Social isolation is an important factor in pain-related appraisal and coping. The impact of pain is reduced in individuals who perceive a greater sense of inclusion from, and engagement with others.
Keywords: Social isolation, Pain interference, Physical function, Chronic pain
Abstract
Background
Evidence suggests social interactions play an important role in pain perception.
Purpose
The aim of this study was to determine whether social isolation (SI) in people with persistent pain determines pain interference (PI) and physical function over time.
Methods
Patients seeking care at a tertiary pain management referral center were administered the Patient Reported Outcome Measurement Information System (PROMIS®) SI, PI, physical function, depression, and average pain intensity item banks at their initial consultation and subsequent visits as part of their routine clinical care. We used a post hoc simulation of an experiment using propensity score matching (n = 4,950) and carried out a cross-lagged longitudinal analysis (n = 312) of retrospective observational data.
Results
Cross-lagged longitudinal analysis showed that SI predicted PI at the next time point, above and beyond the effects of pain intensity and covariates, but not vice versa.
Conclusions
These data support the importance of SI as a factor in pain-related appraisal and coping and demonstrate that a comprehensive assessment of the individuals’ social context can provide a better understanding of the differential trajectories for a person living with pain. Our study provides evidence that the impact of pain is reduced in individuals who perceive a greater sense of inclusion from and engagement with others. This study enhances the understanding of how social factors affect pain and have implications for how the effectiveness of therapeutic interventions may be improved. Therapeutic interventions aimed at increasing social connection hold merit in reducing the impact of pain on engagement with activities.
Introduction
Experts within the fields of health sociology and population health are debating the extent to which socioeconomic position drives physical and mental health, or vice versa [1]. For example, a large-scale Swedish study examined the effects of the social environment at a population health level and demonstrated that low neighborhood social capital and general trust were associated with higher rates of psychosomatic symptoms, musculoskeletal pain, and depression [2]. Through the lens of social health selection, differences in socioeconomic situation (i.e., social isolation [SI], emotional support, ability, and satisfaction to participate in social activities) occur when the quality of intermediary factors are unevenly distributed between the different socioeconomic classes. In this scenario, the degree of SI, for example, would induce a higher or lower prevalence of physical or mental health problems. In contrast, through the lens of physical and mental health selection, differences in physical health (i.e., physical function [PF], pain interference [PI], fatigue, sleep disturbance) or mental health (i.e., depression, anxiety, anger, self-efficacy) occur when the quality of intermediary factors is unevenly distributed between the different physical and mental health classes. In this scenario, the degree of PF (physical health domain) or depression (mental health domain), for example, would induce a higher or lower prevalence of one’s ability and satisfaction to participate in social activities (social health/situation). While the inter-relationship between physical, mental, and social health is likely nonlinear and context dependent, the purpose of this study was to help inform this “causal relationship” debate.
Evidence within the fields of pain and rehabilitation science suggests social interactions play an important role in both the stress response and perception of pain. SI in early life leads to altered hormonal stress responses and more pronounced anxiety in adult (rodent) individuals, and social support acts as a buffer against stress [3]. Aslund et al. [2] identified that social distress heightened sensitivity to physical pain. Eisenberger et al. [4] found that socially excluded individuals responded to unpleasant heat stimuli with lower pain thresholds. People with rheumatoid arthritis and low levels of social support at baseline experienced higher levels of pain intensity at the 3- and 5-year follow-up [5], and in people with chronic musculoskeletal pain, an association was found between perceived social support and PI [6].
Depressive symptoms are an important factor to consider in interpreting the link between social and physical health. Individuals with low back pain (LBP) and higher levels of baseline depression experienced a slower recovery process [7]. Individuals with depressive symptoms and acute and subacute episodes of LBP were more likely to have persistent pain in the 6-month follow-up [8]. A lagged reciprocal link was also discovered between LBP and depressive symptoms [9]. Collectively, these studies, which have examined data across population health, preclinical, experimental pain, and clinical pain levels, lend support to the perspective that SI has detrimental effects on an individual’s perception of pain and subsequent coping responses.
Our primary aim of this study was to characterize the relationship between the level of SI in people with persistent musculoskeletal pain with the level of PI and PF over time. Our secondary aim was to identify the processes that help to explain the relationship between SI and physical health. Our primary hypothesis was that people living with persistent pain who reported higher levels of SI at the initiation of treatment would have higher levels of PI and lower levels of PF over the course of short-term care. Given the expectation that depressive symptoms have shown relationships with both pain and function in prior studies, our secondary hypothesis was that depressive symptoms would mediate the relationship between SI and physical health.
