Abstract
Introduction
Hypertension is a recognised risk factor for lacunar stroke. However, their association has been evaluated using static blood pressure (BP) assessment in supine or sitting position alone. We hypothesised that impaired dynamic (orthostatic) BP control may associate with lacunar strokes.
Patients and methods
Consecutive subjects with mitral regurgitation (MR) confirmed, lacunar strokes were compared with two control groups. Firstly “normal”, age and sex matched, population controls (1:3 ratio) and then ‘at risk’ controls matched for age, sex, hypertension history and antihypertensive medication (1:2 ratio). Orthostatic BP control was assessed by active stand tests with continuous, phasic, beat-to-beat BP measurement.
Findings
Thirty-six subjects (mean 69.9 years) were compared with 108 controls in group 1 and 72 in group 2. Prevalence of syncope was higher among those with lacunar stroke (cases: 44.4%, group 1: 17.6%, p = 0.003, group 2: 12.5%, p = 0.0004, Fisher’s exact). Mean baseline systolic BP (SBP) was significantly higher in cases (cases: 150 mm Hg, group 1: 140 mm Hg, p = 0.03, group 2: 143 mm Hg, p = 0.1). Ten seconds after standing, SBP dropped significantly less in cases (cases: −14.1 mm Hg, group 1: −31.4 mm Hg, p < 0.0005, group 2: −27.3 mm Hg, p = 0.001, t test). Diastolic BP also fell significantly less in cases. Cases’ SBP and DBP recovered to, then persistently overshot baseline levels.
Discussion and conclusion
Subjects with MR-defined lacunar stroke, of likely small vessel aetiology, exhibit orthostatic hypertension compared with population norms.
Keywords: Active stand test, case–control study, lacunar infarction, orthostatic hypertension
Introduction
Lacunar strokes account for one-quarter of all infarcts.1 C Miller Fisher’s pathological samples of the 1950s confirmed that such infarcts were related to disease of the small perforating arteries and subsequent research has unequivocally linked hypertension with such small vessel disease, and with lacunar stroke.2 However, modifying BP in lacunar stroke patients has had a lower than expected impact on the incidence of lacunar stroke.3
Narrowing the diameter of a diseased artery is associated with an exponential reduction in flow proportional to the forth power of the vessel radius. Autoregulation may ameliorate precipitous decline in flow, but may be inadequate in some cases to compensate for dynamic blood pressure changes like those which occur with orthostasis.
Autoregulation of blood flow to the white matter is impaired in patients with small vessel disease states. The small and medium sized cerebral arteries supplying the white matter normally change diameter rapidly to modulate systemic and cerebral blood flow. This normal physiologic response has been shown to be impaired when small vessel disease is present.4 Normal physiological changes in tone or diameter are usually observed when dynamic BP stressors occur such as during prolonged standing, changes in posture from sitting or lying to standing or following valsalva may either be slowed or reduced in extent thus potentially resulting in abnormalities in flow and tissue perfusion of the white matter. We hypothesise that such sudden changes in blood flow caused by impaired dynamic BP control may also contribute to incident infarction and that these abnormalities in dynamic control of blood pressure may contribute to the association between blood pressure and lacunar stroke.
We also sought to investigate whether lacunar strokes might differ in their dynamic blood pressure profile depending on the location of the infarct within the white matter; specifically whether they are a border-zone lacunar infarct or a non-border-zone lacunar infarct.
Methods
Ethical approval for the study was granted by the Joint Hospitals’ Research Ethics Committee.
Cases
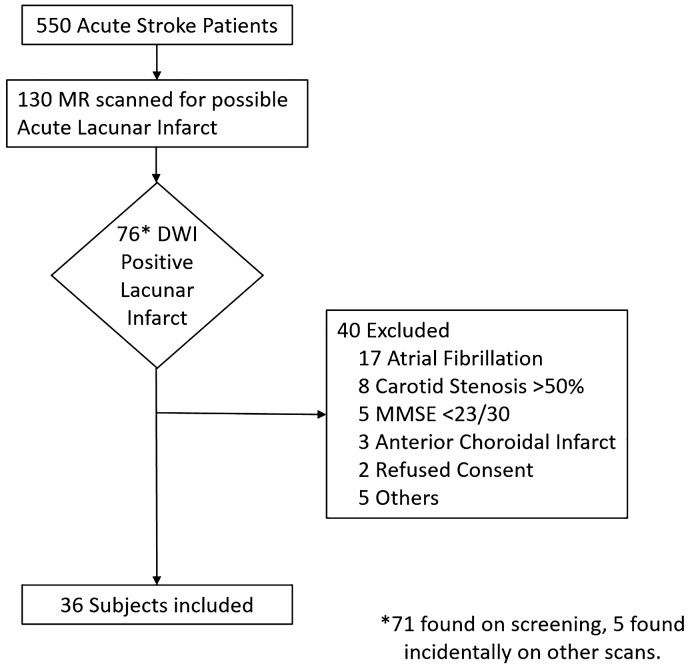
A consecutive sample of acute lacunar stroke patients was prospectively recruited over 18 months from the Acute Stroke Service of a university teaching hospital and for six months from a second associated teaching hospital to increase recruitment. Patients with suspected possible lacunar infarction (based on clinical criteria) underwent magnetic resonance (MR) brain scanning as part of routine assessment. Cognitively intact (MMSE>22) patients who were identified as having confirmed acute lacunar infarction on MR scan were recruited. Written informed consent was obtained. Subjects were excluded if their stroke was likely of thromboembolic aetiology such as those with internal carotid stenosis of>50% on either side (based on Strandness criteria)5 or evidence of atrial fibrillation (Figure 1).
