Abstract
Introduction
Poststroke epilepsy (PSE) is the most common form of acquired epilepsy after middle age. The primary aim of this study was to study the impact of PSE on prognosis. A secondary aim was to validate recent findings from smaller studies on the risk of developing PSE on a nationwide scale.
Patients and methods
We performed a nationwide cohort study based on comprehensive national registries and included patients without a prior epilepsy diagnosis surviving more than 2 months after stroke, identified by the Swedish Stroke Register (Riksstroke) and linked to the National Patient Register and Cause of Death Register. Cox proportional time-updated hazard model was used to assess the risk of death, with or without multivariable adjustment for possible confounders, and multiple Cox regression was used to examine associations between PSE and clinical characteristics.
Results
In 106,455 patients, PSE (defined as a seizure diagnosis more than 7 days after stroke) was detected in 7.3%, with lower cumulative incidence after ischemic stroke (6.4%) than after intracerebral haemorrhage (12.4%). Stroke severity, intracerebral haemorrhage and young age were associated with a risk of PSE. The risk of death was increased in patients with PSE (hazard ratio: 1.68, 95% confidence interval: 1.25–1.53). Also with adjustments for age, comorbidities and stroke severity, an increased risk of death associated with PSE remained.
Discussion
Studies are needed on potential causes of increased mortality in PSE, such as a direct seizure-related mortality, less ambitious secondary stroke prophylaxis or rehabilitation, or impact of antiepileptic drugs on cardiovascular risk.
Keywords: Cohort study, stroke, seizures
Introduction
Cerebrovascular disease is the most common cause of new-onset epilepsy in older adults.1 The long-term cumulative incidence of poststroke epilepsy (PSE) was recently reported as 6.4% and 8.2%,2,3 and an even higher incidence was described in a smaller study on younger patients.4 There is however relatively little information on the prognosis of PSE once established – often the clinically relevant scenario for epileptologists. In a recent study on young patients, an increased risk of death was associated with PSE,5 but other small studies have reached conflicting results.5–8 In theory, there are several ways in which PSE may negatively impact the prognosis after stroke. Seizure-related risks apart, treatment with antiepileptic drugs (AEDs) may interact with secondary stroke prophylaxis and several AEDs have a detrimental impact on lipid profiles.9 In addition, side-effects of AEDs or real or imagined seizure-related risks may interfere with rehabilitation.
The prevalence of PSE is likely to increase with an ageing population and improvements in stroke care. Generally, epilepsy carries a small but significant increase in mortality, but these risks are not readily extrapolated to PSE given the medical context with cerebrovascular disease as a major comorbidity. On one hand, PSE is considered easy-to-treat and many patients have focal non-life threatening seizures. On the other hand, seizure-related risks may be increased in patients with sequelae after stroke and the issues regarding AED treatment and cardiovascular risk outlined above is a cause for concern.
We asked if PSE has an impact on prognosis and used cross-linking of comprehensive national registries to perform a large cohort study with the aims to (a) investigate the association between PSE and mortality and (b) validate the recent estimates of PSE risk on a national scale.
Methods
The study population was identified using the Swedish stroke register (Riksstroke), a national quality register established in 1994.10 All hospitals admitting acute stroke patients participate in the registry. Details are available at http://www.riksstroke.org/eng/. The Riksstroke register has an estimated coverage of 94% for acute stroke in adults, and includes information on living conditions, comorbidities prior to stroke and medical treatment. A Riksstroke follow-up is performed 3 months after the stroke and includes information on rehabilitation and support.
For the purpose of this study, patients with stroke during the years 2005–2010 were identified in Riksstroke. The years were chosen to reflect current practice, yet allow sufficient follow-up time to assess risks of epilepsy and mortality. The registry includes patients with intracerebral haemorrhage (ICH), ischemic stroke and unspecified stroke (International Classification of Diseases 10th revision (ICD-10) codes I61, I63 and I64, respectively). The number of unclassified strokes was low (n = 3325, 3.1%), and since these patients had similar basic characteristics as patients with ischemic stroke, they were treated as having ischemic strokes for the purpose of this study.
