Abstract
Purpose
Autophagy has emerged in recent years as a critical cellular survival mechanism for cell homeostasis and may play a protective role in atherosclerosis. We aimed to review here the role autophagy plays in different cell types present in carotid atherosclerotic plaques and that may be associated with the development of unstable carotid atheroma plaque.
Methods
We performed a thorough literature exploration in this area of research covering the three main cell types present in carotid atheroma plaques.
Findings
Reviewed reports indicate that the role of autophagy in stable or unstable carotid atherosclerotic plaques depends on the different cell types and phenotypes, the stage and morphology of the plaque and the specific autophagy factor/s involved.
Discussion
Although defective autophagy could be one of the causes for carotid atheroma plaques to become unstable, it is important to take into account that autophagic players can act differentially in different cell types and different stages of the developed plaque.
Conclusion
This review provides an overview of the role of autophagy in the main cell types in carotid atherosclerosis (i.e. macrophages, endothelial cells and smooth muscle cells).
Keywords: Atherosclerosis, autophagy carotid, unstable plaque
Atherosclerosis in the carotid arteries is very common in the aging population. It is also one of the main risk factors to develop a cerebrovascular accident (CVA),1 and recent research shows that CVA prevalence is increasing in the younger population.2 CVA is one of the leading causes of morbidity/mortality and disability in the world with a high impact in societies.3
Atherosclerosis is a chronic inflammatory disease that results in the progressive formation of atherosclerotic plaques. In this gradual process, several different cell types are implicated, and a sequence of events will take place that may lead ultimately to plaque rupture. The exact mechanisms involved in plaque rupture are not known but include lipid deposition, oxidative stress, inflammation, endothelial dysfunction, foam cell formation and smooth muscle cell (SMC) differentiation. In addition, plaque necrosis is a characteristic of the atherosclerotic lesions that cause cerebrovascular disease4 and that are promoted by impaired phagocytic elimination of apoptotic cells.
Stenosis is the end result of atheroma plaque deposition in the artery. These atheroma plaques can be unstable, with a thin fibrous cap and a large fatty core (prone to rupture) or stable, with a thick fibrous cap and small fatty core (unlikely to rupture). Most clinicians correlate the instability of the atherosclerotic plaque with the development of cerebrovascular events.5 Patients who suffer severe asymptomatic carotid stenosis higher than 70%–90% or symptomatic with stenosis higher than 70% would be routinely recommended for carotid endarterectomy (CEA) surgery.6 Recently, due to the improved medical therapies, the benefit of CEA for asymptomatic patients has been questioned.7 Although it is true that a proportion of patients with asymptomatic carotid disease will never become symptomatic, CEA would be crucial for those at high risk to become symptomatic. For that reason, understanding the molecular basis by which a plaque becomes unstable may be of relevance to identify patients who are at higher risk.
The mechanisms involved in plaque stability are complex, and the complete processes taking place are not completely understood. However, autophagy is known to play a role in the process of the atherosclerotic plaque development.8 In this review, we will discuss current evidence for the role of autophagy in the development of an unstable atherosclerotic plaque.