Figure 1.
Screening and recruitment process.
Classification of infarcts
Both centres used a 1.5 T MR scanner. An acute lacunar infarct was defined as a small infarct, 2–20 mm in diameter, located in the basal ganglia, the deep cerebral white matter or the brainstem and perfused by a single small perforating cerebral artery and that exhibited increased signal intensity on diffusion weighted magnetic resonance imaging (MRI) brain.6 Infarcts in the distribution of the anterior choroidal artery were also excluded as their source is likely thromboembolic.6
Assessment of phasic blood pressure response to active stand
Assessment took place at least six weeks post-stroke to minimise the confounding effect of post-stroke dysfunction of autoregulation.7 Continuous, non-invasive beat-to-beat blood pressure measurement was performed with a Finometer device (Finopres Pro; Philips Medical), which utilizes digital photoplethysmography (see supplementary data). The device adhered to the standard of the Association for the Advancement of Medical Instrumentation and was assigned an A/B grading by the British Hypertension Society.8 This compares favourably with gold-standard and intra-arterial recordings9 and has been shown to have comparable results in day-to-day home testing in stroke patients.10 Assessments took place in a tertiary-referral, syncope unit.
Subjects underwent a validated protocol for phasic, beat-to-beat active stand assessment.11 Participants rested in the supine position in a temperate environment for a 10-min period and were then prompted to stand rapidly, with assistance if necessary, and to remain standing unaided for 3 min. During this process continuous blood pressure recordings were taken (see supplementary data).
Demographic data such as co-morbid disease, medication history, smoking status, etc. were obtained. Cases were also asked the The Irish LondituDinal study of Ageing (TILDA)-validated question; whether they had “ever blacked out or fainted” as an indicator of syncope prevalence.12
Controls
Controls were identified from the TILDA database. TILDA is a prospective cohort study of community-dwelling adults aged ≥50 years who reside in the Republic of Ireland.12 A nationally representative, equal probability sample (“epsem”) was selected as described by Whelan13 such that selection bias was minimized and each member of the population of Ireland aged ≥50 years living at a private residential address were equally likely to be invited to participate.12 TILDA participants undertook a computer-assisted personal interview in their own homes and were subsequently invited to a physical health assessment, performed by trained study nurses in one of two dedicated health centres. Controls underwent phasic, beat-to-beat blood pressure assessment using the same protocol and instrument as cases.
Case–control comparison
To compare orthostatic blood pressure responses among lacunar stroke cases and TILDA-derived controls, two separate statistical models were generated. In the first model cases (lacunar stroke patients) were compared with population-based (TILDA-derived), normal, controls matched for age (within 1 year) and gender, in a 3:1 ratio (group 1 or “normals”).
In the second model efforts were made to control for potential confounding factors such as hypertension, etc. In this model cases (lacunar stroke patients) were compared with TILDA-derived controls matched for age (within 1 year), gender, the absence/presence of anti-hypertensive medication and the absence/presence of prior hypertension (group 2 or “at risk”) in a 2:1 ratio and that did not report a prior history of transient ischaemic attack or stroke. The difference in ratio of cases to control was due to the greater challenge in identifying control subjects in group 2 due to the greater number of factors being matched.
Case to case comparison
To compare orthostatic blood pressure behaviour between putative subtypes of lacunar stroke a third model was generated. Cases themselves were subcategorised into border-zone lacunar strokes and non-border-zone lacunar strokes. A border-zone lacunar infarct (BLI) was defined as a lacunar infarct occurring in the deep white matter areas of the corona radiata or centrum semiovale region.6,14–16 Maps of border-zone regions, generated by Damasio and Bogousslavski were used to identify border-zone infarcts, a technique utilised in previous studies.15,16 A non-border-zone lacunar infarct (NBLI) was defined as a lacunar infarct that occurs in the territory of a deep perforating artery with origin from a major cerebral artery such as a lateral striate or pontine perforating vessel (internal capsule, thalamus, caudate, lentiform, pons).
