Abstract
Background
Aberrant expression of microRNAs (miRNAs) is closely involved in cancer development. Downregulation of miR-29a-3p and its tumor suppressive roles in cancer have been revealed by multiple reporters. However, study of its expression pattern and function in papillary thyroid carcinoma (PTC) is rare.
Materials and methods
The expression of miR-29a-3p in PTC tissues and cells was detected by qPCR. CCK-8, plate clone formation, transwell invasion, Western blot, immunohistochem-istry, and luciferase reporter assays were carried out to identify the target of miR-29a-3p and explore its roles and mechanisms in PTC.
Results
Deregulated miR-29a-3p in PTC tissues and cell lines were revealed by qPCR. Restoring miR-29a-3p expression significantly inhibited growth, proliferation, and invasion of PTC cells demonstrated by CCK-8, plate clone formation, and transwell assays. Luciferase reporter assays showed miR-29a-3p can directly target OTUB2 in PTC cells. Ectopic expression of OTUB2 can antagonize the effects of miR-29a-3p on cell growth, proliferation, and invasion of PTC. Mechanistically, OTUB2 overexpression can activate NF-κB signaling mostly by stabilizing TRAF6. Upregulated OTUB2 expression was observed in PTC tissues via immunohistochemistry analysis. Moreover, OTUB2 showed a positive correlation to metastatic status and showed a negative correlation to the overall survival rate in PTC patients.
Conclusion
Deregulated miR-29a-3p can promote cell growth, proliferation, and invasion in PTC. OTUB2 is a direct downstream target of miR-29a-3p in PTC, and it mediates the effects of deregulated miR-29a-3p by activating TRAF6-associated NF-κB signaling in PTC.
Keywords: miR-29a-3p, PTC, OTUB2, NF-κB signaling
Introduction
Thyroid cancer (TC) is the most common malignant tumor of the endocrine system. In spite of the popularity of early screening, its incidence rate is still growing. TC can be divided into papillary, follicular, medullary, and anaplastic subtype.1–3 Among them, papillary thyroid carcinoma (PTC) is the most common type, accounting for >80% of all TCs. Generally, the efficacy and prognosis of PTC are satisfactory. However, some PTC patients, especially those with malignant pathological characteristics including poor differentiation, lymph node, and distant metastasis, demonstrate unfavorable outcome.4 Therefore, it is of great importance to explore the molecular events during malignant progression of PTC, which would lay a solid foundation for developing more effective diagnostic and prognostic biomarkers and therapeutic targets.
In addition to genetic regulation, epigenetic regulations have vital roles in almost all physiological and pathological processes, of course, including carcinogenesis. As one of epigenetic regulators, microRNAs (miRNAs), a class of endogenous, non-coding, small (18–22 nucleotides) RNAs, have proved their key role in cancer. By binding to the 3′-untranslated region (3′-UTR) of its target mRNA transcripts, miRNAs can cause target mRNA degradation or translational suppression, which subsequently modulates the development and progression of cancers.5,6 Multiple miRNAs including both oncogenic and inhibitory ones have been reported to exert vital roles in markers of PTC progression such as proliferation, invasion, and metastasis.7,8 However, the functions of multiple miRNAs whose abnormal expressions have been revealed by studies focusing on differential expression miRNA profiles based on next-generation sequencing in PTC have not been done.9,10
Recently, OTUB2, a member of the ovarian tumor (OTU) superfamily, has been proved to mediate the ubiquitination and degradation of TRAF6, a key positive regulator for NF-κB.11 TRAF6 can activate NF-κB signaling by mediating the K63 polyubiquitination of IKK.12 Meanwhile, aberrant activation of NF-κB has been proved to promote growth, proliferation, and metastasis in PTC.13,14 Although the roles of OTUB2 in cancers have not been reported, it may have important functions in PTC as can be concluded from the above studies. miR-29a-3p, belonging to the miR-29 family and which is downregulated in majority of solid tumors and hematologic neoplasms as indicated by transcript profiling or northern blot analysis, mainly exhibits tumor suppressive roles by targeting multiple downstream genes.15,16 A set of miRNA microarray data from a recent study suggests that miR-29a-3p may also be downregulated in PTC.17 In this study, we explored the expression and functions of miR-29a-3p in PTC. Indeed, miR-29a-3p is downregulated in PTC and positively correlates to the prognosis of PTC. Moreover, we proved that miR-29a-3p can suppress PTC progression by directly targeting OTUB2, which results in inactivation of TRAF6/NF-κB signaling. Thus, our study revealed the tumor suppressor roles of miR-29a-3p in PTC and thus lays the experimental foundations for clinical treatment of PTC by targeting OTUB2/TRAF6/NF-κB signaling.
Materials and methods
Patients and tissue specimens
Between March 2016 and April 2017, 98 paired tissue samples including PTC tissues and corresponding adjacent normal tissues were obtained from newly diagnosed patients who received no chemotherapy or radiotherapy, at the department of general surgery of our hospital. All specimens were divided into two parts, one for immediate snap freezing in liquid nitrogen, and one for fixation and paraffin-embedded specimen production. The pathological characteristics of all tissues were confirmed by two experienced histopathologists. Written informed consent was obtained from all patients for use of their tissue samples and clinical records. The study protocol was performed under the approval of the Ethics Committee of The Second Affiliated Hospital of Harbin Medical University. Moreover, the study was also conducted in accordance with the Declaration of Helsinki.
