exclusion criteria.
| 1. | Severely disabling stroke (modified Rankin score ≥4 at screening) |
| 2. | Patent foramen ovale with plans for closure |
| 3. | Known serious infection or inflammatory disease that may be the cause of stroke |
| 4. | Patient has or is intended to receive an implantable ECG loop recorder |
| 5. | Indication for chronic anticoagulation |
| 6. | Indication for chronic antiplatelet therapy |
| 7. | Active bleeding/major bleeding within last six months/ previous nontraumatic intracranial hemorrhage (any type, ever)/high risk for serious bleeding |
| 8. | Hepatic disease associated with coagulopathy |
| 9. | Renal disease with estimated GFR < 30 ml/min/1.73 m2 |
| 10. | Life expectancy less than six months |
| 11. | Use of strong inhibitors of both cytochrome P450 isoenzyme 3A4 (CYP3A4) and P-glycoprotein (e.g. protease inhibitors and several azole-antimycotic agents) |
| 12. | Female of childbearing potential who is not surgically sterile or who is sexually active and not using reliable contraception |
| 13. | Chronic, regular use of a conventional nonsteroidal anti-inflammatory drug |
Patients are randomly allocated by an interactive voice/web response system to either rivaroxaban or aspirin in a 1:1 ratio, with the block size sequestered until the end of recruitment. Randomization is stratified by country and by age <60 and ≥60 years.
Follow-up
Participants return for outpatient office visits at one, six, and 12 months and then every six months until a common end-study date. At three months, participants are contacted by telephone, and a telephone contact is done one month after the end-of-treatment visit. Participants are assessed for the occurrence of safety and efficacy events, adherence, adverse events and vital signs, and quality of life, functional status and cognitive function are recorded. Adherence to assigned therapy is assessed by interview and pill counts at each visit. Low-dose (≤100 mg daily) aspirin in addition to study-assigned therapy is permitted if a new indication develops during follow-up (e.g. stable coronary artery disease), but dual antiplatelet therapy mandates cessation of study drug for the duration of use.
Outcome events
The primary efficacy outcome is time to recurrent stroke (ischemic, hemorrhagic, and undefined stroke, including TIAs with positive neuroimaging) or systemic embolism. This outcome was chosen in part to align with trials demonstrating a substantial reduction in the risk of embolic events by DOACs in atrial fibrillation patients. Secondary efficacy outcomes are outlined in Table 3. The primary safety outcome is major bleeding according to the criteria of the International Society of Thrombosis and Haemostasis.12 We anticipate that the annual rate of intracerebral hemorrhage will be relatively low (<0.4%/yr) due to prohibition of concomitant dual antiplatelet therapy, exclusion of lacunar strokes as qualifying events, and delayed initiation of anticoagulation for a least seven days after the qualifying stroke, and be equal in both treatment arms. Extracranial major hemorrhage is anticipated to be increased among those assigned rivaroxaban versus aspirin (2%/year versus 1%/year, respectively).13,14
Table 3.
Trial outcome events.
| Primary efficacy outcome: |
| • Stroke (ischemic, hemorrhagic, and undefined stroke, TIA with positive neuroimaging) and systemic embolism |
| Secondary efficacy outcomes:a |
| • Cardiovascular death (including death due to all types of hemorrhage), recurrent stroke, systemic embolism, and myocardial infarction |
| • All-cause mortality |
| • Individual components of the primary and secondary efficacy outcomes (stroke, cardiovascular death, and myocardial infarction) as well as |
| • Recurrent ischemic stroke (including TIA with positive neuroimaging) |
| • Disabling/fatal stroke (modified Rankin score ≥4) at 90 days after recurrence |
| Primary safety outcome: |
| • Major bleeding meeting ISTH criteria12 |
| Secondary safety outcomes: |
| • Life-threatening bleeding |
| • Clinically relevant nonmajor bleeding |
| • Intracranial hemorrhage |
Listed in the order of sequential testing.
ISTH: International Society on Thrombosis and Haemostasis; TIA: transient ischemic attack.
Statistical analysis plan
The primary efficacy analyses will be based on the intent-to-treat population. Rivaroxaban-assigned patients will be compared with the aspirin control group using a log–rank test. Kaplan–Meier estimates will be used to plot the cumulative incidence risk over time. Risk reduction will be estimated with the Cox proportional hazards model. Secondary efficacy outcomes will be analyzed using similar methods as for the primary efficacy analysis, with testing performed in hierarchical order to control the global type 1 error level (Table 3). The statistical analysis plan includes several prespecified subgroup analyses based on age, sex, global region, and time from index stroke to randomization of <30 days, 31 days to 3 months, and >3 months.