Statistical analysis
The data were analysed in Stata 12. Chi square and Fisher’s exact tests were utilized for case, control comparison of categorical variables according to their background history, medication and history of syncope. Student’s t test was used to compare continuous parametric data and the Wilcoxon rank sum test was used to compare non-parametric data. In the analysis of active stand data, differing baseline BP and HR values were controlled for by measuring the change in BP and change in heart rate in response to active stand rather than absolute values. Baseline BP was defined as mean BP between 30 and 60 s before standing.11 The study had power to detect a difference of 8 mm Hg in mean SBP between cases and control group 1 and a difference of 10 mm Hg between cases and control group 2 (alpha = 0.8, p = 0.05).
Results
Cases
Five hundred and fifty subjects were admitted with stroke during the study period. Of these 130 subjects were clinically diagnosed with a possible lacunar stroke and underwent MRI. Of these 76 were found to have an acute, Diffusion Weighted Imaging (DWI)-positive, lacunar infarct. Forty were excluded, the majority because of the concomitant presence of a potential alternate thromboembolic source, such as significant carotid stenosis or atrial fibrillation. Others were excluded because impaired cognition, declined consent or because the distribution of the infarct was felt to be in the anterior choroidal artery territory rather than a small perforating vessel.
Thirty-six subjects (mean age 69.9 years (SD 11.6)) were thus considered suitable for the study (n = 16 female (43.2%)). Demographic characteristics of cases are listed in Table 1. Median NIHSS was 2.7 (SD 1.5). The locations of the lacunar infarcts were as follows: the internal capsule in 11, the corona radiata in 10, the centrum semiovale in 8, the lentiform nucleus in 4, the thalamus or caudate in 3 and the pons in 1.
Table 1.
The demographics including medication use and past medical history for cases, controls in group 1 (controls matched for age and gender) and controls in group 2 (non-stroke controls matched for age, gender, anti-hypertensive medication, hypertension).
| Controls |
Controls |
p value |
p value |
||
|---|---|---|---|---|---|
| Cases | Group 1 | Group 2 | |||
| Description | (N = 36) | (N = 108) | (N = 72) | Group 1 | Group 2 |
| Age, mean (SD) | 69.9 (11.6) | 69.6 (11.5) | 69.7 (11.1) | 1 | 1 |
| Female gender, n (%) | 15 (41.7) | 46 (42.6) | 30 (41.7) | 1 | 1 |
| Demographics, n (%) | |||||
| History hypertension, n (%) | 21 (58.3) | 31 (27.7) | 42 (58.3) | <0.0005 | 1 |
| Anti-hypertensive med., n (%) | 21 (58.3) | 47 (43.5) | 42 (58.3) | 0.12 | 1 |
| Number anti-hypertens. med., median (IQR) | 1 (2) | 0 (1) | 1 (2) | 0.16 | 0.96 |
| Beta blocker, n (%) | 7 (19.4) | 19 (17.6) | 11 (15.2) | 0.8 | 0.58 |
| Diuretic, n (%) | 5 (13.5) | 11 (10.2) | 11 (15.3) | 0.54 | 0.85 |
| Syncope, n (%) | 16 (44.4) | 19 (17.6) | 9 (12.5) | <0.0005 | <0.0005 |
| Ischaemic heart disease*, n (%) | 7 (19.4) | 9 (8.3) | 4 (5.6) | 0.04 | 0.02 |
| Diabetes, n (%) | 4 (11.1) | 5 (4.6) | 8 (11.1) | 0.12 | 0.89 |
| Current smoker, n (%) | 10 (27) | 5 (4.6) | 5 (6.9) | 0.005 | 0.005 |
*History of myocardial infarction, angina or coronary revascularisation.
Case–control comparison
These thirty-six cases were compared with 108 controls in group 1 (“normals”) and 72 controls in group 2 (“at risk”), see Table 1. The prevalence of syncope was higher among cases than among controls in both groups (cases: n = 16 (44.4%), group 1: n = 19 (17.6%), p = 0.003, group 2: n = 9 (12.5%), p = 0.0004, Fisher’s exact). The prevalence of diabetes was not significantly higher in either stroke group (cases: n = 4 (11.1%), group 1: n = 5 (4.6%), p = 0.23, group 2: n = 5 (6.9), p = 0.48, Fisher’s exact).
