Abstract
Background
The aim of this study was to compare the alterations in intraocular pressure (IOP) values during the early postoperative period after intravitreal ranibizumab, aflibercept, or dexamethasone implant injections.
Material/Methods
In this retrospective study, a total of 188 patients were grouped into 3 groups: the ranibizumab group, the aflibercept group, and the dexamethasone group. Ocular axial length (AXL) and anterior chamber depth (ACD) were measured in the pre-injection period. IOP was measured just before the injection at 1 minute,10 minutes, 1 hour, 1 day, and 1 month after injection.
Results
There was a transient peak in the ranibizumab group and the aflibercept group at 1 minute that started to decrease at 10 minutes and IOP values returned to preoperative values at approximately 1 hour. Similar alterations were also determined for the dexamethasone group with a lesser increase noted. In the correlation analysis, only alterations in IOP levels at 1 minute were negatively correlated with preoperative AXL values. There was not any correlation between preoperative AXL or ACD values and IOP alterations at any other time points.
Conclusions
There was a sudden, transient increase in IOP values after intravitreal ranibizumab or aflibercept injections; which return to normal values in a short time without requirement of any medical treatments. This transient peak was determined to be negatively correlated with the preoperative AXL.
MeSH Keywords: Axial Length, Eye; Intraocular Pressure; Intravitreal Injections
Background
Nowadays, intravitreal anti-vascular endothelial growth factors (VEGFs), such as ranibizumab, aflibercept, and bevacizumab injection therapy, are commonly used to treat many disorders associated with inflammation such as retinal vein occlusion, age-related macular degeneration, and diabetic macular edema [1,2]. Owing to their large clinical usage, there is accumulating data about the adverse effects of intravitreal anti-VEGF injections [3].
Intravitreal injection of anti-VEGF agents was associated with an increase in the intraocular pressure (IOP), most probably due to volume effect [4]. Transient increases in IOP values have been defined commonly in especially the first 30 minutes after injection that were returned to baseline levels in follow-ups [5–7]. On the other hand, sustained IOP elevations might also be seen after anti-VEGF injections especially after multiple injections in patients with a history of glaucoma [8]. Moreover, in patients with age-related macular degeneration, the risk of sustained IOP elevations was also reported to be increased [9,10].
In this study, we aimed to compare the alterations in IOP values during early postoperative period after intravitreal ranibizumab, aflibercept, and dexamethasone implant injections; and to determine the association of IOP alterations with preoperative axial length (AXL) and anterior chamber depth (ACD). To the best of our knowledge, the data comparing these 3 agents in the literature is limited. We aimed to define a prognostic marker in preoperative period that might aid us to define the patients who require IOP follow-up after intravitreal anti-VEGF injections.
Material and Methods
The study was approved by the local ethics committee of Erzincan University Clinical Research Ethics Committee Erzincan, Turkey (Ethics Committee Number: 33216249-604.01.02-E.47674, Date: November 22, 2016). The study was performed in Erzincan Mengücekgazi Education and Research Hospital between January 2016 and November 2016
In this retrospective study, a total of 188 patients (106 male and 82 female) who were scheduled for intra-vitreal ranibizumab, aflibercept, or dexamethasone implant injections and who did not have any intravitreal injections before were included. Patients with the history of glaucoma, history of ocular hypertension, patients with preop IOP bigger than 22 mm Hg, corneal diseases affecting IOP measurement, narrow iridocorneal angle, and patients having any surgical or medical ocular treatment were excluded from the study. Patients with the diagnosis of age-related macular degeneration, central retinal vein thrombosis, branch retinal vein obstruction, or diabetic retinopathy who were having edema or leakage on macula in fundus fluorescein angiography and optical coherence tomography were included in the study. The patients were grouped into 3 groups: the ranibizumab group (n=81; 0.5 mg/0.05 mL ranibizumab was injected), the aflibercept group (n=53; 2 mg/0.05 mL aflibercept was injected) and the dexamethasone group (n=54; 0.7 mg dexamethasone implant was injected). All patients underwent a detailed ocular examination. Ocular AXL and ACD were measured in pre-injection period with optic biometry (AL Scan, Nidek). IOP was measured with i-care tonometry (Icare TAO1i, Icare Finland) just before the injection at 1 minute, 10 minutes, 1 hour, 1 day, and 1 month after injection in a sitting position. All of the drugs were injected by one experienced surgeon in the operating room. After topical anesthetic proparacaine 0.5% (Alcaine, Alcon-Belgium) a solution of 10% povidone iodine was used for ocular disinfection. Sterile drape and lid speculum were applied before injection. Ranibizumab and aflibercept injections were performed using a 30-gauge needle; dexamethasone implant was performed using a special 23-gauge needle from superotemporal pars plana at 4 mm from the limbus throughout the displaced conjunctiva in a tunneled way. After that the injection site was compressed by a rigid sponge at least 5 seconds to prevent vitreous or drug reflux. A subconjunctival blep formation at the injection site was considered positive for reflux. And all reflux positive patients were excluded from the study. After the injection light perception was checked in all patients.
