Abstract
Objective
We analysed the Qatar stroke registry for ethnic variations in patients admitted with cerebrovascular disease at Hamad General Hospital, Qatar.
Methods
Patients admitted with acute stroke from January 2014 to December 2015, enrolled in the registry were included in the study. We evaluated the clinical presentation, risk factors, and outcome at discharge and 90 days post-discharge in relation to the patient’s ethnic background.
Results
A total of 1727 patients were enrolled in the Hamad General Hospital stroke registry (Middle Eastern 594 (34.4%), South East Asian 924 (53.5%) and Far Eastern 209 (12.1%)). There were significant differences in risk factors, clinical presentation and prognosis. Compared to Middle Eastern patients, Far Eastern patients were younger (62.8 ± 13.7 vs. 48.9 ± 9.1 years; p < 0.001). Diabetes and hypertension were significantly more common in Middle Eastern patients (358 (60.3%), 458 (77.1%)) compared to South East Asian patients (420 (45.5%), 596 (64.5%)) and Far Eastern patients (57 (27.3%), 154 (73.7%)), respectively (p < 0.001). Stroke was more severe in the Far Eastern group (median (interquartile range) – 5.0 (2–11.5)) compared to the Middle Eastern group (median (interquartile range) – 4.0 (1–8)) and South East Asian (median (interquartile range) – 4.0 (2–9)), p = 0.011. Mortality at 90 days was highest in patients from the Far East (15/209 (8.2%)) compared to the Middle East (35/594 (6.5%)) and South East Asia (33/924 (4.0)), p = 0.028. Patients from the Far East had significantly higher rates of intracranial hemorrhage compared to the Middle East and South East Asia (70/209 (33.5%), 77/594 (13.0%), and 169/924 (18.3%)), respectively (p < 0.001).
Conclusion
The early age at presentation and the poor control of risk factors, especially in patients from South East Asia and the Far East requires attention.
Keywords: Acute stroke, epidemiology, ethnicity, prognosis, risk factors, stroke registry
Introduction
Cerebrovascular disease is the second most common cause of death worldwide1 and the leading cause of acquired disability in adults.2–4 Compared to the developed countries, the risk is particularly high in developing countries where: (i) stroke presents at a younger age,5 (ii) there is a lack of awareness and treatment of risk factors and (iii) poor management of the acute event causes higher mortality.5–7
There has been a shift in populations across the world, leading to a major change in the demographic profile of several countries.8 In many regions of the world, ethnically diverse populations are living together and in some countries, especially in the Middle East where, the expatriates outnumber the local population. Qatar has 2.4 million inhabitants (December 2014) comprised 88% by expatriates;9 and 272,000 native Qataris. Major expatriate populations include 945,000 Southeast Asians, 400,000 non-Qatari Arabs and 200,000 people from the Far East.9 In Qatar acute medical services, including hospital admissions are free of charge for all residents. However, comprehensive primary healthcare is lacking for most expatriates and many Qatari nationals. Higher education is lacking, especially in the South East Asian labour community, and vascular risk factors are poorly controlled. Studying vascular disease in such genetically distinct populations can provide pertinent information – about risk profiles, pathogenesis and understanding the severity of the disease.
We report our experience with acute stroke in relationship to the ethnicity, risk factors, type and severity of stroke, risk of complications, 90-day prognosis and early recurrence in the state of Qatar.
Methods
Data from patients admitted with a stroke or transient ischemic attack (TIA) to Hamad General Hospital (HGH), Doha, Qatar from 1 January 2014 through 30 December 2015 were analysed from a hospital-based prospective stroke registry. HGH is a Joint Commission International (JCI) accredited 600-bed hospital, with 200 beds reserved for medical patients. It is the only tertiary care medical facility in Qatar where the stroke service is located, 95% of all strokes in Qatar requiring admission to hospital are admitted to HGH. The stroke program, also certified by JCI, is a consultation service responsible for evaluation of all stroke patients admitted to the hospital. HGH is equipped with all the necessary laboratory, neuro-radiological and neurosurgical facilities and infrastructure required to manage acute stroke patients; HGH has a 24-hr thrombolysis and endovascular service.10,11
Patient characteristics
Patient characteristics including age, sex, nationality, medical comorbidities and prior medication were collected into the Stroke Registry. Ethnicity was defined based on social groups sharing cultural and language traits within the broad categories of Arabs (Qatari and not-Qatari), South East Asians (Indian, Pakistani, Bangladeshi, Sri Lankans Nepalese and Myanmar) and the Far East (predominantly from Philippines). Data from the ambulance service, emergency department (ED), door-to-needle time (for thrombolysis patients), NIH Stroke Scale (NIHSS) score, length of stay (LoS) in ED, LoS in the stroke ward (SW) and general medical ward (GMW), completed investigations, timing of investigations, neuroimaging data, post-stroke complications, in-hospital mortality, and post-discharge disposition were entered into the registry. Ischemic stroke, TIAs and intracranial haemorrhage (ICH) were diagnosed according to the WHO criteria12 and stroke subtypes by the TOAST criteria.13 Thrombolysis-related hemorrhagic complications were defined according to the SITS-MOST criteria.14 Symptomatic haemorrhage was defined by the SITS-MOST criteria as local or remote parenchymal hemorrhage on the 22–36 h post-treatment imaging scan, combined with a neurological deterioration of 4 points or more on the NIHSS from baseline, or from the lowest NIHSS value between baseline and 24 h, or leading to death. The modified Rankin scale (mRS) measurements were done at discharge and at 90 days following onset of symptoms. The patients were classified as good (mRS of ≤ 0–2) or poor (mRS 3–6) outcome. We used the dichotomised mRS scale as it the most commonly method in use to evaluate recovery at 90 days.15