Blood pressure and heart rate response to active stand
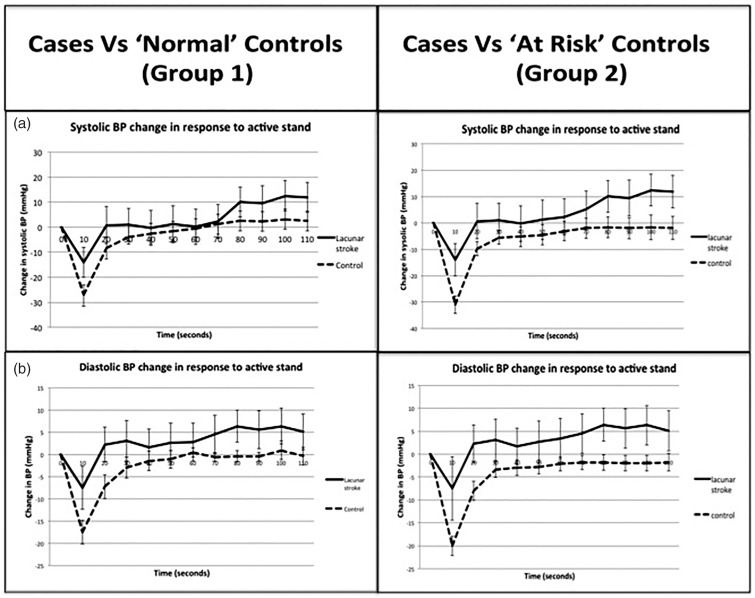
Mean assessment time post-stroke was 10.1 (SD 8) weeks. Mean baseline SBP was significantly higher in cases than in group 1 but not in group 2 (cases mean 10 mm Hg higher than group 1, p = 0.03, and 7 mm Hg higher than group 2, p = 0.1). Ten seconds after standing SBP dropped significantly less in cases than in either group of controls (cases: 17.3 mm Hg less than group 1, p = 0.0001, and 13.2 mm Hg less than group 2, p = 0.001, t test). For the last 30 s of the assessment (80–110 s) cases significantly overshot the baseline BP levels, when compared with both control groups and remained above baseline BP levels for the remainder of assessment (Table 2, Figure 2).
Table 2.
Active stand results for cases, controls in group 1 (‘normal’ controls matched for age and gender) and controls in group 2 (‘at risk’ non-stroke controls matched for age, gender, anti-hypertensive medication, hypertension).a
| Cases | Control |
Control |
p value |
p value |
|
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 1 | Group 2 | ||
| Systolic BP change from baseline, mm Hg: | |||||
| Baseline BP, mean (SD) | 150 (18.3) | 140 (25) | 143 (27.2) | 0.03 | 0.1 |
| 10 s, mean (SD) | −14.1 (18.5) | −31.4 (16.9) | −27.3 (19.6) | 0.0001 | 0.001 |
| 20 s, mean (SD) | 0.7 (22.5) | −9.7 (19.2) | −8.3 (22.5) | 0.008 | 0.03 |
| 30 s, mean (SD) | 1 (19.4) | −5.5 (18.8) | −3.8 (18.3) | 0.07 | 0.2 |
| 40 s, mean (SD) | −0.25 (20) | −5.2 (19.6) | −2.5 (17.5) | 0.2 | 0.55 |
| 50 s, mean (SD) | 1.18 (22.1) | −4.5 (20.3) | −1.67 (16.7) | 0.15 | 0.45 |
| 60 s, mean (SD) | 2.3 (21) | −3 (20) | −1.1 (16.3) | 0.16 | 0.73 |
| 70 s, mean (SD) | 5.3 (20.1) | −2 (20.2) | 1.5 (17.1) | 0.06 | 0.3 |
| 80 s, mean (SD) | 10 (18.4) | 1.6 (21) | 2.5 (17.3) | 0.004 | 0.04 |
| 90 s, mean (SD) | 9.5 (19.5) | −2 (21.7) | 2.3 (16.6) | 0.007 | 0.05 |
| 100 s, mean (SD) | 12.5 (17.8) | −1.7 (21.7) | 3.2 (17.4) | 0.0007 | 0.01 |
| 110 s, mean (SD) | 11.9 (17.9) | −1.8 (22.7) | 2.5 (17.1) | 0.002 | 0.01 |
| Diastolic BP change from baseline, mm Hg | |||||
| Baseline BP. mean (SD) | 75.7 (12.1) | 74 (12.3) | 73.3 (14) | 0.07 | 0.06 |
| 10 s, mean (SD) | −7.5 (14.4) | −19.9 (10.4) | −17.5 (11.7) | <0.0001 | 0.002 |
| 20 s, mean (SD) | 2.25 (12.1) | −8 (11) | −7.22 (10.9) | <0.0001 | 0.0001 |
| 30 s, mean (SD) | 3.1 (13.4) | −3.4 (9) | −2.95 (10.1) | 0.001 | 0.01 |
| 40 s, mean (SD) | 1.7 (11.9) | −2.9 (9.3) | −1.4 (9.2) | 0.02 | 0.13 |
| 50 s, mean (SD) | 2.7 (12.9) | −2.9 (12.9) | −1.08 (8.3) | 0.01 | 0.07 |
| 60 s, mean (SD) | 3.4 (12.7) | −2.1 (8.5) | −0.2 (8.1) | 0.003 | 0.08 |
| 70 s, mean (SD) | 4.5 (12.7) | −1.8 (8.6) | −0.54 (8.5) | 0.001 | 0.02 |
| 80 s, mean (SD) | 6.4 (10.6) | −1.8 (8.9) | −0.36 (8.3) | <0.0001 | 0.0005 |
| 90 s, mean (SD) | 5.6 (12.3) | −2 (9.2) | −0.5 (8.7) | 0.0002 | 0.004 |
| 100 s, mean (SD) | 6.3 (12.4) | −2 (9.4) | −0.2 (9) | 0.0001 | 0.003 |
| 110 s, mean (SD) | 5.1 (12.8) | −1.8 (9.9) | −0.34 (8.4) | 0.001 | 0.01 |