Statistical analyses
Statistical analyses were performed using SPSS software version 21.0 (SPSS Inc, Chicago, IL, USA). Descriptive statistics for the continuous variables were presented as mean ± standard deviation; while count and percentages for categorical variables. One-way ANOVA was used to compare the group means. Tukey multiple comparison test was also used to identify different group means followed by ANOVA. In addition, chi-square test was performed to determine the relationship between categorical variables. Pearson correlation analysis was performed to define the association of IOP alterations with the AXL and ACD. Statistical significance level was considered as 0.05.
Results
A total of 188 patients who were treated with 3 different intravitreal injections were included in the study. There was not any statistically significant difference regarding age or gender among the 3 groups (Table 1). Preoperative diagnoses of patients were age-related macular degeneration (49 patients in ranibizumab group and 33 patients in aflibercept group), central retinal vein thrombosis (6 patients in aflibercept group and 40 patients in dexamethasone group), branch retinal vein obstruction (17 patients in ranibizumab group, 14 patients in aflibercept group and 7 patients in dexamethasone group) and diabetic retinopathy (15 patients in ranibizumab group and 7 patients in dexamethasone group).
Table 1.
Demographic features, preoperative AXL and ACD measurements and IOP values at different time periods during study.
| Ranibizumab (n: 81) | Aflibercept (n: 53) | Dexamethasone (n: 54) | p | |
|---|---|---|---|---|
| Age | 62.94±10.07 | 65.70±10.59 | 59.47±8.76 | 0.08 |
| Gender (M/F) | 40/41 | 30/23 | 36/18 | 0.16 |
| Right/left | 52/29 | 32/21 | 32/22 | 0.84 |
| Preop AXL | 22.95±.73 | 22.94±0.82 | 23.31±0.79 | 0.51 |
| Preop ACD | 3.08±0.35 | 2.99±0.40 | 3.11±0.25 | 0.32 |
| Preop IOP | 15.14±2.95 | 15.64±3.18 | 16.13±3.13 | 0.29 |
| 1st min IOP | 37.20±6.41 | 36.75±5.98 | 22.93±3.44 | 0.001 |
| 10th min IOP | 23.62±5.77 | 22.96±5.46 | 18.78±2.71 | 0.004 |
| 1st hour IOP | 18.22±3.67 | 17.92±4.31 | 16.96±2.74 | 0.48 |
| 1st day IOP | 15.83±3.20 | 14.83±2.68 | 16.09±2.38 | 0.18 |
| 1st month IOP | 15.23±2.16 | 15.04±2.01 | 15.31±2.05 | 0.32 |
M – Male; F – Female; Preop – preoperative; AXL – axial length; ACD – anterior chamber depth; IOP – intraocular pressure.
In ranibizumab or aflibercept injected groups; IOP was not over 40 mm Hg in any patients and in dexamethasone implant group, IOP was not higher than 25 mm Hg in any patients. There were not any problems observed in any patients in the control of retinal artery occlusion immediately performed after injection in all patients. Therefore, in any patients included in the study, postoperative paracentesis or anti-glaucomatous medication were not required (Figure 1).
Figure 1.
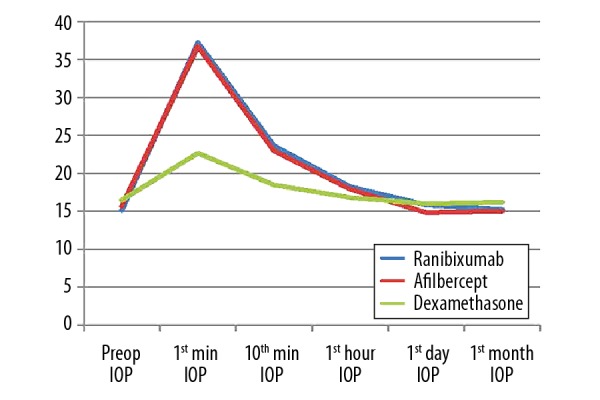
Alterations in IOP levels in different treatment groups.
