Abstract
Background
Curcumin has clear anti-tumor activity in various carcinomas. It regulates various signaling pathways like Wnt/β-catenin and JAK2/STAT3, which play vital roles in cell proliferation of several carcinomas, but to the best of our knowledge, there are currently no published reports on human glioma CHME cells. Therefore, the aim of this study was to explore the effect of curcumin on human glioma CHME cells.
Material/Methods
The CHME cell line was purchased from American Type Culture Collection (ATCC). The expressions of caspases 3, caspases 9, PARP, BAX, and BCL2 were detected by Western blot. Annexin V FITC, mitochondrial membrane potential, and reactive oxygen species were detected by flow cytometry. DAPI staining was detected by fluorescence microscopy. Cell viability was assessed by 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay.
Results
We found that curcumin has cytotoxic activity in human glioma CHME cells, as shown by DAPI staining, annexin V/PI, and nuclear morphology. We found that cell growth decreased with increased concentration of curcumin, as well as sowing effects on expression of caspase-3, caspase-9, and cleavage of PARP, which suggests apoptotic cascade activity. The increase in reactive oxygen species and loss of mitochondrial membrane potential (Δψmt) in concentration-dependent manners suggests biochemical induction of apoptosis in CHME cells.
Conclusions
Curcumin has effective anticancer activity in human glioma CHME cells by inducing the apoptotic pathway.
MeSH Keywords: Apoptosis; Caspases; Curcumin; Glioma; Membrane Potential, Mitochondrial; Reactive Oxygen Species
Background
Among various cancers, glioma is known as the most antagonistic human cancers in which central nervous system is predominantly affected and there are high mortality and incidence rate of malignant tumors in a central nervous system [1]. The World Health Organization classifies glioma according to degree of malignancy into levels, among which the fourth level is most aggressive, and the patients with fourth-level glioma have an average post-diagnosis survival of only 14 months, despite treatment with radiotherapy, surgery, and chemotherapy [2,3]. The proliferation rate of malignant glioma cells is very high and these cells also provide a favorable microenvironment for tumor formation [4], in which the tumor is protected from therapeutic radiation and slowly invades the brain [5,6]. This favorable tumor microenvironment supports the progression of glioma. Cancer cells are not as genetically stable as the healthy cells that create the microenvironment. Therefore, it is important to discover new drugs with fewer adverse effects and high efficacy for treatment of human glioma [7]. Other novel agents are needed to obstruct tumor progression and prevent creation of a favorable tumor microenvironment [8,9]. Therefore, drug discovery programs are now focusing on phytochemicals as potent candidates for the treatment of various cancers [10–13]. In previous studies, curcumin, a Chinese traditional medicine that is isolated from the rhizome of Curcuma longa, has shown various biological activities, including antioxidant, anti-inflammatory, antidiabetic, and anticancer effects [14,15]. Curcumin, a natural compound, has anticancer activity by modulating the expression of growth factors, cytokines, oncogenes, and transcription factors [16–18]. Curcumin has shown to inhibit cell proliferation and induction of apoptosis in different types of cancer cells [19–22]. Curcumin has broad molecular targets, including cell invasion related to gene products, transcription factors, and inflammatory cytokines [23–28]. Curcumin therapy is now in phase II clinical trials after successful completion of phase I clinical trials in patients with colon cancer [29–31]. Besides having multiple effects in the cell, apoptosis and antiproliferative mechanisms are induced by curcumin, and studies have reported that curcumin is effective against melanomas and carcinomas [32–35]. Therefore, the aim of this study was to evaluate the effect of curcumin on antiproliferative and apoptosis in human glioma CHME cells.
Material and Methods
Chemicals and reagents
MTT, penicillin G sodium salt, streptomycin sulphate, sodium pyruvate, DMSO (dimethyl sulfoxide), RPMI 1640 Roswell Park Memorial Institute medium, Rhodamaine-123, curcumin, DCFH-DA (2,7-Dichlorodihydrofluorescein diacetate), radioimmunoprecipitation assay buffer (RIPA), phosphate-buffered saline, and BSA were purchased from Sigma. All antibodies (Beta-actin, PARP, Caspases 9, Caspases 3, BAX and BCL2) and annexin V/PI were obtained from Cell Signaling Technology. Fetal bovine serum (FBS) was purchased from Invitrogen. Immobilon Western chemiluminescent horse radish peroxidase (HRP) substrate and PVDF membrane were purchased from Merck Millipore.