For information on epilepsy and mortality, Riksstroke was cross-linked to the National Patient Register (NPR) and Cause of Death Register (CDR).11 These registers are based on personal identification numbers and run by the National board of Health and Welfare. NPR contains information on all diagnoses registered at hospital-based care from 1987 and some hospital-based outpatient care since 2001, and has been used to identify cases of epilepsy in cross-linking studies.12 NPR was the source of information on seizure-related diagnoses; epilepsy, status epilepticus and seizures and CDR contributed with date of death. The patients were followed until death or end of study (31 December 2014).
Study population and definition of PSE
The Riksstroke output contained 131,453 unique individuals with valid personal identification numbers. Patients that had a prior seizure-related diagnosis (ICD-10: G40, G41 and R56.8; ICD-9: 345 and 780C, n = 4272), died during the first 2 months after stroke (n = 20,847), or registration anomalies (age > 110, death before stroke, n = 14, or incomplete date of death, n = 65) were excluded. Based on the current ILAE definition of acute symptomatic seizures as seizures occurring within 1 week of acute stroke, PSE was defined as a seizure-related diagnosis registered at least 7 days after the date of the index stroke (ICD-10: G40, G41 or R56.8; ICD-9 was not used during the study years).
Analysis and statistics
Data were analysed using descriptive statistics and Cox proportional hazard ratio models, with epilepsy as a time-dependent explanatory variable (since inclusion in the study was at the time of the index stroke). For sensitivity analyses, the association between epilepsy and death was examined also with patients identified as PSE based solely on a single seizure or status epilepticus code excluded or counted as not having PSE. Similarly, an analysis was performed without patients with a diagnosis of brain tumour (ICD-10 C71). In the adjusted Cox model, multivariable adjustments were made for the following potential explanatory variables (attempting to adjust for stroke severity and general health); age, sex, living arrangements at admission and follow-up, mobility at admission and follow-up, atrial fibrillation, diabetes, thrombolytic therapy, length of admission, level of consciousness at admission and smoking. Patients with missing data were excluded in these analyses and no imputations made.
Approvals and patient consent
This study, performed in agreement with privacy legislation in Sweden, was approved by the regional ethics committee in Gothenburg (Approval number 187-15). Data were obtained by linking the above registries using the unique personal identification number of every Swedish citizen. The National Board of Health and Welfare anonymised all data after linkage and before we were given access to them. Patients are informed that registration in Riksstroke is voluntary.
Results
A total of 106,455 patients, who survived 2 months after stroke and did not have an epilepsy diagnosis prior to the stroke, were included. Baseline characteristics are found in Table 1. The mean age was 74.6 years with 1.9% of patients under 45 years of age and 48.7% of female gender.
Table 1.
Baseline characteristics at time of stroke.
