Abstract
Objective
To evaluate the efficacy, safety and economics of levetiracetam (LEV) for epilepsy.
Materials and methods
PubMed, Scopus, the Cochrane Library, OpenGrey.eu and ClinicalTrials.gov were searched for systematic reviews (SRs), meta-analyses, randomized controlled trials (RCTs), observational studies, case reports and economic studies published from January 2007 to April 2018. We used a bubble plot to graphically display information of included studies and conducted meta-analyses to quantitatively synthesize the evidence.
Results
A total of 14,803 records were obtained. We included 30 SRs/meta-analyses, 34 RCTs, 18 observational studies, 58 case reports and 2 economic studies after the screening process. The included SRs enrolled patients with pediatric epilepsy, epilepsy in pregnancy, focal epilepsy, generalized epilepsy and refractory focal epilepsy. Meta-analysis of the included RCTs indicated that LEV was as effective as carbamazepine (CBZ; treatment for 6 months: 58.9% vs 64.8%, OR=0.76, 95% CI: 0.50–1.16; 12 months: 54.9% vs 55.5%, OR=1.24, 95% CI: 0.79–1.93), oxcarbazepine (57.7% vs 59.8%, OR=1.34, 95% CI: 0.34–5.23), phenobarbital (50.0% vs 50.9%, OR=1.20, 95% CI: 0.51–2.82) and lamotrigine (LTG; 61.5% vs 57.7%, OR=1.22, 95% CI: 0.90–1.66). SRs and observational studies indicated a low malformation rate and intrauterine death rate for pregnant women, as well as low risk of cognitive side effects. But psychiatric and behavioral side effects could not be ruled out. LEV decreased discontinuation due to adverse events compared with CBZ (OR=0.52, 95% CI: 0.41–0.65), while no difference was found when LEV was compared with placebo and LTG. Two cost-effectiveness evaluations for refractory epilepsy with decision-tree model showed US$ 76.18 per seizure-free day gained in Canada and US$ 44 per seizure-free day gained in Korea.
Conclusion
LEV is as effective as CBZ, oxcarbazepine, phenobarbital and LTG and has an advantage for pregnant women and in cognitive functions. Limited evidence supports its cost-effectiveness.
Registered number
PROSPERO (No CRD 42017069367).
Keywords: seizure freedom, responder rate, quality of life, malformations, neurological development, psychiatric side effects, cost-effectiveness
Background
Epilepsy ranks fourth after tension-type headache, migraine and Alzheimer disease in the world’s neurological disorders burden.1 A systematic review (SR) and meta-analysis of international studies reported that the point prevalence of active epilepsy was 6.38 per 1,000 people, while the lifetime prevalence was 7.60 per 1,000 people. The annual cumulative incidence of epilepsy was 67.77 per 100,000 people, while the incidence rate was 61.44 per 100,000 person-years.2 As a fairly common clinical condition affecting all ages and requiring long-term, sometimes lifelong, treatment, epilepsy incurs high health care costs for the society.1 In 2010, the total annual cost for epilepsy was 13.8 billion and the total cost per patient was €5,221 in Europe.3 Meanwhile, in the USA, epilepsy-related costs ranged from $1,022 to $19,749 per person annually.4 What is more, drug-refractory epilepsy was a major cost driver,5 with main costs from anticonvulsants, hospitalization and early retirement.6
Currently, antiepileptic drugs (AEDs) are the main treatment method for epilepsy patients, and it was reported that approximately two-thirds of epileptic seizures were controlled by AEDs.7 Conventional AEDs such as carbamazepine (CBZ) and sodium valproate (VPA) have been proven to have good therapeutic effects and low treatment cost. However, some adverse events (AEs) related to these drugs, such as Stevens–Johnson syndrome, menstrual disorder and memory deterioration seriously affect the tolerance and compliance of patients. Compared with conventional AEDs, new AEDs have the potential to be safer, but also more expensive.8
Levetiracetam (LEV) is a novel AED that has been approved as an adjunctive therapy for adults with focal epilepsy since 1999 in the US. In 2006, it was licensed as monotherapy for adults and adolescents above 16 years of age with newly diagnosed focal-onset seizures with or without secondary generalization in Europe. Also, it has been indicated as an adjunctive therapy for partial-onset seizures in patients above 4 years of age in China since 2007. Although the precise mechanism of LEV is still unclear, current researches suggest that its pharmacological mechanism is different from those of other AEDs. It may bind to the synaptic vesicle protein 2A (SV2A), which presents on the synaptic vesicles and some neuroendocrine cells. SV2A may participate in the exocytosis of synaptic vesicles and regulate the release of neurotransmitters, especially the release of excitatory amino acids, and thus depress the epilepsy discharge.9,10 Other possible mechanisms of LEV include the following: selective inhibition of voltage-dependent N-type calcium channels in hippocampal pyramidal cells and reduction of the negative allosteric agents’ inhibition, such as zinc ions and B-carbolines, on glycine and γ-aminobutyric acid neurons, which results in indirectly increasing central nervous system inhibition.11
LEV is almost completely absorbed after oral administration and the absorption is unaffected by food. The bioavailability is nearly 100% and the steady-state concentrations are achieved in 2 days if LEV is taken twice daily. Sixty-six percent of LEV is renally excreted unchanged and its major metabolic pathway is enzymatic hydrolysis of the acetamide group, which is independent of liver CYP/CYP450; so, no clinically meaningful drug–drug interactions with other AEDs were found.12 One published SR of LEV suggested LEV has an equal efficacy compared with conventional AEDs and it is well tolerated for long-term therapy without significant effect on the immune system.13 But in recent years, apart from the most frequent AEs of LEV, such as nausea, gastrointestinal symptoms, dizziness, irritability and aggressive behavior, some rare AEs of LEV have been reported, including eosinophilic pneumonia, rhabdomyolysis, thrombocytopenia, elevated kinase and reduced sperm quality.14–17
Thus, we conducted a mapping review to evaluate the efficacy, safety and economic profiles of LEV compared with all other AEDs for epilepsy, to provide evidence-based information for the rational use of LEV and research agendas.
Materials and methods
Search strategy
We searched PubMed, Scopus, Cochrane Central Register of Controlled Trials, ClinicalTrials.gov and OpenGrey.eu from Jan 1, 2007 to April 30, 2017 and updated the search results till April 23, 2018. The following keywords were used in search terms: “anticonvulsant*”, “anticonvulsive”, “antiepileptic*”, “antiepilepsirin*”, “epileps*”, “epileptic*”, “seizure*”, “convulsion*”, “trial”, “comparative effectiveness research”, “cohort study”, “case-control study”, “case report*”, “case series”, “cost-benefit analysis”, “cost-effectiveness analysis”, “cost-utility analysis”, “cost-minimization analysis”, “systematic review”, “meta-analysis” and “health technology assessment”. The search terms “Keppra”, “Levetiracetam”, “Desitrend”, “Spritam”, “Kepcet”, “Kevtam” and “Levitam” were used to search relevant literature to LEV. The study was registered on PROSPERO (No CRD 42017069367).
Study selection and outcome measures
Four independent investigators manually screened the references of all retrieved records for potentially eligible studies through the title and abstract screening in the first stage and the full-text screening in the second. For the title and abstract screening, studies appearing to meet the inclusion criteria or with insufficient information to make a clear judgment, judged by either authors or both, were included in the full-text screening process. We obtained full texts of all these studies for the full-text screening. We included studies if they 1) enrolled patients diagnosed with epilepsy, 2) compared the efficacy, safety or economic profiles of LEV, without restricting to dosage and duration and 3) SR, meta-analysis, randomized controlled trials (RCTs), observational studies, case reports and economic studies were considered. We resolved the disagreements through discussion, and if necessary, a third party was consulted and discussed.
The primary efficacy outcomes focused on seizure freedom. The secondary efficacy outcomes included 50% responder rate, quality of life (QoL), discontinuation due to AEs, serious AEs, total AEs, single AEs and cost-effectiveness.
Data extraction and quality assessment
Data extraction was performed by two independent investigators according to a predesigned data collection form. Extracted information included authors, publication year, search time frame, number of LEV trials, participant characteristic (seizure type, gender and age), intervention information (the dosage and duration), treatment duration, outcome of interest and dropout rate.
Two investigators independently assessed the methodological quality of included studies. We assessed the quality of included SRs using the Assessment of Multiple Systematic Reviews tool (range, 0–11).18 We assessed the risk of bias in the eligible RCTs with the Cochrane risk of bias assessment tool.19 The methodological quality of eligible observational studies was evaluated with the Newcastle–Ottawa Scale.20 We evaluated the quality of the eligible pharmacoeconomic study with consolidated health economic evaluation reporting standard.21 We did not conduct quality assessment of case reports. In the case of missing data, we contacted the authors of eligible studies for clarifications. All disagreements about data extraction and quality assessment were resolved through discussion among all authors.
Statistical analysis
We compared the treatment effect through meta-analyses in an intention-to-treat manner (following the allocation of participants in studies) of newly included RCTs. Results of RCTs evaluating similar interventions in similar participants were pooled. We calculated the OR for categorical outcomes. We performed meta-analyses of newly included RCTs with RevMan 5.3 software using random-effect model. Statistical heterogeneity was assessed with the Mantel–Haenszel chi-squared test and quantified with the I2 test. P<0.05 was considered statistically significant. Analyses of evidence mapping were conducted in R version 3.4.3. We used a bubble plot to graphically display the evidence regarding seizure type, control vs LEV and outcome measures. Seizure type was classified based on the type of patients and type of epilepsy. Controls were classified based on the class of antiepileptic drug. Outcomes were classified into efficacy and safety outcomes. The number of included studies in SRs and the number of included patients in RCTs were presented as the size of the circles. We described the safety outcomes of observational studies and pooled the numbers of case reports by classification of diseases.
Results
Study selection
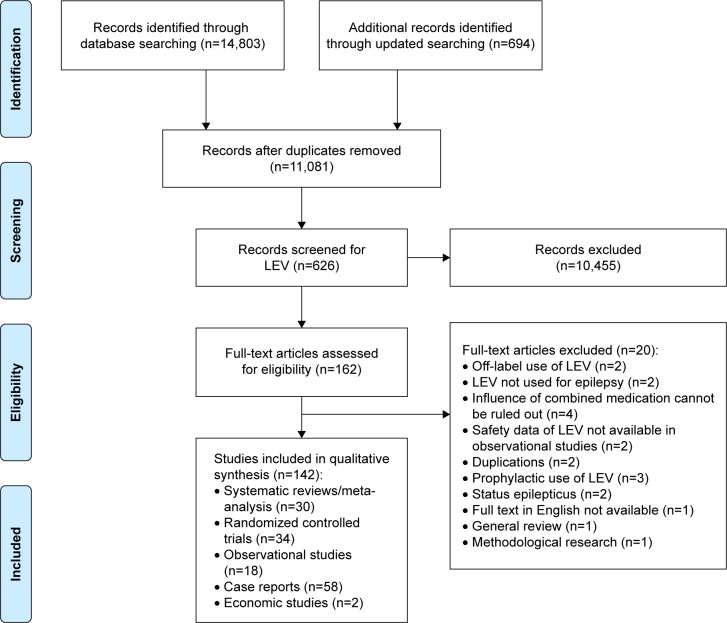
The initial search identified 14,803 relevant records and the updated search identified 694 records. Also, 11,801 records remained after duplicates were removed. Of these, 10,455 records were excluded after LEV search and title/abstract screening and 162 reports were eligible for full-text review. After full-text review, we included 142 reports: 30 SRs/meta-analyses,22–51 34 RCTs,52–85 18 observational studies,86–103 58 case reports104–161 and 2 economic studies162,163 (Figure 1).
Figure 1.
Flow diagram for literature search and study selection.
Abbreviation: LEV, levetiracetam.