Diabetes was diagnosed according to the American Diabetes Association (ADA) and WHO recommendation16 and included patients with a previous diagnosis of diabetes, on medication for diabetes or an HbA1c of more than 6.5%, and the diagnosis of pre-diabetes was based on an HbA1c of 5.7–6.4% as per the 2015 ADA clinical practice recommendations. Hypertension was defined as systolic blood pressure (BP) ≥ 140 mm Hg or a diastolic pressure ≥ 90 mm Hg, or on current treatment with antihypertensive drugs. We kept track of the number of patients with hypertension or diabetes who were unaware of these conditions at the time of presentation to the hospital. Dyslipidemia was defined as low-density lipoprotein-cholesterol level ≥ 3.62 mmol/L, high-density lipoprotein-cholesterol level ≤ 1.03 mmol/L, triglycerides ≥ 1.69 mmol/L, or current treatment with a cholesterol-lowering drug. Atrial fibrillation was diagnosed based on electrocardiographic findings on admission or on Holter monitoring during hospitalisation. Smoking was defined as current cigarette smoking. BP and body mass index were measured on admission. Thrombolytic therapy was defined as intravenous administration of thrombolytic agents such as recombinant tissue type plasminogen activator.
Patients were admitted to the SW or the GMW and received acute care by a multidisciplinary team comprised stroke neurologists, clinical nurse specialists, coordinators, a nursing team trained in the care of stroke patients, stroke-trained physical, occupational, speech and language therapists and acute rehabilitation physicians. Specific attention was paid to preventative measures to minimise the risk of aspiration pneumonia, bladder infection, venous thrombosis and pressure ulcers. Approximately 75% of stroke patients were admitted to the geographically defined SW. The multidisciplinary team was also involved in the care of patients who were admitted to the GMW and the same stroke-care protocols as the SW were implemented in these patients.
Data collection
Upon identification and confirmation of diagnosis using the International Classification of Disease, 10th Edition, definitions (H34.1, I63.x, I64.x, I61.x, I60.x, G45.x), patients’ data were collected by trained stroke coordinators. The ethnicities of the patients were recorded at admission.
Data analysis and statistics
Descriptive results for all continuous variables were reported as mean ± standard deviation (SD) (for normally distributed data) or median with range (for data not normally distributed). Numbers and percentages were reported for all categorical variables. The distribution of continuous variables was assessed before using statistical tools. Mean level comparisons between patients from different ethnic backgrounds were assessed using ANOVA test and multiple comparison analysis was performed using Scheffe test. If assumption of an ANOVA test was violated, then an alternative non-parametric Kruskal Wallis test was performed. Pearson Chi-square test or Fisher exact test whenever appropriate was used to compare the proportion of all categorical variables between the groups. Multiple logistic regression analysis was performed to assess for risk factors associated with ICH compared to ischemic stroke after selecting important and significant variables at bivariate analysis. Odds ratio (OR) and the 95% confidence interval for the OR were reported. A ‘p’ value < 0.05 (two-tailed) was considered significant. SPSS 21.0 statistical package was used for the analysis.
Results
Patient characteristics
A total of 1727 patients (Middle Eastern 594 (34.4%), South East Asian 924 (53.5%) and Far Eastern 209 (12.1%)) were admitted to HGH with a diagnosis of acute stroke or TIA between January 2014 and December 2015. The age, sex, and ethnicity of the patients are shown in Table 1. The majority of patients were men 1395 (80.8%). This is reflective of the demographics of the country with a large male expatriate community.9 Qataris constituted 333/594 (56.1%) of the total Middle Eastern patients. The mean age of patients presenting with stroke was 54.5 ± 13.7 years. Patients from the Far East had the youngest age at presentation compared to the Middle Eastern group (48.9 ± 9.1 vs. 62.8 ± 13.7 years; p < 0.001). Amongst the patients from the Middle East, the Qatari patients were significantly older than the non-Qataris (64.9 ± 14.5 vs.60.1 ± 14.3 years; p < 0.001). The body mass index (BMI) was significantly higher in the Middle East group (29.7 ± 5.3) compared to South East Asians (26.4 ± 4.2) or those from the Far East (26.8 ± 3.9); p < 0.001 (Table 1). With regard to the BMI, there were no differences between the Qatari and non-Qatari Arabs ((29.7 ± 6.7), (29.6 ± 6.1); p: 0.879), respectively.
Table 1.