Study development and committees
The trial coprincipal investigators from the Population Health Research Institute (RGH, SJC) initially proposed the trial to Bayer Pharma AG (SDB). The Steering Committee is responsible for the scientific leadership and consists of the national leaders (n = 32) of each participating country, the coprincipal investigators, sponsor representatives, and additional experts in thrombosis and clinical trials. The Publications Committee approves all manuscripts prior to submission and consists of four national leaders (rotating annually), the coprincipal investigators, and a sponsor representative. Sponsor representatives (who comprise <15% of these two committees) have voting rights. Outcome event verification is overseen by a central Adjudication Committee that will assess whether reported outcome events meet study criteria.
An independent Data Monitoring Committee monitors participant safety on an ongoing basis and may recommend modification of the study protocol in case unexpected safety concerns arise or may terminate the study for safety concerns that are not offset by benefits related to stroke reduction. Two formal interim analyses will occur when approximately 50 and 67% of primary efficacy outcome events have accrued, and the trial will be stopped for overwhelming efficacy by one treatment relative to the other if not mitigated by safety issues.
Two substudies
The primary objective of the MIND MRI Substudy is to determine the effect of rivaroxaban versus aspirin on MRI-defined clinically silent (or covert) brain infarcts and clinical ischemic strokes. Covert infarcts are neither asymptomatic nor benign and result in considerable morbidity including cognitive decline, loss of independence, gait impairment, and falls. While stroke recurrence increases the likelihood of dementia, poststroke cognitive decline often occurs in the absence of clinical recurrence. The mechanism is thought to be either a manifestation of the increasing vascular burden or an interaction between subclinical ischemia and coexisting neurodegenerative pathology, principally Alzheimer’s disease. Therapies aimed at interrupting the accumulating vascular pathology and its consequences may improve the cognitive and functional trajectory of ESUS patients.
The incidence of covert brain infarcts is expected to exceed recurrent clinical ischemic strokes, but owing to the possible heterogeneous pathogenesis of these lesions, covert infarcts may be less sensitive to anticoagulants regarding prevention. About 1000 participants will undergo brain MRI using a standardized acquisition protocol near the time of randomization that will be repeated near study end. MRIs will be interpreted at a core MRI facility by experts unaware of treatment assignment.
The Biomarker, Genetics, Gene Expression Substudy will collect plasma, RNA, and DNA samples from up to 3000 participants for exploratory analyses aimed at establishing whether ESUS is a distinct clinical entity or the result of multiple unrelated causes that have in common a propensity for thrombosis. The substudy will integrate biomarker information, comprehensive genomics characterization, and gene expression (including miRNA) data to identify pathways linked to ESUS and stroke recurrence. Integration of these approaches has the potential to be more revealing than the use of any one alone. Anticipated analyses include the predictive value of D-dimer for recurrent stroke, NT-proBNP levels as predicting atrial fibrillation during follow-up, and several as yet undefined, exploratory biomarkers for predicting clinical events.
Design issues
Choice of aspirin as the control antiplatelet therapy
Aspirin is the best characterized and most widely used antiplatelet therapy for secondary stroke prevention. Most experts believe that there is no compelling evidence that other antiplatelet agents offer important benefits over aspirin, and hence many major guidelines include aspirin as acceptable chronic antiplatelet therapy for secondary stroke prevention, including for patients with cryptogenic ischemic strokes.15–17 However, in some countries, aspirin monotherapy is not the preferred antiplatelet therapy for secondary stroke prevention.18 If investigators were allowed to choose the antiplatelet comparator and dose based on local preference, a double-blind comparison with rivaroxaban would not be feasible, and it was elected to choose a single antiplatelet comparator. For ESUS patients, there are no existing data about relative efficacy of different antiplatelet therapies. Hence, aspirin is the most reasonable choice for the antiplatelet control arm of NAVIGATE ESUS and in-line with current global practice.5,18
Choice of 15 mg once daily dose of rivaroxaban
The selection of a dose of anticoagulant for a new indication often involves a phase 2 dose-ranging study. However, such a study was not considered feasible due to the relatively low rate of recurrent stroke in ESUS patients and the absence of an established surrogate biomarker. Consequently, the dosage was extrapolated from clinical evidence on the use of rivaroxaban in other patient populations. Rivaroxaban 20 mg daily (15 mg daily for those with an estimated creatinine clearance between 30 and 49 ml/min) was efficacious and safe in patients with atrial fibrillation, in whom embolism is the dominant cause of stroke.19 Rivaroxaban 15 mg daily dose appears to be efficacious for stroke prevention in Japanese patients with atrial fibrillation.20 Due to concern about a potentially higher rate of intracerebral hemorrhage in patients with recent ischemic stroke, a dosage of 15 mg once daily in ESUS patients, without dose reduction for moderate renal impairment, was deemed appropriate. Modeling data show an overlap of exposure (i.e. of rivaroxaban plasma concentrations over time) for the 15 and 20 mg doses.21 For this patient population, rivaroxaban 15 mg daily was chosen to optimally balance efficacy with safety.