Molecular basis of autophagy
Autophagy can be differentiated in three route types used to deliver cellular components to the lysosome: microautophagy, chaperone-mediated autophagy and macroautophagy. In this review, we focus mainly on macroautophagy, which routinely is termed as autophagy. It is a regulated mechanism required for cellular metabolism and homeostasis, and while basal autophagy is considered a housekeeping function to degrade intracellular harmful proteins and organelles,9 excessive autophagy could lead to cell death.10
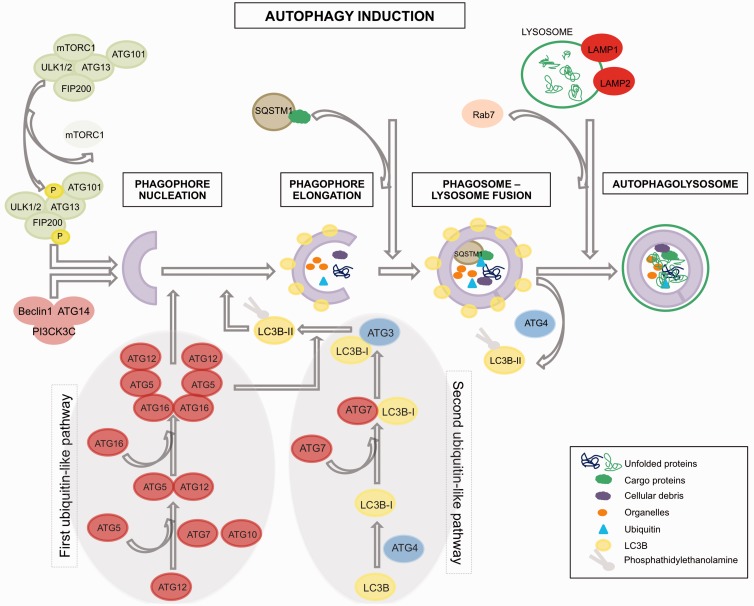
Autophagy is an evolutionary conserved pathway regulated by autophagy-related genes (ATGs). It is a catabolic intracellular process associated with the formation of double-membrane structures called autophagosomes.11 These are involved in the degradation of bulk cytoplasmic contents, aggregated or damaged proteins and damaged organelles. The material is transferred to the lysosomes by fusion with the autophagosomes, and degraded elements are subsequently released back into the cytosol where they can be recycled for biosynthesis or energy production. Autophagosome formation is driven by the cooperation of a group of proteins called autophagy-related proteins (abbreviated as ATGs) that can be classified into four groups (Figure 1). Autophagy initiation is controlled by the Atg1/Unc-51-like kinase (ULK) complex that is composed of ULK1/2, Atg13, FIP200 and Atg101 12,13 and can be regulated by different signals through the mTOR pathway (i.e. MAPK signalling, Akt signalling, ERK, TP53, PI3KI). Upon mTOR inhibition (i.e. activation of autophagy), mTOR is released from this complex and will subsequently activate the ULK kinase activity leading to the phosphorylation and activation of Atg13 and FIP200. Then, a complex composed of Beclin-1, class-III-phosphatidylinositol-3-kinase (PICK3C3) and Atg14, will be recruited by the ULK signalling pathway14 leading to the activation of the first two ubiquitin-like pathways, which are responsible for vesicle formation.15 The first of these, Atg12-Atg5 complex, is formed when Atg12 is activated by Atg7 and conjugates with Atg5 prior to the formation of a homodimer together with Atg16L1. This event is a requisite for the second ubiquitin-like pathway initiation (MAPLC3B (I/II)-phosphatidylethanolamine), which starts with the conjugation of MAP1LC3B-I to phosphatidylethanolamine through activation by Atg7, leading to the closure of the autophagosome formation. Once the autophagosome is completed, the Atg-5-Atg12-Atg16L complex dissociates and Atg4 releases MAP1LC3B-II thus allowing the fusion of the phagosome with the lysosome. Completion of this process requires the function of the lysosomal proteins LAMP2, LAMP1 and GTP-binding protein Rab7.16 The degradation of the autophagic load, which will be released into the cytoplasm, takes place in these autophagolysosomes. Nevertheless, it is important to note that mTOR signalling constitutes not the only route towards activate autophagy, since for example inhibition of Inositol 1,4,5-Trisphosphate Receptor Type 1 (ITPR 1) provokes as well an up-regulation of autophagic activity by activating Activate Kinase Alpha 1 Sub-unit (AMPK) in a mTOR-independent manner,17 which will activate the ULK complex. Other autophagy mTOR-independent routes can be revealed by autophagy regulators, which work thorough mTOR-independent pathways, such us threalose, spermidine, TLR7.18–20
Figure 1.
Autophagy overview.