Preop AXL or preop ACD values were also not significantly different among the 3 groups. IOP values at different time periods are summarized in Table 1. Regarding these data, in the post-injection period, in the dexamethasone group the IOP values were significantly lower compared with the other 2 groups at 1 minute and at 10 minutes. There was not any significant difference between the groups regarding the IOP levels obtained on later periods.
Alterations in IOP levels are summarized in Figure 1 and Table 2. Regarding these data, there was a transient peak in ranibizumab and aflibercept groups on the first minute that started to decrease at 10 minutes and IOP values returned to preoperative values in approximately 1 hour. Similar alterations were also determined on dexamethasone group with a lesser increase. The differences compared with the preoperative period, at 1 minute, 10 minutes, and 1 hour were statistically significant lower in dexamethasone group compared with the other 2 groups. At those 3 time points, the IOP alterations were not statistically significantly different between the ranibizumab group and the aflibercept group. On the first day measurements, regarding the alterations in IOP values compared with the preoperative data; in the aflibercept group and the dexamethasone group there was a decrease in mean IOP levels, but there was an increase in the ranibizumab group; however, the differences were statistically significant only between the ranibizumab group and the aflibercept group. When the differences between the IOP values on thirtieth day (1 month) and preoperative data were compared among the 3 groups, there was not any statistically significant difference.
Table 2.
Alterations in IOP values among different treatment groups during study period.
| Ranibizumab (n: 81) | Aflibercept (n: 53) | Dexamethasone (n: 54) | p | |
|---|---|---|---|---|
| ΔIOP 1 | 22.04±6.79 | 21.11±6.51 | 6.15±3.42 | <0.001 |
| ΔIOP 2 | 8.46±5.68 | 7.32±5.66 | 2.12±2.04 | <0.001 |
| ΔIOP 3 | 3.07±3.98 | 2.28±3.67 | 0.36±3.42 | 0.034 |
| ΔIOP 4 | 0.67±2.87 | −0.81±2.90 | −0.44±1.82 | 0.01 |
| ΔIOP 5 | 0.08±2.58 | −0.60±2.47 | −0.28±2.16 | 0.32 |
ΔIOP 1 – the difference between the IOP values on 1st minute and preoperative data; ΔIOP 2 – the difference between the IOP values on 10th minute and preoperative data; ΔIOP 3 – the difference between the IOP values on 1st hour and preoperative data; ΔIOP 4 – the difference between the IOP values on 1st day and preoperative data; ΔIOP 5 – the difference between the IOP values on 1st month and preoperative data.
When the alterations in IOP values were investigated among different disease groups, only the ΔIOP 1 values (the difference between the IOP values at 1 minute and preoperative data) were statistically significantly lower in patients with central retinal vein thrombosis compared with the other diseases (P: 0.001). There was not any statistically significant difference between different diseases groups regarding IOP alterations (Table 3).
Table 3.
Alterations in IOP values among different preoperative disease groups during study period.
| AMD | CRVT | BRVO | DRP | P | |
|---|---|---|---|---|---|
| ΔIOP 1 | 22.45±6.76 | 10.68±8.12 | 19.64±6.22 | 18.82±9.12 | 0.001 |
| ΔIOP 2 | 7.69±5.51 | 5.22±7.24 | 8.24±4.68 | 6.24±5.74 | 0.22 |
| ΔIOP 3 | 2.45±3.52 | 2.12±3.23 | 3.46±3.24 | 1.18±5.42 | 0.26 |
| ΔIOP 4 | 0.20±2.58 | −1.12±3.14 | −0.06±2.14 | 0.42±4.34 | 0.34 |
| ΔIOP 5 | −0.09±2.55 | −0.74±2.44 | −0.24±1.68 | −0.12±3.32 | 0.82 |
ΔIOP 1 – the difference between the IOP values on 1st minute and preoperative data; ΔIOP 2 – the difference between the IOP values on 10th minute and preoperative data; ΔIOP 3 – the difference between the IOP values on 1st hour and preoperative data; ΔIOP 4 – the difference between the IOP values on 1st day and preoperative data; ΔIOP 5 – the difference between the IOP values on 1st month and preoperative data.