Screening participants for covert paroxysmal atrial fibrillation
When the trial was designed in 2013–2014, the approach to detection of atrial fibrillation at many stroke centers was an ECG at hospital admission and 24 h of inpatient telemetry or Holter monitoring.5,22 In mid-2014, two influential studies reported that brief episodes (several minutes) of previously unrecognized atrial fibrillation could be detected in 10–20% of patients with cryptogenic ischemic stroke if the duration of cardiac monitoring was prolonged to 30 days.23,24 However, it is unknown whether brief (≤6 min) episodes of atrial fibrillation detected weeks or months after stroke identify patients who benefit from anticoagulation. Further, short episodes of atrial fibrillation are not necessarily temporally associated with stroke risk, raising further doubt about their relevance to the pathogenesis of stroke.25
For NAVIGATE ESUS eligibility, screening for covert paroxysmal atrial fibrillation with ≥20 h of cardiac rhythm monitoring is required, with episodes >6 min duration mandating exclusion based on the ASSERT trial.26 Sites are permitted to monitor for longer periods (e.g. 7–30 days) based on local clinical practice, but monitoring must be completed prior to randomization. Randomization of patients undergoing prolonged (e.g. months to years) cardiac rhythm monitoring using implanted recorders is an exclusion criterion because of the uncertain implications of subgroup analysis in those with implanted monitoring devices and the likelihood of cross-over based on current enthusiasm in some countries for the use of anticoagulants if even brief episodes of atrial fibrillation are detected.
If NAVIGATE ESUS results demonstrate a reduction in recurrent ischemic stroke with anticoagulation, could this overall result be driven by a large treatment effect among a subgroup of subjects with covert paroxysmal atrial fibrillation? While this is unlikely based on predicted frequencies, this possibility will be assessed by routine clinical screening to determine if atrial fibrillation is present at the time of primary efficacy events and comparing the frequency of atrial fibrillation between treatment arms. Further, if rivaroxaban shows superior efficacy versus aspirin and is relatively safe for ESUS patients, it will challenge the need for prolonged cardiac rhythm monitoring for most patients with cryptogenic stroke.
Relative effects of rivaroxaban versus aspirin on different embolic sources
ESUS includes multiple sources (cardiac, arterial, paradoxical). Are different embolic sources likely to respond similarly to rivaroxaban versus aspirin? Clinical trials have not shown a benefit of anticoagulation over antiplatelet therapy for secondary prevention of stroke due to arterial sources (including intracranial stenosis and aortic arch atheroma), but subgroup analyses support the biologic plausibility if a consistent anticoagulant effect can be maintained.27–29 Further, often two or more potential embolic sources are detected in individual ESUS patients, and the specific culprit cannot be identified, so that it is pragmatic to assess the relative effect of antithrombotic therapies among all ESUS patients. A series of exploratory subgroup analyses will examine recurrent stroke rates and response to anticoagulation associated with individual potential embolic sources as well as the presence of prothrombotic disorders (e.g. active cancer).30,31
Should TEE be required for eligibility?
Minor-risk embolic sources are more frequently detected using TEE than with precordial echocardiography,32 and TEE is sometimes recommended routinely for young patients with cryptogenic stroke. However, global surveys indicate that TEE is infrequently done in routine clinical practice,5,22 and with uncertain evidence-based management implications.33 While it would be ideal to more accurately characterize minor-risk embolic sources, mandating TEE (mildly invasive and costly) for trial eligibility would severely restrict the number of participating sites and bias recruitment to patients willing to undergo this procedure.