Demographics and clinical characteristics among different ethnicities.
| Overall n = 1727 | Middle Eastern n = 594 | South East Asian n = 924 | Far Eastern n = 209 | Overall significance | Middle Eastern vs. South East Asian | Middle Eastern vs. Far Eastern | South East Asian vs. Far Eastern | |
|---|---|---|---|---|---|---|---|---|
| Total patients | 1727 | 594 (34.4) | 924 (53.5) | 209 (12.1) | – | |||
| Age | 54.5 ± 13.7 | 62.8 ± 14.6 | 50.4 ± 11.2 | 48.9 ± 9.1 | <0.001 | <0.001 | <0.001 | 0.276 |
| Gender | <0.001 | <0.001 | 0.004 | <0.001 | ||||
| Male | 1395 (80.8) | 384 (64.6) | 853 (92.3) | 158 (75.6) | ||||
| Female | 332 (19.2) | 210 (35.4) | 71 (7.7) | 51 (24.4) | ||||
| Initial diagnosis | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Intracerebral bleed | 316 (18.3) | 77 (13.0) | 169 (18.3) | 70 (33.5) | ||||
| Ischemic strokes | 1192 (69.0) | 408 (68.7) | 671 (72.6) | 113 (54.1) | ||||
| TIA | 203 (11.8) | 103 (17.3) | 75 (8.1) | 25 (12.0) | ||||
| CVST | 16 (0.9) | 6 (1.0) | 9 (1.0) | 1 (0.5) | ||||
| Risk factors | ||||||||
| Diabetes | 835 (48.3) | 358 (60.3) | 420 (45.5) | 57 (27.3) | <0.001 | <0.001 | <0.001 | <0.001 |
| Hypertension | 1208 (69.9) | 458 (77.1) | 596 (64.5) | 154 (73.7) | <0.001 | <0.001 | 0.318 | 0.012 |
| Dyslipidaemia (n = 1558) | 891 (57.2) | 310 (58.4) | 474 (56.4) | 107 (57.2) | 0.776 | – | – | – |
| Smoking (n = 1642) | 347 (21.1) | 96 (17.3) | 211 (23.8) | 40 (20.0) | 0.011 | 0.003 | 0.388 | 0.248 |
| Stroke (n = 1650) | 179 (10.8) | 116 (20.5) | 55 (6.2) | 8 (4.0) | <0.001 | <0.001 | <0.001 | 0.232 |
| CAD | 172 (10.0) | 95 (16.0) | 71 (7.7) | 6 (2.9) | <0.001 | <0.001 | <0.001 | 0.013 |
| Atrial fibrillation | 67 (4.0) | 48 (8.1) | 15 (1.6) | 4 (1.9) | <0.001 | <0.001 | 0.002 | 0.768 |
| BMI (n = 1379) | 27.6 ± 5.3 | 29.7 ± 6.4 | 26.4 ± 4.2 | 26.8 ± 3.9 | <0.001 | <0.001 | <0.001 | 0.578 |
| DM not on treatment (n = 835) | 495 (59.3) | 141 (39.4) | 313 (74.5) | 41 (71.9) | 0.001 | <0.001 | <0.001 | 0.635 |
| HTN not on treatment (n = 1208) | 944 (78.1) | 334 (72.9) | 491 (82.4) | 119 (77.3) | 0.001 | <0.001 | 0.287 | 0.147 |
| TOAST (n = 1237) | 0.001a | <0.001 | 0.296 | 0.890 | ||||
| Small vessel disease | 816 (66.0) | 272 (63.8) | 464 (67.3) | 80 (65.6) | ||||
| Large vessel disease | 239 (19.3) | 71 (16.7) | 142 (20.6) | 26 (21.3) | ||||
| Cardioembolic | 104 (8.4) | 56 (13.1) | 40 (5.8) | 8 (6.6) | ||||
| Determined aetiology | 35 (2.8) | 8 (1.9) | 24 (3.5) | 3 (2.5) | ||||
| Undetermined aetiology | 43 (3.5) | 19 (4.5) | 19 (2.8) | 5 (4.1) | ||||
| TPA given | 131/1192 (11.0) | 28/408 (6.9) | 87/671 (13.0) | 16/113 (14.2) | 0.004 | 0.002 | 0.014 | 0.728 |
| DNT (in min) | 46.6 ± 26.1 | 55.7 ± 29.4 | 44.3 ± 23.8 | 43.0 ± 29.9 | 0.113 | _ | _ | _ |
| Post tPA bleed (n = 131) | 7/131 (5.3) | 4/28 (14.3) | 2/87 (2.3) | 1/16 (6.3) | 0.031 | 0.030 | 0.638 | 0.401 |
| HbA1c levels (n = 1707) | 7.2 ± 2.3 | 7.5 ± 2.2 | 7.4 ± 2.3 | 6.4 ± 1.9 | <0.001 | 0.704 | <0.001 | <0.001 |
| RBS | 9.6 ± 4.9 | 10.2 ± 5.0 | 9.6 ± 5.1 | 7.9 ± 3.1 | <0.001 | 0.055 | <0.001 | <0.001 |
| Cholesterol levels (n = 1453) | 5.0 ± 1.4 | 4.9 ± 1.4 | 5.0 ± 1.4 | 5.2 ± 1.3 | 0.005 | 0.077 | 0.009 | 0.246 |
| Triglyceride levels (n = 1404) | 1.7 ± 1.1 | 1.71 ± 1.1 | 1.7 ± 1.1 | 1.52 ± 0.8 | 0.114 | _ | _ | _ |
| HDL levels (n = 1406) | 1.04 ± 0.5 | 1.1 ± 0.6 | 0.97 ± 0.36 | 1.2 ± 0.37 | <0.001 | <0.001 | 0.462 | <0.001 |
| LDL levels (n = 1440) | 3.2 ± 1.2 | 3.1 ± 1.1 | 3.3 ± 1.2 | 3.4 ± 1.2 | <0.001 | 0.002 | 0.001 | 0.304 |
Note: Results are expressed as mean ± standard deviation, and number (percentage); based on history of DM, treatment. For DM and HbA1c criteria.