Immunohistochemistry
Immunohistochemical staining was done by using the streptavidin-peroxidase IHC Kit (CWBIO, Beijing, China) according to the manufacturer’s instruction. Briefly, after successive processing, which included dewaxing, antigen retrieval, and H2O2 treatment, the paraffin-embedded tissue slides were incubated with the OTUB2 antibody (BBI, Shanghai, China; dilution ratio: 1:50) overnight at 4°C. Then, the slides were washed with PBS and incubated with a biocatalytic secondary antibody followed by incubation with the avidin-biotin peroxidase complex. Finally, the tissue sections were treated with 3′, 3′-diaminobenzidine until a brown color developed and were counterstained with Harris’ modified hematoxylin. The immunoreactions were evaluated independently by two pathologists. A semiquantitative IHC-score, which ranges from 0 to 300, was calculated for each specimen by multiplying the distribution areas (0%–100%) at each staining intensity level by the intensities, according to the previous reports.17,18
Cell culture and transfection
Human PTC cell lines K1 and TPC-1, the human thyroid follicular epithelial cell line, Nthy-ori3-1, and HEK 293 T cells were purchased from the Shanghai Cell Bank (Shanghai, China). All cell lines were grown in RPMI 1640 medium (BI, Jerusalem, Israel), supplemented with 10% fetal bovine serum (BI), and incubated at 37°C in a humidified incubator containing 5% CO2. miR-29a-3p mimics, control mimics, specific small interfering RNA (siRNA) for OTUB2, and control siRNA were synthesized by RiboBio Inc (Guangzhou, China) according to the published work.19,20 miRNA mimics and siRNAs with final concentration at 50 nM were transfected into PTC cells using Lipofectamine™ 2000 (Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer’s instructions. Briefly, nucleic acids, which includes the plasmids and siRNAs, and the Lipofectamine reagent were diluted with RPMI 1640 medium, for 5 minutes. Then the mediums containing nucleic acids and Lipofectamine reagent were mixed and incubated at room temperature to form liposomes. After 15 minutes, each mixture was added into the corresponding culture medium and 6 hours later, the culture medium was removed and replaced with fresh medium.
RNA isolation and quantitative reverse transcription polymerase chain reaction (qPCR)
Total RNAs of snap-frozen tissue samples and cells were extracted by using TRIzol reagent (Thermo Fisher Scientific) according to the manufacturer’s instruction. Subsequently, 1 µg RNA was reverse transcribed into cDNA using HiScript first Strand cDNA Synthesis Kit (Vazyme, Nanjing, China) and Bulge-LoopTM miRNA qRT-PCR Kit (RiboBio, Guangzhou, China), respectively. Then, the relative expression of miR-29a-3p and OTUB2 were detected by Applied Biosystems 7900HT real-time PCR Systems (Thermo Fisher Scientific) using U6 small nuclear RNA and GAPDH as internal controls. The relative expression was determined by 2−ΔΔCT method. The primer sequences were listed as follow: OTUB2, forward: 5′-ACACTTGGAACCGGCTTGAC-3′, reverse: 5′-AGCACACGGACTGTCCTGA-3′; GAPDH, forward: 5′-CAGCAAGAGCACAAGAGGAA-3′, reverse: 5′-ATGG-TACATGACAAGGTGCGG-3′.21 The primers for miR-29a-3p and U6 small nuclear RNA were purchased from RiboBio Inc.
Luciferase reporter assay
The 3′-UTR sequence of OTUB2 containing the predicted potential miR-29a-3p binding sites cloned into downstream of the luciferase gene in the psiCHECK2 luciferase reporter vector (Promega Corporation, Fitchburg, WI, USA), was named as OTUB2 wild type (WT). The mutant type of OTUB2 lacking complementarity with miR-29a-3p binding sequence in 3′-UTR region was also constructed and named as OTUB2 mutant (MUT). Then, luciferase reporter vectors, miR-29a-3p or matched controls were introduced into 293 T cells by using Lipofectamine 2000 (Thermo Fisher Scientific). After 48 hours, the luciferase activity was measured using a Dual-Luciferase Reporter detection System (Promega Corporation). The relative luciferase activity was expressed as the ratio of firefly luciferase to Renilla luciferase activity.
CCK-8 assay
Cell growth was monitored by using a CCK-8 kit (Beyotime, Shanghai, China) referring to the manufacturer’s instruction. Briefly, PTC cells were plated at 5×103 cells/well in 96-well plates and cultured for 96 hours. After every 24 hours, the CCK-8 reagent was added and the absorbance value at 450 nm of every well was obtained using the Epoch microplate spectrophotometer (Bio-Tek, Winooski, VT, USA). Eventually, the growth rate was reflected by the absorbance values. The cck-8 assay was performed for three times in triplicate.
Plate clone formation assay
Plate clone formation assay was performed as described in the published paper. Simply, PTC cells were trypsinized and seeded in 6-well plates at the density of 1,000 cells/well. After continuous culture for 8 days, the cell clones were fixed with methanol and incubated with crystal violet. Clones containing >50 cells were counted under an inverse microscope (Nikon, Tokyo, Japan). The plate clone formation assay was performed for three times in triplicate
Transwell invasion assay
Transwell assay was done following the manufacturer’s instructions to analyze the invasive ability of PTC cells. 8-mm pore size transwell chambers precoated with Matrigel (Corning Incorporated, Corning,, NY, USA) were adopted. In brief, 0.5 mL RPMI 1640 medium containing 2.5×104 cells were added into the chamber, which was embedded and immersed in a 24-well plate filled with 0.75 mL RPMI 1640 medium containing 10% FBS per well. After 48 hours of culture, the medium was removed and the cells were fixed with methanol and stained with crystal violet. The cells which failed to invade the Matrigel were removed with a swab, then the invasive cells were pictured and their numbers calculated from five randomly selected microscopic fields.