Although autophagy has been considered for long time as an unspecific process for degradation of components of the cytoplasm, recent evidence has revealed that autophagy pathways can differentiate selective load for delivery to the lysosome.21,22 This is guaranteed by the aide of an assistant (p62/SQSTM1), which contains a LC3-interaction domain and an ubiquitin-binding domain, which allows the incorporation of p62 into the autophagosome. p62/SQSTM1 is required for the aggregation of polyubiquitinilated proteins followed by their autophagic degradation.22 During this process, p62 is also efficiently degraded in the autophagosomes, in contrast to autophagy-deficient cells in which p62 is accumulated intracellularly rather than being reduced. Therefore, it can be said that low levels of p62/SQSTM1 correlate with presence of autophagic activity.23
Autophagy in atherosclerosis
The mechanisms involved in atherosclerosis are multifactorial, but it is known that various pro-atherogenic factors (i.e. oxidized lipids, reactive oxygen species, ER stress, cytokines release, hypoxia) can stimulate autophagy during atherosclerosis progression.24 However, while it is accepted that autophagy can be observed in atherosclerosis, its precise role remains poorly understood (Table 1).25 Indications for autophagy in atherosclerosis in vivo are restricted to few studies and have been obtained mainly from animal models,26,27 but Perrota28 reported evidences that autophagy is activated in human cell types (aorta) in cerebrovascular disease. The extent to which autophagy in atherosclerosis may exert ‘detrimental’ or ‘beneficial’ effects has to still be comprehensively clarified, since in the literature, both labels have been associated with atherosclerosis processes.24 Autophagy may initially be an adaptative mechanism occurring in atherosclerotic plaque to recycle cellular damaged components for cell survival, and for that reason, autophagy in atherosclerosis has been traditionally tagged as ‘beneficial’. Autophagic activity is important to (1) maintain the plasticity and survival of vascular smooth muscle cells (VSMC), to (2) control macrophage content in the plaque by autophagic-repair mechanism and to (3) provoke specific cell death (induced by autophagy) in order to reduce the content of macrophages promoting the stability of the atherosclerotic plaque.29,30 However, under conditions of continuous increased oxidative stress, the autophagic machinery may become unable to clear proteins destined for degradation or damaged organelles.31 It can be anticipated that some cell types (i.e. SMCs and macrophages) in asymptomatic stable plaques may benefit from a regular autophagic activity by which the unwanted proteins/foam cells are removed without provoking undesired cellular death. If this regular autophagic activity is not present, for example, in macrophages from mice, cells will be conducted towards apoptotic death causing plaque instability.27 Similarly, when autophagic activity becomes continuously activated in an excessive manner, the plaque may become unstable due to detrimental cell death occurring in some cells, such SMCs and macrophages.20,30 Thus, balanced autophagy activity is needed in atherosclerosis in order to preserve/promote stable atherosclerotic plaques. In a recent study, we showed that human carotid unstable symptomatic plaques exhibit lower expression levels of the autophagic activity marker, MAP1LC3B, compared with stable asymptomatic plaques.32 It could be hypothesized that those unstable plaques probably contained SMCs/macrophages where low levels or even absence of autophagic activity in early stages could have provoked cell death causing plaque disruption. However, testing of additional markers is warranted to ensure this hypothesis.23
Table 1.
Autophagy modifier biomarkers in atherosclerotic cells and/or plaque.
| Gene | Cell type/plaque | Cell origin | Observations | References |
|---|---|---|---|---|
| ATG16L1 | SMCs | Human and mice/carotid | Increased in early phase vs. later phase lesions | Magné et al.33 |
| ATG16L1 | Macrophages | Human/carotid | Increased in foam cells found in late phase lesions | Magné et al.33 |
| Visfatin | Macrophages | Human/carotid | Increased in unstable vs. stable | Dahl et al.34 |
| Visfatin | Plaque | Human/carotid | Increased in unstable vs. stable | Dahl et al.34 |
| MAP1LC3B | Plaque | Human/carotid | Increased in stable plaques | Swaminathan et al.32 |
| MAP1LC3B | Endothelial cells | Human (HUVEC) | Decreased in aging cell vs. normal tissue | Menghini et al.35 |
| BECN1 | Endothelial cells | Human (HUVEC) and carotid plaque | Decreased in aging cell vs. normal tissue | Menghini et al.35 |
| ATG5 | Endothelial cells | Human (HUVEC) | Decreased in aging cell vs. normal tissue | Menghini et al.35 |
| p62 | SMCs | ATG7 knockout mouse model/aorta | Increased in ATG7−/− vs. ATG7+/+ | Grootaert et al.36 |
| p62 | Macrophages | ApoE null mice/aorta | Increased in late stage lesions | De Meyer et al.20 |
| IGF-1 | SMCs | Human/carotid | Protective effect in asymptomatic by inhibition of autophagy | Jia et al.37,38 |
| TLR7 | Macrophages | Human/carotid | Increased in relation with plaque progression | De Meyer et al.20 |
| TLR7 | SMCs | Human/carotid | Not expressed | De Meyer et al.20 |
| TLR7 | Endothelial cells | Human/carotid | Not expressed | De Meyer et al.20 |
| p53 | Plaque | Human/carotid | Increased in unstable plaques | Yuan et al.39 and Miah et al.40 |
| NPY | SMCs | Human/carotid | Increased in unstable plaques | Lagraauw et al.41 |
SMCs: smooth muscle cells.