TIA: transient ischemic attack; LDL: low-density lipoprotein; HDL: high-density lipoprotein; HbA1c: haemoglobin A1c; tPA: tissue plasminogen activator; BMI: body mass index; CAD: coronary artery disease; HTN: hypertension.
P-value has been measured using Fisher Exact test.
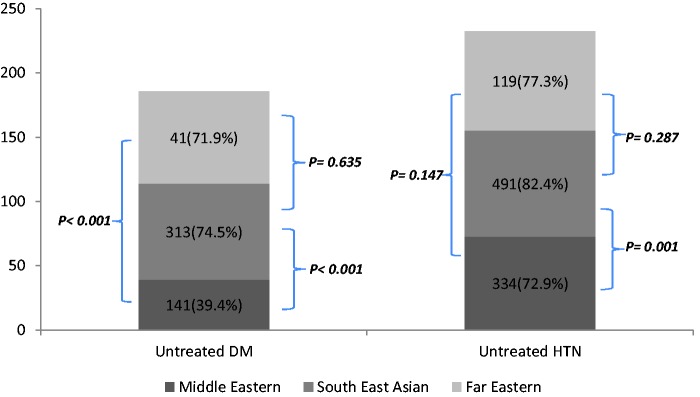
There were several differences in the risk factor profiles of the three ethnic groups (Table 1). The number of untreated diabetics was found to be more in South East Asians 313/420 (74.5%) and patients from the Far East 41/57 (71.9%) compared to the Middle Eastern group 141/358 (39.4%), p < 0.001. Untreated hypertension was seen more frequently in patients from South East Asia 491/596 (82.4%) and the Far East 119/154 (77.3%) compared to the Middle East 334/458 (72.9%), p < 0.001 (Figure 1).
Figure 1.
Percentage of patients with untreated Diabetes (DM) and hypertension (HTN) at the index event.
Within the Middle Eastern group, diabetes and hypertension was highest in the Qatari patients. Diabetes was present in 225/333 (67.6%) of Qatari and 133/261 (51%) of non-Qataris (p < 0.001); 84/225 (37.3%) of Qatari and 57/133 (42.9%) of non-Qataris were not on any treatment for diabetes (p = 0.301). Hypertension was present in 272/333 (81.7%) of Qataris and 186/261 (71.3%) of non-Qataris (p =0.003). 195/272 (71.7%) of Qatari and 139/186 (74.7%) of non-Qataris were not on treatment for hypertension, p = 0.472.
Diagnosis
Stroke diagnosis in the various ethnic groups is shown in Table 1. Small vessel ischemic stroke was found in similar frequency in Middle Eastern patients 272/426 (63.8%) and in the Far Eastern group 80/122 (65.6%), but was significantly higher in South East Asian patients 464/689 (67.3%); p: 0.001. Cortical strokes were also significantly more common in Middle Eastern patients 110/594 (18.5%) and South East Asian patients 183/924 (19.8%). In patients from the Far East, cortical stroke was significantly lower 25/209 (12.0%); p < 0.001. A history of stroke, TIAs and coronary artery disease (CAD) was most commonly seen in the Middle Eastern patients (Table 1). Previous stroke or CAD was rare in Far Eastern patients. ICH was significantly higher in patients from the Far East (33.5%) compared to the Middle East (13.0%) and South East Asia (18.3%), p ≤ 0.001 (Table 1). Stroke severity was found to be greater in the Far Eastern group (median (interquartile range (IQR)) – 5.0 (2–11.5)) as compared to Middle Eastern group (median (IQR)– 4.0 (1–8)) and South East Asian patients (median (IQR)– 4.0 (2–9)), p = 0.011 (Table 2).
Table 2.
Comparison of complication and outcomes data among the different ethnicities.
| Overall n = 1727 | Middle Eastern n = 594 | South East Asian n = 924 | Far Eastern n = 209 | Overall significance | Middle Eastern vs. South East Asian | Middle Eastern vs. Far Eastern | South East Asian vs. Far Eastern | |
|---|---|---|---|---|---|---|---|---|
| NIHSS severity | 4.0 (2–9) | 4.0 (1–8) | 4.0 (2–9) | 5.0 (2–11.5) | 0.011 | 0.095 | 0.014 | 0.280 |
| Length of stay | 4 (2–7) | 4 (2–8) | 4 (2–6) | 4 (2–9) | 0.799 | _ | _ | _ |
| Mortality at discharge | 64 (3.7) | 19 (3.2) | 30 (3.2) | 15 (7.2) | 0.018 | 0.959 | 0.014 | 0.009 |
| Mortality at 90 days (n = 1543) | 83 (5.4) | 35 (6.5) | 33 (4.0) | 15 (8.2) | 0.028 | 0.043 | 0.415 | 0.016 |
| Prognosis at DC (mRS 3–6) | 642 (37.2) | 223 (37.5) | 334 (36.1) | 85 (40.7) | 0.462 | _ | _ | _ |
| Prognosis at 90 days (mRS 3–6) (n = 1604) | 382 (23.8) | 156 (27.9) | 176 (20.5) | 50 (26.7) | 0.004 | 0.001 | 0.767 | 0.062 |
Note: Results are expressed as mean ± standard deviation, median (interquartile range), and number (percentage).