Cell line and growth conditions
MCF-10A, HL60, SNU-5, LS180, BV2, CHME, and THP1 was purchased from ATCC (American Type Culture Collection) (Manassas, VA) was grown in RPMI media completed with 10% FBS, including penicillin G (70 mg/L), streptomycin (100 mg/L), and NaHCO3 (3.7 g/L) in an incubator at 37°C, 98% humidity, and 5% CO2. Treatment of cells with curcumin was dose- and time-dependent and curcumin was dissolved in DMSO (<0.1% DMSO).
Cell viability assay
MTT assay was used to assess cell viability. At a density of 0.35×105, cells were seeded in 96-well plates for 24 h in a humidified incubator. After 24 h, CHME cells were treated with curcumin at various times and concentrations. MTT dye (2.5 mg/ml) was added into each well 4 h before termination of the experiment. Media were discarded and DMSO (150 μl) was added into each well to dissolve formazan. Absorbance was measured at 570 nm using a plate reader (Synergy MX), and viability of cells was calculated.
Mitochondrial membrane potential (MMP) assay
To observe mitochondrial membrane potential change, rhodamine-123 fluorescence was used. Cells were plated in 6-well plates at a density of 0.35×105 for 24 h. After 24 h, cells were treated with curcumin at various concentrations for 24 h. Before 30 min of termination of the experiment, Rhodamine-123 was added into incomplete media and placed in an incubator at 37°C. Cells were trypsinized, washed 3 times with PBS, and 30 000 cells were analyzed by flow cytometry.
Nuclear morphology
CHME cells were grown in 60-mm dishes at a density of 0.85×105 for 24 h. Curcumin at different concentrations was added into each plate and DMSO (0.2%) alone was used as a vehicle. Cells were trypsinized and centrifuged at 400 g and pellets were resuspended in (3: 1 ratio) acetic acid: methanol for 4 h, then cells were plated on chilled glass slides. After 20 min, cells plated on slides were dried and 1 ug/ml DAPI was used to stain cells for 30 min in the dark. Cells were washed 3 times with PBS and images were taken with a fluorescence microscope.
Reactive oxygen species
To observe the effect of curcumin on generation of reactive oxygen species, CHME cells at a density of 0.35×105 were seeded in 6 well plates for 24 h. Curcumin at different concentrations was added into each well for 24 h. Before 30 min of termination, DCFH2DA dye was added at a concentration of 5 μM per ml in the dark. After 30 min, cells were washed 3 times with PBS and collected by trypsinization into flow tubes by adding 400 μl of PBS. All steps were done in the dark after adding DCFH2DA dye into samples. Samples were analyzed by flow cytometry, and 30 000 cells/event were captured for analysis.
Detection of apoptosis via Annexin V/PI
We seeded 0.35×105 cells into 6-well plate for 24 h or until the cells entered log phase. After 24 h, cells were treated with curcumin at different concentrations for 24 h. To assess apoptosis by annexin V/PI, cells were trypsinized and washed with PBS in flow tubes, and 300 μL of binding buffer including annexin antibody was added in the dark for 10 min, according to the manufacturer’s protocol. The samples were analyzed by flow cytometry, and 20 000 cells were captured for analysis.
Western blot
CHME cells at a 0.85×105 plated into 60-mm dishes for 24 h for Western blot analysis. After 24 h, cells were treated with curcumin at different concentrations for 24 h. Cells were washed 3 times with PBS, followed by trypsinization, then centrifuged at 100 g for 5 min. These cells were collected and lysed with complete radioimmunoprecipitation assay (RIPA) buffer containing sodium orthovanadate, sodium fluoride, PMSF, and cocktail for 45 min. Next, cells were centrifuged at 12 000 rpm for 10 min and supernatant was collected. The total protein in the supernatant was estimated by Bradford reagent and absorbance was measured at 594 nm. Then, dye was added to each supernatant sample containing proteins. The protein samples at a concentration of 90 μg were loaded into SDS PAGE and run for 3 h at 80 V. These separated proteins in SDS PAGE were transferred onto PVDF membranes (0.45 pore size) for 2 h at 100 V. The protein membranes were blocked for 1 h with non-fat skimmed milk. Primary antibody was incubated overnight, then membranes were washed 3 times with Tris-buffered saline-Tween 20 (TBST) buffer for 5 min each time. HRP-conjugated secondary antibody was added into each protein membrane for 1 h at room temperature, followed by washing in TBST 3 times for 5 min each time. ECL chemiluminescence kits were for protein bands on X-ray film.