Western blot
Briefly, after disrupting cells using RIPA buffer (Beyotime), total protein content of the PTC cells was obtained via high-speed centrifugation at 4°C. Then, the protein samples were subjected to concentration detection and denaturation. Next, the protein samples were separated by 12% SDS PAGE (40 µg/lane), electrophoretically transferred to polyvinylidene difluoride membranes (PVDF). Subsequently, the membranes were subjected to blocking with 5% non-fat milk, incubation with the corresponding primary antibodies at 4˚C overnight: OTUB2 (BBI), TRAF6, GAPDH (Abclonal, Woburn, MA, USA), p-NF-κB p65 (Ser468), and p65 (CST, Danvers, MA, USA); washing with TBST for three times (5 minutes each time); incubation with the secondary antibody for 1 hour at room temperature; and washing with TBST for three times (5 minutes each time) again. Finally, the immunoreactive bands were developed with a chemiluminescent HRP substrate (EMD Millipore, Burlington, MA, USA) and imaged by a FluorChem FC3 system (ProteinSimple, San Jose, CA, USA).
Statistical analysis
All experiments were performed in triplicate. Statistical analysis was conducted using SPSS18.0 software and Graph-Pad prism version 6. For comparisons between two groups, a Student’s t test or chi-squared test was used. P<0.05 were considered to be statistically significant.
Results
miR-29a-3p is downregulated in PTC tissues and cell lines
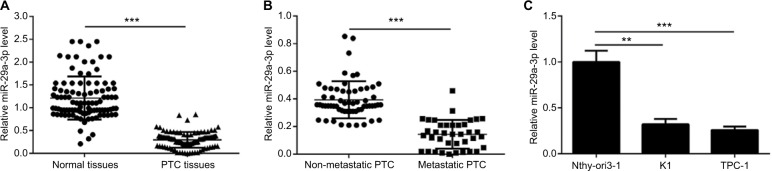
Firstly, we detected the expression of miR-29a-3p in 98 cases of paired cancer and paracancerous tissues by qPCR. As shown in Figure 1A, the expression of miR-29a-3p is significantly downregulated in PTC tissues. Moreover, miR-29a-3p levels are inversely correlated to the metastatic status of PTC (Figure 1B). Similarly, compared to the expression in human thyroid follicular epithelial cells, downregulation of miR-29a-3p is observed in PTC cells also (Figure 1C). Thus, our study revealed that miR-29a-3p expression is downregulated and inversely correlated to the metastatic stage of PTC suggesting it may have vital functions in PTC progression.
Figure 1.
miR-29a-3p is deregulated in PTC tissues and cells.
Notes: (A) Decreased miR-29a-3p levels in PTC tissues were demonstrated by qPCR. (B) Compared with non-metastatic PTC (n=60 cases), metastatic PTC (n=38 cases) showed lower level of miR-29a-3p. (C) Decreased miR-29a-3p was also observed in PTC cells indicated by qPCR results. **P<0.01 and ***P<0.001.
Abbreviations: PTC, papillary thyroid carcinoma; qPCR, quantitative reverse transcription polymerase chain reaction.
Restoring miR-29a-3p expression impaired the malignancy of PTC cells
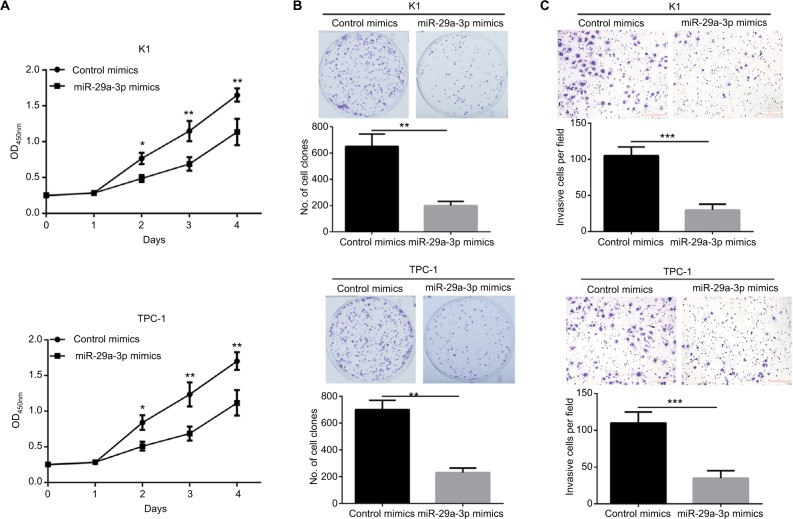
To explore the functions of miR-29a-3p in PTC, we successfully restored the level of miR-29a-3p by introducing miR-29a-3p mimics into K1 and TPC cells. Subsequently, the effects of miR-29a-3p on growth, proliferation, and invasion of PTC cells were detected by CCK-8, plate clone formation, and transwell invasion assays. Restoration of miR-29a-3p levels significantly inhibited the growth and proliferation of PTC cells reflected by the impaired cell viability, and fewer and smaller clones (Figure 2A, B). miR-29a-3p restoration also inhibited the invasive ability of PTC cells demonstrated by the fewer invasive cells (Figure 2C). Therefore, we proved that miR-29a-3p can inhibit the malignancy of PTC cells.
Figure 2.
Restoring miR-29a-3p expression suppressed growth, proliferation, and invasive ability of PTC cells.
Notes: (A) The growth of K1 and PTC-1 cells was inhibited by transfection with miR-29a-3p mimics, indicated by the CCK-8 assay. (B) The proliferation of K1 and PTC-1 cells was inhibited by transfection with miR-29a-3p mimics, indicated by plate clone formation assay. (C) The invasion of K1 and PTC-1 cells was inhibited by transfection with miR-29a-3p mimics, indicated by plate clone formation assay. *P<0.05, **P<0.01, and ***P<0.001.