Study characteristics and quality assessment
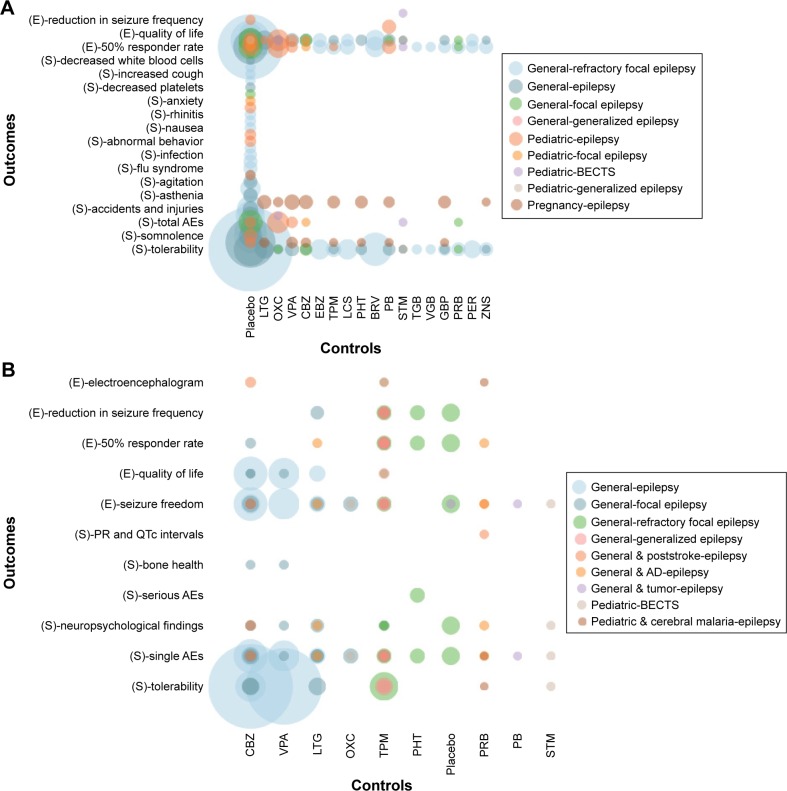
The included SRs were published between 2007 and 2018, enrolling patients with pediatric epilepsy, epilepsy in pregnancy, focal epilepsy, generalized epilepsy and refractory focal epilepsy. Twenty SRs compared LEV with placebo,22–35,38,40,44,46,49,50 19 SRs compared LEV with other AEDs23,24,30,34,36–43,45–51 and 8 SRs were network meta-analyses that compared LEV with other AEDs23,30,37,45–48,50 as well as placebo.23,30,46,50 Outcome measures included seizure freedom, 50% responder rate, reduction in seizure frequency, neuropsychological findings, congenital malformation, serious AEs, total AEs, single AEs and other outcomes (Figure 2A). Among the included RCTs, 12 compared LEV with placebo,52,55,56,58,60–63,65,66,68,78 9 compared LEV with CBZ,53,69,70,73,74,79–82 4 compared LEV with lamotrigine (LTG),57,64,71,81 3 compared LEV with phenobarbital (PB),64,75,85 3 compared LEV with VPA,70,74,82 2 compared LEV with oxcarbazepine (OXC),54,83 2 compared LEV with sulthiame,72,84 1 compared LEV with pregabalin,77 1 compared LEV with phenytoin59 and 1 compared LEV with topiramate.67 Outcome measures included seizure freedom, 50% responder rate, reduction in seizure frequency, QoL, serious AEs, total AEs, single AEs and other outcomes (Figure 2B).
Figure 2.
Evidence mapping of included systematic reviews (A) and randomized controlled trials (B).
Abbreviations: AD, Alzheimer’s disease; AEs, adverse events; BECTS, benign childhood epilepsy with centrotemporal spikes; BRV, brivaracetam; CBZ, carbamazepine; E, efficacy outcomes; EBZ, eslicarbazepine; GBP, gabapentin; LCS, lacosamide; LTG, lamotrigine; OXC, oxcarbazepine; PB, phenobarbital; PER, perampanel; PHT, phenytoin; PRB, pregabalin; S, safety outcomes; STM, sulthiame; TGB, tiagabine; TPM, topiramate; VGB, vigabatrin; VPA, sodium valproate; ZNS, zonisamide.
The two economic studies were from Canada and Korea, both of which focus on add-on therapy for refractory epilepsy.162,163 The two studies used a decision-tree model from the social perspective and payer perspective, respectively.
Study characteristics of the included observational studies and case reports are shown in Tables 1 and 2, respectively.
Table 1.
The characteristics of included observational studies
| Study, year | Intervention
|
Duration | Safety outcomes | ||
|---|---|---|---|---|---|
| Patients | LEV | Control | |||
|
| |||||
| Bootsma et al, 200886 | Patients with chronic refractory epilepsies | LEV | TPM | 24 months | Drug discontinuation, adverse events |
| Andersohn et al, 201087 | Patients with epilepsy | AEDs including LEV | No AEDs | 5.5 years | Self-harm/suicidal behavior |
| Arif et al, 201088 | Above 55 years old with epilepsy | LEV | CBZ/CLB/GBP/LTG/OXC/PHT/TPM/VPA/ZNS | 12 months | Most common intolerable adverse effects |
| Merrell et al, 201089 | Patients with glioma and seizures | LEV | PHT | 18 months | Adverse side effects |
| Rauchenzauner et al, 201090 | Prepubertal children with idiopathic epilepsy | LEV | VPA | 6 months | Sex steroid hormone |
| Veiby et al, 201491 | Children exposed prenatally to AEDs | AEDs including LEV | No AEDs | During pregnancy | Risk of growth restriction, major congenital malformations |
| Xiao et al, 201492 | Children with typical BECTS | LEV | VPA | 18 months | Adverse events |
| Javed et al, 201593 | Adult outpatients with epilepsy | LEV | CBZ/CLB/FBM/GBP/LCM/LTG/OXC/PB/PGB/PHT/PRM/RFM/TGB/TPM/VGB/VPA/ZNS | 12 years | Cognitive side effects |
| Tinchon et al, 201594 | Patients with glioblastoma multiforme and symptomatic seizures | LEV | No AEDs/VPA | 4–8 weeks | Hematological toxicity |
| Tomson et al, 201595 | Children exposed prenatally to AEDs | LEV | CBZ/LTG/OXC/PB/polytherapy/VPA | During pregnancy | Intrauterine death rates |
| Bektaş et al, 201796 | Children with new-onset partial seizures | LEV | VPA | 3 months | Psychiatric and behavioral side effects |
| Chen et al, 201797 | Patients with epilepsy | LEV | CBZ/CLB/FBM/GBP/LCM/LTG/OXC/PB/PGB/PHT/PRM/RFM/TGB/TPM/VGB/VPA/ZNS | At least 1 year | Psychiatric and behavioral side effects |
| Frey et al, 201799 | New user of AEDs | LEV | CBZ/CLB/LMG//PB/PHT/PRB/VPA | ≤84 days prior to the index date | Stevens–Johnson syndrome and toxic epidermal necrolysis |
| Maschio et al, 2017101 | Patients with brain tumor-related epilepsy | LEV | LCM | 6 months | Adverse events |
| Shih et al, 2017102 | Patients with epilepsy | LEV | CBZ/LTG/OXC/PB/PHT/polytherapy/TPM/VPA | NR | Thyroid function |
| Stephen et al, 2017103 | Patients with uncontrolled seizures | LEV | ESL/LCM/PER/PRB/RTG/TPM/ZNS | 6–8 weeks | Psychiatric side effects |
| Egunsola et al, 201898 | Children receiving AEDs | LEV | CLB/CBZ/ESM/LCM/LTG/PHT/PB/TPM/VGB/VPA/ZNS | 3 months | Adverse drug reactions |
| Lee et al, 2018100 | Patients with drug-induced seizures | LEV | No control | NR | Adverse events |
Abbreviations: AED, antiepileptic drugs; BECTS, benign childhood epilepsy with centrotemporal spikes; CBZ, carbamazepine; CLB, clobazam; ESL, eslicarbazepine acetate; ESM, ethosuximide; FBM, felbamate; GBP, gabapentin; LCM, lacosamide; LEV, levetiracetam; LMG, lamotrigine; LTG, lamotrigine; NR, not reported; OXC, oxcarbazepine; PB, phenobarbital; PER, perampanel; PGB, pregabalin; PHT, phenytoin; PRB, pregabalin; PRM, primidone; RFM, rufinamide; RTG, retigabine; TGB, tiagabine; TPM, topiramate; VGB, vigabatrin; VPA, sodium valproate; ZNS, zonisamide.
Table 2.
The characteristics of included case reports
| Psychiatric and behavioral side effects (n=17) | Hematological side effects (n=10) | Skin (n=10) | Kidney (n=4) | Liver (n=4) | Seizure aggravation (n=3) | Others (n=10) |
|---|---|---|---|---|---|---|
|
| ||||||
| Tamarelle et al, 2009109 | Gallerani et al, 2009105 | Gómez-Zorrilla et al, 2012123 | Hurwitz et al, 2009107 | Broli et al, 2010111 | Caraballo et al, 2010112 | Newsome et al, 2007104 |
| vande Griend et al, 2009110 | Hacquard et al, 2009106 | Zou et al, 2012125 | Chau et al, 2012122 | Xiong et al, 2012124 | Babtain, 2012118 | Alkhotani and Mclachlan, 2012117 |
| Givon et al, 2011116 | Peer Mohamed et al, 2009108 | Karadag et al, 2013127 | Isaacson et al, 2014136 | Sethi et al, 2013130 | Makke et al, 2015149 | Akiyama et al, 2014131 |
| Bishop-Freeman et al, 2012119 | Oghlakian et al, 2010113 | Zou et al, 2014142 | Spengler et al, 2014140 | Azar and Aune, 2014133 | Aksoy et al, 2014132 | |
| Calabrò et al, 2012120 | Sahaya et al, 2010114 | Eleni, 2015144 | Koklu et al, 2014137 | |||
| Camacho et al, 2012121 | Bachmann et al, 2011115 | Gencler et al, 2015147 | Arı et al, 2015143 | |||
| Hommet et al, 2013126 | Flannery et al, 2015145 | Bayram et al, 2016153 | Ju et al, 2016157 | |||
| Kaufman et al, 2013128 | Peyrl et al, 2015151 | Dar et al, 2016154 | Turati et al, 2017158 | |||
| Metin et al, 2013129 | Taberner Bonastre et al, 2015152 | Jones et al, 2016156 | Kubota et al, 2017159 | |||
| Bui et al, 2014134 | García et al, 2016155 | Sereflican et al, 2017161 | Ozdemir et al, 2018160 | |||
| Hwang et al, 2014135 | ||||||
| Kumar et al, 2014138 | ||||||
| Park et al, 2014139 | ||||||
| Zaki and Gupta, 2014141 | ||||||
| Fujikawa et al, 2015146 | ||||||
| Kawakami et al, 2015148 | ||||||
| Molokwu et al, 2015150 | ||||||
In general, the quality of included SRs and economic studies was good. The included RCTs were generally of low risk of bias. Sixteen RCTs used the double-blind design and 24 adopted the intention-to-treat principle to analyze data (Table 3).
Table 3.