| All (n = 106,455, 100%) | ICH (n = 10,195, 9.6%) | Infarction (n = 96,260, 90.4%) | |
|---|---|---|---|
| Demographics | |||
| Mean age at stroke (SD) | 74.6 (12.0) | 70.9 (13.4) | 75.0 (11.7) |
| Age | |||
| <45 | 2031 (1.9%) | 400 (3.9%) | 1631 (1.7%) |
| Gender | |||
| Male | 55,015 (51.7%) | 5588 (54.8%) | 49,427 (51.3%) |
| Female | 51,440 (48.3%) | 4607 (45.2%) | 46,833 (48.7%) |
| Living at arrival | |||
| Living at home without assistance | 84,893 (80.0%) | 8405 (82.8%) | 76,488 (79.7%) |
| Living at home with assistance | 15,091 (14.2%) | 1192 (11.7%) | 13,900 (14.5%) |
| Other housing (i.e. nursing home) | 6138 (5.8%) | 558 (5.5%) | 5580 (5.8%) |
| Missing | 332 | 40 | 292 |
| Mobility at arrival | |||
| Independent mobility | 96,850 (91.6%) | 9368 (92.5%) | 87,482 (91.5%) |
| Independent mobility indoors but not outdoors | 5964 (5.6%) | 506 (5.0%) | 5458 (5.7%) |
| Assistance by at least one other person or bedridden. | 2892 (2.7%) | 249 (2.5%) | 2643 (2.8%) |
| Missing | 749 | 72 | 677 |
| Stroke severity | |||
| Level of consciousness | |||
| RLS 1 | 95,203 (90.1%) | 7800 (77.5%) | 87,403 (91.5%) |
| RLS 2–3 | 8890 (8.4%) | 1818 (18.1%) | 7072 (7.4%) |
| RLS 4–8 | 1531 (1.4%) | 443 (4.4%) | 1088 (1.1%) |
| Missing | 831 | 134 | 697 |
| Thrombolysis | 3938 (3.7%) | 22 (0.2%) | 3916 (4.1%) |
| Missing | 785 | 71 | 714 |
| Living at follow-up | |||
| Living at home without assistance | 53,698 (57.7%) | 4458 (51.8%) | 49,240 (58.3%) |
| Living at home with assistance | 19,130 (20.6%) | 1672 (19.4%) | 17,458 (20.7%) |
| Other housing (i.e. nursing home) | 20,168 (21.7%) | 2470 (28.7%) | 17,698 (21.0%) |
| Missing | 13,459 | 1595 | 11,864 |
| Mobility at follow-up | |||
| Independent mobility | 60,789 (67.9%) | 4947 (59.8%) | 55,842 (68.7%) |
| Independent mobility indoors but not outdoors | 14,558 (16.3%) | 1449 (17.5%) | 13,109 (16.1%) |
| Needs assistance by at least one other person or bedridden | 14,177 (15.8%) | 1871 (22.6%) | 12,306 (15.1%) |
| Missing | 16,931 | 1928 | 15,003 |
| Comorbidities | |||
| Diabetes | 20,771 (19.6%) | 1438 (14.2%) | 19,333 (20.2%) |
| Missing | 589 | 83 | 506 |
| Atrial fibrillation | 26,387 (25.0%) | 1533 (15.3%) | 24,854 (26.1%) |
| Missing | 1065 | 145 | 920 |
| Smoking | 15,243 (15.8%) | 1214 (13.5%) | 14,029 (16.1%) |
| Missing | 10,209 | 1219 | 8990 |
Numbers in brackets are percentages if not otherwise stated. RLS: Reaction level scale.
During follow-up (mean 4.8 years), we identified 7740 (7.3%) cases that developed PSE. For ischemic stroke, the cumulative incidence of PSE was 6.7% and for ICH, the cumulative incidence of PSE was 12.4%. Median time to occurrence of a seizure diagnosis was approximately 1 year in both groups, 1.16 years for ischemic stroke and 0.9 years for ICH (Table 2).
Table 2.
Cumulative incidence of PSE and time to PSE.
| Total (n = 106,455) | ICH (n = 10,195) | Infarction (n = 96,260) | |
|---|---|---|---|
| PSE (diagnosis > 7 days from stroke) | |||
| PSE, n (%) | 7740 (7.3%) | 1263 (12.4%) | 6477 (6.7%) |
| Time from stroke to PSE (years) | |||
| Mean (SD) | 1.86 (1.90) | 1.65 (1.82) | 1.90 (1.91) |
| Median (min, max) | 1.11 (0.02, 9.80) | 0.90 (0.02, 9.80) | 1.16 (0.02, 9.41) |
PSE: poststroke epilepsy; ICH: intracerebral haemorrhage.