| Heart rate change from baseline, bpm | |||||
| Baseline, mean (SD) | 70 (13.8) | 65 (10.2) | 65 (8.6) | 0.03 | 0.06 |
| 10 s, mean (SD) | 7.1 (12) | 12.6 (6.8) | 12.8 (7.2) | 0.0008 | 0.002 |
| 20 s, mean (SD) | 7.3 (10.5) | 7.8 (7) | 8.8 (5.5) | 0.74 | 0.32 |
| 30 s, mean (SD) | 6.3 (9) | 7 (6.9) | 7.6 (5.5) | 0.65 | 0.36 |
| 40 s, mean (SD) | 6.9 (8.5) | 7.3 (6.8) | 8.7 (6.1) | 0.77 | 0.2 |
| 50 s, mean (SD) | 7.6 (9.9) | 7.9 (6.9) | 9.5 (6.6) | 0.83 | 0.23 |
| 60 s, mean (SD) | 8.7 (7.3) | 8.1 (7.1) | 9.1 (6.3) | 0.65 | 0.78 |
| 70 s, mean (SD) | 8.8 (8.2) | 7 (7) | 8.5 (6.3) | 0.28 | 0.57 |
| 80 s, mean (SD) | 8.8 (8.2) | 7 (7.1) | 7.4 (6.4) | 0.21 | 0.32 |
| 90 s, mean (SD) | 8.3 (8.8) | 6.5 (6.7) | 6.8 (5.8) | 0.21 | 0.29 |
| 100 s, mean (SD) | 9.5 (8) | 6.4 (7) | 6.9 (6) | 0.03 | 0.06 |
| 110 s, mean (SD) | 8.3 (8) | 6.5 (6.5) | 6.7 (5.2) | 0.2 | 0.23 |
Values reflect change in systolic and diastolic BP and heart rate in response to active stand. Positive integers represent values above the baseline.
Figure 2.
Cases compared to age and gender matched, population-derived controls (group 1) and age, gender, hypertension history and anti-hypertensive medication use matched controls (group 2). The graph represents phasic, beat-to-beat recording of systolic BP response to active stand. It demonstrates the change in BP from baseline BP. Baseline BP is defined as average between between 30 and 60 s before standing. A negative value indicates the BP at that time point is below the baseline value. Error bars reflect 95% confidence intervals. (a) Represents systolic BP change and (b) represents diastolic BP change.
Mean baseline diastolic BP (DBP) in cases was not significantly higher than in either control group. Ten seconds after standing DBP also fell significantly less in cases than in either group of controls (cases 12.4 mm Hg less than group 1, p < 0.0001, and 10 mm Hg less than group 2, p = 0.002, t test). For the last 60 s of the assessment (50–110 s) cases again significantly overshot the baseline BP levels, compared with controls and remained above baseline BP levels for the remainder of assessment (Table 2, Figure 2).
Mean resting heart rate in cases was slightly higher in cases than in controls (cases 5 bpm more than both group 1 and 2, p = 0.03 and p = 0.06, respectively). Ten seconds after standing cases exhibited significantly lower increase in heart rate than controls (cases rose by 5.5 bpm less than both group 1, and group 2, p = 0.0008 and p = 0.002, respectively). Thereafter no significant difference in heart rate was observed between the groups (Table 2).
Case–case comparison
Of the 36 cases, 17 (47%) were identified as BLIs and 19 as NBLIs. The BLI groups were significantly older (73.9 years versus 66.5 years, p = 0.05). There was no significant difference in gender, mean blood pressure values, or antihypertensive medication use. The prevalence of ipsilateral carotid disease, hypertension, previous stroke and ischaemic heart disease was similar in both groups.