Statistical analysis
All data are the means of 3 independent experiments. Statistical analysis was done by Bonferroni method and statistical significance was set at * p<0.05, ** p<0.01, *** P<0.005.
Results
Curcumin inhibits cell proliferation in human CHME cells
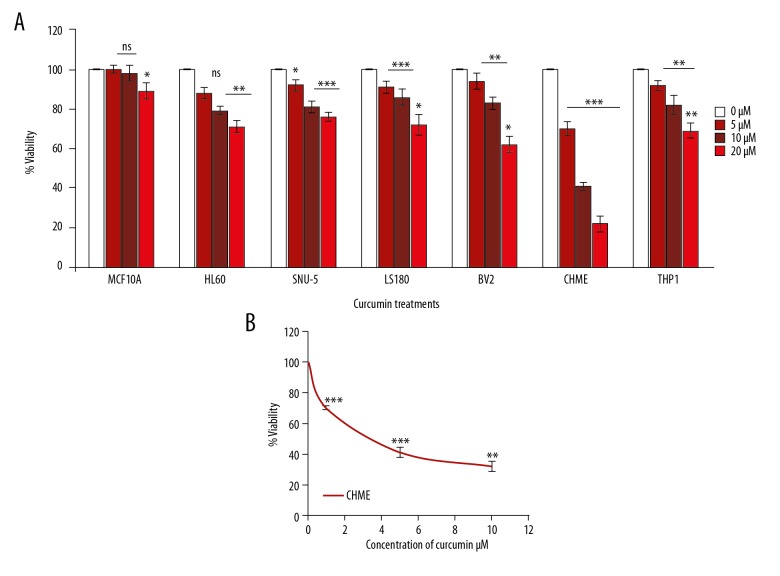
Various cell lines were treated with 0 μM, 1 μM, 5 μM, and 10 μM of curcumin for 24 h for assessment of initial cytotoxicity. The percentage of cell viability was decreased by increasing concentration of curcumin in different cell lines (Figure 1A), but, interestingly, MCF10A remained unaffected. Moreover, curcumin-treated CHME cells that are actually a human brain glioma cell line were most sensitive among all cell lines; therefore, the CHME cell line was chosen for further studies. The cell viability was decreased by curcumin at 24 h in a concentration-dependent manner (Figure 1B). The results suggest that curcumin decreases the proliferation/viability of human glioma CHME cells.
Figure 1.
Cell vibility inhibition by curcumin in different cell lines. (A) The cell viability of different cell lines was inhibited by treating the cells with curcumin at 0 μM, 5 μM, 10 μM, and 20 μM for 24 h. (B) Cell viability was reduced by 50% (IC50) in human CHME cells. The data represented here are the means of 3 independent experiments. Statistical analysis was done by Bonferroni method and statistical significance was set at * p<0.05, ** p<0.01, *** P<0.05.
Induction of apoptosis by curcumin in human CHME cells
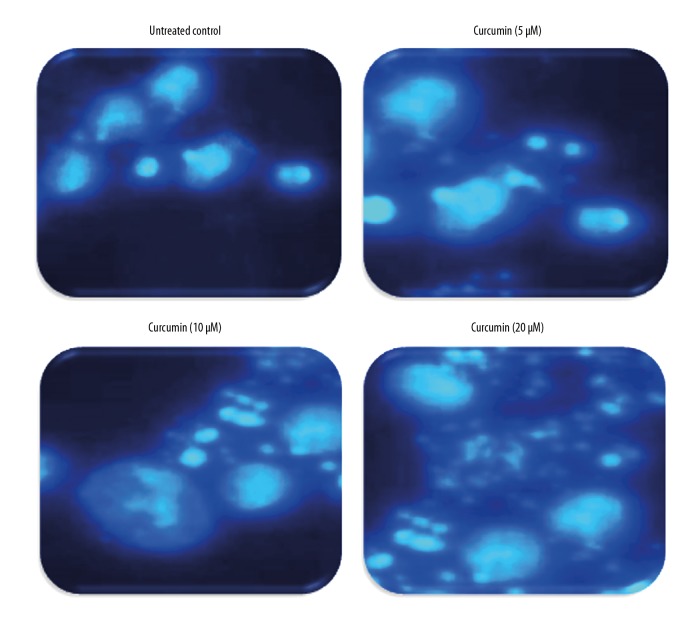
DAPI staining was used for detection of apoptosis in curcumin-treated CHME cells at various concentrations (0 μM, 5 μM, 10 μM, and 20 μM). Apoptotic bodies were observed in curcumin-treated cells, with fragmented nuclei compared with control cells treated with DMSO as a vehicle, which had bright nuclei as shown by fluorescence microscopy (Figure 2). These results suggest that curcumin potentiates cell death by apoptosis in a dose-dependent manner.