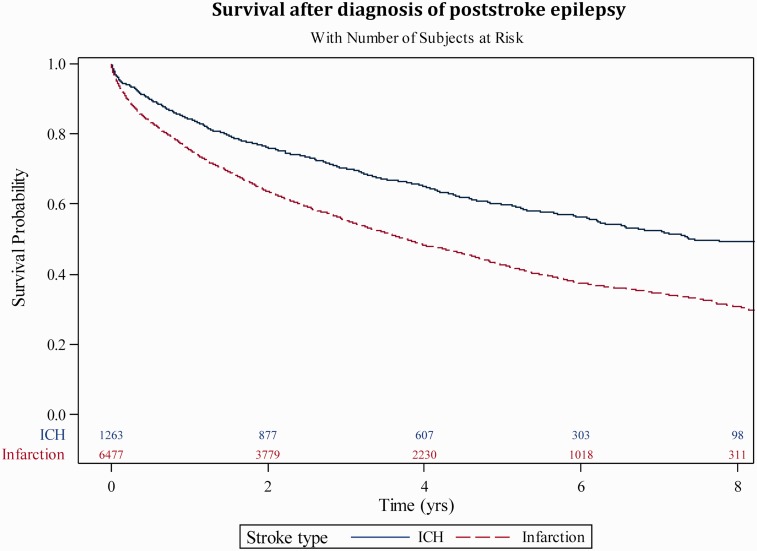
After the first seizure diagnosis, the 2- and 5-year survival was 66% and 45%. For comparison, after the index stroke, the 2- and 5-year survival for patients with ICH (regardless if PSE or not) were 82% and 64%, and the corresponding numbers for patients with ischemic stroke were 79% and 59%. Kaplan–Mayer curves for survival of patients with PSE after different stroke types are shown in Figure 1.
Figure 1.
Kaplan–Meyer curve of survival after PSE diagnosis.
We analysed the association between death and PSE by a time-updated Cox proportional Hazard model with PSE as the time-dependent explanatory variable (Table 3). In the total population, PSE was associated with an increased risk of death (hazard ratio (HR): 1.69, 95% confidence interval (CI): 1.64–1.74). When stratified by stroke type and age, the risk of death was significantly increased (HR: 1.54, 95% CI: 1.38–1.72) in patients aged 45 or above with PSE after ischemic stroke. For other subgroups, the number of observations was smaller and the findings not statistically significant, although increased HR for death was seen in all groups (Table 3).
Table 3.
Cox proportional time-updated hazard model for risk of death depending on presence of epilepsy, stratified by type and age.
| Age | Stroke type | n | HR (95% CI) |
|---|---|---|---|
| All | All | 106,455 | 1.69 (1.64–1.74) |
| <45 | ICH | 400 | 1.66 (0.22–12.34) |
| <45 | Infarction | 1631 | 3.23 (0.79–13.20) |
| ≥45 | ICH | 9795 | 1.17 (0.92–1.49) |
| ≥45 | Infarction | 94,629 | 1.54 (1.38–1.72) |
ICH: intracerebral haemorrhage; HR: hazard ratio; CI: confidence interval.
To ensure that the increased mortality was not due to acute symptomatic seizures or status epilepticus, we also performed sensitivity analyses. The increased risk of death remained when patients identified as PSE based solely on a single seizure code were excluded. This was also the case in an even more conservative analysis, where single seizure patients were counted as not having PSE. The same analyses were performed for patients identified as PSE based on a single code for status epilepticus, with the same results. Similarly, the increased HR for death remained when patients with codes for brain tumours before or after the stroke were excluded.
We finally asked if epilepsy per se conferred an extra risk of death and performed a Cox hazard model with multivariable adjustments for clinical characteristics that influenced mortality independently of PSE (age, gender, diabetes, atrial fibrillation, anticoagulation, stroke type, thrombolysis, length of admission, level of consciousness on arrival, living arrangements and mobility at admission and follow-up and smoking). The analysis was based on 79,777 individuals with complete data sets. The HR for death in patients with PSE in the adjusted model was 1.36 (95% CI: 1.20–1.55). An increased HR for death associated with PSE remained in the adjusted model when smoking and living arrangements and mobility at 3 months (parameters with many missing values) were not taken into account, which resulted in an analysis based on 102,978 patients.