Abbreviation: PTC, papillary thyroid carcinoma.
miR-29a-3p directly targets OTUB2 in PTC
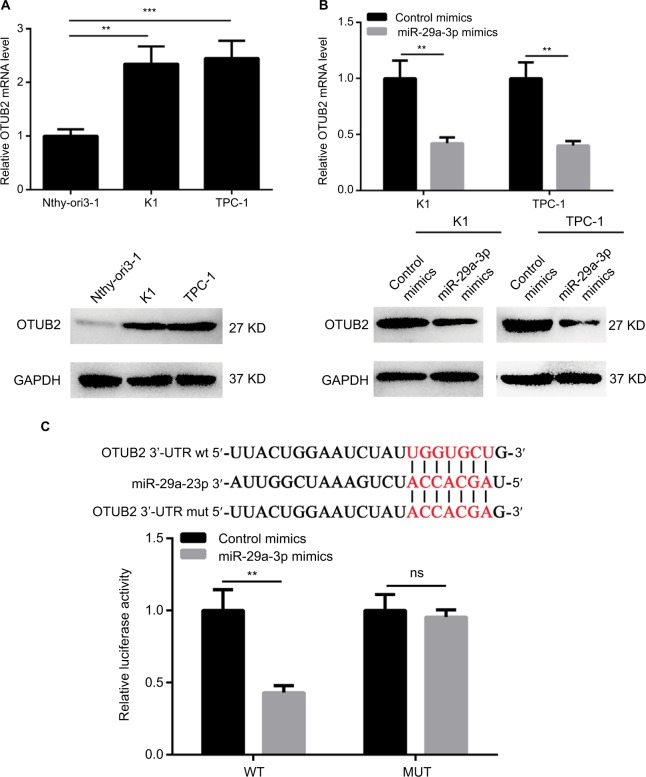
In order to reveal the downstream effector of miR-29a-3p in PTC, several miRNA target prediction tools were used to identify the targets of miR-29a-3p. As predicted by TargetScan and RNA22Sites databases, the 1329–1335 bases of OTUB2 3′-UTR exhibit perfect complementarity to the seed sequences of miR-29a-3p. Therefore, some experiments were performed to validate this hypothesis. The inverse correlation between miR-29a-3p and OTUB2 expression was observed in human thyroid follicular epithelial cells and PTC cells (Figure 3A). Furthermore, the mRNA and protein levels of OTUB2 were both decreased by miR-29a-3p mimics transfection in PTC cells (Figure 3B). Subsequently, luciferase reporter assay was used to confirm that miR-29a-3p can directly bind to the 3′-UTR of OTUB2 mRNA. As shown in Figure 3C, miR-29a-3p mimics can significantly suppress the luciferase activity in cells transfected with WT OTUB2 3′-UTR reporter plasmid, while mutated OTUB2 3′-UTR exhibited a complete restoration of the luciferase activity inhibited by the miR-29a-3p mimics. Thus, we validated that miR-29a-3p can directly target OTUB2 in PTC cells.
Figure 3.
miR-29a-3p directly targets OTUB2 in PTC cells.
Notes: (A) The expression of OTUB2 is upregulated in PTC cells indicated by qPCR and Western blot. (B) miR-29a-3p mimics decreased the expression of OTUB2 in PTC cells indicated by qPCR and Western blot. (C) Luciferase reporter assay showed that miR-29a-3p can directly bind to the 3′UTR region of OTUB2. **P<0.01, ***P<0.001.
Abbreviations: ns, not significant; MUT, mutant; PTC, papillary thyroid carcinoma; qPCR, quantitative reverse transcription polymerase chain reaction; WT, wild type; 3′-UTR, 3′-untranslated region.
OTUB2 knockdown can mimic, while OTUB2 ectopic expression can antagonize, the effects of miR-29a-3p in PTC
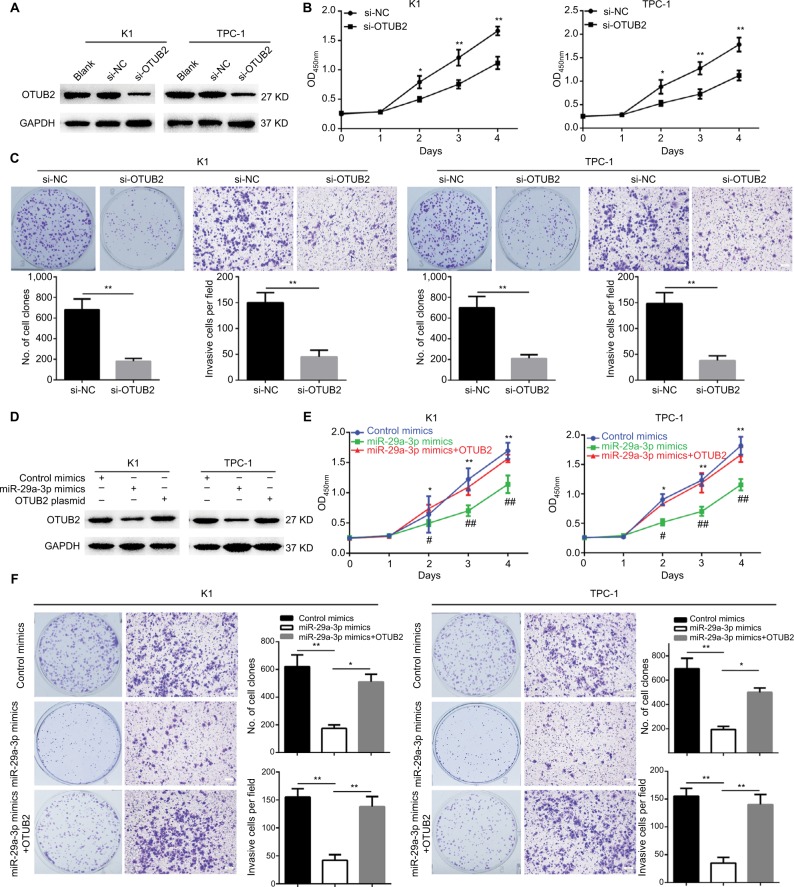
Since miR-29a-3p can target OTUB2 in PTC cells, the role of OTUB2 in miR-29a-3p-related functions were studied. Firstly, we explored the effects of OTUB2 knockdown on the malignant characteristics of PTC. As shown in Figure 4A, successful OTUB2 knockdown was obtained using siRNA. The effect of this was similar to the effects of the miR-29a-3p mimics, and OTUB2 knockdown significantly inhibited the growth, proliferation, and invasive ability of PTC cells (Figure 4B, C). Moreover, we showed that the inhibitory effects of miR-29a-3p mimics can be nearly antagonized by ectopic expression of OTUB2 plasmid without the 3′-UTR sequence (Figure 4D–F). miR-29a-3p inhibited PTC progression by suppressing OTUB2-associated NF-κB signaling.