Risk of bias of included randomized controlled trials
| Study, year | Random sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selecting reporting | Other source of bias |
|---|---|---|---|---|---|---|
| Berkovic et al, 200752 | Low | Low | Low | Low | Low | High |
| Borggraefe et al, 201372 | Low | Low | Low | Low | Unclear | Unclear |
| Brodie et al, 200753 | Unclear | Unclear | Low | Low | Low | Unclear |
| Consoli et al, 201269 | Low | High | High | Low | Unclear | Low |
| Coppola et al, 200754 | Low | High | High | Low | Unclear | Unclear |
| Cumbo and Ligori, 201064 | Unclear | Unclear | Low | Low | Unclear | Low |
| de La Loge et al, 201065 | Unclear | Unclear | Low | Low | Low | High |
| Fattore et al, 201168 | Low | Unclear | Low | Low | Unclear | Unclear |
| Hakami et al, 201682 | Low | Unclear | High | Low | Low | Low |
| Hakami et al, 201270 | Low | Unclear | High | Low | Low | Low |
| Inoue et al, 201578 | Unclear | Unclear | Low | Low | Low | Unclear |
| Labiner et al, 200957 | Unclear | Unclear | Low | Low | Unclear | Low |
| Jung et al, 201579 | Low | Low | High | Unclear | Low | Low |
| Kim et al, 201783 | Unclear | Unclear | High | Unclear | Low | Unclear |
| Levisohn et al, 200958 | Low | Unclear | Low | Low | Low | High |
| Lim et al, 200959 | Low | Unclear | Unclear | Low | Unclear | Unclear |
| Peltola et al, 200960 | Unclear | Unclear | Low | Low | Low | High |
| Piña-Garza et al, 200961 | Unclear | Unclear | High | Unclear | Low | Unclear |
| Rosenow et al, 201271 | Low | Unclear | High | Low | Low | Low |
| Rossetti et al, 201476 | Low | Low | High | Low | Low | Unclear |
| Siniscalchi et al, 201485 | Unclear | Unclear | High | Low | Unclear | Low |
| Suresh et al, 201580 | Unclear | Unclear | High | Unclear | Low | Low |
| Tacke et al, 201784 | Low | Low | Low | Unclear | Low | Unclear |
| Trinka et al, 201374 | Low | Low | High | Unclear | Low | High |
| Werhahn et al, 201581 | Low | Low | Low | Low | Low | Low |
| Wu et al, 200962 | Unclear | Unclear | Low | Low | Low | Low |
| Xiao et al, 200963 | Low | Low | Low | Low | Unclear | Unclear |
| Zaccara et al, 201477 | Low | Unclear | Low | Low | Low | Unclear |
| Zhou et al, 200856 | Low | Unclear | High | Unclear | Unclear | Unclear |
| Noachtar et al, 200855 | Low | Low | Low | Low | Low | Unclear |
| NCT0122874766 | Unclear | Unclear | Low | Low | Low | Unclear |
| NCT0198281275 | Unclear | Unclear | High | Low | Low | Low |
| NCT0195412173 | Unclear | Unclear | High | Low | Low | Unclear |
| NCT0122973567 | Unclear | Unclear | High | Low | Low | Unclear |
Efficacy
Seizure freedom
Thirteen SRs evaluated rates of seizure freedom23,26,31,37,40,41,43–46,49–51 (Figure 2A) and indicated that LEV increased the rates of seizure freedom compared with placebo,23,26,31,40,44,46,49,50 but there was no difference when LEV was compared with OXC,41,49 LTG23,37,45,51 and brivaracetam.40
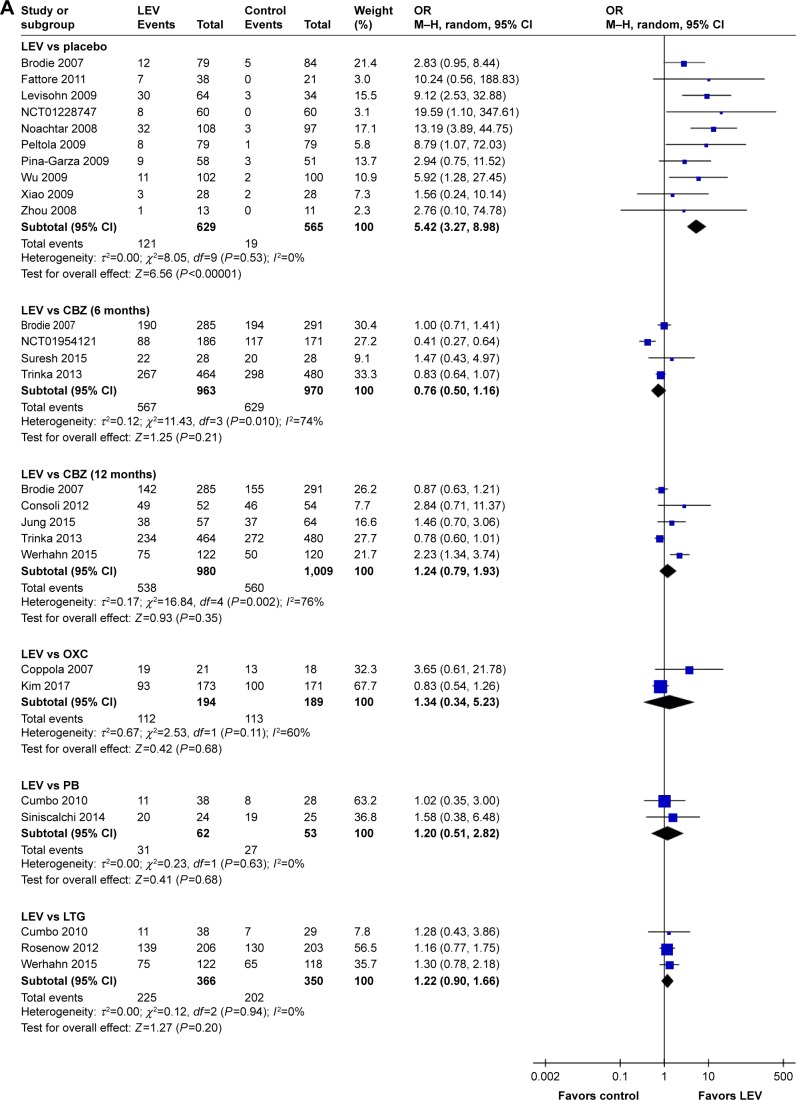
Meta-analysis of newly included RCTs indicated that LEV increased the rates of seizure freedom compared with placebo (19.2% [121/629] vs 3.4% [19/565], OR=5.42, 95% CI: 3.27–8.98). Meta-analyses of newly included RCTs showed that there was no difference when LEV was compared with CBZ (treatment for 6 months: 58.9% [567/963] vs 64.8% [629/970], OR=0.76, 95% CI: 0.50–1.16; treatment for 12 months: 54.9% [538/980] vs 55.5% [560/1,009], OR=1.24, 95% CI: 0.79–1.93), OXC (57.7% [112/194] vs 59.8% [113/189], OR=1.34, 95% CI: 0.34–5.23), PB (50.0% [31/62] vs 50.9% [27/53], OR=1.20, 95% CI: 0.51–2.82) and LTG (61.5% [225/366] vs 57.7% [202/350], OR=1.22, 95% CI: 0.90–1.66). We observed significant heterogeneity across included studies in the subgroup of CBZ (I2=74% for 6 months treatment and I2=76% for 12 months treatment), as shown in Figure 3A.
Figure 3.
Rate of seizure freedom of included randomized controlled trials (A) and ≥50% responder rates of included randomized controlled trials (B).
Abbreviations: CBZ, carbamazepine; df, degrees of freedom; LEV, levetiracetam; LTG, lamotrigine; M–H, Mantel–Haenszel; OXC, oxcarbazepine; PB, phenobarbital; random, random-effect model.
≥50% responder rates
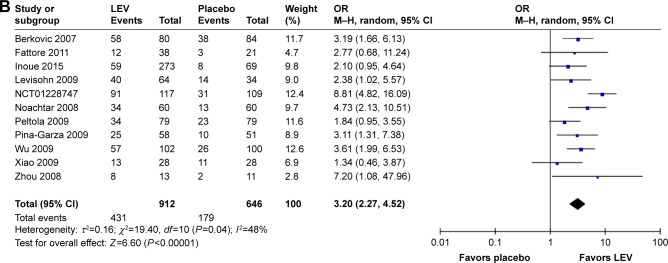
Sixteen SRs evaluated ≥50% responder rates23,24,26,27,29–31,36,40–43,46,49–51 (Figure 2A) and 12 SRs indicated that LEV increased the rates of ≥50% responder rates compared with placebo,23,24,26,27,29–31,36,40,42,46,49 but there was no difference when LEV was compared with brivaracetam.40
Meta-analysis of newly included RCTs indicated that LEV increased the rates of ≥50% responder rates compared with placebo (n=1,558, 47.3% [431/912] vs 27.7% [179/646], OR=3.20, 95% CI: 2.27–4.52), as shown in Figure 3B.
Improvement of QoL
One SR suggested that LEV had a positive effect on some aspects of QoL in adults.27
Meta-analysis of newly included RCTs showed that there was no difference between LEV and placebo in improvement of QoL (n=224, OR=2.76, 95% CI: 0.85–8.94). We observed significant heterogeneity (I2=72%) across included studies.
Safety
Discontinuation due to AEs
SRs indicated that there was no difference in risk of discontinuation due to AEs when LEV was compared with placebo.24
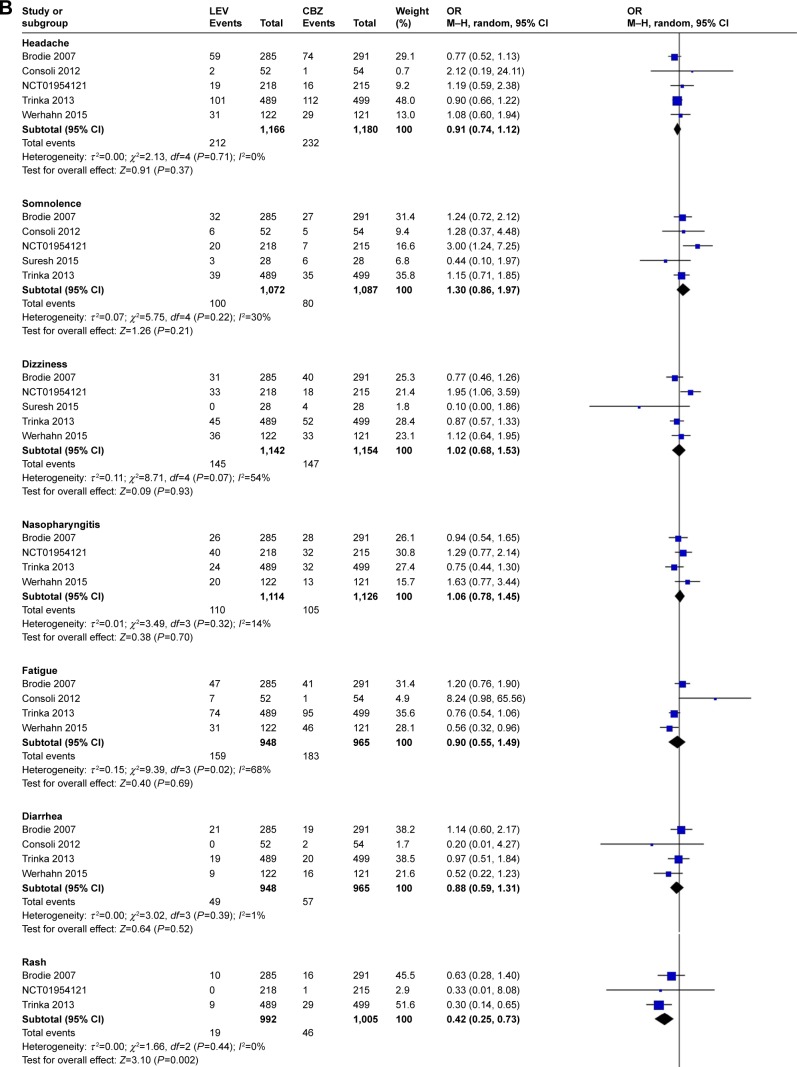
Meta-analysis of newly included RCTs indicated that LEV decreased discontinuation due to AEs compared with CBZ (OR=0.52, 95% CI: 0.41–0.65), while there was no difference when LEV was compared with placebo (OR=1.16, 95% CI: 0.92–1.46) and LTG (OR=1.24, 95% CI: 0.55–2.83). We observed significant heterogeneity (I2=74%) across included studies in the subgroup of LTG.
Serious AEs
Meta-analysis of newly included RCTs showed that there was no difference when LEV was compared with placebo (OR=1.10, 95% CI: 0.59–2.05), CBZ (OR=0.83, 95% CI: 0.35–1.95) and LTG (OR=1.40, 95% CI: 0.74–2.62) in the rates of serious AEs.
Total AEs
SRs indicated that AEs were not significantly different between the LEV group and the placebo group.31
Meta-analysis of newly included RCTs showed that there was no difference when LEV was compared with placebo (OR=1.16, 95% CI: 0.92–1.46) and OXC (OR=0.73, 95% CI: 0.47–1.15) in the rates of total AEs.
Single AEs
Malformations and prenatal outcomes
Two SRs reported the safety of AEDs during pregnancy, both of which indicated that LEV was not associated with a higher risk compared to control (RR=0.32, 95% CI: 0.10–1.07 and OR=0.72, 95% CI: 0.43–1.16, respectively).39,47
Two observational studies used data from deliveries recorded in the compulsory Medical Birth Registry of Norway 1999–2011 and International Registry of Antiepileptic Drugs and Pregnancy (EURAP) registry, respectively.91,95 While data in the Norway registry showed LEV had a low malformation rate for pregnant women (OR=0.63, 95% CI: 0.16–2.55 for monotherapy and OR=1.08, 95% CI: 0.27–4.43 for polytherapy), data in the EURAP registry indicated low intrauterine death rates (8.6%, 95% CI: 5.8%–12.3%).