In addition to the association between PSE and death, we analysed the risk of developing PSE depending on baseline characteristics by Cox analysis (Table 4). Age <45, stroke subtype (ICH), thrombolytic therapy, poor mobility at 3 months follow-up, assisted living arrangements at 3 months follow-up and level of consciousness at arrival were associated with increased risk of PSE.
Table 4.
Clinical variables and hazard ratios for poststroke epilepsy.
| Variable | % observation used | Value | HR (95% CI) | Pr > χ2 |
|---|---|---|---|---|
| Age | 100% | Age < 45 vs. age ≥ 45 | 1.41 (1.24–1.60) | <.0001 |
| Gender | 100% | Male vs. female | 1.10 (1.05–1.15) | <.0001 |
| Stroke type | 100.0% | ICH vs. infarction | 1.86 (1.76–1.98) | <.0001 |
| Consciousness at arrival | 99.2% | RLS 1 vs. RLS 2–3 | 0.24 (0.21–0.27) | <.0001 |
| RLS 2–3 vs. RLS 4–8 | 0.67 (0.59–0.76) | <.0001 | ||
| Thrombolysis | 99.3% | Yes vs. No | 1.77 (1.62–1.94) | <.0001 |
| Living at follow-up | 87.4% | Living at home without vs. with assistance | 0.38 (0.36–0.40) | <.0001 |
| Living at home with assistance vs. other housing (i.e. nursing home) | 0.65 (0.61–0.70) | <.0001 | ||
| Mobility at follow-up | 84.1% | Independent mobility, indoors and outdoors vs. dependence outdoors | 0.36 (0.34–0.38) | <.0001 |
| Independent mobility indoors vs. needs assistance by at least one other person or bedridden | 0.61 (0.56–0.65) | <.0001 | ||
| Atrial fibrillation | 99.0% | Yes vs. No | 1.36 (1.29–1.43) | <.0001 |
| Warfarin/NOAC | 99.0% | Yes vs. No | 1.22 (1.12–1.33) | <.0001 |
| Diabetes | 99.4% | Yes vs. No | 0.98 (0.92–1.04) | n.s. |
| Smoking | 90.4% | Yes vs. No | 1.02 (0.96–1.06) | n.s. |
n.s.: not significant.
Discussion
We present findings from a pragmatic large nationwide cohort study on the prognosis of PSE. The main finding of our study was that PSE is associated with an increased risk of death compared with stroke that is not followed by PSE. Furthermore, we were able to validate findings of previous investigators regarding the current incidence of PSE. Our results provide quantitative estimates useful for information to patients with newly diagnosed PSE and raise important questions for stroke and epilepsy health care providers.
The strength of our study lies in its nationwide approach. As all registry-based investigations, our study also has limitations. Reporting to the comprehensive national registries (NPR and CDR) is mandatory and the registries have been validated,11 but data are not present for patients that are never treated at a hospital. For dramatic symptoms like seizures, this should be a minor concern and similar approaches have been used previously to identify cases of stroke and seizures in Sweden.10,12 The similarity of the incidence of PSE in our material and that a recent large study2 indicates that we identified most patients with PSE. We decided to use a long time requirement for survival of the acute phase of stroke (2 months) to minimise contribution of short-term case fatality. To avoid including patients with acute symptomatic seizures (which indicate a poor prognosis), we included only patients with a seizure code 7 days or more after the index stroke.
We decided to identify patients with PSE by codes for single seizures as well as epilepsy. This strategy was chosen as the high recurrence risk for patients with a single seizure and a structural brain lesion like stroke is increasingly recognised6 and since 2014 encompassed in the ILAE definition of epilepsy.13 For this reason, patients with a single seizure in our cohort most likely had a recurrence risk motivating an epilepsy diagnosis. The vast majority (>83%) of our cases were not based on a single seizure code. Misdiagnosis of stroke recurrence and seizures is common and we decided against trying to identify acute symptomatic seizures due to stroke recurrence. For this reason, and for assurance that acute symptomatic seizures were not the explanation for the association between PSE and death, we performed several sensitivity analyses demonstrating that the increased HR for death did not depend on single codes of seizures, status epilepticus or brain tumours.