NIHSS: NIH Stroke Scale; mRS: modified Rankin scale.
Reperfusion therapy
A total of 131 (11.0%) of ischemic stroke patients received reperfusion therapy with tissue plasminogen activator. It was highest for patients from South East Asia 87 (13.0%) and the Far East 16 (14.2%) and least frequent for Middle Eastern patients 28 (6.9%); p ≤ 0.004 (Table 1). There were no differences in means of transportation to the hospital (55% by ambulance), time from onset to arrival in hospital and ‘door-to-needle’ times within the ethnic groups. Reluctance to consent for thrombolysis from patient or family was seen in Middle Eastern patients. South East Asian patients were most frequently single men and ‘two physicians consent’ facilitated treatment in eligible patients. Overall, the risk of post tissue plasminogen activator (tPA) symptomatic ICH was 5.3% (7/131) (Middle Eastern 4 (14.3%), Far Eastern 1 (6.3%), and 2 (2.3%), p = 0.031). Outcome in thrombolysed patients at discharge (mRS ≤ 2 – (Middle East 14/28 (50.0%), South East Asian 42/87 (48.3%), Far East8/16 (50.0%)), p = 0.983) and at 90 days (mRS ≤ 2 – (Middle East 15/24 (62.5%), South East Asian 49/78 (62.8%), Far East 10/15 (66.7%)), p = 0.957) was also similar in the three ethnic groups.
Prognosis at discharge and 90-day outcome
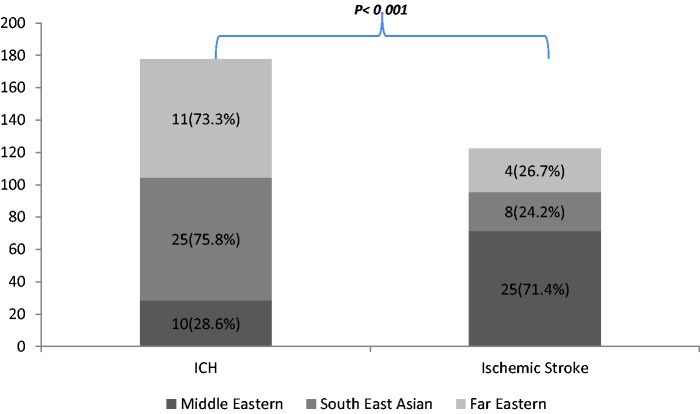
Only 64/1727 (3.7%) patients died during hospitalisation and another 19 patients died during the following 90 days. Mortality at 90 days was highest in the patients from the Far East (15 (8.2%)) versus the Middle East (35 (6.5%)) and South East Asia (33 (4.0)), p = 0.028 (Table 2). At discharge, 13.2% (40/303) of patients with ICH and 1.7% (24/1407) of ischemic stroke died during hospitalisation (p < 0.0001), while at 90 days, the overall mortality was found to be 16.8% (44/262) for ICH and 3.1% (39/1266) (p < 0.0001) for ischemic stroke patients. This was secondary to the higher rates of ICH among patients from the Far East compared to the Middle East and South East Asia (Figure 2). The mRS at discharge was not significantly different between the three ethnic groups. Evaluation at 90 days revealed that patients from South East Asian had significantly better outcome (p = 0.004) (Table 2), (also see supplemental Table 1). Compared to other ethnic groups, recurrent stroke was found to be more frequent in patients from the Middle East 22 (3.7%) compared to 14 (1.5%) in South East Asian patients and 3 (1.4%) in the Far Eastern group, p = 0.019).
Figure 2.
Percentage of patients with ICH and ischemic stroke between Middle Eastern, South East Asian, and Far Eastern among those who died at 90 days.
Risk factors associated with ischemic stroke versus intra-cerebral haemorrhage
Table 3 details a multiple binary logistic regression model to identify significant independent factors associated with ICH versus ischemic stroke after adjusting for demography in the three main ethnic groups. The adjusted OR and 95% confidence intervals are shown in Table 3. The odds of having ICH among males were 1.57 times higher than females (p = 0.057), while patients from the Far East have 2.84 times more likelihood of having ICH compared to patients from the Middle East (p < 0.001). The presence of hypertension was the most significant factor contributing to ICH with adjusted OR of 3.04 (95% CI: 1.99–4.61) after adjusting for age, gender and ethnicity. The lowering of serum cholesterol levels had significant adverse associated with ICH, while the presence of diabetes, prior stroke, CAD, and smoking significantly increases the risk of developing ischemic stroke (Table 3).
Table 3.