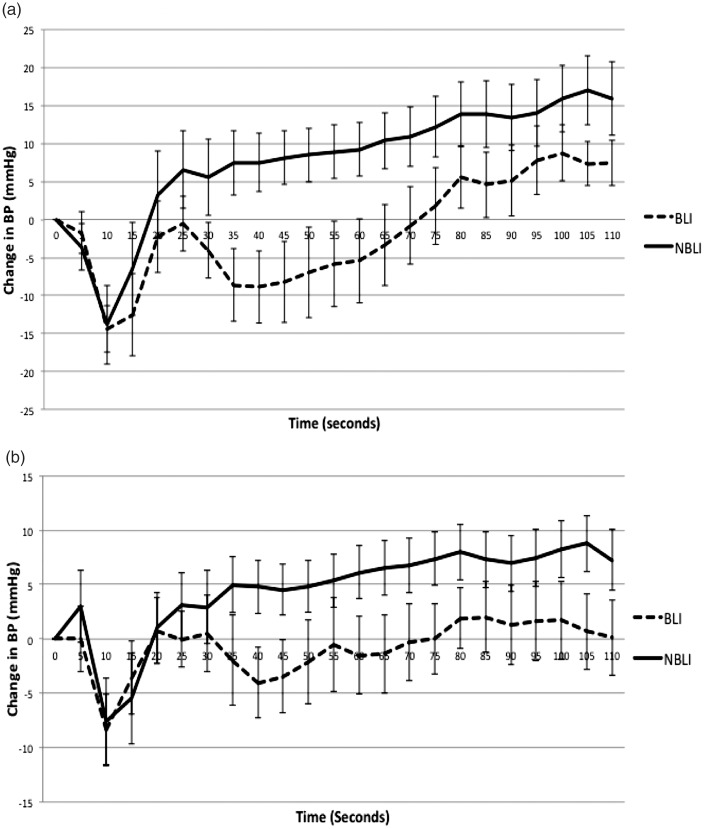
In the active stand assessment the BLI group exhibited significant delay in recovering baseline SBP compared with the NBLI group. This delay first became significant at 35 s, at which point the BLI group BP level was 16.1 mm Hg lower than the NBLI group, p = 0.02. This significant delay persisted for 40 s (p = 0.01), 45 s (p = 0.02), 50 s (p = 0.03), 55 s (p = 0.03), 60 s (p = 0.03) and 65 s (p = 0.04) (Figure 3).
Figure 3.
Phasic, beat-to-beat recording of BP response to active stand for border-zone lacunar infarcts (BLI) and for non-border-zone lacunar infarcts (NBLI). It demonstrates the change in BP from baseline BP. Baseline BP is defined as average between 30 and 60 s before standing. A negative value indicates the BP at that time point is below the baseline value. (a) represents systolic BP change and (b) represents diastolic BP change.
The BLI group also exhibited a significant delay in recovery of DBP at 40 s. The BLI group was 8 mm Hg below the NBLI group, p = 0.04. Otherwise there was no significant difference between the DBP recovery in both groups (Figure 3).
Controlling for the effects of age and the use of anti-hypertensive agents the distal lacunar stroke group still exhibited significant delay in recovery of SBP at 35, 40 and 45 s (p = 0.02, p = 0.04, p = 0.04, respectively).
Discussion
In our study, subjects with carefully identified lacunar infarcts differed in their dynamic blood pressure control compared with a representative sample of the normal population and compared with an “at risk” population, matched for cardiovascular risk. Notably, subjects with lacunar infarcts exhibited a blunted dip in blood pressure immediately post-stand compared with “normals” and with those “at risk”. In the next phase, that of blood pressure recovery, lacunar infarct subjects recovered BP and overshot the baseline blood pressure. This orthostatic hypertension persisted throughout the assessment. Seemingly contradictory is that lacunar stroke subjects exhibited an increased prevalence of syncope compared with both control groups.
The phenomenon of orthostatic hypotension is well described and found in about 17% of the elderly population.17 Orthostatic hypertension is less well characterised yet has been reported in 11% of the Japanese elderly population and 22% of the US veteran population.18,19 Orthostatic hypertension is arbitrarily defined as SBP rise of 20 mm Hg on standing though no clear medical consensus exists20 and is defined in a recent paper a post-stand BP higher than baseline BP.21 It represents a dysautonomia and patients exhibit high levels of noradrenalin and vasopressin during upright posture.18 It commonly co-exists with other dysautonomias such as orthostatic hypotension and postural orthostatic tachycardia syndrome,22 an overlap which might explain our finding that lacunar stroke patients reported more syncope than controls.
A study by Kario et al.23 of subjects ≥60 years diagnosed as hypertensive on ambulatory blood pressure monitoring showed an association between orthostatic hypertension and silent cerebral infarction. The same study found a less significant increase in silent infarction in those with orthostatic hypotension but a higher rate of orthostatic hypertension in a population of ‘extreme dippers’ who drop their blood pressure abnormally at night.24 It is not clear if any of the subjects had suffered a previous clinical stroke.