Since stroke severity is a well-known risk factor for PSE, a poor prognosis associated with PSE was somewhat expected. Indeed, the 2-year survival for patients with PSE (66%) was close to the 5-year survival of the population as a whole (64% for ICH and 59% for infarctions) and the increased risk of death associated with PSE was decreased when multivariable adjustments were made for variables reflecting stroke severity and outcome. Interestingly, a non-negligible increase in risk of death (HR: 1.36, 95% CI: 1.20–1.55) remained also after these adjustments. This indicates that some excess mortality may be linked to PSE itself and raises important questions about the health care provided to patients with PSE.
What might cause the increased risk of death associated with PSE? Excess mortality could be due to seizures. This seems unlikely, given the short time that most patients in our cohort were exposed to seizure-related risks (mean follow-up time of 4.8 years) and the relatively high proportion of focal non-life threatening seizures generally seen after stroke.14 In a study on young patients with PSE, where exposure to seizure-related risks should be the greatest, the authors reported an increased risk of death associated with PSE, but no difference in causes of death compared with non-PSE stroke patients.5 In general, cerebro- or cardiovascular disease is a quantitatively substantial cause of death in older patients with epilepsy.14,15 These constituted the majority of our cohort, so that at least some excess risk of death was due to vascular disease seems plausible.
To date, this is the largest study on mortality associated with PSE. Our findings are in line with an earlier small study demonstrating low 10-year survival (approximately 35%) after a first unprovoked seizure in 101 stroke patients6 and with a demonstrated increased risk of death in young patients with PSE,5 but contrasts with two smaller studies (one of which was our own) that found no association with mortality in PSE.7,8 Most likely, these differences reflect inadequate sample size in the previous investigations. Regarding risk of developing PSE, our findings are well in line with estimates of the cumulative incidence of PSE reported by Graham and Jungehulsing2,3 and our data provide validation of these important studies. The associations between risk of PSE and ICH, young age and more severe stroke have been described previously.2,3,16,17
Our findings highlight the importance of further research on PSE. Could there be more to do for patients with seizures and cerebrovascular disease? Are patients with PSE subject to less ambitious secondary cardiovascular prophylaxis after stroke, are there detrimental interactions between this prophylaxis and AEDs, or do our findings reflect a negative impact of treatment with AEDs on lipid profiles?9 Or does PSE perhaps lead to difficulties in participation in rehabilitation and activation? We plan future studies on the identified cohort regarding cause of death and prescribed antiepileptic treatment, which will hopefully bring some clarity to these issues.
Acknowledgements
The authors are very grateful to Mattias Molin, Statistiska konsultgruppen, Gothenburg for statistical services and The Riksstroke Collaboration (www.riksstroke.org).
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Åsberg receives institutional research funding from AstraZeneca NordicBaltic, received research support from The Swedish Stroke Register and The National Association for Stroke Patients in Sweden, and receives research funding from The Swedish Research Council. Dr. Kumlien has received research support from the Thureus foundation. Dr. Zelano and Dr. Redfors reports no disclosures.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Apart from the authors institutions, this study was funded by Jeanssons foundation and the Swedish society of medicine.
Ethical approval
This study, performed in agreement with privacy legislation in Sweden, was approved by the regional ethics committee in Gothenburg (Approval number 187-15).
Informed consent
Patients are informed that inclusion in Riksstroke registry is for research purposes and voluntary (an opt-out option exists). The ethical committee waived the need for informed consent for this particular study.
Guarantor
JZ.
Contributorship
Dr Zelano: Idea, study design, data collection, data analysis, preparation of manuscript. Dr Åsberg: study design, data analysis, preparation of manuscript. Dr Redfors: data analysis, preparation of manuscript. Dr Kumlien: idea, study design, data analysis, preparation of manuscript.