Discussion
Although an embolic etiology for most cryptogenic ischemic strokes was proposed more than two decades ago,4 there has been little progress in secondary stroke prevention for this large fraction of patients with ischemic stroke. Among such patients meeting criteria for ESUS, we anticipate that rivaroxaban will be associated with a reduced risk of recurrent embolic events, similar rates of intracranial hemorrhage, and only modestly increased (and potentially acceptable) rates of major extracranial hemorrhage relative to aspirin. NAVIGATE ESUS is a multinational, randomized, double-blind, superiority trial comparing antithrombotic therapies for secondary stroke prevention in a well-defined cohort of patients with nonlacunar cryptogenic stroke with embolic features. In order to be widely applicable globally, the trial is designed pragmatically (e.g. intracranial imaging is not required because this would exclude participation of many sites where this is not standard practice, and the duration of cardiac rhythm monitoring over 20 h is flexible). Anticipated subgroup analyses comparing rivaroxaban with aspirin for stroke prevention in participants with PFO, left ventricular systolic dysfunction, active cancer, or nonstenotic atherosclerotic plaques in the aorta, carotid arteries, and intracranial arteries are likely to be underpowered but still of considerable clinical interest. NAVIGATE ESUS is likely to be a landmark clinical trial impacting clinical management of large numbers of patients with cryptogenic ischemic stroke attributed to embolism.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All of the coauthors have received financial remuneration from Bayer Pharma AG for service on the Steering Committee except H. Mundl, S. Berkowitz, C. Pater, G. Peters, and B. Kirsch who are all employees of the sponsors. All coauthors receive stipend and research support for participation in NAVIGATE ESUS from the industry sponsors.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Bayer Pharma AG and Janssen Research and Development LLC; partial funding for the Biomarker, Genetics, Gene Expression Substudy from the Canadian Stroke Prevention Intervention Network.
Informed consent
Not applicable.
Ethical approval
The trial has been approved by local research ethics boards at all clinical sites.
Guarantor
RGH guarantees the accuracy of the information.
Contributorship
All coauthors have contributed to the trial design, have reviewed the manuscript with critical revision, and approved the manuscript.
References
- 1.Hart RG, Diener H-C, Coutts SB, et al. on behalf of the Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014; 13: 429–438. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Yiin GS, Geraghty OC, et al. Oxford vascular study. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol 2015; 14: 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher CM. Concerning strokes. Canadian Med Assoc J 1953; 69: 257–268. [PMC free article] [PubMed] [Google Scholar]
- 4.Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol 1989; 25: 382–390. [DOI] [PubMed] [Google Scholar]
- 5.Perera KS, Vanassche T, Bosch J, et al. Embolic strokes of undetermined source: prevalence and patient features in the ESUS Global Registry. Int J Stroke 2016; 11: 526–533. [DOI] [PubMed] [Google Scholar]
- 6.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146: 857–867. [DOI] [PubMed] [Google Scholar]
- 7.Connolly S, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364: 806–817. [DOI] [PubMed] [Google Scholar]
- 8.Ruff CT, Giugliano RP, Brauwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomized trials. Lancet 2014; 383: 955–962. [DOI] [PubMed] [Google Scholar]
- 9.NAVIGATE ESUS. https//clinicaltrials.gov/ct2/show/nct02313909 (accessed May 2016).
- 10.Diener HC, Easton JD, Granger CB, et al. Design of Randomized, double-blind, Evaluation in secondary Stroke Prevention comparing the EfficaCy and safety of the oral Thrombin inhibitor dabigatran etexilate vs. acetylsalicyclic acid in patient with Embolic Stroke of Undetermined Source (RE-SPEcT ESUS). Int J Stroke 2015; 10: 1309–1312. [DOI] [PubMed] [Google Scholar]
- 11.ATTICUS. https//clinicaltrials.gov/ct2/show/nct02427126 (accessed 6 July 2016).