In correlation analysis, only alterations in IOP levels at first minute were negatively correlated with preoperative AXL values. There was not any correlation between preoperative AXL or ACD values and IOP alterations at any other time points (Table 4).
Table 4.
Correlation analysis between preoperative AXL or ACD levels and IOP alterations.
| DIOP 1 | DIOP 2 | DIOP 3 | DIOP 4 | DIOP 5 | |
|---|---|---|---|---|---|
| Preop AXL | |||||
| r | −0.44 | −0.09 | −0.12 | −011 | −011 |
| p | 0.001 | 0.31 | 0.12 | 0.12 | 0.12 |
| Preop ACD | |||||
| r | 0.01 | −0.05 | 0.09 | −0.06 | 0.06 |
| p | 0.84 | 0.58 | 0.28 | 0.93 | 0.64 |
Preop – preoperative; AXL – axial length; ACD – anterior chamber depth; IOP – intraocular pressure.
Discussion
In this study, we investigated the alterations in IOP values after anti-VEGF or corticosteroid injections and determined that there was a transient peak in all groups on the first minute which returned to preoperative values in approximately 1 hour. After anti-VEGF injections, the alterations in IOP at 1 minute, 10 minutes, and 1 hour were significantly higher than that of the dexamethasone group. In correlation analysis, only alterations at 1 minute were negatively correlated with the preoperative AXL values; there were not any other significant correlations between IOP alterations and preoperative AXL or ACD values at any time periods. To the best of our knowledge, the data comparing these 3 drugs is limited in the literature. After injection of these drugs, the complaints of the patients are generally reported in the first minutes and for that reason alterations in IOP in the first minutes were investigated. When we compared the 3 medications, we found a similar increase in IOP after the acute phase. This showed us that this increase was not related to the drug substance, suggesting that there was no difference between these 3 drugs in terms of safety except for the group of risky patients.
Volume of injected fluid, method of application (with or without tunnel), amount of reflux, size of needle, scleral thickness, eye size, posterior vitreous detachment, and vitreous liquefaction have been shown to be the main determinants of IOP alterations after intravitreal injections [11,12]. Similar with our results, transient alterations in IOP values after anti-VEGF injections have been reported before. Omay et al. [13] reported significant increases in IOP values at the end of 30 minutes after bevacizumab or ranibizumab injections which returned to pre-injection values at the end of the first day. In the same way, Trehan et al. [14] reported a transient rise in IOP immediately after the intravitreal injection of anti-VEGF agents that returned to normal within 30 minutes in all cases. Lee et al. [15] also defined a transient increase in IOP just after intravitreal injection of anti-VEGF agents which normalized in 30 minutes without any significant effects on retinal blood flow. Lim et al. [16] reported significant alterations in IOP in the first 25 minutes after intravitreal injections, which were correlated with the alterations in visual acuity. In the same way, Pece et al. [17] reported that after intravitreal injection of ranibizumab, the mean IOP at baseline was significantly higher than at both 5 minutes and 60 minutes, and at 5 minutes, IOP spikes >40 mm Hg were determined in 18 of 50 patients. We did not determine a peak of >40 mm Hg in our study at any time. In light of these data, we suggest that transient alterations in IOP values is common after intravitreal anti-VEGF injections but frequent IOP monitoring or any prophylactic treatment is not required in a normal healthy population.
We determined a negative correlation between the IOP alterations on the first day and preoperative AXL. Hollands et al. [18] reported a considerable short-term transient rise in IOP at 0 to 30 minutes after intravitreal injection of ranibizumab in both phakic and pseudophakic eyes; they also reported a significant relationship between shorter AXL and IOP increase after 5 seconds. Cacciamani et al. [19] defined that every 1 mm shortening in AXL caused a 5 mm Hg increase in IOP. However, Hoang et al. [20] did not determine AXL as a predictor of transient or sustained IOP elevation in patients with age-related macular degeneration receiving anti-VEGF injections. Similarly, Fuest et al. [21] did not determine any significant associations between AL or scleral thickness and early IOP elevations in their study; but they also included the patients with reflux which was reported as a limitation of that study. In concordance with our study, they reported that there were also not any associations between AC depth and IOP alterations. Thus, we can say that shorter eyes have an augmented IOP increase in the first few minutes compared with longer ones. A simple measurement of refraction might give an idea about the AXL and by this way, in patients with hyperopic error having shorter AXL, higher IOP alterations might be expected.