Figure 4.
OTUB2 knockdown can mimic, while OTUB2 overexpression can antagonize, the effects of miR-29a-3p on PTC cell growth, proliferation, and invasion.
Notes: (A) OTUB2 was successfully knocked down by specific siRNAs. OTUB2 knockdown suppressed cell growth (B), proliferation, and invasion (C), in PTC cells. (D) OTUB2 ectopic expression rescued the level of OTUB2 in PTC cells transfected by miR-29a-3p mimics. OTUB2 ectopic expression released the inhibitory effects of miR-29a-3p on growth (E), proliferation, and invasion (F), in PTC cells. *,#P<0.05, **,##P<0.01. # and ## stand for the statistical difference between miR-29a-3p mimics group and miR-29a-3p mimics+OTUB2 group. * and ** stand for the statistical difference between miR-29a-3p mimics group and control mimics group.
Abbreviations: NC, negative control; PTC, papillary thyroid carcinoma; siRNA, small interfering RNA.
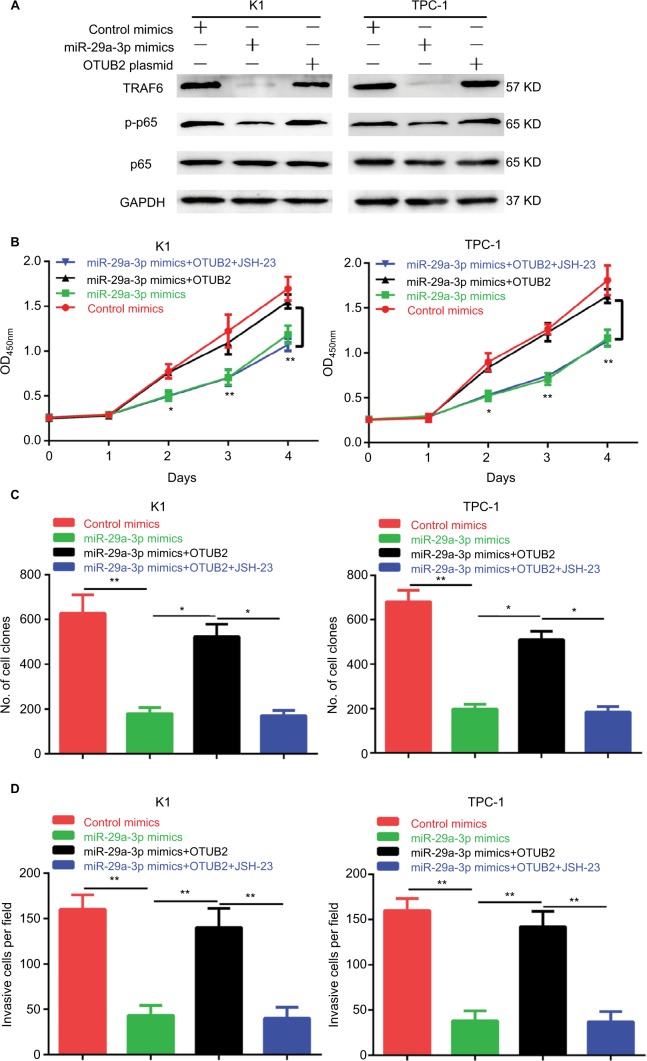
Furthermore, we explored the mechanism of miR-29a-3p/ OTUB2 action in PTC. By analyzing the previous studies on OTUB2, we found that OTUB2 can stabilize TRAF6,11 a positive regulator of NF-κB signaling by mediating its deubiquitination, suggesting that miR-29a-3p/OTUB2 may regulate the activity of NF-κB in PTC. Indeed, we observed that miR-29a-3p mimics markedly decreased the protein levels of TRAF6 and p-p65 (Figure 5A). Moreover, OTUB2 overexpression can rescue the inhibitory effects of miR-29a-3p mimics on TRAF6 and p-p65 levels (Figure 5A). Next, we explored the role of TRAF6/NF-κB signaling in miR-29a-3p/OTUB2-associated functions in PTC. As shown in Figure 5B–D, when TRAF6/NF-kB signaling was blocked by JSH-23, a specific inhibitor for NF-kB, the antagonistic effects of OTUB2 overexpression, which involved the rescue of the inhibitory effects of miR-29a-3p mimics in PTC, were impaired. Therefore, we revealed that miR-29a-3p can inhibit PTC progression by suppressing OTUB2-associated TRAF6/ NF-κB signaling.
Figure 5.