Figure 2.
Curcumin induces apoptosis in human glioma CHME cells in a concentration-dependent manner. Cells were treated with curcumin (0 μM, 5 μM, 10 μM, and 20 μM) for 24 h and stained with DAPI for 30 min. Apoptosis was observed under a fluorescence microscope.
Effect of curcumin on reactive oxygen species in CHME cells
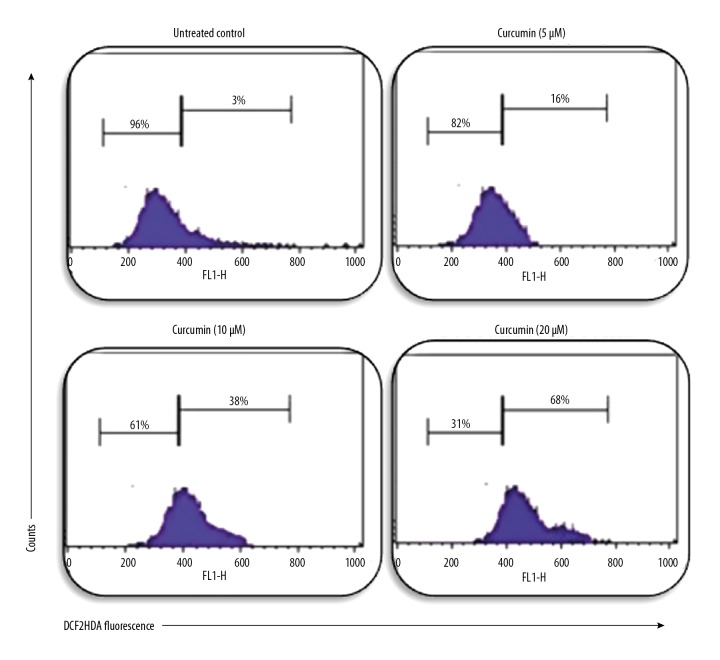
It is well established that reactive oxygen species induce apoptosis in cancer cells; therefore, we examined curcumin-treated CHME cells for reactive oxygen species generation. Interestingly, cells treated with different concentrations of curcumin had increased reactive oxygen species in all curcumin-treated cells, as shown in Figure 3. Our results clearly indicate that reactive oxygen species generation increases with increased concentration of curcumin, which increased apoptosis of human glioma CHME cells.
Figure 3.
Effect of curcumin on ROS generation in human glioma CHME cells. CHME cells were treated with curcumin at concentrations of 0 μM, 5 μM, 10 μM, and 20 μM for 24 h. DCFH2DA dye was used to measure the level of ROS through flow cytometry. We found that the generation of ROS increased in a dose-dependent manner.