Neurological development
One SR showed that LEV did not increase the risk for delayed development of children (cognitive development delay: OR=3.42, 95% Credible Interval: 0.65–16.40; psychomotor development delay: OR=0.27, 95% Credible Interval: 0.00–4.65).48
An observational study by Javed et al93 indicated a low risk of cognitive side effects of LEV (OR=0.68, 95% CI: 0.48–0.99 in patients newly started on polypharmacy).
Psychiatric and behavioral side effects (PBSEs)
One SR showed from various types of studies that LEV administration was associated primarily with adverse psychotropic effects including anxiety, irritability and depression.28 One SR32 indicated that LEV increased the risk of developing several behavioral side effects (RR=2.18, 95% CI: 1.42–3.37) such as aggression, hostility and nervousness, while the other SR reported lower rates of behavioral effects.33 Another SR indicated that LEV may have a relationship with suicidality in epilepsy (Figure 2A).34
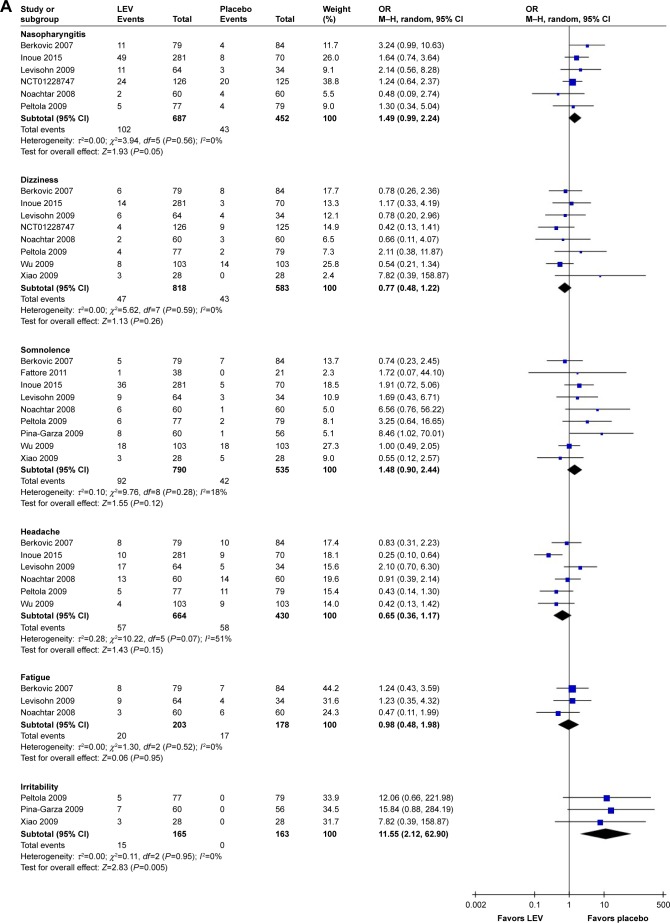
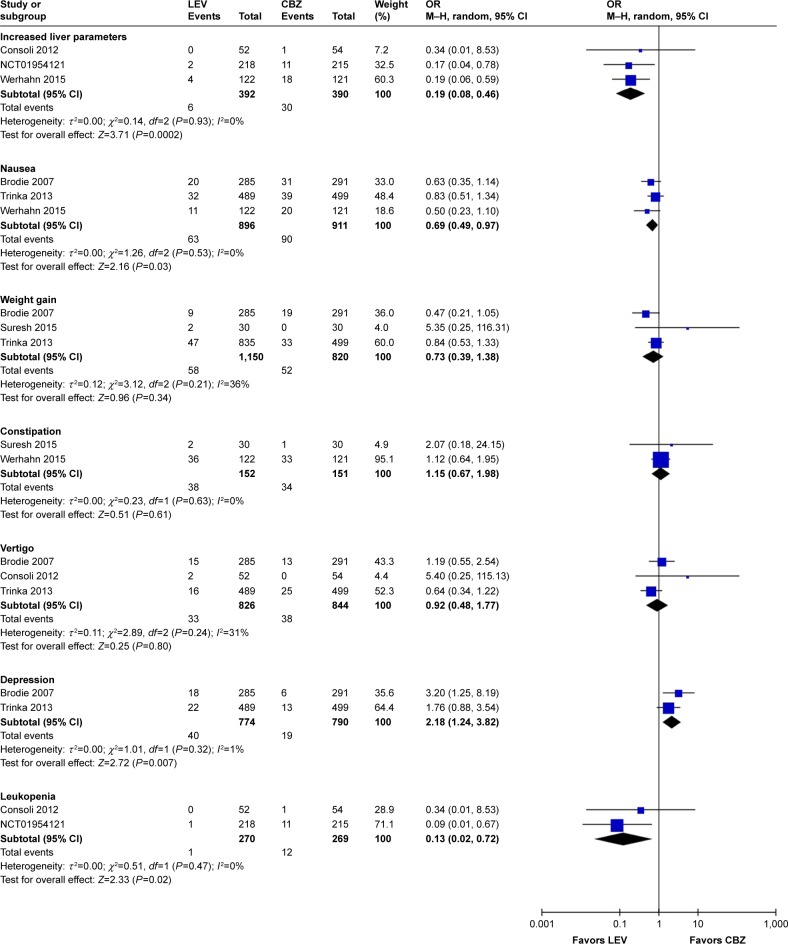
Meta-analysis of newly included RCTs indicated that LEV increased the risk of irritability compared with placebo (n=328, OR=11.55, 95% CI: 2.12–62.90; Figure 4A) and the risk of depression compared with CBZ (n=1,564, OR=2.18, 95% CI: 1.24–3.82; Figure 4B). But no difference was found in the risk of depression when LEV was compared with LTG (n=673, OR=1.80, 95% CI: 0.82–3.97).
Figure 4.
Risk of single adverse events (LEV vs placebo, A; LEV vs CBZ, B).
Abbreviations: CBZ, carbamazepine; df, degrees of freedom; LEV, levetiracetam; M–H, Mantel–Haenszel; random, random-effect model.
For observational studies, Bootsma et al86 indicated the most prevalent AEs for LEV were activating mood disorders (8.1% for 6 months, 5.2% for 12 months and 10.6% for 18 months), Arif et al88 indicated psychiatric AEs were the most common adverse effects leading to intolerability and Andersohn et al87 indicated LEV was associated with an increased risk of self-harm or suicidal behavior. Chen et al97 indicated that LEV had the greatest PBSE rate in adults with epilepsy. However, Bektaş et al96 indicated that psychosocial and behavioral side effects of LEV treatment are not frequent and they do not emerge in most of the children at lower doses, and Stephen et al103 indicated a lower rate of psychiatric side effects for LEV than sodium channel blocking AEDs.
Among the 58 case reports, 17 reported PBSEs, including depression, suicidality and hypersexuality.
Other AEs
SRs indicated that LEV did not increase the risk of imbalance,22 but increased the risk of diplopia (Figure 2A).25
Meta-analysis of newly included RCTs indicated LEV had a lower risk of leukopenia (OR=0.13, 95% CI: 0.02–0.72), rash (OR=0.42, 95% CI: 0.25–0.73), increased liver parameters (OR=0.19, 95% CI: 0.08–0.46) and nausea (OR=0.69, 95% CI: 0.49–0.97) compared with CBZ (Figure 4B). LEV had a lower risk of nausea (OR=0.62, 95% CI: 0.39–0.98) and a higher risk of fatigue (OR=1.87, 95% CI: 1.26–2.77) compared with LTG. Meta-analyses of newly included RCTs showed that there was no difference when LEV was compared with placebo, CBZ, LTG and OXC in headache (Figure 4A). No difference was found in somnolence and dizziness when LEV was compared with placebo, CBZ and LTG (Figure 4A).
Among the observational studies, Merrell et al indicated LEV had fewer side effects than phenytoin.89 Rauchenzauner et al indicated LEV did not seem to induce changes in reproductive endocrine functions and clinically relevant endocrine side effects in prepubertal children.90 Tinchon et al indicated LEV has no additional impact on medium-term hematological toxicity in glioblastoma multiforme patients.94 Xiao et al reported all AEs of LEV were either mild or transient and thus did not lead to withdrawal from drug treatment.92
Other case reports were related to side effects in the hematological system, skin, kidney, liver and other systems (Table 2).
Cost-effectiveness
Two cost-effectiveness evaluations for refractory epilepsy with the decision-tree model were conducted in Canada and Korea, respectively.
The Canadian study showed the incremental cost- effectiveness ratio (ICER) was US$ 76.18 per seizure-free day (SFD) gained for the base-case scenario; when the cost of surgical investigation and surgery was included in the model, the ICERs decreased to US$ 39.18, which was the most cost-effective situation.162
The Korean study showed that LEV add-on therapy gained 18.3 SFDs per patient per year and the ICERs were US$ 44 per SFD per patient and US$ 11,084 per quality-adjusted life year gained from the third-party payer perspective.163
Discussion
In our evidence map, the included SRs and newly conducted meta-analyses showed consistent results regarding clinical benefits and potential harms of LEV. Our evidence map indicated that LEV had similar efficacy in seizure freedom compared with conventional AEDs and was superior to placebo in seizure freedom and ≥50% responder rates. What is more, LEV had a lower risk of discontinuation due to AEs compared with CBZ and did not increase the risk of malformations and prenatal outcomes as well as neurological development. Limited evidence suggested it was cost-effective in certain settings.
LEV has been classified by the US Food and Drug Administration as a category C drug, with the caution that it should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. A Cochrane review included in our study analyzed the incidence of congenital malformations in pregnant women during AED treatment and reported that LEV and LTG exposure carried the lowest risk of overall malformation.39 A recently published prospective cohort study based on the EURAP international registry reported the lowest prevalence of major congenital malformations of LEV (2.8%, 17/599 pregnancies) compared with other seven commonly used AEDs.164 Two observational studies91,95 included in this evidence map drew similar conclusions. A published study found that compared with VPA, LEV did not cause apoptosis in immature rat brain neurons, which may be the reason of its safety for pregnant women.165 Neurologists are also concerned with the effect of AEDs on cognitive function, which significantly affects the QoL of patients, especially children and the elderly. No AEs of LEV on cognitive function were found in our study, which was consistent with the guidelines. However, there are some RCTs, observational studies and case reports indicating the AEs of mood disorders of LEV. We should monitor these AEs during the course of medication.
A number of guidelines included LEV as a main drug for antiepileptic treatment. The National Institute for Health and Care Excellence (NICE; 2017) recommended that LEV could be used as a monotherapy and in the adjunctive treatment of focal epilepsy (with or without secondary generalization) and adjunctive therapy of myoclonic seizures in patients with juvenile myoclonic epilepsy and generalized tonic clonic seizures.7 The Scottish Intercollegiate Guidelines Network gave a similar recommendation and further suggested that LEV or LTG may be a reasonable alternative for women of childbearing age. Moreover, the guideline also suggested that LEV was better tolerated than sustained-release CBZ in poststroke seizures and produced fewer cognitive AEs than LTG or PB in the elderly with epilepsy and Alzheimer disease.166 The Biopharmaceutics Drug Disposition Classification System predicted that the risk of skin rash by LEV is not as high as by CBZ or LTG,167 and that human leukocyte antigen testing is not necessary. With the increasing number of studies on LEV, guideline recommendations need to update the evidence for LEV.168 Our research provides supplements for evidence update in future guidelines.
The economic evaluation of LEV showed that LEV appeared to be cost-effective when the costs of surgical investigation were discounted. Besides, when LEV is added to the usual treatment of patients with refractory epilepsy, the increase in drug costs may at least be partially offset by savings in other medical costs due to an increase in SFDs and improvement of QoL.169 But until now, the NICE guideline still has suggested LEV monotherapy as a second-line drug and LEV is considered when the standard first-line drugs such as CBZ and LTG are unsuitable or develop intolerance in the newly diagnosed focal seizure. The economic profiles of our research can help with the cost-effectiveness decision making in certain conditions.