Role of autophagy in atherosclerotic cell types
Autophagic markers have been identified in the main cell types in atherosclerotic plaques from animals with experimental atherosclerosis (i.e. macrophages, endothelial cells (ECs) and SMCs).26,42
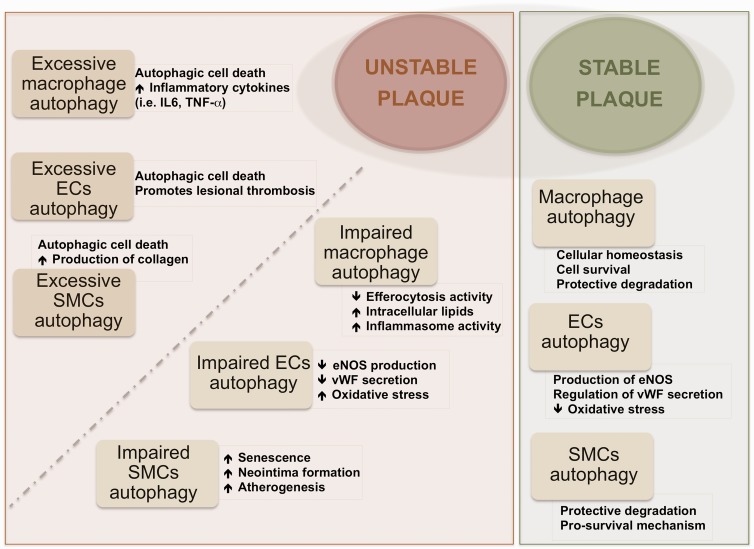
Macrophages are known to play an important role in atherosclerosis. At early stages of atherosclerosis, macrophages are involved in the clearance of cholesterol deposits in vascular tissue. Then, when lipids continue to accumulate inside the cells, macrophages are converted in what is called foam cells. However, macrophage phenotype heterogeneity has to be taken into account for the complete understanding of their clinical significance in atherosclerosis. Various macrophage phenotypes present in human atherosclerotic plaques have been recently reviewed43 and, to the complexity of distinct subpopulations present in the different developmental stages of the plaque, is to be added the specific distribution of subtypes populating the different zones of atheroma plaque. At any rate, macrophage autophagy is known to play a role in atherosclerosis.44 Liao et al.27 reported that inhibition of autophagy in macrophages induced plaque necrosis and destabilization in mice. Also, activation of autophagy in macrophages through mTOR inhibition was reported to lead to stabilization of atherosclerotic plaque in mice.45 These data support also the protective role of autophagic activity in macrophages in atherosclerosis (Figure 2). Macrophage subtype named Mox, presented in murine atherosclerosis different patterns of gene expression than other subtypes (M1, classically classified as pro-inflammatory and M2, anti-inflammatory) including redox-regulated genes; furthermore, Mox showed decreased autophagic activity compared with M1 and M2 in mice.46 Since the Mox type appeared to represent only 30% of the macrophages present in the advanced plaque, caution is needed when interpreting the contribution of macrophage autophagy to atherosclerosis.
Figure 2.
Effects of autophagy on plaque stability.
ECs: endothelial cells; SMCs: smooth muscle cells.