Loss of mitochondrial membrane potential by curcumin in human glioma CHME cells
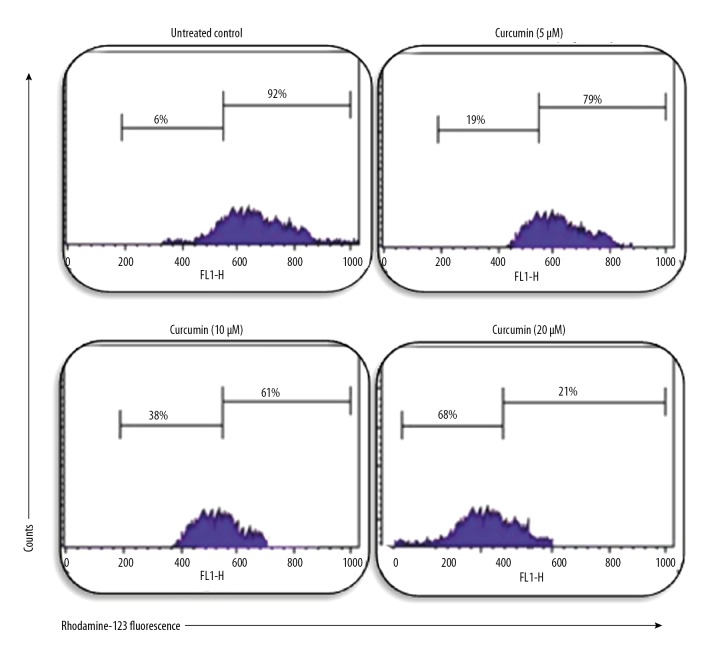
Early reports established that mitochondria play a significant role in apoptosis. In this study, using a fluorescent dye rhodamine-123, we explored whether curcumin induces mitochondrial membrane potential loss. We found that the fluorescence of Rhodamine-123 was gradually decreased with increasing concentrations of curcumin, as shown in Figure 4. Our results clearly indicate that curcumin induces apoptosis by the loss of mitochondrial membrane potential in the human glioma CHME cell line.
Figure 4.
Curcumin induces mitochondrial membrane potential loss in CHME cells. Cells treated with curcumin at 0 μM, 5 μM, 10 μM, and 20 μM for 24 h had gradual mitochondrial membrane potential loss compared with vehicle cells.
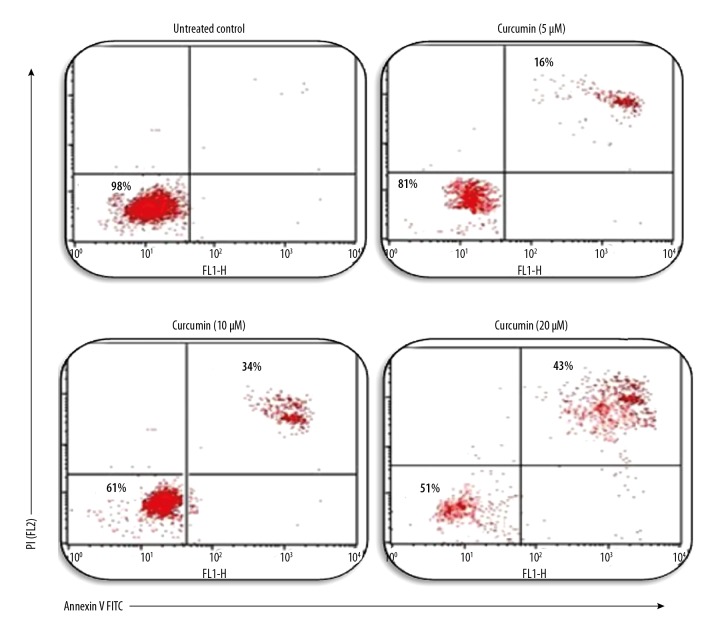
Annexin V/PI assay for apoptosis
Annexin V/PI assay was used to confirm apoptotic cell death caused by curcumin. Human glioma CHME cells were treated with different concentrations of curcumin, and displayed an increase in apoptotic population compared with the vehicle group (DMSO control), as shown in Figure 5. Our results clearly show that curcumin induces apoptosis in a dose-dependent manner.
Figure 5.
Detection of apoptosis by Annexin V PI in cells treated with curcumin. Apoptotic cells were detected by curcumin at a dose-dependent manner and results were analyzed by flow cytometry using Annexin V PI.
Induction of caspases activation and PARP cleavage in human glioma CHME cells
Apoptosis is programmed cell death in which the activation of caspases and cleavage of PARP takes place to trigger apoptotic cell death. We treated CHME cells with curcumin at different concentrations for 24 h to investigate activation of caspases. Surprisingly, the activation of caspases like caspase 9 and caspase 3 (indicated by pro-caspase 9 and pro-caspase 3), as well as PARP cleavage, takes place with increasing concentration of curcumin as compared with vehicle cells (DMSO 0.2%), the expression of BAX was increased, and BCL2 was decreased significantly, and the ratio of BAX: BCL2 is also increased, as shown in Figure 6. These results demonstrate that curcumin induces apoptosis in human glioma CHME cells in a concentration-dependent manner.