Bivariate and multiple logistic regression analysis to identify risk factors associated with ICH vs. ischemic stroke/TIA.
| Ischemic stroke/TIA |
ICH |
Bivariate logistic regression
analysis |
Multiple logistic regression
analysis |
|||
|---|---|---|---|---|---|---|
| n (%) | n (%) | Odds ratio (95% CI for odds ratio) | p | Adjusted odds ratio (95% CI for adjusted odds ratio) | p | |
| Gender | ||||||
| Female | 273 (84.8%) | 49 (15.2%) | 1 | 1 | ||
| Male | 1134 (81.7%) | 254 (18.3%) | 1.2 (0.89–1.74) | 0.193 | 1.57 (0.98–2.49) | 0.057 |
| Age in years (mean ± SD) | 55.4 ± 13.7 | 51.2 ± 12.5 | 0.97 (0.97–0.98) | <0.001 | 0.991 (0.97–1.01) | 0.226 |
| Ethnicity | ||||||
| Middle East | 512 (87.2%) | 75 (12.8%) | 1 | 1 | – | |
| South East Asian | 753 (82.3%) | 162 (17.7%) | 1.47 (1.09–1.97) | 0.011 | 1.30 (0.86–1.97) | 0.212 |
| Far Eastern | 142 (68.3%) | 66 (31.7%) | 3.17 (2.17–4.64) | <0.001 | 2.84 (1.71–4.72) | <0.001 |
| Diabetic | ||||||
| No | 669 (76.3%) | 208 (23.7%) | 1 | 1 | ||
| Yes | 738 (88.6%) | 95 (11.4%) | 0.41 (0.32–0.54) | <0.001 | 0.65 (0.46–0.91) | 0.011 |
| Hypertension | ||||||
| No | 447 (88.3%) | 59 (11.7%) | 1 | 1 | ||
| Yes | 960 (79.7%) | 244 (20.3%) | 1.93 (1.42–2.61) | <0.001 | 3.04 (1.99–4.61) | <0.001 |
| Stroke | ||||||
| No | 1182 (81.3%) | 272 (18.7%) | 1 | 1 | ||
| Yes | 161 (89.9%) | 18 (10.1%) | 0.49 (0.29–0.81) | 0.005 | 0.46 (0.24–0.87) | 0.017 |
| CAD | ||||||
| No | 1251 (81.3%) | 288 (18.7%) | 1 | 1 | ||
| Yes | 156 (91.2%) | 15 (8.8%) | 0.42 (0.24–0.72) | 0.002 | 0.39 (0.19–0.79) | 0.009 |
| Smoking | ||||||
| No | 1023 (79.9%) | 257 (20.1%) | 1 | 1 | ||
| Yes | 314 (91.0%) | 31 (9.0%) | 0.39 (0.26–0.58) | <0.001 | 0.37 (0.23–0.59) | <0.001 |
| Cholesterols | 5.1 ± 1.3 | 4.7 ± 1.4 | 0.80 (0.71–0.90) | <0.001 | 0.73 (0.64–0.84) | <0.001 |
CAD: coronary artery disease; ICH: intracranial haemorrhage; TIA: transient ischemic attack.
Discussion
To the best of our knowledge, this is the first comprehensive prospective study reviewing the risk factors profile, clinical features and prognosis in a large, ethnically diverse population of stroke patients from the Middle East, South East Asia and the Far East. The age at presentation of stroke was significantly younger than reports from North America and Europe. There was a wide variance in the risk factor profile between the different ethnic groups. The combination of hypertension and diabetes (in Middle Eastern and South East Asian patients) was associated with increased risk of cortical and subcortical ischemic stroke, whereas the high rates of hypertension (with significantly lower rates of diabetes) were seen in patients from the Far East explained the rates of ICH. Symptoms on admission tended to be mild in most patients, including in patients presenting with ICH, accounting for the low mortality in our cohort.
Higher rates of subcortical lacunar strokes with milder symptoms were likely secondary to poorly controlled hypertension and diabetes and may explain the better prognosis at 90 days. The results may be biased as we were able to conduct 90-day mRS telephone interviews in approximately 50% of patients. We cannot exclude the possibility that expatriate patients with severe deficits may have left Qatar following discharge from hospital.
Stroke presentation at a young age in the Middle East and South East Asia has previously been reported.17,18 Poor awareness and inadequate management of hypertension and diabetes may be important contributing factors. A lack of awareness of vascular risk factors has also been reported in the Qatar population with less than 30% aware that hypertension and diabetes were important risk factors.19 A striking finding in our study was the high prevalence of dysglycaemia, especially in the Qatari population. The 67.6% is nearly double that of any previously reported prevalence of diabetes in stroke patients, which varied from 11% to 43%.20 The prevalence of hypertension, especially untreated, was also very high in all the groups. The higher rates of sub-cortical ‘lacunar’ strokes presenting with milder symptoms, at a younger age is likely to be a result of the poor awareness and control of diabetes and hypertension. Significant difference in age and BMI between the three ethnic groups may be additional factors accounting for the higher rates of diabetes in Arabs compared to South East Asians.