References
- 1.Forsgren L, Beghi E, Oun A, et al. The epidemiology of epilepsy in Europe - a systematic review. Eur J Neurol 2005; 12: 245–253. [DOI] [PubMed] [Google Scholar]
- 2.Graham NS, Crichton S, Koutroumanidis M, et al. Incidence and associations of poststroke epilepsy: the prospective South London Stroke Register. Stroke 2013; 44: 605–611. [DOI] [PubMed] [Google Scholar]
- 3.Jungehulsing GJ, Heuschmann PU, Holtkamp M, et al. Incidence and predictors of post-stroke epilepsy. Acta Neurol Scand 2013; 127: 427–430. [DOI] [PubMed] [Google Scholar]
- 4.Arntz RM, Maaijwee NA, Rutten-Jacobs LC, et al. Epilepsy after TIA or stroke in young patients impairs long-term functional outcome: the FUTURE Study. Neurology 2013; 81: 1907–1913. [DOI] [PubMed] [Google Scholar]
- 5.Arntz RM, Rutten-Jacobs LC, Maaijwee NA, et al. Poststroke epilepsy is associated with a high mortality after a stroke at young age: Follow-up of transient ischemic attack and stroke patients and unelucidated risk factor evaluation study. Stroke 2015; 46: 2309–2311. [DOI] [PubMed] [Google Scholar]
- 6.Hesdorffer DC, Benn EK, Cascino GD, et al. Is a first acute symptomatic seizure epilepsy? Mortality and risk for recurrent seizure. Epilepsia 2009; 50: 1102–1108. [DOI] [PubMed] [Google Scholar]
- 7.Serafini A, Gigli GL, Gregoraci G, et al. Are early seizures predictive of epilepsy after a stroke? Results of a population-based study. Neuroepidemiology 2015; 45: 50–58. [DOI] [PubMed] [Google Scholar]
- 8.Zelano J, Lundberg RG, Baars L, et al. Clinical course of poststroke epilepsy: a retrospective nested case-control study. Brain Behav 2015; 5: e00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vyas MV, Davidson BA, Escalaya L, et al. Antiepileptic drug use for treatment of epilepsy and dyslipidemia: Systematic review. Epilepsy Res 2015; 113: 44–67. [DOI] [PubMed] [Google Scholar]
- 10.Asplund K, Hulter Asberg K, Appelros P, et al. The Riks-Stroke story: building a sustainable national register for quality assessment of stroke care. Int J Stroke 2011; 6: 99–108. [DOI] [PubMed] [Google Scholar]
- 11.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattsson P, Tomson T, Eriksson O, et al. Sociodemographic differences in antiepileptic drug prescriptions to adult epilepsy patients. Neurology 2010; 74: 295–301. [DOI] [PubMed] [Google Scholar]
- 13.Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014; 55: 475–482. [DOI] [PubMed] [Google Scholar]
- 14.Phabphal K, Geater A, Limapichat K, et al. Risk factors of recurrent seizure, co-morbidities, and mortality in new onset seizure in elderly. Seizure 2013; 22: 577–580. [DOI] [PubMed] [Google Scholar]
- 15.Keezer MR, Bell GS, Neligan A, et al. Cause of death and predictors of mortality in a community-based cohort of people with epilepsy. Neurology 2016; 86: 704–712. [DOI] [PubMed] [Google Scholar]
- 16.Bladin CF, Alexandrov AV, Bellavance A, et al. Seizures after stroke: a prospective multicenter study. Arch Neurol 2000; 57: 1617–1622. [DOI] [PubMed] [Google Scholar]
- 17.Arntz R, Rutten-Jacobs L, Maaijwee N, et al. Post-stroke epilepsy in young adults: a long-term follow-up study. PLoS One 2013; 8: e55498. [DOI] [PMC free article] [PubMed] [Google Scholar]