Figure 6.
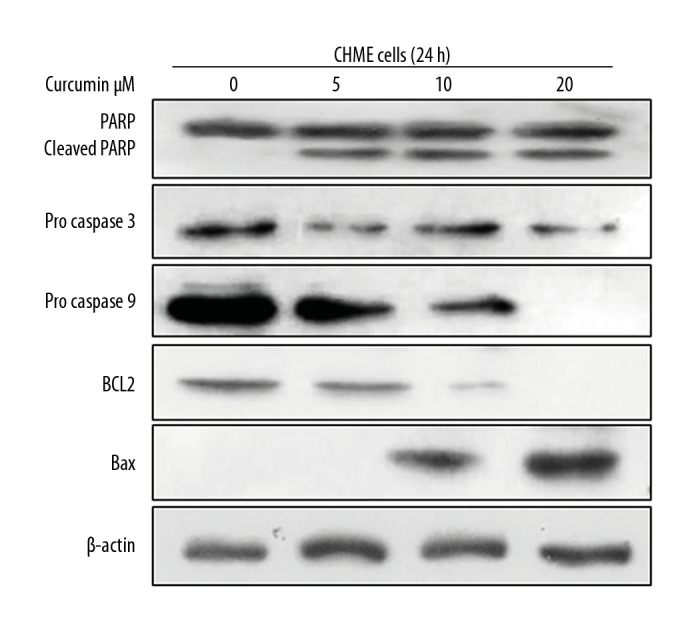
Western blot analysis of induction of apoptosis in human glioma CHME cells. Activation of caspases and cleavage of PARP and ratio of BAX: BCL2 were increased in curcumin-treated cells compared with control cells. Therefore, curcumin induces apoptosis in a dose-dependent manner.
Discussion
Curcumin contributes to immune response, cell cycle regulation, antioxidant response, epithelial-mesenchymal transition (EMT), and tumor progression in various cancers by various pathways [36–39]. Curcumin affects apoptosis and G2/M arrest, but the mechanism is still unclear [40]. The few published clinical trials on the anti-tumor activity of curcumin show high metabolic and chemical stability [17]. Furthermore, there is no evidence of anti-tumor activity in human brain CHME cells. In China, curcumin is used to treat burns and is a traditional Chinese medicine [41]. Previous reports have shown the chemo-therapeutic potential of curcumin against various cancers via anti-tumor activity. Therefore, much attention has been focused on its application in different cancers [42]. Our results demonstrate for the first time that curcumin induces apoptosis in human glioma CHME cells in a dose-dependent manner, as detected by cell proliferation assay. After inhibiting cell viability, chromosome condensation, blabbing, and fragmentation of nuclei were observed in the human glioma CHME cell line, as detected by DAPI staining, in curcumin-treated cells compared with control cells. Moreover, production of ROS was increased and MMP was decreased by curcumin in a dose-dependent manner. Interestingly, Annexin V results agree with our previous results indicating that curcumin induces apoptosis in the human glioma CHME cell line. Furthermore, apoptosis was confirmed by Western blot analysis, by which the activation of caspases and cleavage of PARP was observed in curcumin-treated cells.
Conclusions
We found that curcumin induces apoptosis by a series events, including the loss of mitochondrial membrane potential and generation of reactive oxygen species, as well as increased expression of apoptotic proteins and decreased expression of anti-apoptotic proteins. In conclusion, curcumin induces apoptosis in CHME cells. Further research is needed to explore the mechanism by which the signaling pathway is mediated by curcumin.
Acknowledgements
The authors are thankful to Hai-yun wang, Department of Pediatrics, Puai Hospital, Tongii Medical College, Huazhong University of Science and Technology, Wuhan, China for assistance with flow cytometry.