IOP elevations in late periods after ranibizumab or aflibercept injections are due to toxic effects of these drugs on the trabecular meshwork, uveascleral pathway, or schlemm channel [22,23]. However, acute IOP increases as reported in our study were mainly due to volume enhancing effects of these drugs and therefore were recovered in a very short-time period.
Dexamethasone is a corticosteroid with great anti-inflammatory activity having different pharmacologic activity, lipid solubility, and delivery requirements compared with triamcinolone [24]. Dexamethasone is less lipophilic and does not accumulate greatly in the trabecular meshwork, and for that reason carries a lower risk of IOP increases [25,26]. In the literature, the number of studies investigating the effects of dexamethasone implants on IOP levels in acute periods is limited. In a recent study, it was reported that in especially patients with glaucoma suspicion and steroid response, IOP elevations were determined in the first 2 weeks after implantation [27]. Similar with our results, Theodoropulos et al. [28] also reported that after dexamethasone implantation, IOP increases were short-lived, moderate in severity, and readily managed with IOP lowering drugs. In the study of Alagoz et al. [29], after implantation, there was a decrease reported on the first minute in patients with reflux, while there were not any significant alterations in patients without reflux. In our study, we excluded the patients with reflux and we determined a significant elevation in IOP levels after dexamethasone implantation; which was not as high as seen in the ranibizumab group or the aflibercept group. We believe that this increase was not associated with the physical and chemical properties of dexamethasone that play important roles in late effects of the drug but instead it was due to the volume enhancing effect as seen in the other 2 drug groups. As a matter of fact, in this group, the IOP values recovered in a very short period like the other 2 groups.
One other interesting finding in our study was that the alterations in IOP values in the first minute among patients with central retinal vein thrombosis were significantly lower than those of patients treated for age-related macular degeneration, branch retinal vein obstruction, or diabetic retinopathy. In long-term results, any significant differences regarding IOP values between patients with central retinal vein thrombosis or branch retinal vein obstruction was not reported before [30]. But age-related macular degermation was regarded as a risk factor for IOP alterations after intravitreal injections [31]. Larger, clinical studies are warranted to elucidate the different IOP responses after anti-VEGF injections in different disease groups.
During intravitreal injections, in order to block the reflux and give the entire drug into the eye, many eye surgeons prefer to enter the eye by creating a tunnel on the sclera and massage the wound area after injection; to prevent the postoperative hypotonia and to prevent the contamination from the open wound site. In our study, we showed that even in the not-at-risk patient group, all 3 drug groups caused IOP increases in the acute phase. Therefore, more increases can be expected in risky patients. In previous studies, these IOP increases were associated with blockage of axonal transport or vascular occlusive events [32,33]. It should therefore be kept in mind that these drugs might cause eye damage in the early periods as well as known long-term IOP increases and especially those at-risk patients should be followed more closely for IOP increases from early periods on, and should be evaluated for preventive measures such as medications reducing IOP or postoperative paracentesis when required.
There were some limitations in our study that should be emphasized. We could not evaluate the scleral thickness or vitreal conditions since our study was retrospective and those data were not present in our patient records. Moreover, our results were obtained after the first injection of anti-VEGF agents or dexamethasone implant; however, in clinical practice most of the patients required repeated injections and the effects of these repeated injections should also be evaluated.
Conclusions
We determined that there was a sudden, transient increase in IOP values after intravitreal ranibizumab or aflibercept injections, which return to normal values without requirement of any medical treatments in a short-time period. This transient peak was determined to be negatively correlated with the preoperative AXL. Because of the short time to return to normal IOP levels and the similar IOP effects of the 3 drugs, we suggest that the increase in IOP determined in the acute period was due to the volume enhancing effect of the drugs but not to the drug substance. Among the parameters examined, only a negative correlation was found between AL and IOP in very early post-injection periods. This IOP increase returned to normal without the need for any intervention in the group of patients we examined. We might not have encountered a situation that required any intervention because we did not investigate a group of risky patients in terms of IOP increase. However, care should be taken especially in patients with risky eyes in terms of IOP increase, as well as eyes with very short AXLs. Larger prospective studies are warranted to define the effects of preoperative AXL on short-term and long-term alterations in IOP levels in both high- and low-risk patients after intravitreal anti-VEGF injections or dexamethasone implantations.