OTUB2 antagonizes the effects of miR-29a-3p in PTC by activating TRAF6-related NF-κB signaling.
Notes: (A) OTUB2 overexpression antagonized the inhibitory effect of miR-29a-3p mimics on TRAF6 and p-p65. JSH-23 treatment abolished the rescue effects of OTUB2 overexpression on growth (B), proliferation (C), and invasion (D), in PTC cells. *P<0.05, **P<0.01.
Abbreviation: PTC, papillary thyroid carcinoma.
OTUB2 is upregulated in PTC tissues and its level shows inverse correlation to miR-23a-3p levels and prognosis of PTC
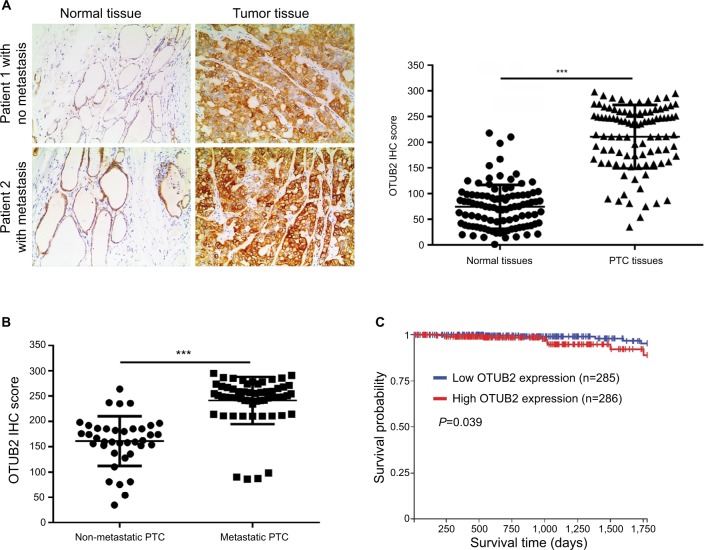
To further explore the relation between OTUB2 and miR-29a-3p in PTC tissues, we assayed the OTUB2 level, using IHC, in the same tissues that were used for miR-29a-3p detection. As shown in Figure 6A, the level of OTUB2 is significantly upregulated in PTC tissues compared with that in paracancerous tissues. Moreover, the OTUB2 level is higher in metastatic PTC tissues than that in non-metastatic PTC tissues (Figure 6B). We also analyzed the relation between OTUB2 level and overall survival rate in PTC. Accordingly, as indicated by Figure 6C, OTUB2 levels showed inverse correlation to OS in PTC on analyzing TCGA data using the UCSC Xena website tool (https://xena.ucsc.edu/). Thus, we also proved that OTUB2 is upregulated in PTC tissues and its level shows inverse correlation to miR-29a-3p levels and prognosis of PTC.
Figure 6.
OTUB2 is upregulated in PTC tissues and its level inversely correlates to the prognosis of PTC.
Notes: (A) PTC tissues showed significantly higher OTUB2 expression than the adjacent normal tissues. (B) Metastatic PTC tissues (n=38 cases) showed higher OTUB2 expression than non-metastatic tissues (n=60 cases). (C) PTC patients with high OTUB2 expression showed less survival probability than those patients with a low OTUB2 expression as indicated by online data from TCGA. ***P<0.001.
Abbreviation: PTC, papillary thyroid carcinoma.
Discussion
Numerous studies have shown that miRNAs play vital roles in cancer. By modulating the mRNA stability of the target gene, miRNAs can regulate almost all processes in cancer. Moreover, due to its distinct expression pattern in tumor patient serum, some miRNAs can also serve as biomarkers for cancer diagnosis and prognosis prediction.5,6 In this study, we proved that miR-29a-3p was downregulated in PTC cell lines and tissue specimens. Functional analyses indicated that restoring miR-29a-3p expression suppressed growth, proliferation, invasion in PTC cells. We also confirmed that OTUB2 was a direct target of miR-29a-3p. Consistent with miR-29a-3p restoration, OTUB2 knockdown mimicked, while OTUB2 overexpression antagonized the effects of miR-29a-3p in PTC cells. We further discovered that TRAF6-associated NF-κB signaling mediated the functions of miR-29a-3p/OTUB2 regulation in PTC. Taken together, the results of this study prove that the miR-29a-3p can exert its suppressive functions in PTC by targeting OTUB2-associated TRAF6/ NF-κB signaling.
Abnormal miRNA expression profiles of PTC have been analyzed by next generation sequencing and miRNA microarray analysis.9,10 Dozens of candidate differential miRNAs, both up- and downregulated, have been identified in PTC suggesting miRNAs may play important roles in PTC.7 Downregulated miRNAs including miR-204-5 p,22 miR-219-5 p,23 miR-125a-5p,17 miR-199a,24 etc, which inhibit PTC progression, and upregulated miRNAs including miR-146b-5p,25 miR-183,26 etc, which promote PTC progression, have been validated. As for miR-29a-3p, its tumor inhibitory effects have been revealed in many studies. Downregulated miR-29a-3p has been observed in gastric cancer,19 clear cell renal cell carcinoma,27 hepatocellular carcinoma,28 as well as chronic myeloid leukemia.29 Moreover, restoration of miR-29a-3p levels in PTC cell lines exhibited significant inhibitory effects on tumor progression characteristics such as cell growth, proliferation, migration, and invasion.19,28 Consistent with the existing studies, downregulation of miR-29a-3p in PTC was observed in our study also and its inhibitory effects on growth, proliferation, and invasion of PTC cells are thus confirmed. These results offer another supporting evidence for the suppressive roles of miR-29a-3p in cancers.