To the best of our knowledge, this study is the most comprehensive evidence of LEV in the following aspects. First, we included various types of studies, such as high-quality RCTs, cohort studies, observational studies, case reports and economic studies. The literature included was comprehensive and involved a large number of patients. Second, we evaluated the clinical application of LEV from three dimensions: efficacy, safety and economy, while the three aspects were studied respectively or the evaluation of LEV was among the overall evaluation of a variety of AEDs in the previous published studies.30,36,163,170 Thus, our study can provide comprehensive evidence of LEV for physicians or policymakers.
Our study still had some limitations. First, only English language studies were included. We tried to include important conference abstracts found in the databases, but failed to find relevant studies. Moreover, the literature included in this study was published after 2007, although previously published studies were included in the SRs of the evidence map. Third, some special types of seizures such as status epilepticus (SE) were excluded and data of LEV in special populations were not assessed separately. Fourth, no subgroup analysis of different types of seizures and/or epilepsy syndromes was conducted.
The NICE guideline suggested that LEV is potentially as effective as PB and safer for SE. Currently available intravenous AEDs are limited, and intravenous LEV may have advantages for patients who cannot be administered orally with SE or in the perioperative period.171,172 A chart review in Germany showed LEV was the first choice for intravenous treatment of SE compared with valproate, phenytoin and lacosamide.173 We can evaluate the role of LEV for SE in future studies.
Conclusion
LEV has been applied for diverse epilepsies, and the evidence map shows that it increases the rates of seizure freedom and ≥50% responder rates compared with placebo, has similar efficacy with CBZ, OXC, PB and LTG, and also has an advantage for pregnant women as well as in cognitive functions. LEV does not increase the risks of serious AEs and discontinuation from studies due to AEs. Limited evidence supports its cost-effectiveness.
Acknowledgments
We extend special thanks to Wei Huang from Zhejiang Province Chinese Medical Hospital, Le Gao, Ji-Chun Yang and Yang Xu from Peking University Health Science Center as well as Jun-Wen Zhou from the Public Health Department, and Aix-Marseille University for their contribution in conducting the literature review and drafting figures for this manuscript. We would like to thank Li Wang from Peking University First Hospital and Yuan Zhang from McMaster University for expert consultation. This study was funded by UCB China Inc. At no point did UCB China Inc. attempt to influence the manuscript. The abstract of this paper was presented as a poster in the ISPE’s 11th Asian Conference on Pharmacoepidemiology with interim findings.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Beghi E. Addressing the burden of epilepsy: many unmet needs. Pharmacol Res. 2016;107:79–84. doi: 10.1016/j.phrs.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Fiest KM, Sauro KM, Wiebe S, et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. 2017;88(3):296–303. doi: 10.1212/WNL.0000000000003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olesen J, Gustavsson A, Svensson M, et al. The economic cost of brain disorders in Europe. Eur J Neurol. 2012;19(1):155–162. doi: 10.1111/j.1468-1331.2011.03590.x. [DOI] [PubMed] [Google Scholar]
- 4.Begley CE, Durgin TL. The direct cost of epilepsy in the United States: a systematic review of estimates. Epilepsia. 2015;56(9):1376–1387. doi: 10.1111/epi.13084. [DOI] [PubMed] [Google Scholar]
- 5.Kortland LM, Alfter A, Bähr O, et al. Costs and cost-driving factors for acute treatment of adults with status epilepticus: a multicenter cohort study from Germany. Epilepsia. 2016;57(12):2056–2066. doi: 10.1111/epi.13584. [DOI] [PubMed] [Google Scholar]
- 6.Willems LM, Richter S, Watermann N, et al. Trends in resource utilization and prescription of anticonvulsants for patients with active epilepsy in Germany from 2003 to 2013 – a ten-year overview. Epilepsy Behav. 2018;83:28–35. doi: 10.1016/j.yebeh.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Epilepsies: diagnosis and management (clinical guideline [CG137]) [Accessed July 18, 2017]. Available from: https://www.nice.org.uk/guidance/cg137.
- 8.Zhu F, Lang SY, Wang XQ, et al. Long-term effectiveness of antiepileptic drug monotherapy in partial epileptic patients: a 7-year study in an epilepsy center in China. Chin Med J. 2015;128(22):3015–3022. doi: 10.4103/0366-6999.168968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matagne A, Margineanu DG, Kenda B, Michel P, Klitgaard H. Anti-convulsive and anti-epileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein, SV2A. Br J Pharmacol. 2008;154(8):1662–1671. doi: 10.1038/bjp.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rigo JM, Hans G, Nguyen L, et al. The anti-epileptic drug levetiracetam reverses the inhibition by negative allosteric modulators of neuronal GABA- and glycine-gated currents. Br J Pharmacol. 2002;136(5):659–672. doi: 10.1038/sj.bjp.0704766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deshpande LS, Delorenzo RJ. Mechanisms of levetiracetam in the control of status epilepticus and epilepsy. Front Neurol. 2014;5:11. doi: 10.3389/fneur.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emswiler MP, Cumpston KL. Second generation anticonvulsants: gabapentin, lamotrigine, levetiracetam, and topiramate. [Accessed July 18, 2017]. Available from: https://www.researchgate.net/publication/312660054_Second_Generation_Anticonvulsants_Gabapentin_Lamotrigine_Levetiracetam_and_Topiramate?ev=auth_pub.
- 13.French J, Edrich P, Cramer JA. A systematic review of the safety profile of levetiracetam: a new antiepileptic drug. Epilepsy Res. 2001;47(1–2):77–90. doi: 10.1016/s0920-1211(01)00296-0. [DOI] [PubMed] [Google Scholar]
- 14.Spencer D. Levetiracetam in men with epilepsy: testosterone is left alone but sperm count is paramount. Epilepsy Curr. 2017;17(2):99–100. doi: 10.5698/1535-7511.17.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fagan A, Fuld J, Soon E. Levetiracetam-induced eosinophilic pneumonia. BMJ Case Rep. 2017;2017:bcr2016219121. doi: 10.1136/bcr-2016-219121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Shin JW. Levetiracetam-induced thrombocytopenia in a patient with status epilepticus. Epileptic Disord. 2017;19(1):104–108. doi: 10.1684/epd.2017.0889. [DOI] [PubMed] [Google Scholar]
- 17.di Lorenzo R, Li Y. Rhabdomyolysis associated with levetiracetam administration. Muscle Nerve. 2017;56(1):E1–E2. doi: 10.1002/mus.25548. [DOI] [PubMed] [Google Scholar]
- 18.Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7(1):10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells GA, Shea B, O’Connell D. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Accessed July 18, 2017]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 21.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health. 2013;16(2):e1–e5. doi: 10.1016/j.jval.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Sirven JI, Fife TD, Wingerchuk DM, Drazkowski JF. Second-generation antiepileptic drugs’ impact on balance: a meta-analysis. Mayo Clin Proc. 2007;82(1):40–47. doi: 10.4065/82.1.40. [DOI] [PubMed] [Google Scholar]
- 23.Costa J, Fareleira F, Ascenção R, Borges M, Sampaio C, Vaz-Carneiro A. Clinical comparability of the new antiepileptic drugs in refractory partial epilepsy: a systematic review and meta-analysis. Epilepsia. 2011;52(7):1280–1291. doi: 10.1111/j.1528-1167.2011.03047.x. [DOI] [PubMed] [Google Scholar]
- 24.Lo BW, Kyu HH, Jichici D, Upton AM, Akl EA, Meade MO. Meta-analysis of randomized trials on first line and adjunctive levetiracetam. Can J Neurol Sci. 2011;38(3):475–486. doi: 10.1017/s0317167100011902. [DOI] [PubMed] [Google Scholar]
- 25.Han H, Qu W, Kang H, et al. Effect of second-generation antiepileptic drugs on diplopia: a meta-analysis of placebo-controlled studies. J Huazhong Univ Sci Technolog Med Sci. 2012;32(4):557–562. doi: 10.1007/s11596-012-0096-5. [DOI] [PubMed] [Google Scholar]
- 26.Maguire M, Marson AG, Ramaratnam S. Epilepsy (generalized) BMJ Clin Evid. 2012;2012:1201. [PMC free article] [PubMed] [Google Scholar]
- 27.Mbizvo GK, Dixon P, Hutton JL, Marson AG. Levetiracetam add-on for drug-resistant focal epilepsy: an updated Cochrane Review. Cochrane Database Syst Rev. 2012;1901;9(9):CD00. doi: 10.1002/14651858.CD001901.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piedad J, Rickards H, Besag FM, Cavanna AE. Beneficial and adverse psychotropic effects of antiepileptic drugs in patients with epilepsy: a summary of prevalence, underlying mechanisms and data limitations. CNS Drugs. 2012;26(4):319–335. doi: 10.2165/11599780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Arya R, Glauser TA. Pharmacotherapy of focal epilepsy in children: a systematic review of approved agents. CNS Drugs. 2013;27(4):273–286. doi: 10.1007/s40263-013-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodalia PN, Grosso AM, Sofat R, et al. Comparative efficacy and tolerability of anti-epileptic drugs for refractory focal epilepsy: systematic review and network meta-analysis reveals the need for long term comparator trials. Br J Clin Pharmacol. 2013;76(5):649–667. doi: 10.1111/bcp.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang Y, Wu X, Xu L, et al. Randomized-controlled trials of levetiracetam as an adjunctive therapy in epilepsy of multiple seizure types. J Clin Neurosci. 2014;21(1):55–62. doi: 10.1016/j.jocn.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 32.Halma E, de Louw AJ, Klinkenberg S, Aldenkamp AP, Ijff DM, Majoie M. Behavioral side-effects of levetiracetam in children with epilepsy: a systematic review. Seizure. 2014;23(9):685–691. doi: 10.1016/j.seizure.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Mbizvo GK, Dixon P, Hutton JL, Marson AG. The adverse effects profile of levetiracetam in epilepsy: a more detailed look. Int J Neurosci. 2014;124(9):627–634. doi: 10.3109/00207454.2013.866951. [DOI] [PubMed] [Google Scholar]
- 34.Fountoulakis KN, Gonda X, Baghai TC, et al. Report of the WPA section of pharmacopsychiatry on the relationship of antiepileptic drugs with suicidality in epilepsy. Int J Psychiatry Clin Pract. 2015;19(3):158–167. doi: 10.3109/13651501.2014.1000930. [DOI] [PubMed] [Google Scholar]