In our cohort, South East Asian subjects had symptom profiles similar to what is reported from India and Pakistan.21,22 Presentation at a younger age and higher rates of subcortical lacunar stroke are likely related to the higher incidence of untreated hypertension and diabetes.3,21 A study with 289 patients from Pakistan showed hypertension (79%) and diabetes (52%) as the most common risk factors.23 Jafar reported that a high salt intake and a higher incidence of diabetes and hypertension may be responsible for the strokes developing at a younger age in Pakistan.24. Direct comparisons of strokes in patients from South East Asia and Northern Europe descent are infrequent. Moussouttas et al.25 compared clinical features in 99 South East Asian and 106 North European patients. Similar to our results, South East Asian patients were younger, had significantly higher rates of diabetes (48%), and subcortical stroke was double the rate observed in Caucasians.25
In our study, patients from the Far East had the highest incidence of ICH. This is similar to the higher incidence of ICH reported in studies from Japan, China, Hong Kong, Taiwan, Philippines and Korea.26,27 Although hypertension being most common risk factor in patients from the Far East, its incidence was comparable to Middle Eastern patients and significantly higher than patients from South East Asia. Patients from the Far East however had significantly lower rates of diabetes when compared to the other ethnic groups in our study. Similar increased risk of ICH has been reported in Far Eastern immigrants settled in USA.28 Amongst patients who died from an acute stroke in USA between 1995 and 1998, the underlying etiology was ICH in Far Eastern patients was 38% and 18% in Caucasian patients.29
It is likely that genetic factors predispose the Far Eastern population to higher risk of ICH. The PRoFESS trial enrolled 20,332 patients in two-by-two factorial design comparing two dual antiplatelet regimens and telmisartan to placebo. Thirty-three percent of patients enrolled were of Far Eastern origin. The risk of ICH was significantly higher in this group, especially in individuals with hypertension (systolic BP > 150) compared to Caucasians.30 Similarly, the risk of warfarin-related ICH was four times higher in Far Eastern patients compared to Caucasians,31 in patients treated for atrial fibrillation in a large cohort study from California. In an interesting comparison of stroke etiology in long-term residents in Hawaii, similar trends were evident.32
There are a number of strengths in our study. This is the first study that reports on the magnitude of cerebrovascular disease in a diverse population. Cerebrovascular disease manifests at a younger age, especially in the patients from Southeast Asia and the Philippines. We report on high rates of undiagnosed or poorly controlled diabetes and hypertension, which was likely to have been a major contributor to the disease manifesting at an early age. The number of patients with pre-diabetes and diabetes was alarming and requires urgent attention. Similarly, despite the young age, there was disturbingly high incidence of hypertension in the patients. The higher rate of subcortical strokes seen in all ethnic groups is likely related to dysglycaemia and hypertension. The higher rates of ICH seen in patients from the Far East are also likely related to the hypertension. The population of Qatar largely consists of expatriates who do not stay in Qatar long term. The majority are blue-collar workers with very little education and lack awareness of the risk factors for vascular diseases. Our study identified the need to be more aggressive in the management of vascular risk factors in this population.
We acknowledge some limitations of this study. We do not know the prevalence of risk factors (diabetes, hypertension, dyslipidemia, atrial fibrillation and BMI) among the normal or non-stroke (control group) population in Qatar. We also do not know the duration of the diabetes and hypertension prior to the acute stroke or how well these conditions were treated prior to the stroke, although such data are often difficult to collect and have inherent inaccuracies. Lack of awareness of risk factors was a significant finding in our study. We did not collect patient data which could inform us about patients’ awareness of stroke risk factors; level of education, household income, geographical location and occupation. These factors would be important to determine for preventive measures of vascular adverse events.
In conclusion, we report that stroke develops at a young age in Qatar. The high rates of subcortical stroke are likely related to the risk factor profile of the study population.
Supplementary Material
Acknowledgments
We would like to thank Andrew Peter Branch at the Academic Health Services (Hamad Medical Corporation) for reviewing the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
Ethical approval to conduct the study and preparing this manuscript was obtained from the Medical Research Centre at Hamad Medical Corporation, Doha, Qatar.
Informed consent
Waiver of informed consent was provided to the IRB at Hamad Medical Corporation in order to use patients’ data from the database.
Guarantor
AS is the guarantor for the study, having access to all the data and the final decision to submit the manuscript article.
Contributorship
Akhtar, Kamran, Deleu and Shuaib contributed to the design, scientific content and review of the final manuscript. Bourke, Joseph and Santos assisted with data collection. Salam conducted the statistical analysis. Irfan contributed to the discussion. Khan assisted in literature search, final editing and review of the article. Malik contributed to the study design and analysis.
References
- 1.Feigin VL. Stroke in developing countries: can the epidemic be stopped and outcomes improved. Lancet Neurol 2007; 6: 94–97. [DOI] [PubMed] [Google Scholar]
- 2.O’Donnell M, Yousef S. Tracking the global burden of stroke: the need for large scale international studies. Lancet 2009; 8: 306–307. [DOI] [PubMed] [Google Scholar]
- 3.Wasay M, Khatri IA, Kaul S. Stroke in South Asian countries. Nat Rev 2014; 10: 135–143. [DOI] [PubMed] [Google Scholar]
- 4.Johnson SC, Mendis S, Mathers CD. Global variations in stroke burden and mortality: estimates from monitoring, surveillance and modelling. Lancet Neurol 2009; 8: 345–354. [DOI] [PubMed] [Google Scholar]
- 5.O’Donell J, Xavier D, Liu L, et al. Risk factors for ischemic and hemorrhagic stroke in 22 countries (the INTER-STROKE study). Lancet 2010; 376: 112–123. [DOI] [PubMed] [Google Scholar]
- 6.Mehndiratta MM, Khan M, Mehndiratta P, et al. Stroke in Asia: geographical variations and temporal trends. J Neurol Neurosurg Psychiatr 2014; 85: 1308–1312. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed E, El-Menyar South Asian ethnicity and cardiovascular risk: the known, the unknown and the paradox. Angiology 2015; 66: 405–415. [DOI] [PubMed] [Google Scholar]
- 8.Development Goals in an Era of Demographic Change, www.worldbank.org/en/publication/global-monitoring-report (accesses 29 February 2016).