Actually, the effects of miRNAs are mainly dependent on its downstream targets. By targeting oncogenic or antitumor genes, the miRNA can promote or inhibit cancer development and progression. For example, oncogenic genes such as SphK2,30 SNAIL1,24 IGFBP5,22 and CD147,17 etc, can be directly targeted by miR-613, miR-199a-5p, miR-204-5 p, and miR-125a, respectively, resulting in progression inhibition in PTC. The downstream targets of miR-29a-3p have been revealed by several studies. miR-29a-3p can modulate CYP2C19,31 ALDH5A1, and SLC22A732 in human liver cells. Up to now, miR-29a-3p can suppress tumor progression by directly decreasing PROM1,33 IGF1R,28 and Notch2 levels.34 In our study, we identified OTUB2 as a new target of miR-29a-3p in PTC. Moreover, we further observed that ectopic expression of OTUB2 can antagonize the inhibitory effects of miR-29a-3p in PTC.
OTUB2, one of OTUB (Otubain) deubiquitinating enzyme family members, plays vital roles in the regulation of DNA repair pathway, virus-triggered signaling, and pancreatic cell survival.10,20,35 In detail, OTUB2 determines the DNA repair pathway choice by modulating RNF8-mediated L3MBTL1 ubiquitination in the early stage of DNA double-strand break response.20 Besides, OTUB2 can suppress virus-triggered type I interferon induction by directly mediating the deubiquitination of TRAF3 and TRAF6, two E3 ubiquitin ligases required for NF-κB activation.10 However, the role of OTUB2 in cancer has been rarely revealed. In our study, we originally confirmed upregulation of OTUB2 and the inverse correlations of OTUB2 with miR-29a-3p expression and prognosis in PTC. Considering the vital roles of NF-κB signaling in PTC,12,13 and the known interaction of OTUB2 with TRAF 3 and TRAF6, we further validated the role of NF-κB signaling in the functions of miR-29a-3p/OTUB2 in PTC. Indeed, we confirmed that miR-29a-3p can inhibit the activity of NF-κB signaling by modulating TRAF6 level in an OTUB2-dependent manner. Thus, our outcomes originally indicate that OTUB2 can exert positive roles in carcinogenesis; of course, more efforts would have to be taken to fully discover its function in tumors.
Conclusion
In all, we originally confirmed the downregulation of miR-29a-3p associated with upregulation of OTUB2, whose expression is inversely correlated to prognosis, can promote cell growth, proliferation, and invasion by activating TRAF6/ NF-κB signaling in PTC.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Carling T, Udelsman R. Thyroid cancer. Annu Rev Med. 2014;65:125–137. doi: 10.1146/annurev-med-061512-105739. [DOI] [PubMed] [Google Scholar]
- 2.Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388(10061):2783–2795. doi: 10.1016/S0140-6736(16)30172-6. [DOI] [PubMed] [Google Scholar]
- 3.Wiltshire JJ, Drake TM, Uttley L, Balasubramanian SP. Systematic review of trends in the incidence rates of thyroid cancer. Thyroid. 2016;26(11):1541–1552. doi: 10.1089/thy.2016.0100. [DOI] [PubMed] [Google Scholar]
- 4.Aragon Han P, Weng CH, Khawaja HT, et al. MicroRNA expression and association with clinicopathologic features in papillary thyroid cancer: a systematic review. Thyroid. 2015;25(12):1322–1329. doi: 10.1089/thy.2015.0193. [DOI] [PubMed] [Google Scholar]
- 5.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20(8):460–469. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Garofalo M, Croce CM. microRNAs: master regulators as potential therapeutics in cancer. Annu Rev Pharmacol Toxicol. 2011;51:25–43. doi: 10.1146/annurev-pharmtox-010510-100517. [DOI] [PubMed] [Google Scholar]
- 7.Chrus´cik A, Lam AK. Clinical pathological impacts of microRNAs in papillary thyroid carcinoma: a crucial review. Exp Mol Pathol. 2015;99(3):393–398. doi: 10.1016/j.yexmp.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Peng Y, Li C, Luo DC, Ding JW, Zhang W, Pan G. Expression profile and clinical significance of microRNAs in papillary thyroid carcinoma. Molecules. 2014;19(8):11586–11599. doi: 10.3390/molecules190811586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang J, Kong D, Cui Q, et al. Bioinformatic analysis and identification of potential prognostic microRNAs and mRNAs in thyroid cancer. PeerJ. 2018;6:e4674. doi: 10.7717/peerj.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazeh H, Deutch T, Karas A, et al. Next-generation sequencing identifies a highly accurate miRNA panel that distinguishes well-differentiated thyroid cancer from benign thyroid nodules. Cancer Epidemiol Biomarkers Prev. 2018;27(8):858–863. doi: 10.1158/1055-9965.EPI-18-0055. [DOI] [PubMed] [Google Scholar]
- 11.Li S, Zheng H, Mao AP, et al. Regulation of virus-triggered signaling by OTUB1- and OTUB2-mediated deubiquitination of TRAF3 and TRAF6. J Biol Chem. 2010;285(7):4291–4297. doi: 10.1074/jbc.M109.074971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen ZJ. Ubiquitination in signaling to and activation of IKK. Immunol Rev. 2012;246(1):95–106. doi: 10.1111/j.1600-065X.2012.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bommarito A, Richiusa P, Carissimi E, et al. BRAFV600E mutation, TIMP-1 upregulation, and NF-κB activation: closing the loop on the papillary thyroid cancer trilogy. Endocr Relat Cancer. 2011;18(6):669–685. doi: 10.1530/ERC-11-0076. [DOI] [PubMed] [Google Scholar]