- 35.Verrotti A, Prezioso G, di Sabatino F, Franco V, Chiarelli F, Zaccara G. The adverse event profile of levetiracetam: a meta-analysis on children and adults. Seizure. 2015;31:49–55. doi: 10.1016/j.seizure.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Weijenberg A, Brouwer OF, Callenbach PM. Levetiracetam monotherapy in children with epilepsy: a systematic review. CNS Drugs. 2015;29(5):371–382. doi: 10.1007/s40263-015-0248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campos MS, Ayres LR, Morelo MR, Marques FA, Pereira LR. Efficacy and tolerability of antiepileptic drugs in patients with focal epilepsy: systematic review and network meta-analyses. Pharmacotherapy. 2016;36(12):1255–1271. doi: 10.1002/phar.1855. [DOI] [PubMed] [Google Scholar]
- 38.Egunsola O, Choonara I, Sammons HM. Safety of levetiracetam in paediatrics: a systematic review. PLoS One. 2016;11(3):e0149686. doi: 10.1371/journal.pone.0149686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weston J, Bromley R, Jackson CF, et al. Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child. Cochrane Database Syst Rev. 2016;11:CD010224. doi: 10.1002/14651858.CD010224.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, Li S, Li H, Zou X. Levetiracetam vs. brivaracetam for adults with refractory focal seizures: a meta-analysis and indirect comparison. Seizure. 2016;39:28–33. doi: 10.1016/j.seizure.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Geng H, Wang C. Efficacy and safety of oxcarbazepine in the treatment of children with epilepsy: a meta-analysis of randomized controlled trials. Neuropsychiatr Dis Treat. 2017;13:685–695. doi: 10.2147/NDT.S130269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song JM, Hahn J, Kim SH, Chang MJ. Efficacy of treatments for infantile spasms: a systematic review. Clin Neuropharmacol. 2017;40(2):63–84. doi: 10.1097/WNF.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 43.Mchugh DC, Lancaster S, Manganas LN. A systematic review of the efficacy of levetiracetam in neonatal seizures. Neuropediatrics. 2018;49(1):012–017. doi: 10.1055/s-0037-1608653. [DOI] [PubMed] [Google Scholar]
- 44.Mohd-Tahir NA, Li SC. Meta-analyses of newer antiepileptic drugs as adjunct for treatment of focal epilepsy in children. Epilepsy Res. 2018;139:113–122. doi: 10.1016/j.eplepsyres.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Nevitt SJ, Sudell M, Weston J, Tudur Smith C, Marson AG. Anti-epileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data. Cochrane Database Syst Rev. 2017;6:CD011412. doi: 10.1002/14651858.CD011412.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosati A, Ilvento L, Lucenteforte E, et al. Comparative efficacy of antiepileptic drugs in children and adolescents: a network meta-analysis. Epilepsia. 2018;59(2):297–314. doi: 10.1111/epi.13981. [DOI] [PubMed] [Google Scholar]
- 47.Veroniki AA, Cogo E, Rios P, et al. Comparative safety of anti-epileptic drugs during pregnancy: a systematic review and network meta-analysis of congenital malformations and prenatal outcomes. BMC Med. 2017;15(1):95. doi: 10.1186/s12916-017-0845-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veroniki AA, Rios P, Cogo E, et al. Comparative safety of antiepileptic drugs for neurological development in children exposed during pregnancy and breast feeding: a systematic review and network meta-analysis. BMJ Open. 2017;7(7):e017248. doi: 10.1136/bmjopen-2017-017248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L, Wang C, Li W. A meta-analysis of randomized controlled trials on levetiracetam in the treatment of pediatric patients with epilepsy. Neuropsychiatr Dis Treat. 2018;14:769–779. doi: 10.2147/NDT.S151413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao T, Feng X, Liu J, Gao J, Zhou C. Evaluate the efficacy and safety of anti-epileptic medications for partial seizures of epilepsy: a network meta-analysis. J Cell Biochem. 2017;118(9):2850–2864. doi: 10.1002/jcb.25936. [DOI] [PubMed] [Google Scholar]
- 51.Zhu LN, Chen D, Xu D, Tan G, Wang HJ, Liu L. Newer antiepi-leptic drugs compared to levetiracetam as adjunctive treatments for uncontrolled focal epilepsy: an indirect comparison. Seizure. 2017;51:121–132. doi: 10.1016/j.seizure.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 52.Berkovic SF, Knowlton RC, Leroy RF, Schiemann J, Falter U, Levetiracetam N01057 Study Group Placebo-controlled study of levetiracetam in idiopathic generalized epilepsy. Neurology. 2007;69(18):1751–1760. doi: 10.1212/01.wnl.0000268699.34614.d3. [DOI] [PubMed] [Google Scholar]
- 53.Brodie MJ, Perucca E, Ryvlin P, Ben-Menachem E, Meencke HJ, Levetiracetam Monotherapy Study Group Comparison of levetiracetam and controlled-release carbamazepine in newly diagnosed epilepsy. Neurology. 2007;68(6):402–408. doi: 10.1212/01.wnl.0000252941.50833.4a. [DOI] [PubMed] [Google Scholar]
- 54.Coppola G, Franzoni E, Verrotti A, et al. Levetiracetam or oxcarbazepine as monotherapy in newly diagnosed benign epilepsy of childhood with centrotemporal spikes (BECTS): an open-label, parallel group trial. Brain Dev. 2007;29(5):281–284. doi: 10.1016/j.braindev.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Noachtar S, Andermann E, Meyvisch P, et al. Levetiracetam for the treatment of idiopathic generalized epilepsy with myoclonic seizures. Neurology. 2008;70(8):607–616. doi: 10.1212/01.wnl.0000297512.18364.40. [DOI] [PubMed] [Google Scholar]
- 56.Zhou B, Zhang Q, Tian L, Xiao J, Stefan H, Zhou D. Effects of levetiracetam as an add-on therapy on cognitive function and quality of life in patients with refractory partial seizures. Epilepsy Behav. 2008;12(2):305–310. doi: 10.1016/j.yebeh.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Labiner DM, Ettinger AB, Fakhoury TA, et al. Effects of lamotrigine compared with levetiracetam on anger, hostility, and total mood in patients with partial epilepsy. Epilepsia. 2009;50(3):434–442. doi: 10.1111/j.1528-1167.2008.01792.x. [DOI] [PubMed] [Google Scholar]
- 58.Levisohn PM, Mintz M, Hunter SJ, Yang H, Jones J, N01103 Levetiracetam Study Group Neurocognitive effects of adjunctive levetiracetam in children with partial-onset seizures: a randomized, double-blind, placebo-controlled, noninferiority trial. Epilepsia. 2009;50(11):2377–2389. doi: 10.1111/j.1528-1167.2009.02197.x. [DOI] [PubMed] [Google Scholar]
- 59.Lim DA, Tarapore P, Chang E, et al. Safety and feasibility of switching from phenytoin to levetiracetam monotherapy for glioma-related seizure control following craniotomy: a randomized Phase II pilot study. J Neurooncol. 2009;93(3):349–354. doi: 10.1007/s11060-008-9781-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peltola J, Coetzee C, Jiménez F, et al. Once-daily extended-release levetiracetam as adjunctive treatment of partial-onset seizures in patients with epilepsy: a double-blind, randomized, placebo-controlled trial. Epilepsia. 2009;50(3):406–414. doi: 10.1111/j.1528-1167.2008.01817.x. [DOI] [PubMed] [Google Scholar]
- 61.Piña-Garza JE, Nordli DR, Rating D, et al. Adjunctive levetiracetam in infants and young children with refractory partial-onset seizures. Epilepsia. 2009;50(5):1141–1149. doi: 10.1111/j.1528-1167.2008.01981.x. [DOI] [PubMed] [Google Scholar]
- 62.Wu XY, Hong Z, Wu X, et al. Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in Chinese patients with refractory partial-onset seizures. Epilepsia. 2009;50(3):398–405. doi: 10.1111/j.1528-1167.2008.01729.x. [DOI] [PubMed] [Google Scholar]
- 63.Xiao Z, Li JM, Wang XF, et al. Efficacy and safety of levetiracetam (3,000 mg/day) as an adjunctive therapy in Chinese patients with refractory partial seizures. Eur Neurol. 2009;61(4):233–239. doi: 10.1159/000197109. [DOI] [PubMed] [Google Scholar]
- 64.Cumbo E, Ligori LD. Levetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer’s disease. Epilepsy Behav. 2010;17(4):461–466. doi: 10.1016/j.yebeh.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 65.de La Loge C, Hunter SJ, Schiemann J, Yang H. Assessment of behavioral and emotional functioning using standardized instruments in children and adolescents with partial-onset seizures treated with adjunctive levetiracetam in a randomized, placebo-controlled trial. Epilepsy Behav. 2010;18(3):291–298. doi: 10.1016/j.yebeh.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 66.UCB Japan Co Ltd A double-blind, placebo-controlled study of levetiracetam in epilepsy patients with generalized tonic-clonic seizures (except partial seizures evolving to secondarily generalized seizures) [Accessed July 18, 2017]. Available from: https://ClinicalTrials.gov/show/NCT01228747.
- 67.UCB Korea Co., Ltd Levetiracetam versus topiramate as adjunctive therapy to evaluate efficacy and safety in subjects with refractory partial onset seizures. [Accessed July 18, 2017]. Available from: https://ClinicalTrials.gov/show/NCT01229735.
- 68.Fattore C, Boniver C, Capovilla G, et al. A multicenter, randomized, placebo-controlled trial of levetiracetam in children and adolescents with newly diagnosed absence epilepsy. Epilepsia. 2011;52(4):802–809. doi: 10.1111/j.1528-1167.2010.02976.x. [DOI] [PubMed] [Google Scholar]
- 69.Consoli D, Bosco D, Postorino P, et al. Levetiracetam versus carbamazepine in patients with late poststroke seizures: a multicenter prospective randomized open-label study (EpIC Project) Cerebrovasc Dis. 2012;34(4):282–289. doi: 10.1159/000342669. [DOI] [PubMed] [Google Scholar]
- 70.Hakami T, Todaro M, Petrovski S, et al. Substitution monotherapy with levetiracetam vs older antiepileptic drugs: a randomized comparative trial. Arch Neurol. 2012;69(12):1563–1571. doi: 10.1001/archneurol.2012.2203. [DOI] [PubMed] [Google Scholar]
- 71.Rosenow F, Schade-Brittinger C, Burchardi N, et al. The LaLiMo Trial: lamotrigine compared with levetiracetam in the initial 26 weeks of monotherapy for focal and generalised epilepsy – an open-label, prospective, randomised controlled multicenter study. J Neurol Neurosurg Psychiatry. 2012;83(11):1093–1098. doi: 10.1136/jnnp-2011-301999. [DOI] [PubMed] [Google Scholar]
- 72.Borggraefe I, Bonfert M, Bast T, et al. Levetiracetam vs. sulthiame in benign epilepsy with centrotemporal spikes in childhood: a double-blinded, randomized, controlled trial (German HEAD Study) Eur J Paediatr Neurol. 2013;17(5):507–514. doi: 10.1016/j.ejpn.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 73.UCB Pharma SA Open-label, randomized, active-controlled study of LEV used as monotherapy in patients with partial-onset seizures. [Accessed July 18, 2017]. Available from: https://ClinicalTrials.gov/show/NCT01954121.
- 74.Trinka E, Marson AG, van Paesschen W, et al. KOMET: an unblinded, randomised, two parallel-group, stratified trial comparing the effectiveness of levetiracetam with controlled-release carbamazepine and extended-release sodium valproate as monotherapy in patients with newly diagnosed epilepsy. J Neurol Neurosurg Psychiatry. 2013;84(10):1138–1147. doi: 10.1136/jnnp-2011-300376. [DOI] [PubMed] [Google Scholar]
- 75.University of Rochester A safety and feasibility study of enteral LVT vs. standard of care for seizure control in pediatric CM (LVT2) [Accessed July 18, 2017]. Available from: https://ClinicalTrials.gov/show/NCT01982812.