Footnotes
This study was presented as a poster presentation on 50th National Congress of Turkish Ophthalmology Society
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.CATT Research Group. Martin DF, Maguire MG, Ying GS, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee YJ, Park HJ, Lee TG, et al. Primary combined photodynamic therapy and intravitreal bevacizumab injection for neovascular age-related macular degeneration. J Korean Ophthalmol Soc. 2010;51(1):35–41. [Google Scholar]
- 3.Sampat KM, Garg SJ. Complications of intravitreal injections. Curr Opin Ophthal. 2010;21(3):178–83. doi: 10.1097/ICU.0b013e328338679a. [DOI] [PubMed] [Google Scholar]
- 4.Hollands H, Wong J, Bruen R, et al. Short-term intraocular pressure changes after intravitreal injection of bevacizumab. Can J Ophthalmol. 2007;42(6):807–11. doi: 10.3129/i07-172. [DOI] [PubMed] [Google Scholar]
- 5.Kim JE, Mantravadi AV, Hur EY, Covert DJ. Short-term intraocular pressure changes immediately after intravitreal injections of anti-vascular endothelial growth factor agents. Am J Ophthalmol. 2008;146(6):930–34.e1. doi: 10.1016/j.ajo.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Falkenstein IA, Cheng L, Freeman WR. Changes of intraocular pressure after intravitreal injection of bevacizumab (avastin) Retina. 2007;27(8):1044–47. doi: 10.1097/IAE.0b013e3180592ba6. [DOI] [PubMed] [Google Scholar]
- 7.Kim YJ, Sung KR, Lee KS, et al. Long-term effects of multiple intravitreal antivascular endothelial growth factor injections on intraocular pressure. Am J Ophthalmol. 2014;157:1266–71.e1. doi: 10.1016/j.ajo.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 8.Mazzulla DA, Hariprasad SM, Jager RD, Mieler WF. Short-term intraocular pressure trends after intravitreal injection of bevacizumab (avastin) Retin Cases Brief Rep. 2008;2:234–35. doi: 10.1097/ICB.0b013e31815e9409. [DOI] [PubMed] [Google Scholar]
- 9.Hoang QV, Mendonca LS, Della Torre KE, et al. Effect on intraocular pressure in patients receiving unilateral intravitreal anti-vascular endothelial growth factor injections. Ophthalmology. 2012;119(2):321–26. doi: 10.1016/j.ophtha.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Segal O, Ferencz JR, Cohen P, et al. Persistent elevation of intraocular pressure following intravitreal injection of bevacizumab. Isr Med Assoc J. 2013;15:352–55. [PubMed] [Google Scholar]
- 11.Kotliar K, Maier M, Bauer S, et al. Effect of intravitreal injections and volume changes on intraocular pressure: Clinical results and biomechanical model. Acta Ophthalmol Scand. 2007;85:777–81. doi: 10.1111/j.1600-0420.2007.00939.x. [DOI] [PubMed] [Google Scholar]
- 12.Frenkel MP, Haji SA, Frenkel RE. Effect of prophylactic intraocular pressure-lowering medication on intraocular pressure spikes after intravitreal injections. Arch Ophthalmol. 2010;128:1523–27. doi: 10.1001/archophthalmol.2010.297. [DOI] [PubMed] [Google Scholar]
- 13.Omay E, Elgin U, Sen E, Yilmazbas P. The early effects of intravitreal anti vascular endothelial growth factor agents on intraocular pressure and central corneal thickness. Int Ophthalmol. 2016;36(5):665–70. doi: 10.1007/s10792-016-0171-1. [DOI] [PubMed] [Google Scholar]
- 14.Trehan HS, Kaushik J, Rangi A, et al. Anterior segment changes on ultrasound biomicroscopy after intravitreal anti vascular endothelial growth factor injection. Med J Armed Forces India. 2017;73(1):58–64. doi: 10.1016/j.mjafi.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JW, Park H, Choi JH, et al. Short-term changes of intraocular pressure and ocular perfusion pressure after intravitreal injection of bevacizumab or ranibizumab. BMC Ophthalmol. 2016;16:69. doi: 10.1186/s12886-016-0255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim HB, Kim MS, Jo YJ, Kim JY. Short-term visual acuity and intraocular pressure changes and their correlation after anti-vascular endothelial growth factor injection. Ophthalmologica. 2016;236(1):36–42. doi: 10.1159/000445038. [DOI] [PubMed] [Google Scholar]