Advanced atherosclerotic plaques have been associated basically with high content of macrophages, whereas unstability of plaques would correlate more strongly with the distinct macrophage phenotypes and their ability to change phenotype than with the quantity of cells. It is indeed likely that in the same plaque different macrophage phenotypes with distinct biological features co-exist, and these may exert contrasting, or even opposing, effects (i.e. macrophages showing autophagic activity or showing deficient autophagy). The overall balance of all the factors playing some role might be determinant of the stability/instability of atherosclerotic plaque. Moore et al.47 reported the capacity of macrophages to promote plaque regression by changing phenotype in atherosclerosis via administration of the M2-driving cytokine IL-13 to mice. At the same time, Tian et al.45 reported that induction of autophagy drives macrophages towards the M2 phenotype. It is known that macrophages with impaired autophagy cannot be cleared by efferocytosis; in contrast, these macrophages become apoptotic, and the inflammasome pathway may be activated leading to lesion progression (Figure 2).48 This thus raises the question whether the observed plaque regression is related with the role of autophagic activity in those specific macrophages.
SMCs are the main component of the vascular system and can experience phenotypic change during atherosclerosis development (i.e. synthetic, macrophage-like or osteochondrogenic phenotypes).48 SMCs are not passive bystanders but are considered crucial player in atherosclerotic processes. Autophagy exerts a critical role in atherosclerosis by regulating vascular SMC phenotype.29 Autophagy within normal levels of activity in SMCs is associated with survival and plaque stability while excessive autophagic activity might lead to SMC death and plaque destabilization as reported in different species.31,49 At early stages of atherosclerosis, exposure of vascular SMCs to relatively modest concentrations of oxLDL was shown to increase autophagy that acts as protective mechanism, whereas exposure to high concentrations, which can happen in later stages of atherosclerosis, resulted in a reduction of protective type of autophagy and augmentation of autophagic-mediated cell death in cells from mice aortas.50 In addition, recently, several studies provided evidence for distinct modes of autophagy induction in vascular SMCs leading to different outcomes. For instance, platelet-derived growth factor, known to be secreted during vascular injury by several cell types, protects against cellular death through activation of autophagy,29 and other factors, such as osteopontin, have been shown to induce autophagic-driven death in vascular cells from human aortas.51 Insulin-like growth factor-1 (IGF-1) promotes cell survival by inhibition of autophagy in SMCs isolated from carotid plaques (Table 1)37; however, this effect was only observed in SMCs from asymptomatic plaques.38 Recently, Grootaert et al.36 observed detrimental effects of autophagy inhibition in vascular SMCs isolated from atg7 knockout animal model (impaired autophagy), which resulted in higher senescence, neointima formation and atherogenesis and as well as accumulation of SQSTM1.
Vascular ECs are one of the most active cells in the human body, and functional changes in these cells are as well correlated with cerebrovascular diseases. It is known that autophagy plays a role in maintaining a correct endothelial function.48 Autophagy in ECs induces eNOS expression, and consequently NO availability would reduce oxidative stress in human cells.52 Also, autophagy regulates the secretion of endothelial von Willebrand factor in human ECs, which is known to play a role in blood coagulation.53 Recent studies have demonstrated that autophagic activity is present also in ECs in atherosclerotic plaques, as implied by high expression levels of the autophagic marker ATG16L1.33,54 Oxidative stress, which occurs in the atherosclerotic environment, has been observed to trigger autophagy also in human endothelial aortic cells.55 Similarly to vascular SMCs and macrophages, vascular ECs exposed to excessive autophagic activity can suffer autophagic-mediated death provoking a disruption in the stability of the plaque.8 Dahl et al.34 have found visfatin expression levels to be increased in instable symptomatic human carotid plaques. Since visfatin induces the PI3K/Akt/mTOR pathway (meaning inhibiting autophagy),56 higher visfatin levels observed in vascular ECs57 could be associated with a decreased autophagic activity.
In summary, evidence is available that the induction of autophagy activity protects against cellular damage in the main cell types involved in atherosclerotic disease (Figure 2).30 The autophagy-associated biomarkers identified so far as differently expressed in unstable and stable plaques are summarized in Table 1.