- 12.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3: 692–694. [DOI] [PubMed] [Google Scholar]
- 13.Haris M, Usman U, Notaro LA, et al. Combination antiplatelet therapy for secondary stroke prevention: enhanced efficacy or double trouble? Am J Cardiol 2009; 103: 1107–1112. [DOI] [PubMed] [Google Scholar]
- 14.Flaker GC, Eikelboom JW, Shestakovska O, et al. Bleeding during treatment with aspirin versus apixaban in patients with atrial fibrillation unsuitable for warfarin: the AVERROES Trial. Stroke 2012; 43: 3291–3296. [DOI] [PubMed] [Google Scholar]
- 15.European Stroke Organization (ESO) Executive Committee and ESO Writing Committee. Guidelines for the management of ischemic stroke and transient ischemic attack 2008. Cerebrovas Dis 2008; 25: 457–505. [DOI] [PubMed] [Google Scholar]
- 16.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: 2160–2236. [DOI] [PubMed] [Google Scholar]
- 17.Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141: e601S–e636S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institute for Health and Care Excellence (U.K.) http://pathways.nice.org.uk/pathways/stroke#path=view%3A/pathways/stroke/acute-stroke.xml&content=view-node%3Anodes-antiplatelets-and-anticoagulant-treatment (accessed 6 July 2016).
- 19.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891. [DOI] [PubMed] [Google Scholar]
- 20.Hori M, Matsumoto M, Tanashashi N, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation. The J-ROCKET AF Study. Circ J 2012; 76: 2104–2111. [DOI] [PubMed] [Google Scholar]
- 21.Stampfuss J, Kubitza D, Becka M, et al. The effect of food on the absorption and pharmacokinetics of rivaroxaban. Int J Clin Pharmacol Ther 2013; 51: 549–561. [DOI] [PubMed] [Google Scholar]
- 22.Giruparajah M, Bosch J, Vanassche T, et al. Global survey of the diagnostic evaluation and management of cryptogenic ischemic stroke. Int J Stroke 2015; 10: 1031–1036. [DOI] [PubMed] [Google Scholar]
- 23.Gladstone DJ, Spring M, Dorian P, et al. for the EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370: 2467–2477. [DOI] [PubMed] [Google Scholar]
- 24.Sanna T, Diener H-C, Passman RS, et al. for the CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014; 370: 2478–2486. [DOI] [PubMed] [Google Scholar]
- 25.Brambatti M, Connolly SJ, Gold MR, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation 2014; 129: 2094–2099. [DOI] [PubMed] [Google Scholar]
- 26.Healy JS, Connolly SJ, Gold MR, et al. for the ASSERT Investigators. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012; 366: 120–129. [DOI] [PubMed] [Google Scholar]
- 27.Amarenco P, Davis S, Jones EF, et al. Aortic Arch Related Cerebral Hazard Trial Investigators. Clopidogrel plus aspirin versus warfarin in patients with stroke and aortic arch plaques. Stroke 2014; 45: 1248–1257. [DOI] [PubMed] [Google Scholar]
- 28.Sacco RL, Prabhakaran S, Thompson JLP, et al. Comparison of warfarin vs. aspirin for the prevention of recurrent stroke or death: subgroup analyses from the Warfarin-Aspirin Recurrent Stroke Study. Cerebrovasc Dis 2006; 22: 4–12. [DOI] [PubMed] [Google Scholar]
- 29.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. for the Warfarin-Aspirin Symptomatic Intracranial Disease Trial Investigator. Comparison of warfarin an aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2005; 352: 1305–1316. [DOI] [PubMed] [Google Scholar]
- 30.Weber R, Goertler M, Bennemann J, et al. German Stroke Study Collaboration. Prognosis after cryptogenic cerebral ischemia in patients with coagulopathies. Cerebrovasc Dis 2009; 28: 611–617. [DOI] [PubMed] [Google Scholar]
- 31.Seok JM, Kim SG, Kim JW, et al. Coagulopathy and embolic signal in cancer patients with ischemic stroke. Ann Neurol 2010; 68: 213–219. [DOI] [PubMed] [Google Scholar]
- 32.Hart RG, Koudstaal PJ, Albers GW. Cardioembolic stroke. In: Bogousslavsky J, Ginsberg M. (eds). Cerebrovascular disease, Cambridge, MA: Blackwell Science, 1998, pp. 1392–1428. [Google Scholar]
- 33.McGrath ER, Paikin JS, Motlagh B, et al. Transeosphageal echocardiography in patients with cryptogenic ischemic stroke: a systematic review. Am Heart J 2014; 168: 706–712. [DOI] [PubMed] [Google Scholar]