- 17.Pece A, Allegrini D, Montesano G, Dimastrogiovanni AF. Effect of prophylactic timolol 0.1% gel on intraocular pressure after an intravitreal injection of ranibizumab: A randomized study. Clin Ophthalmol. 2016;10:1131–38. doi: 10.2147/OPTH.S106096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollands H, Wong J, Bruen R, Campbell RJ, et al. Short-term intraocular pressure changes after intravitreal injection of bevacizumab. Can J Ophthalmol. 2007;42(6):807–11. doi: 10.3129/i07-172. [DOI] [PubMed] [Google Scholar]
- 19.Cacciamani A, Oddone F, Parravano M, et al. Intravitreal injection of bevacizumab: changes in intraocular pressure related to ocular axial length. Jpn J Ophthalmol. 2013;57(1):63–67. doi: 10.1007/s10384-012-0194-8. [DOI] [PubMed] [Google Scholar]
- 20.Hoang QV, Jung JJ, Mrejen S, Freund KB. Influence of axial length and postinjection reflux on sustained intraocular pressure elevation as a result of intravitreal anti-vascular endothelial growth factor therapy. Retina. 2014;34(3):519–24. doi: 10.1097/IAE.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 21.Fuest M, Kotliar K, Walter P, Plange N. Monitoring intraocular pressure changes after intravitreal Ranibizumab injection using rebound tonometry. Ophthalmic Physiol Opt. 2014;34:438–44. doi: 10.1111/opo.12134. [DOI] [PubMed] [Google Scholar]
- 22.Bakri SJ, Beer PM. The effect of intravitreal triamcinolone acetonide on intraocular pressure. Ophthalmic Surg Lasers Imaging. 2003;34:386–90. [PubMed] [Google Scholar]
- 23.Sniegowski M, Mandava N, Kahook MY. Sustained intraocular pressure elevation after intravitreal injection of bevacizumab and ranibizumab associated with trabeculitis. Open Ophthalmol J. 2010;4:28–29. doi: 10.2174/1874364101004010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang-Lin JE, Attar M, Acheampong AA, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52:80–86. doi: 10.1167/iovs.10-5285. [DOI] [PubMed] [Google Scholar]
- 25.Edelman JL. Differentiated intraocular glucocorticoids. Ophthalmologica. 2010;224:25–30. doi: 10.1159/000315158. [DOI] [PubMed] [Google Scholar]
- 26.Thakur A, Kadam R, Kompella UB. Trabecular meshwork and lens partitioning of corticosteroids: Implications for elevated intraocular pressure and cataracts. Arch Ophthalmol. 2011;129:914–20. doi: 10.1001/archophthalmol.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivasan R, Sharma U, George R, et al. Sankara Nethralaya Vitreoretinal Study Group (SNVR Study Group) Intraocular pressure changes after dexamethasone implant in patients with glaucoma and steroid responders. Retina. 2017 doi: 10.1097/IAE.0000000000001924. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Alagoz N, Alagoz C, Yılmaz I, et al. Immediate intraocular pressure changes following intravitreal dexamethasone implant. J Ocul Pharmacol Ther. 2016;32:44–49. doi: 10.1089/jop.2015.0087. [DOI] [PubMed] [Google Scholar]
- 29.Papadia M, Misteli M, Jeannin B, Herbort CP. The influence of anti-VEGF therapy on present day management of macular edema due to and CRVO: A longitudinal analysis on visual function, injection time interval and complications. Int Ophthalmol. 2014;34(6):1193–201. doi: 10.1007/s10792-014-0002-1. [DOI] [PubMed] [Google Scholar]
- 30.Wen JC, Reina-Torres E, Sherwood JM, et al. Intravitreal anti-VEGF injections reduce aqueous outflow facility in patients with neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58(3):1893–98. doi: 10.1167/iovs.16-20786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quigley HA, Anderson DR. Distribution of axonal transport blockade by acute intraocular pressure elevation in the primate optic nerve head. Invest Ophthalmol Vis Sci. 1977;16:640–44. [PubMed] [Google Scholar]
- 32.Michelson G, Groh MJ, Langhans M. Perfusion of the juxtapapillary retina and optic nerve head in acute ocular hypertension. Ger J Ophthalmol. 1996;5(6):315–21. [PubMed] [Google Scholar]