- 76.Rossetti AO, Jeckelmann S, Novy J, Roth P, Weller M, Stupp R. Levetiracetam and pregabalin for antiepileptic monotherapy in patients with primary brain tumors. A Phase II randomized study. Neuro Oncol. 2014;16(4):584–588. doi: 10.1093/neuonc/not170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zaccara G, Almas M, Pitman V, Knapp L, Posner H. Efficacy and safety of pregabalin versus levetiracetam as adjunctive therapy in patients with partial seizures: a randomized, double-blind, noninferiority trial. Epilepsia. 2014;55(7):1048–1057. doi: 10.1111/epi.12679. [DOI] [PubMed] [Google Scholar]
- 78.Inoue Y, Yagi K, Ikeda A, et al. Efficacy and tolerability of levetiracetam as adjunctive therapy in Japanese patients with uncontrolled partial-onset seizures. Psychiatry Clin Neurosci. 2015;69(10):640–648. doi: 10.1111/pcn.12300. [DOI] [PubMed] [Google Scholar]
- 79.Jung DE, Yu R, Yoon JR, et al. Neuropsychological effects of levetiracetam and carbamazepine in children with focal epilepsy. Neurology. 2015;84(23):2312–2319. doi: 10.1212/WNL.0000000000001661. [DOI] [PubMed] [Google Scholar]
- 80.Suresh SH, Chakraborty A, Virupakshaiah A, Kumar N. Efficacy and safety of levetiracetam and carbamazepine as monotherapy in partial seizures. Epilepsy Res Treat. 2015;2015(4):1–6. doi: 10.1155/2015/415082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Werhahn KJ, Trinka E, Dobesberger J, et al. A randomized, double-blind comparison of antiepileptic drug treatment in the elderly with new-onset focal epilepsy. Epilepsia. 2015;56(3):450–459. doi: 10.1111/epi.12926. [DOI] [PubMed] [Google Scholar]
- 82.Hakami T, O’Brien TJ, Petty SJ, et al. Monotherapy with levetiracetam versus older AEDs: a randomized comparative trial of effects on bone health. Calcif Tissue Int. 2016;98(6):556–565. doi: 10.1007/s00223-016-0109-7. [DOI] [PubMed] [Google Scholar]
- 83.Kim JH, Lee SK, Loesch C, et al. Comparison of levetiracetam and oxcarbazepine monotherapy among Korean patients with newly diagnosed focal epilepsy: a long-term, randomized, open-label trial. Epilepsia. 2017;58(4):e70–e74. doi: 10.1111/epi.13707. [DOI] [PubMed] [Google Scholar]
- 84.Tacke M, Gerstl L, Heinen F, et al. Effect of anticonvulsive treatment on neuropsychological performance in children with BECTS. Eur J Paediatr Neurol. 2016;20(6):874–879. doi: 10.1016/j.ejpn.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 85.Siniscalchi A, Scaglione F, Sanzaro E, et al. Effects of phenobarbital and levetiracetam on PR and QTc intervals in patients with post-stroke seizure. Clin Drug Investig. 2014;34(12):879–886. doi: 10.1007/s40261-014-0243-9. [DOI] [PubMed] [Google Scholar]
- 86.Bootsma HP, Ricker L, Diepman L, et al. Long-term effects of levetiracetam and topiramate in clinical practice: a head-to-head comparison. Seizure. 2008;17(1):19–26. doi: 10.1016/j.seizure.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 87.Andersohn F, Schade R, Willich SN, Garbe E. Use of antiepileptic drugs in epilepsy and the risk of self-harm or suicidal behavior. Neurology. 2010;75(4):335–340. doi: 10.1212/WNL.0b013e3181ea157e. [DOI] [PubMed] [Google Scholar]
- 88.Arif H, Buchsbaum R, Pierro J, et al. Comparative effectiveness of 10 antiepileptic drugs in older adults with epilepsy. Arch Neurol. 2010;67(4):408–415. doi: 10.1001/archneurol.2010.49. [DOI] [PubMed] [Google Scholar]
- 89.Merrell RT, Anderson SK, Meyer FB, Lachance DH. Seizures in patients with glioma treated with phenytoin and levetiracetam. J Neurosurg. 2010;113(6):1176–1181. doi: 10.3171/2010.5.JNS091367. [DOI] [PubMed] [Google Scholar]
- 90.Rauchenzauner M, Bitsche G, Svalheim S, et al. Effects of levetiracetam and valproic acid monotherapy on sex-steroid hormones in prepubertal children – results from a pilot study. Epilepsy Res. 2010;88(2–3):264–268. doi: 10.1016/j.eplepsyres.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 91.Veiby G, Daltveit AK, Engelsen BA, Gilhus NE. Fetal growth restriction and birth defects with newer and older antiepileptic drugs during pregnancy. J Neurol. 2014;261(3):579–588. doi: 10.1007/s00415-013-7239-x. [DOI] [PubMed] [Google Scholar]
- 92.Xiao F, An D, Deng H, Chen S, Ren J, Zhou D. Evaluation of levetiracetam and valproic acid as low-dose monotherapies for children with typical benign childhood epilepsy with centrotemporal spikes (BECTS) Seizure. 2014;23(9):756–761. doi: 10.1016/j.seizure.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 93.Javed A, Cohen B, Detyniecki K, et al. Rates and predictors of patient-reported cognitive side effects of antiepileptic drugs: an extended follow-up. Seizure. 2015;29:34–40. doi: 10.1016/j.seizure.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 94.Tinchon A, Oberndorfer S, Marosi C, et al. Haematological toxicity of valproic acid compared to levetiracetam in patients with glioblastoma multiforme undergoing concomitant radio-chemotherapy: a retrospective cohort study. J Neurol. 2015;262(1):179–186. doi: 10.1007/s00415-014-7552-z. [DOI] [PubMed] [Google Scholar]
- 95.Tomson T, Battino D, Bonizzoni E, et al. Antiepileptic drugs and intrauterine death: a prospective observational study from EURAP. Neurology. 2015;85(7):580–588. doi: 10.1212/WNL.0000000000001840. [DOI] [PubMed] [Google Scholar]
- 96.Bektaş G, Tekin U, Özkan MU, et al. The influence of levetiracetam on psychosocial and behavioral functioning in children: a case–control and follow-up study. Epilepsy Behav. 2017;72:39–42. doi: 10.1016/j.yebeh.2017.04.042. [DOI] [PubMed] [Google Scholar]
- 97.Chen B, Choi H, Hirsch LJ, et al. Psychiatric and behavioral side effects of antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2017;76:24–31. doi: 10.1016/j.yebeh.2017.08.039. [DOI] [PubMed] [Google Scholar]
- 98.Egunsola O, Choonara I, Sammons HM, Whitehouse WP. Safety of antiepileptic drugs in children and young people: a prospective cohort study. Seizure. 2018;56:20–25. doi: 10.1016/j.seizure.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 99.Frey N, Bodmer M, Bircher A, et al. The risk of Stevens–Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptic drugs. Epilepsia. 2017;58(12):2178–2185. doi: 10.1111/epi.13925. [DOI] [PubMed] [Google Scholar]
- 100.Lee T, Warrick BJ, Sarangarm P, et al. Levetiracetam in toxic seizures. Clin Toxicol (Phila) 2018;56(3):175–181. doi: 10.1080/15563650.2017.1355056. [DOI] [PubMed] [Google Scholar]
- 101.Maschio M, Zarabla A, Maialetti A, et al. Quality of life, mood and seizure control in patients with brain tumor related epilepsy treated with lacosamide as add-on therapy: a prospective explorative study with a historical control group. Epilepsy Behav. 2017;73:83–89. doi: 10.1016/j.yebeh.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 102.Shih FY, Chuang YC, Chuang MJ, et al. Effects of antiepileptic drugs on thyroid hormone function in epilepsy patients. Seizure. 2017;48:7–10. doi: 10.1016/j.seizure.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 103.Stephen LJ, Wishart A, Brodie MJ. Psychiatric side effects and anti-epileptic drugs: observations from prospective audits. Epilepsy Behav. 2017;71(Pt A):73–78. doi: 10.1016/j.yebeh.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 104.Newsome SD, Xue LY, Jennings T, Castaneda GY. Levetiracetam-induced diffuse interstitial lung disease. J Child Neurol. 2007;22(5):628–630. doi: 10.1177/0883073807302602. [DOI] [PubMed] [Google Scholar]
- 105.Gallerani M, Mari E, Boari B, Carletti R, Marra A, Cavallo M. Pancytopenia associated with levetiracetam treatment. Clin Drug Investig. 2009;29(11):747–751. doi: 10.2165/11319450-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 106.Hacquard M, Richard S, Lacour JC, Lecompte T, Vespignani H. Levetiracetam-induced platelet dysfunction. Epilepsy Res. 2009;86(1):94–96. doi: 10.1016/j.eplepsyres.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 107.Hurwitz KA, Ingulli EG, Krous HF. Levetiracetam induced interstitial nephritis and renal failure. Pediatr Neurol. 2009;41(1):57–58. doi: 10.1016/j.pediatrneurol.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 108.Peer Mohamed B, Mohamed BP, Prabhakar P. Thrombocytopenia as an adverse effect of levetiracetam therapy in a child. Neuropediatrics. 2009;40(5):243–244. doi: 10.1055/s-0030-1247524. [DOI] [PubMed] [Google Scholar]
- 109.Tamarelle C, Pandit F, Mazarati A, Riquet A, Vallée L, Auvin S. Levetiracetam-induced depression in a 5-year-old child with partial epilepsy. Seizure. 2009;18(3):235–236. doi: 10.1016/j.seizure.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 110.vande Griend JP, Linnebur SA, Bainbridge JL. Probable levetiracetam-associated depression in the elderly: two case reports. Am J Geriatr Pharmacother. 2009;7(5):281–284. doi: 10.1016/j.amjopharm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 111.Broli M, Provini F, Naldi I, et al. Unexpected gamma glutamyltransferase rise increase during levetiracetam monotherapy. Epileptic Disord. 2010;12(1):81–82. doi: 10.1684/epd.2010.0291. [DOI] [PubMed] [Google Scholar]
- 112.Caraballo RH, Cersósimo R, de Los Santos C. Levetiracetam-induced seizure aggravation associated with continuous spikes and waves during slow sleep in children with refractory epilepsies. Epileptic Disord. 2010;12(2):146–150. doi: 10.1684/epd.2010.0305. [DOI] [PubMed] [Google Scholar]
- 113.Oghlakian R, Nock C, Koubeissi M. A case of levetiracetam-induced thrombocytopenia. Epileptic Disord. 2010;12(4):335–337. doi: 10.1684/epd.2010.0343. [DOI] [PubMed] [Google Scholar]
- 114.Sahaya K, Goyal MK, Sarwal A, Singh NN. Levetiracetam-induced thrombocytopenia among inpatients: a retrospective study. Epilepsia. 2010;51(12):2492–2495. doi: 10.1111/j.1528-1167.2010.02788.x. [DOI] [PubMed] [Google Scholar]
- 115.Bachmann T, Bertheussen KH, Svalheim S, et al. Haematological side effects of antiepileptic drug treatment in patients with epilepsy. Acta Neurol Scand Suppl. 2011;191(191):23–27. doi: 10.1111/j.1600-0404.2011.01539.x. [DOI] [PubMed] [Google Scholar]
- 116.Givon L, Porter S, Padmanabhan B, Goren J, Cohen PA. Levetiracetam, seizures, and suicidality. Harv Rev Psychiatry. 2011;19(1):47–55. doi: 10.3109/10673229.2011.549777. [DOI] [PubMed] [Google Scholar]
- 117.Alkhotani A, Mclachlan RS. Levetiracetam induced angioedema in a patient with previous anticonvulsant hypersensitivity reaction to phenytoin and lamotrigine. Seizure. 2012;21(5):407–408. doi: 10.1016/j.seizure.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 118.Babtain FA. Levetiracetam may worsen myoclonus in patients with juvenile myoclonic epilepsy: case reports. Clin Neuropharmacol. 2012;35(4):201–202. doi: 10.1097/WNF.0b013e31825eed8c. [DOI] [PubMed] [Google Scholar]
- 119.Bishop-Freeman SC, Kornegay NC, Winecker RE. Postmortem levetiracetam (Keppra®) data from North Carolina. J Anal Toxicol. 2012;36(6):422–428. doi: 10.1093/jat/bks052. [DOI] [PubMed] [Google Scholar]
- 120.Calabrò RS, Italiano D, Militi D, Bramanti P. Levetiracetam-associated loss of libido and anhedonia. Epilepsy Behav. 2012;24(2):283–284. doi: 10.1016/j.yebeh.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 121.Camacho A, Espín JC, Nuñez N, Simón R. Levetiracetam-induced reversible autistic regression. Pediatr Neurol. 2012;47(1):65–67. doi: 10.1016/j.pediatrneurol.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 122.Chau K, Yong J, Ismail K, Griffith N, Liu M, Makris A. Levetiracetam-induced severe acute granulomatous interstitial nephritis. Clin Kidney J. 2012;5(3):234–236. doi: 10.1093/ckj/sfs023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gómez-Zorrilla S, Ferraz AV, Pedrós C, Lemus M, Peña C. Levetiracetam-induced drug reaction with eosinophilia and systemic symptoms syndrome. Ann Pharmacother. 2012;46(7–8):e20. doi: 10.1345/aph.1R084. [DOI] [PubMed] [Google Scholar]