Conclusions
Recently, autophagy has been receiving considerable attention since its associated pathways may constitute potential targets for therapeutical interference in atherosclerosis disease. Several drugs have been reported that act to either activate or inhibit autophagy activity.58,59 Particularly, mTOR inhibitors (i.e. rapamycin and derivatives) have been tested as potential plaque stabilizing drugs in animal models. For example, Verheye et al.60 found that rapamycin analogue everolimus reduced macrophage content in cholesterol-treated rabbits while SMCs remained unaffected. However, while several works have hallmarked autophagy as a potential strategic therapeutical target,59,60 others have pointed to the harmful effects that stimulation of autophagy may exert on pathogenetic processes.8,20,31 In addition, caution is needed when evaluating the effects of specific drugs used to induce/inhibit autophagy activity (for instance, imiquimod exerts autophagy-independent effects).20 Cell death may be triggered by excessive autophagic activity, such as that observed in macrophages, SMCs and ECs in specific circumstances. Could the unwanted dysfunctional autophagy be targeted to become active again? Several reports have observed that autophagic inducers through mTOR inhibition are capable of reducing plaque progression in advanced atherosclerosis.61
To gain a more coherent understanding of the role of autophagy in stable or unstable carotid atherosclerotic plaque, it is essential to take into consideration the following variables: (1) the diverse cell phenotypes existing in the plaque at different stages; (2) the specific morphology and stage of the plaque (stable/unstable); (3) the autophagy activity level (inexistent/regular/excessive) found in the distinct phenotypes and in different plaque types; (4) the specific autophagy pathway/s involved in each specific cell type or phenotype, type of plaque (i.e. mTOR dependent or independent).
Acknowledgements
None.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Guarantor
IA.
Contributorship
IA researched literature and conceived the report. IA wrote the first draft of the manuscript. KV was involved in the first draft reviewing. IA, HG, KV and MMF reviewed and approved the final version of the manuscript.
References
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011; 473: 317–325. [DOI] [PubMed] [Google Scholar]
- 2.Tibæk M, Dehlendorff C, Jørgensen HS, et al. Increasing incidence of hospitalization for stroke and transient ischemic attack in young adults: a registry-based study. J Am Heart Assoc 2016; 5: e003158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2197–2223. [DOI] [PubMed] [Google Scholar]
- 4.Virmani R, Burke AP, Farb A, et al. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006; 47: C13–C18. [DOI] [PubMed] [Google Scholar]
- 5.NASCET Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1991; 325: 445–453. [DOI] [PubMed] [Google Scholar]
- 6.Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: 3754–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spence JD, Naylor AR. Endarterectomy, stenting, or neither for asymptomatic carotid-artery stenosis. N Engl J Med 2016; 374: 1087–1088. [DOI] [PubMed] [Google Scholar]
- 8.Martinet W, De Meyer GRY. Autophagy in atherosclerosis: a cell survival and death phenomenon with therapeutic potential. Circ Res 2009; 104: 304–317. [DOI] [PubMed] [Google Scholar]
- 9.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 2004; 6: 463–477. [DOI] [PubMed] [Google Scholar]
- 10.Bergmann A. Autophagy and cell death: no longer at odds. Cell 2007; 131: 1032–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizushima N, Yoshimori T, Ohsumi Y. The role of atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 2011; 27: 107–132. [DOI] [PubMed] [Google Scholar]
- 12.Hosokawa N, Hara T, Kaizuka T, et al. Nutrient-dependent mTORC1 association with the ULK1–Atg13–FIP200 complex required for autophagy. Mol Biol Cell 2009; 20: 1981–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol 2010; 22: 132–139. [DOI] [PubMed] [Google Scholar]