- 14.Lv N, Shan Z, Gao Y, et al. Twist1 regulates the epithelial-mesenchymal transition via the NF-κB pathway in papillary thyroid carcinoma. Endocrine. 2016;51(3):469–477. doi: 10.1007/s12020-015-0714-7. [DOI] [PubMed] [Google Scholar]
- 15.Ślusarz A, Pulakat L. The two faces of miR-29. J Cardiovasc Med (Hagerstown) 2015;16(7):480–490. doi: 10.2459/JCM.0000000000000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang H, Zhang G, Wu JH, Jiang CP. Diverse roles of miR-29 in cancer (review) Oncol Rep. 2014;31(4):1509–1516. doi: 10.3892/or.2014.3036. [DOI] [PubMed] [Google Scholar]
- 17.Huang P, Mao LF, Zhang ZP, et al. Down-regulated miR-125a-5p promotes the reprogramming of glucose metabolism and cell malignancy by increasing levels of CD147 in thyroid cancer. Thyroid. 2018;28(5):613–623. doi: 10.1089/thy.2017.0401. [DOI] [PubMed] [Google Scholar]
- 18.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48(9):876–878. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Z, Wang L, Song W, et al. Reduced miR-29a-3p expression is linked to the cell proliferation and cell migration in gastric cancer. World J Surg Oncol. 2015;13:101. doi: 10.1186/s12957-015-0513-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato K, Nakajima K, Ui A, Muto-Terao Y, Ogiwara H, Nakada S. Fine-tuning of DNA damage-dependent ubiquitination by OTUB2 supports the DNA repair pathway choice. Mol Cell. 2014;53(4):617–630. doi: 10.1016/j.molcel.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 21.Gorovets D, Kannan K, Shen R, et al. IDH mutation and neuroglial developmental features define clinically distinct subclasses of lower grade diffuse astrocytic glioma. Clin Cancer Res. 2012;18(9):2490–2501. doi: 10.1158/1078-0432.CCR-11-2977. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Wang J, Li X, et al. MiR-204-5p suppresses cell proliferation by inhibiting IGFBP5 in papillary thyroid carcinoma. Biochem Biophys Res Commun. 2015;457(4):621–626. doi: 10.1016/j.bbrc.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 23.Huang C, Cai Z, Huang M, et al. miR-219-5p modulates cell growth of papillary thyroid carcinoma by targeting estrogen receptor α. J Clin Endocrinol Metab. 2015;100(2):E204–E213. doi: 10.1210/jc.2014-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma S, Jia W, Ni S. miR-199a-5p inhibits the progression of papillary thyroid carcinoma by targeting SNAI1. Biochem Biophys Res Commun. 2018;497(1):181–186. doi: 10.1016/j.bbrc.2018.02.051. [DOI] [PubMed] [Google Scholar]
- 25.Lima CR, Geraldo MV, Fuziwara CS, Kimura ET, Santos MF. MiRNA-146b-5p upregulates migration and invasion of different papillary thyroid carcinoma cells. BMC Cancer. 2016;16:108. doi: 10.1186/s12885-016-2146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei C, Song H, Sun X, et al. miR-183 regulates biological behavior in papillary thyroid carcinoma by targeting the programmed cell death 4. Oncol Rep. 2015;34(1):211–220. doi: 10.3892/or.2015.3971. [DOI] [PubMed] [Google Scholar]
- 27.He H, Wang N, Yi X, Tang C, Wang D. Long non-coding RNA H19 regulates E2F1 expression by competitively sponging endogenous miR-29a-3p in clear cell renal cell carcinoma. Cell Biosci. 2017;7:65. doi: 10.1186/s13578-017-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Liu S, Cao L, et al. miR-29a-3p suppresses cell proliferation and migration by downregulating IGF1R in hepatocellular carcinoma. Oncotarget. 2017;8(49):86592–86603. doi: 10.18632/oncotarget.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salati S, Salvestrini V, Carretta C, et al. Deregulated expression of miR-29a-3p, miR-494-3p and miR-660-5p affects sensitivity to tyrosine kinase inhibitors in CML leukemic stem cells. Oncotarget. 2017;8(30):49451–49469. doi: 10.18632/oncotarget.17706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu W, Yang Z, Fan Y, Zheng Q. MicroRNA-613 inhibits cell growth, migration and invasion of papillary thyroid carcinoma by regulating SphK2. Oncotarget. 2016;7(26):39907–39915. doi: 10.18632/oncotarget.9530. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Yu D, Green B, Tolleson WH, et al. MicroRNA hsa-miR-29a-3p modulates CYP2C19 in human liver cells. Biochem Pharmacol. 2015;98(1):215–223. doi: 10.1016/j.bcp.2015.08.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu D, Tolleson WH, Knox B, et al. Modulation of ALDH5A1 and SLC22A7 by microRNA hsa-miR-29a-3p in human liver cells. Biochem Pharmacol. 2015;98(4):671–680. doi: 10.1016/j.bcp.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su J, Lu E, Lu L, Zhang C. MiR-29a-3p suppresses cell proliferation in laryngocarcinoma by targeting prominin 1. FEBS Open Bio. 2017;7(5):645–651. doi: 10.1002/2211-5463.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catanzaro G, Sabato C, Russo M, et al. Loss of miR-107, miR-181c and miR-29a-3p promote activation of notch2 signaling in pediatric high-grade gliomas (pHGGs) Int J Mol Sci. 2017;18(12):2742. doi: 10.3390/ijms18122742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beck A, Vinik Y, Shatz-Azoulay H, et al. Otubain 2 is a novel promoter of beta cell survival as revealed by siRNA high-throughput screens of human pancreatic islets. Diabetologia. 2013;56(6):1317–1326. doi: 10.1007/s00125-013-2889-x. [DOI] [PubMed] [Google Scholar]