- 124.Xiong N, Hou L, Lu N, Mohamed AA, Wang T, Huang Y. Probable levetiracetam-related serum alkaline phosphatase elevation. BMC Neurol. 2012;12:97. doi: 10.1186/1471-2377-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zou LP, Ding CH, Song ZJ, Li XF. Stevens–Johnson syndrome induced by levetiracetam. Seizure. 2012;21(10):823–825. doi: 10.1016/j.seizure.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 126.Hommet C, Beaufils E, Roubeau V, et al. Encephalopathy induced by levetiracetam in an elderly woman. Aging Clin Exp Res. 2013;25(1):111–113. doi: 10.1007/s40520-013-0009-x. [DOI] [PubMed] [Google Scholar]
- 127.Karadag AS, Bilgili SG, Calka O, Onder S, Kosem M, Burakgazi-Dalkilic E. A case of levetiracetam induced bullous pemphigoid. Cutan Ocul Toxicol. 2013;32(2):176–178. doi: 10.3109/15569527.2012.725444. [DOI] [PubMed] [Google Scholar]
- 128.Kaufman KR, Bisen V, Zimmerman A, Tobia A, Mani R, Wong S. Apparent dose-dependent levetiracetam-induced de novo major depression with suicidal behavior. Epilepsy Behav Case Rep. 2013;1:110–112. doi: 10.1016/j.ebcr.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Metin SZ, Ozmen M, Ozkara C, Ozmen E. Hypersexuality in a patient with epilepsy during treatment of levetiracetam. Seizure. 2013;22(2):151–152. doi: 10.1016/j.seizure.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 130.Sethi NK, Sethi PK, Torgovnick J, Arsura E, Cukierwar F. Asymptomatic elevation of liver enzymes due to levetiracetam: a case report. Drug Metabol Drug Interact. 2013;28(2):123–124. doi: 10.1515/dmdi-2013-0006. [DOI] [PubMed] [Google Scholar]
- 131.Akiyama H, Haga Y, Sasaki N, Yanagisawa T, Hasegawa Y. A case of rhabdomyolysis in which levetiracetam was suspected as the cause. Epilepsy Behav Case Rep. 2014;2:152–155. doi: 10.1016/j.ebcr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aksoy D, Cevik B, Kurt S, Pekdas E, Solmaz V. Hypokalemia and hypomagnesaemia related to levetiracetam use. J Clin Neurosci. 2014;21(11):1989–1990. doi: 10.1016/j.jocn.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 133.Azar NJ, Aune P. Acute pancreatitis and elevated liver transaminases after rapid titration of oral levetiracetam. J Clin Neurosci. 2014;21(6):1053–1054. doi: 10.1016/j.jocn.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 134.Bui M, Baslet G, Weisholtz D, Mcelrath T. Levetiracetam-induced psychosis in a pregnant woman with prior substance abuse. Harv Rev Psychiatry. 2014;22(3):193–200. doi: 10.1097/HRP.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 135.Hwang ES, Siemianowski LA, Sen S, Patel R. Levetiracetam: an unusual cause of delirium. Am J Ther. 2014;21(6):e225–e228. doi: 10.1097/MJT.0b013e31828fdaed. [DOI] [PubMed] [Google Scholar]
- 136.Isaacson JE, Choe DJ, Doherty MJ. Creatine phosphokinase elevation exacerbated by levetiracetam therapy. Epilepsy Behav Case Rep. 2014;2:189–191. doi: 10.1016/j.ebcr.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koklu E, Ariguloglu EA, Koklu S. Levetiracetam-induced anaphylaxis in a neonate. Pediatr Neurol. 2014;50(2):192–194. doi: 10.1016/j.pediatrneurol.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 138.Kumar N, Swaroop HS, Chakraborty A, Chandran S. Levetiracetam induced acute reversible psychosis in a patient with uncontrolled seizures. Indian J Pharmacol. 2014;46(5):560–561. doi: 10.4103/0253-7613.140599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Park EM, Holmes JA, Reeder-Hayes KE. Acute mania associated with levetiracetam treatment. Psychosomatics. 2014;55(1):98–100. doi: 10.1016/j.psym.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Spengler DC, Montouris GD, Hohler AD. Levetiracetam as a possible contributor to acute kidney injury. Clin Ther. 2014;36(8):1303–1306. doi: 10.1016/j.clinthera.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 141.Zaki SA, Gupta S. Levetiracetam-induced acute psychosis in a child. Indian J Pharmacol. 2014;46(3):341–342. doi: 10.4103/0253-7613.132195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zou X, Hong Z, Zhou D. Hair loss with levetiracetam in five patients with epilepsy. Seizure. 2014;23(2):158–160. doi: 10.1016/j.seizure.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 143.Arı H, Kahraman F, Acaban MB. The first case of levetiracetam-induced and tolvaptan-resistant hyponatremia. Turk Kardiyol Dern Ars. 2015;43(3):284–287. doi: 10.5543/tkda.2015.45735. [DOI] [PubMed] [Google Scholar]
- 144.Eleni K. Dress syndrome induced by levetiracetam. J Eur Acad Dermatol Venereol. 2015;29(2):377–378. doi: 10.1111/jdv.12346. [DOI] [PubMed] [Google Scholar]
- 145.Flannery AH, Willey MD, Thompson Bastin ML, Buch KP, Bensadoun ES. Eosinophilia and fever with levetiracetam: a case report. Pharmacotherapy. 2015;35(8):e131–e135. doi: 10.1002/phar.1617. [DOI] [PubMed] [Google Scholar]
- 146.Fujikawa M, Kishimoto Y, Kakisaka Y, et al. Obsessive–compulsive behavior induced by levetiracetam. J Child Neurol. 2015;30(7):942–944. doi: 10.1177/0883073814541471. [DOI] [PubMed] [Google Scholar]
- 147.Gencler OS, Gencler B, Altunel CT, Arslan N. Levetiracetam induced psoriasiform drug eruption: a rare case report. Saudi Pharm J. 2015;23(6):720–722. doi: 10.1016/j.jsps.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kawakami Y, Okazaki T, Takase M, Fujino O, Itoh Y. A girl with idiopathic epilepsy showing forced normalization after levetiracetam administration. J Nippon Med Sch. 2015;82(5):250–253. doi: 10.1272/jnms.82.250. [DOI] [PubMed] [Google Scholar]
- 149.Makke Y, Hmaimess G, Nasreddine W, Fawaz A, Beydoun A. Paradoxical exacerbation of myoclonic-astatic seizures by levetiracetam in myoclonic astatic epilepsy. BMC Pediatr. 2015;15:6. doi: 10.1186/s12887-015-0330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Molokwu OA, Ezeala-Adikaibe BA, Onwuekwe IO. Levetiracetam-induced rage and suicidality: two case reports and review of literature. Epilepsy Behav Case Rep. 2015;4:79–81. doi: 10.1016/j.ebcr.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Peyrl A, Weichert N, Kühl JS, Ebell W, Hernáiz Driever P. Levetiracetam as a possible cause of secondary graft failure after allogenic hematopoietic stem cell transplantation. Eur J Paediatr Neurol. 2015;19(1):75–77. doi: 10.1016/j.ejpn.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 152.Taberner Bonastre MT, Peralta Muñoz S, Boza FM, Gumà I, Padró J. Neutropenia secondary to exposure to levetiracetam. Tumori. 2015;101(5):145–146. doi: 10.5301/tj.5000312. [DOI] [PubMed] [Google Scholar]
- 153.Bayram AK, Canpolat M, Çınar SL, et al. Drug reaction with eosinophilia and systemic symptoms syndrome induced by levetiracetam in a pediatric patient. J Emerg Med. 2016;50(2):e61–e66. doi: 10.1016/j.jemermed.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 154.Dar WR, Sofi N, Latief M, Dar IA, Kasana BA. Levetiracetam induced drug reaction with eosinophilia and systemic symptom syndrome. Indian J Dermatol. 2016;61(2):235. doi: 10.4103/0019-5154.177777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.García Carretero R, Romero Brugera M, Olid-Velilla M, Salamanca-Ramirez I. Pancytopenia associated with levetiracetam in an epileptic woman. BMJ Case Rep. 2016;2016:bcr2016217407. doi: 10.1136/bcr-2016-217407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jones RT, Evans W, Mersfelder TL, Kavanaugh K. Rare red rashes: a case report of levetiracetam-induced cutaneous reaction and review of the literature. Am J Ther. 2016;23(3):e944–e946. doi: 10.1097/MJT.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 157.Ju J, Zou LP, Shi XY, Hu LY, Pang LY. Levetiracetam: probably associated diurnal frequent urination. Am J Ther. 2016;23(2):e624–e627. doi: 10.1097/MJT.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 158.Turati M, Glard Y, Afonso D, Griffet J, Bigoni M. Osteochondral alteration in a child treated with levetiracetam: a rare case of juvenile osteochondritis dissecans of the talar head. J Pediatr Orthop B. 2017;26(2):189–192. doi: 10.1097/BPB.0000000000000354. [DOI] [PubMed] [Google Scholar]
- 159.Kubota K, Yamamoto T, Kawamoto M, et al. Levetiracetam-induced rhabdomyolysis: a case report and literature review. Neurol Asia. 2017;22:275–278. [Google Scholar]
- 160.Ozdemir H, Sumer S, Karabagli H, et al. B cell aplasia and hypogammaglobulinemia associated with levetiracetam. Ann Saudi Med. 2018;38(1):545–548. doi: 10.5144/0256-4947.2018.09.01.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sereflican B, Karapinar T, Duzcu SE, Turkoglu ŞA. Disseminated eruptive granuloma annulare induced by levetiracetam. Cutan Ocul Toxicol. 2017;36(3):300–301. doi: 10.1080/15569527.2016.1269336. [DOI] [PubMed] [Google Scholar]
- 162.Beghi E, Atzeni L, Garattini L. Economic analysis of newer antiepileptic drugs. CNS Drugs. 2008;22(10):861–875. doi: 10.2165/00023210-200822100-00006. [DOI] [PubMed] [Google Scholar]
- 163.Suh GH, Lee SK. Economic evaluation of add-on levetiracetam for the treatment of refractory partial epilepsy in Korea. Psychiatry Investig. 2009;6(3):185–193. doi: 10.4306/pi.2009.6.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tomson T, Battino D, Bonizzoni E, et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17(6):530–538. doi: 10.1016/S1474-4422(18)30107-8. [DOI] [PubMed] [Google Scholar]
- 165.Kim J, Kondratyev A, Gale K. Antiepileptic drug-induced neuronal cell death in the immature brain: effects of carbamazepine, topiramate, and levetiracetam as monotherapy versus polytherapy. J Pharmacol Exp Ther. 2007;323(1):165–173. doi: 10.1124/jpet.107.126250. [DOI] [PubMed] [Google Scholar]
- 166.Cumbo E, Ligori LD. Levetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer’s disease. Epilepsy Behav. 2010;17(4):461–466. doi: 10.1016/j.yebeh.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 167.Chan R, Wei CY, Chen YT, Benet LZ. Use of the Biopharmaceutics Drug Disposition Classification System (BDDCS) to help predict the occurrence of idiosyncratic cutaneous adverse drug reactions associated with antiepileptic drug usage. AAPS J. 2016;18(3):757–766. doi: 10.1208/s12248-016-9898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fong JK, Chan EL, Leung H, et al. An update of the Hong Kong Epilepsy Guideline: consensus statement on the use of antiepileptic drugs in Hong Kong. Hong Kong Med J. 2017;23(1):74–88. doi: 10.12809/hkmj166027. [DOI] [PubMed] [Google Scholar]
- 169.Wijnen BFM, van Mastrigt G, Evers S, et al. A systematic review of economic evaluations of treatments for patients with epilepsy. Epilepsia. 2017;58(5):706–726. doi: 10.1111/epi.13655. [DOI] [PubMed] [Google Scholar]
- 170.Kazerooni R, Bounthavong M. Cost-effectiveness analysis of intravenous levetiracetam versus intravenous phenytoin for early onset seizure prophylaxis after neurosurgery and traumatic brain injury. Clinicoecon Outcomes Res. 2010;2:15–23. doi: 10.2147/ceor.s8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lyttle MD, Gamble C, Messahel S, et al. Emergency treatment with levetiracetam or phenytoin in status epilepticus in children – the EcLiPSE study: study protocol for a randomised controlled trial. Trials. 2017;18(1):283. doi: 10.1186/s13063-017-2010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bähr O, Hermisson M, Rona S, et al. Intravenous and oral levetiracetam in patients with a suspected primary brain tumor and symptomatic seizures undergoing neurosurgery: the HELLO trial. Acta Neurochir (Wien) 2012;154(2):229–235. doi: 10.1007/s00701-011-1144-9. [DOI] [PubMed] [Google Scholar]
- 173.Kellinghaus C, Stögbauer F. Treatment of status epilepticus in a large community hospital. Epilepsy Behav. 2012;23(3):235–240. doi: 10.1016/j.yebeh.2011.12.020. [DOI] [PubMed] [Google Scholar]