- 9.Qatar Ministry of Planning and Development 2014. www.gsdp.gov.qa/ (accessed 29 February 2016).
- 10.Akhtar N, Kamran S, Singh R, et al. Beneficial effects of implementing stroke protocols require establishment of a geographically distinct unit. Stroke 2015; 46: 3494–3501. [DOI] [PubMed] [Google Scholar]
- 11.Akhtar N, Kamran S, Singh R, et al. Prolonged stay of stroke patients in the emergency department may lead to an increased risk of complications, poor recovery, and increased mortality. J Stroke Cerebrovasc Dis 2016; 25: 672–678. [DOI] [PubMed] [Google Scholar]
- 12.Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ 1976; 54: 541–553. [PMC free article] [PubMed] [Google Scholar]
- 13.Adams HP, Jr, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 14.Wahlgren N, Ahmed N, Dávalos A, et al. SITS-MOST investigators. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007; 369: 275–282. [DOI] [PubMed] [Google Scholar]
- 15.Touma L, Filion KB, Sterling LH, et al. Stent retrievers for the treatment of acute ischemic stroke: a systematic review and meta-analysis of randomized clinical trials. JAMA Neurol 2016; 73: 275–281. [DOI] [PubMed] [Google Scholar]
- 16.d’Emden MC, Shaw JE, Jones GR, et al. Guidance concerning the use of glycated hemoglobin (HbA1c) for the diagnosis of diabetes mellitus. Med J Aust 2015; 203: 89–90. [DOI] [PubMed] [Google Scholar]
- 17.Al Rajeh S, Awada A. Stroke in Saudi Arabia. Cerebrovasc Dis 2002; 13: 3–8. [DOI] [PubMed] [Google Scholar]
- 18.Benamer HT, Grosset D. Stroke in Arab countries: a systematic literature review. J Neurol Sci 2009; 284: 18–23. [DOI] [PubMed] [Google Scholar]
- 19.Kamran S, Bener AB, Deleu D, et al. The level of awareness of stroke risk factors and symptoms in the Gulf Cooperation Council Countries: Gulf Cooperation Council Stroke Awareness Study. Neuroepidemiology 2007; 29: 235–242. [DOI] [PubMed] [Google Scholar]
- 20.Behrouz R, Powers CJ. Epidemiology of classical risk factors in stroke patients in the Middle East. Eur J Neurol 2016; 23: 262–269. [DOI] [PubMed] [Google Scholar]
- 21.Gunarathne A, Patel JV, Gammon B, et al. Ischemic stroke in South Asians: a review of the epidemiology, pathophysiology, and ethnicity-related clinical features. Stroke 2009; 40: e415–e423. [DOI] [PubMed] [Google Scholar]
- 22.Pandian JD, Singh G, Kaur P, et al. Incidence, short-term outcome, and spatial distribution of stroke patients in Ludhiana, India. Neurology 2016; 86: 425–433. [DOI] [PubMed] [Google Scholar]
- 23.Khan H, Shuaib U, Khatri I, et al. Clinical features and management of ischemic stroke in Pakistan. Neurology 2013; 80(Suppl): P02.031. [Google Scholar]
- 24.Jafar TH. Blood pressure, diabetes, and increased dietary salt associated with stroke – results from a community-based study in Pakistan. J Hum Hypertension 2006; 20: 83–85. [DOI] [PubMed] [Google Scholar]
- 25.Moussouttas M, Aguilar L, Fuentes K, et al. Cerebrovascular disease among patients from the Indian subcontinent. Neurology 2006; 67: 894–896. [DOI] [PubMed] [Google Scholar]
- 26.Okada H, Horibe H, Yoshiyuki O, et al. A prospective study of cerebrovascular disease in Japanese rural communities, Akabane and Asahi. Part 1: evaluation of risk factors in the occurrence of cerebral hemorrhage and thrombosis. Stroke 1976; 7: 599–607. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka H, Ueda Y, Hayashi M, et al. Risk factors for cerebral hemorrhage and cerebral infarction in a Japanese rural community. Stroke 1982; 13: 62–73. [DOI] [PubMed] [Google Scholar]
- 28.Klatsky AL, Friedman GD, Sidney S, et al. Risk of hemorrhagic stroke in Asian American ethnic groups. Neuroepidemiology 2005; 25: 26–31. [DOI] [PubMed] [Google Scholar]
- 29.Ayala C, Croft JB, Greenlund KJ, et al. Sex differences in US mortality rates for stroke and stroke subtypes by race/ethnicity and age, 1995–1998. Stroke 2002; 33: 1197–1201. [DOI] [PubMed] [Google Scholar]
- 30.Estol CJ, Bath PM, Gorelick PB, et al. PRoFESS Publications Committee and PRoFESS Investigators. Differences in ischemic and hemorrhagic recurrence rates among race-ethnic groups in the PRoFESS secondary stroke prevention trial. Int J Stroke 2014; 9(Suppl A100): 43–47. [DOI] [PubMed] [Google Scholar]
- 31.Shen AY, Yao JF, Brar SS, et al. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol 2007; 50: 309–315. [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa K, Koenig MA, Seto TB, et al. Racial disparities among Native Hawaiians and Pacific Islanders with intracerebral hemorrhage. Neurology 2012; 79: 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.