Footnotes
Source of support: Depertmental sources
Conflict of interests
None.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumors of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuller GN. The WHO classification of tumors of the central nervous system. 4th edition. Arch Pathol Lab Med. 2008;132:906. doi: 10.5858/2008-132-906-TWCOTO. [DOI] [PubMed] [Google Scholar]
- 4.Rock K, McArdle O, Forde P, et al. A clinical review of treatment outcomes in glioblastomamultiforme-the validation in a non-trial population of the results of a randomised Phase III clinical trial: Has a more radical approach improved survival? Br J Radiol. 2012;85:729–33. doi: 10.1259/bjr/83796755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zong H, Verhaak RG, Canoll P. The cellular origin for malignant glioma and prospects for clinical advancements. Expert Rev Mol Diagn. 2012;12(4):383–94. doi: 10.1586/erm.12.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alves TR, Lima FR, Kahn SA, et al. Glioblastoma cells: A heterogeneous and fatal tumor interacting with the parenchyma. Life Sci. 2011;89(15):532–39. doi: 10.1016/j.lfs.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Fan CD, Fu XY, Zhang ZY, et al. Selenocysteine induces apoptosis in human glioma cells: Evidence for TrxR1-targeted inhibition and signaling crosstalk. Sci Rep. 2017;7(1):6465. doi: 10.1038/s41598-017-06979-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–37. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goubran HA, Kotb RR, Stakiw J, et al. Regulation of tumor growth and metastasis: the role of tumor microenvironment. Cancer Growth Metastasis. 2014;7:9–18. doi: 10.4137/CGM.S11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Alessandro N, Poma P, Montalto G. Multifactorial nature of hepatocellular carcinoma drug resistance: could plant polyphenols be helpful? World J Gastroenterol. 2007;13:2037–43. doi: 10.3748/wjg.v13.i14.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudolf E, Andelova H, Cervinka M. Polyphenolic compounds in chemoprevention of colon cancer – targets and signaling pathways. Anticancer Agents Med Chem. 2007;7:559–75. doi: 10.2174/187152007781668670. [DOI] [PubMed] [Google Scholar]
- 12.Stevenson DE, Hurst RD. Polyphenolic phytochemicals – just antioxidants or much more? Cell Mol Life Sci. 2007;64:2900–16. doi: 10.1007/s00018-007-7237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fresco P, Borges F, Diniz C, Marques MP. New insights on the anticancer properties of dietary polyphenols. Med Res Rev. 2006;26:747–66. doi: 10.1002/med.20060. [DOI] [PubMed] [Google Scholar]
- 14.Yin SY, Wei WC, Jian FY, Yang NS. Therapeutic applications of herbal medicines for cancer patients. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/302426. 302426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Priyadarsini KI. Chemical and structural features influencing the biological activity of curcumin. Curr Pharm Des. 2013;19:2093–100. doi: 10.2174/138161213805289228. [DOI] [PubMed] [Google Scholar]
- 16.Lin JK. Suppression of protein kinase C and nuclear oncogene expression as possible action mechanisms of cancer chemoprevention by curcumin. Arch Pharm Res. 2004;27:683–92. doi: 10.1007/BF02980135. [DOI] [PubMed] [Google Scholar]
- 17.Yu LL, Wu JG, Dai N, et al. Curcumin reverses chemoresistance of human gastric cancer cells by downregulating the NF-κB transcription factor. Oncol Rep. 2011;26:1197–203. doi: 10.3892/or.2011.1410. [DOI] [PubMed] [Google Scholar]
- 18.Lin SS, Lai KC, Hsu SC, et al. Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and −9 and vascular endothelial growth factor (VEGF) Cancer Lett. 2009;285:127–33. doi: 10.1016/j.canlet.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 19.Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009;11(3):495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan F, Ding Y, Zhang Y, et al. Curcumin suppresses proliferation and migration of MDA-MB-231 breast cancer cells through autophagy-dependent Akt degradation. PLoS One. 2006;11(1):e0146553. doi: 10.1371/journal.pone.0146553. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Sa G, Das T. Anti-cancer effects of curcumin: Cycle of life and death. Cell Div. 2008;3(1):14. doi: 10.1186/1747-1028-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park W, Amin AR, Chen ZG, Shin DM. New perspectives of curcumin in cancer prevention. Cancer Prev Res. 2013;6(5):387–400. doi: 10.1158/1940-6207.CAPR-12-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: A review of anticancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10(1):12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aggarwal BB, Kunnumakkara AB, Harikumar KB, et al. Signal transducer and activator of transcription-3, inflammation, and cancer. Ann NY Acad Sci. 2009;1171(1):59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nosrati N, Bakovic M, Paliyath G. Molecular mechanisms and pathways as targets for cancer prevention and progression with dietary compounds. Int J Mol Sci. 2017;18(10) doi: 10.3390/ijms18102050. pii: E2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samadi AK, Bilsland A, Georgakilas AG, et al. A multi-targeted approach to suppress tumor-promoting inflammation. Semin Cancer Biol. 2015;35:S151–84. doi: 10.1016/j.semcancer.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee DS, Lee MK, Kim JH. Curcumin induces cell cycle arrest and apoptosis in human osteosarcoma (HOS) cells. Anticancer Res. 2009;29(12):5039–44. [PubMed] [Google Scholar]
- 28.Tan TW, Tsai HR, Lu HF, et al. Curcumin-induced cell cycle arrest and apoptosis in human acute promyelocytic leukemia HL-60 cells via MMP changes and caspase-3 activation. Anticancer Res. 2006;26(6B):4361–71. [PubMed] [Google Scholar]
- 29.Kim EJ, Semrad TJ, Bold RJ. Phase II clinical trials on investigational drugs for the treatment of pancreatic cancers. Expert Opin Invest Drugs. 2015;24(6):781–94. doi: 10.1517/13543784.2015.1026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhillon N, Aggarwal BB, Newman RA, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14(14):4491–99. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 31.Bimonte S, Barbieri A, Leongito M, et al. Curcumin anticancer studies in pancreatic cancer. Nutrients. 2016;8(7) doi: 10.3390/nu8070433. pii: E433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen H-W, Lee J-Y, Huang J-Y, et al. Curcumin inhibits lung cancer cell invasion and metastasis through the tumor suppressor HLJ1. Cancer Res. 2008;68:7428–38. doi: 10.1158/0008-5472.CAN-07-6734. [DOI] [PubMed] [Google Scholar]
- 33.Somers-Edgar TJ, Scandlyn MJ, Stuart EC, et al. The combination of epigallocatechin gallate and curcumin suppresses Era-breast cancer cell growth in vitro and in vivo. Int J Cancer. 2008;122:1966–71. doi: 10.1002/ijc.23328. [DOI] [PubMed] [Google Scholar]
- 34.Shankar S, Srivastava RK. Involvement of Bcl-2 family members, phophatidylinositol 3′-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int J Oncol. 2007;30:905–18. [PubMed] [Google Scholar]
- 35.Shi M, Cai Q, Yao L, et al. Antiproliferation and apoptosis induced by curcumin in human ovarian cancer cells. Cell Biol Int. 2006;30:221–26. doi: 10.1016/j.cellbi.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 36.Jordan BC, Mock CD, Thilagavathi R, Selvam C. Molecular mechanisms of curcumin and its semisynthetic analogues in prostate cancer prevention and treatment. Life Sci. 2016;152:135–44. doi: 10.1016/j.lfs.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan H, Liang Y, Jiang B, et al. Curcumin inhibits intracellular fatty acid synthase and induces apoptosis in human breast cancer MDA-MB-231 cells. Oncol Rep. 2016;35:2651–56. doi: 10.3892/or.2016.4682. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Chen H, Xu C, et al. Curcumin inhibits tumor epithelialmesenchymal transition by downregulating the Wnt signaling pathway and upregulating NKD2 expression in colon cancer cells. Oncol Rep. 2016;35:2615–23. doi: 10.3892/or.2016.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang YT, Lin YW, Chiu HM, Chiang BH. Curcumin induces apoptosis of colorectal cancer stem cells by coupling with CD44 Marker. J Agric Food Chem. 2016;64:2247–53. doi: 10.1021/acs.jafc.5b05649. [DOI] [PubMed] [Google Scholar]
- 40.Luthra PM, Kumar R, Prakash A. Demethoxycurcumin induces Bcl-2 mediated G2/M arrest and apoptosis in human glioma U87 cells. Biochem Biophys Res Commun. 2009;384:420–25. doi: 10.1016/j.bbrc.2009.04.149. [DOI] [PubMed] [Google Scholar]
- 41.Shi Tsi S. Use of combined traditional Chinese and Western medicine in the management of burns. Panminerva Med. 1983;25:197–202. [PubMed] [Google Scholar]
- 42.Aggarwal BB, Kunnumakkara AB, Harikumar KB, et al. Signal transducer and activator of transcription-3, inflammation, and cancer. Ann NY Acad Sci. 2009;1171(1):59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]