Orthostatic hypertension correlates with morning BP surge and greater BP variability on 24-h monitoring.18 A recent study, also involving the TILDA population, has reported an association between orthostatic hypertension, on phasic BP assessment, and impaired visual acuity. As the retinal vascular bed is similar to the white matter this may reflect a common disease mechanism.25
In the context of dysautonomia the observed “overshoot” in baseline BP during recovery period may reflect dysfunctional physiological compensatory mechanisms. Given that we controlled for blood pressure therapy, and in particular Beta antagonist and diuretic therapy that particularly associate with abnormal orthostatic BP behaviour,11 we hypothesise that the abnormal BP behaviour may be physiological and intrinsic to the lacunar stroke subjects.
It is also theoretically possible that the acute cerebral infarcts experienced by our subjects may have influenced the impaired orthostatic response through damage to central autonomic control26 although the volume of such infarcts are small. Alternatively it is plausible that our observations merely reflect endothelial dysfunction, which in turn causes both autonomic dysfunction and lacunar infarction and we did not assess endothelial function in this study.
There are some limitations in how our study was conducted. We waited at least six weeks post-stroke for assessment. The purpose of this was to reduce post-stroke dysfunctional cerebral autoregulation, but we acknowledge that it may not have eliminated it. The overall number of cases are small and exclusion rate was high, primarily because of the efforts we took to eliminate confounding factors such as atrial fibrillation and carotid stenosis. We did this to characterise as precisely as possible a group with lacunar strokes arising from non-embolic, small vessel disease. We did not simultaneously assess dynamic cerebral autoregulation using transcranial Doppler ultrasound due to technical challenges in an older population with poor Doppler windows maintain an adequate and consistent Doppler signal, whist they were actively changing posture as in an active stand test. No intracranial imaging was conducted on our control population.
Our control groups were identified from a meticulously characterised population12,13 recruited from a longitudinal study of older people who underwent a comprehensive health screen and dynamic BP as part of their evaluation. The nature of recruitment to this longitudinal study is such that we can state our “normal” control group is highly representative of the Irish population, for that age category. Thus we can be confident that our cases had lacunar stroke of small vessel aetiology, that our matched normal controls were highly representative of the population in general and that our means of assessment met best international standards for assessment of orthostatic blood pressure changes.
In conclusion subjects with lacunar infarction appear to have impairments in dynamic blood pressure control compared with normal control subjects such that they exhibit a blunted initial orthostatic hypotension followed by an orthoshoot in recovery manifesting as relative orthostatic hypertension. They also exhibit an increased tendency for syncope. All may point towards dysautonomia, endothelial dysfunction or both. Further research involving larger sample sizes is needed in this area.
Supplementary Material
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Health Research Board, Ireland. HR/2008/28, Lundbeck Neuroscience Bursary 2010.
Ethical approval
Ethical approval was granted by the Joint AMNCH & St James’s Hospital Ethics Committee. All subjects gave written informed consent to be included in the study.
Informed consent
Written informed consent was obtained from all participants before inclusion in this study.
Guarantor
JH.
Contributorship
JH and RAK conceived the study. DJR conducted the study. JH, SW and DRC identified and recruited subjects. JFM designed the MR protocol and reported the scans. All authors contributed to the design and writing of the paper. JH was primary author.
References
- 1.Chen X, Wen W, Anstey KJ, et al. Prevalence, incidence, and risk factors of lacunar infarcts in a community sample. Neurology 2009; 73: 266–272. [DOI] [PubMed] [Google Scholar]
- 2.Fisher CM, Lacunes CMF. Small deep lacunar infarcts. Neurology 1965; 15: 774–784. [DOI] [PubMed] [Google Scholar]
- 3.Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382: 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park KH, Han SJ, Kim HS, et al. Endothelial function and cardiovascular autonomic activity in neurally mediated syncope. Cardiology 2016; 134: 65–71. [DOI] [PubMed] [Google Scholar]
- 5.Moneta GL, Edwards JM, Papanicolaou G, et al. Screening for asympomatic internal artery stenosis: duplex criteria for discriminationg 60% to 99% stenosis. J Vasc Surg 1995; 21: 989–994. [DOI] [PubMed] [Google Scholar]
- 6.Donnan GA, Norrving B, Bamford J, et al. Classification of subcortical infarcts. In: Donnan GA, Norrving B, Bamford J, et al. (eds) Subcortical stroke. 2nd ed. Oxford: Oxford Medical Publications, 2002, pp.27–36.