- 14.Russell RC, Tian Y, Yuan H, et al. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol 2013; 15: 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng J, Klionsky DJ. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. EMBO Rep 2008; 9: 859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jäger S, Bucci C, Tanida I, et al. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci 2004; 117: 4837–4848. [DOI] [PubMed] [Google Scholar]
- 17.Decuypere J-P, Bultynck G, Parys JB. A dual role for Ca2+ in autophagy regulation. Cell Calcium 2011; 50: 242–250. [DOI] [PubMed] [Google Scholar]
- 18.Sarkar S, Davies JE, Huang Z, et al. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and α-synuclein. J Biol Chem 2007; 282: 5641–5652. [DOI] [PubMed] [Google Scholar]
- 19.Eisenberg T, Knauer H, Schauer A, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol 2009; 11: 1305–1314. [DOI] [PubMed] [Google Scholar]
- 20.De Meyer I, Martinet W, Schrijvers DM, et al. Toll-like receptor 7 stimulation by imiquimod induces macrophage autophagy and inflammation in atherosclerotic plaques. Basic Res Cardiol 2012; 107: 1–13. [DOI] [PubMed] [Google Scholar]
- 21.Stolz A, Ernst A, Dikic I. Cargo recognition and trafficking in selective autophagy. Nat Cell Biol 2014; 16: 495–501. [DOI] [PubMed] [Google Scholar]
- 22.Rogov V, Dötsch V, Johansen T, et al. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol Cell 2014; 53: 167–178. [DOI] [PubMed] [Google Scholar]
- 23.Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2016; 12: 1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinet W, De Meyer I, Verheye S, et al. Drug-induced macrophage autophagy in atherosclerosis: for better or worse? Basic Res Cardiol 2013; 108: 1–11. [DOI] [PubMed] [Google Scholar]
- 25.Lavandero S, Chiong M, Rothermel BA, et al. Autophagy in cardiovascular biology. J Clin Invest 2015; 125: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Razani B, Feng C, Coleman T, et al. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab 2012; 15: 534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao X, Sluimer JC, Wang Y, et al. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab 2012; 15: 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perrotta I. Ultrastructural features of human atherosclerosis. Ultrastruct Pathol 2013; 37: 43–51. [DOI] [PubMed] [Google Scholar]
- 29.Salabei JK, Hill BG. Implications of autophagy for vascular smooth muscle cell function and plasticity. Free Radic Biol Med 2013; 65: 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrijvers DM, De Meyer GRY, Martinet W. Autophagy in atherosclerosis: a potential drug target for plaque stabilization. Arterioscler Thromb Vasc Biol 2011; 31: 2787–2791. [DOI] [PubMed] [Google Scholar]
- 31.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest 2005; 115: 2679–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swaminathan B, Goikuria H, Vega R, et al. Autophagic marker MAP1LC3B expression levels are associated with carotid atherosclerosis symptomatology. PLoS One 2014; 9: e115176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magné J, Gustafsson P, Jin H, et al. ATG16L1 expression in carotid atherosclerotic plaques is associated with plaque vulnerability. Arterioscler Thromb Vasc Biol 2015; 35: 1226–1235. [DOI] [PubMed] [Google Scholar]
- 34.Dahl TB, Yndestad A, Skjelland M, et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis possible role in inflammation and plaque destabilization. Circulation 2007; 115: 972–980. [DOI] [PubMed] [Google Scholar]
- 35.Menghini R, Casagrande V, Marino A, et al. MiR-216a: a link between endothelial dysfunction and autophagy. Cell Death Dis 2014; 5: e1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grootaert MOJ, da Costa Martins PA, Bitsch N, et al. Defective autophagy in vascular smooth muscle cells accelerates senescence and promotes neointima formation and atherogenesis. Autophagy 2015; 11: 2014–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia G, Cheng G, Gangahar DM, et al. Insulin-like growth factor-1 and TNF-alpha regulate autophagy through c-jun N-terminal kinase and Akt pathways in human atherosclerotic vascular smooth cells. Immunol Cell Biol 2006; 84: 448–454. [DOI] [PubMed] [Google Scholar]
- 38.Jia G, Cheng G, Agrawal DK. Differential effects of insulin-like growth factor-1 and atheroma-associated cytokines on cell proliferation and apoptosis in plaque smooth muscle cells of symptomatic and asymptomatic patients with carotid stenosis. Immunol Cell Biol 2006; 84: 422–429. [DOI] [PubMed] [Google Scholar]
- 39.Yuan X-M, Osman E, Miah S, et al. p53 expression in human carotid atheroma is significantly related to plaque instability and clinical manifestations. Atherosclerosis 2010; 210: 392–399. [DOI] [PubMed] [Google Scholar]
- 40.Miah S, Zadeh SNM, Yuan X-M, et al. Expression of Egr1 and p53 in human carotid plaques and apoptosis induced by 7-oxysterol or p53. Exp Toxicol Pathol 2013; 65: 677–682. [DOI] [PubMed] [Google Scholar]
- 41.Lagraauw HM, Westra MM, Bot M, et al. Vascular neuropeptide Y contributes to atherosclerotic plaque progression and perivascular mast cell activation. Atherosclerosis 2014; 235: 196–203. [DOI] [PubMed] [Google Scholar]
- 42.Zhang T, Tian F, Wang J, et al. Endothelial cell autophagy in atherosclerosis is regulated by miR-30-mediated translational control of ATG6. Cell Physiol Biochem 2015; 37: 1369–1378. [DOI] [PubMed] [Google Scholar]
- 43.Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev 2014; 262: 153–166. [DOI] [PubMed] [Google Scholar]
- 44.Ouimet M, Franklin V, Mak E, et al. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab 2011; 13: 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian F, Yu B, Hu J. mTOR mediates the cross-talk of macrophage polarization and autophagy in atherosclerosis. Int J Cardiol 2014; 177: 144–145. [DOI] [PubMed] [Google Scholar]
- 46.Kadl A, Meher AK, Sharma PR, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res 2010; 107: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 2013; 13: 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Meyer GRY, Grootaert MOJ, Michiels CF, et al. Autophagy in vascular disease. Circ Res 2015; 116: 468–479. [DOI] [PubMed] [Google Scholar]
- 49.De Meyer GRY, Martinet W. Autophagy in the cardiovascular system. Biochim Biophys Acta Mol Cell Res 2009; 193: 1485–1495. [DOI] [PubMed] [Google Scholar]
- 50.Ding Z, Wang X, Schnackenberg L, et al. Regulation of autophagy and apoptosis in response to ox-LDL in vascular smooth muscle cells, and the modulatory effects of the microRNA hsa-let-7g. Int J Cardiol 2013; 168: 1378–1385. [DOI] [PubMed] [Google Scholar]
- 51.Zheng Y-H, Tian C, Meng Y, et al. Osteopontin stimulates autophagy via integrin/CD44 and p38 MAPK signaling pathways in vascular smooth muscle cells. J Cell Physiol 2012; 227: 127–135. [DOI] [PubMed] [Google Scholar]
- 52.LaRocca TJ, Henson GD, Thorburn A, et al. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol 2012; 590: 3305–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torisu T, Torisu K, Lee IH, et al. Autophagy regulates endothelial cell processing, maturation and secretion of von Willebrand factor. Nat Med 2013; 19: 1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muller C, Salvayre R, Nègre-Salvayre A, et al. Oxidized LDLs trigger endoplasmic reticulum stress and autophagy: prevention by HDLs. Autophagy 2011; 7: 541–543. [DOI] [PubMed] [Google Scholar]
- 55.Shen W, Tian C, Chen H, et al. Oxidative stress mediates chemerin-induced autophagy in endothelial cells. Free Radic Biol Med 2013; 55: 73–82. [DOI] [PubMed] [Google Scholar]
- 56.Wang P, Guan Y-F, Du H, et al. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy 2012; 8: 77–87. [DOI] [PubMed] [Google Scholar]
- 57.Adya R, Tan BK, Punn A, et al. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc Res 2008; 78: 356–365. [DOI] [PubMed] [Google Scholar]
- 58.Fleming A, Noda T, Yoshimori T, et al. Chemical modulators of autophagy as biological probes and potential therapeutics. Nat Chem Biol 2011; 7: 9–17. [DOI] [PubMed] [Google Scholar]
- 59.Vindis C. Autophagy: an emerging therapeutic target in vascular diseases. Br J Pharmacol 2015; 172: 2167–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verheye S, Martinet W, Kockx MM, et al. Selective clearance of macrophages in atherosclerotic plaques by autophagy. J Am Coll Cardiol 2007; 49: 706–715. [DOI] [PubMed] [Google Scholar]
- 61.Martinet W, De Loof H, De Meyer GRY. mTOR inhibition: a promising strategy for stabilization of atherosclerotic plaques. Atherosclerosis 2014; 233: 601–607. [DOI] [PubMed] [Google Scholar]