- 7.Dawson SL, Panerai RB, Potter JF. Serial changes in static and dynamic cerebral autoregulation after acute ischaemic stroke. Cerebrovasc Dis 2003; 16: 69–75. [DOI] [PubMed] [Google Scholar]
- 8.Schutte AEHH, van Rooyen JM, Malan NT, et al. Validation of the finometer device for measurement of blood pressure in black women. J Hum Hypertens 2004; 18: 79–84. [DOI] [PubMed] [Google Scholar]
- 9.Kaltoft N, Hobolth L, Moller S. Non-invasive measurement of cardiac output by finometer in patients with cirrhosis. Clin Physiol Funct Imaging 2010; 30: 230–233. [DOI] [PubMed] [Google Scholar]
- 10.Webb AJ, Rothwell PM. Physiological correlates of beat-to-beat, ambulatory, and day-to-day home blood pressure variability after transient ischemic attack or minor stroke. Stroke 2014; 45: 533–538. [DOI] [PubMed] [Google Scholar]
- 11.Finucane C, O'Connell MD, Fan CW, et al. Age-related normative changes in phasic orthostatic blood pressure in a large population study: findings from The Irish Longitudinal Study on Ageing (TILDA). Circulation 2014; 130: 1780–1789. [DOI] [PubMed] [Google Scholar]
- 12.Kearney PM, Cronin H, O'Regan C, et al. Cohort profile: the Irish longitudinal study on ageing. Int J Epidemiol 2011; 40: 877–884. [DOI] [PubMed] [Google Scholar]
- 13.Whelan BJ. RANSAM: a national random sampling design for Ireland Economic and Social Research Institute. Econ Soc Rev 1979; 10: 169–174. [Google Scholar]
- 14.Bladin CF, Chambers BR. Clinical features, pathogenesis, and computed tomographic characteristics of internal watershed infarction. Stroke 1993; 24: 1925–1932. [DOI] [PubMed] [Google Scholar]
- 15.Damasio H. A computed tomographic guide to the identification of cerebral vascular territories. Arch Neurol 1983; 40: 138–142. [DOI] [PubMed] [Google Scholar]
- 16.Bogousslavsky J, Regli F. Centrum ovale infarcts: subcortical infarction in the superficial territory of the middle cerebral artery. Neurology 1992; 42: 1992–1998. [DOI] [PubMed] [Google Scholar]
- 17.Applegate WB, Davis BR, Black HR, et al. Prevalence of postural hypotension at baseline in the Systolic Hypertension in the Elderly Program (SHEP) cohort. J Am Geriatr Soc 1991; 39: 1057–1064. [DOI] [PubMed] [Google Scholar]
- 18.Kario K, Eguchi K, Hoshide S, et al. U-curve relationship between orthostatic blood pressure change and silent cerebrovascular disease in elderly hypertensives: orthostatic hypertension as a new cardiovascular risk factor. J Am Coll Cardiol 2002; 40: 133–141. [DOI] [PubMed] [Google Scholar]
- 19.Wecht JM, Weir JP, Martinez S, et al. Orthostatic hypotension and orthostatic hypertension in American veterans. Clin Auton Res 2016; 26: 49–58. [DOI] [PubMed] [Google Scholar]
- 20.Fessel J, Robertson D. Orthostatic hypertension: when pressor reflexes overcompensate. Nat Clin Pract Nephrol 2006; 2: 424–431. [DOI] [PubMed] [Google Scholar]
- 21.Weiss A, Beloosesky Y, Grossman A, et al. The association between orthostatic hypertension and all-cause mortality in hospitalized elderly persons. J Geriatr Cardiol 2016; 13: 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu JS, Yang YC, Lu FH, et al. Population-based study on the prevalence and correlates of orthostatic hypotension/hypertension and orthostatic dizziness. Hypertens Res 2008; 31: 897–904. [DOI] [PubMed] [Google Scholar]
- 23.Kario K, Eguchi K, Hoshide S, et al. U-curve relationship between orthostatic blood pressure change and silent cerebrovascular disease in elderly hypertensives: orthostatic hypertension as a new cardiovascular risk factor. J Am Coll Cardiol 2002; 40: 133–141. [DOI] [PubMed] [Google Scholar]
- 24.Kario K, Hoshide S, Shimada K. Extreme-dipper, abnormal diurnal blood pressure variation in the elderly hypertensive, and orthostatic hypertension. J Hum Hypertens 1998; 12: 141–142. [DOI] [PubMed] [Google Scholar]
- 25.Ní Bhuachalla B, McGarrigle CA, Akuffo KO, et al. Phenotypes of orthostatic blood pressure behaviour and association with visual acuity. Clin Auton Res 2015; 25: 373–381. [DOI] [PubMed] [Google Scholar]
- 26.Panayiotou B, Reid J, Fotherby M, et al. Orthostatic haemodynamic responses in acute stroke. Postgrad Med J 1999; 75: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.