Methods
Participants and Clinical Environment
People seeking treatment at a specialty pain management center within an academic medical institution were enrolled in a learning health care system platform Collaborative Health Outcomes Information Registry (CHOIR: http://choir.stanford.edu), which administered the Patient Reported Outcome Measurement Information System (PROMIS®) SI, PI, PF, and depression (D) item banks, and pain intensity (average intensity over the last 7 days), at the time of their initial consultation visit and on subsequent visits as part of their routine clinical care. The patient population was a heterogeneous mix of people with various persistent, noncancer (musculoskeletal) pain disorders.
Routine clinical care would involve consultation and treatment recommendations from an interdisciplinary pain medicine team of physicians, psychologists, physical therapists, nurse practitioners, physician assistants, and complex care managers. Typical treatments would include optimization of analgesic medications, psychological therapies (e.g., group and individual cognitive-behavioral therapy, acceptance and commitment therapy, biofeedback training), physical therapy (e.g., recommendations for an individualized therapeutic exercise program, yoga, and Tai Chi), interventional procedures (e.g., nerve blocks, radiofrequency ablation, spinal cord stimulators), complementary approaches (e.g., acupuncture, nutraceuticals), and self-management approaches through pain education and experiential training (e.g., action planning, problem-solving, and goal setting).
Psychometric Properties of PROMIS®
Substantial evidence supports the content, cross-sectional, and clinical validity of PROMIS measures [10]. The PROMIS item banks (SI, PI, PF, and D) were administered using computerized adaptive testing (CAT) [11, 12] based on an item response theory approach for each patient care visit across 90 days of treatment. Rather than assessing a set number of items per subscale, the CAT approach identifies the optimal items within each domain based on prior responses from the respondent. CAT assessments are often considered desirable compared with traditional standard scale assessments due to the smaller number of items needed for effective assessment of each construct, as well as increased reliability of measurement [13, 14]. CHOIR includes CAT versions of the PROMIS measures adapted with an in-house algorithm (CHOIR-CAT). CHOIR-CAT was implemented using the same CAT algorithm as the Northwestern University Assessment Center, which has provided open access to PROMIS instruments [12]. PROMIS measures are normed against the U.S. population and have a mean of 50 points and a standard deviation (SD) of 10 points [15].
The PROMIS-SI items assess an individual’s perception of being excluded, detached, disconnected from, unknown, or avoided by others and include the perceived quality of interpersonal relationships, social network, companionship, feeling cared for, valued, of belonging, and trust [16]. Validation testing of the PROMIS-Social Health domain framework identified seven unidimensional factors (using exploratory factor analysis, confirmatory factor analysis, and item response theory) with good model fit and good evidence of criterion and construct validity [17]. The PROMIS-PI item bank assesses the extent to which pain hinders engagement with physical, cognitive, emotional, and recreational activities, as well as sleep and enjoyment in life [18]. The PROMIS-PI has been linked for comparison with other PI legacy instruments [19, 20] such as the 15-item brief pain inventory [21, 22] and the two-item Short-Form 36® Bodily Pain scale [23]. Results indicate that the PROMIS-PI item bank is a psychometrically sound instrument with regards to reliability (0.96 to 0.99 for T-score range 50–80), construct validity, and discriminant validity across pain intensity, disability levels, and persistent conditions (p < .0001) [18].
The PROMIS-PF item bank assesses the ability to carry out activities that require physical actions, ranging from self-care to more complex activities that require a combination of skills, often within a social context [24]. The PROMIS-PF item bank scores have been linked for comparison with the Medical Outcome Study Short-Form 36 Survey (Legacy PF-10) and Health Assessment Questionnaire (Legacy HAQ) [20]. Compared with the Legacy PF-10 and HAQ, the PROMIS-PF item bank has demonstrated superior or equal reliability (precision) and sensitivity to change [25]. Specifically, the PROMIS-PF instrument demonstrated greater precision of 0.90 or better in comparison with the Legacy PF-10 with a higher number of SD range of values covered. For example, the PROMIS-PF covered 4.8 SD (20-item static version) to 6.3 SD (10-item CAT version), in comparison with the Legacy PF-10, which covered 2.4 SD [25].
Study Design and Analysis
The design of this study incorporated a longitudinal assessment of retrospective observational data, which was approved by the Institutional Review Board at the Stanford University School of Medicine. We have attempted to address the analytical challenges involved with using an observational data set through a series of methodological approaches. Appreciating that the identification of correlations cannot be interpreted as a causal relationship, and the direction of causality is usually unclear, we have implemented a study design that allows for causal inference. First, we used a post hoc simulation of an experiment using propensity score matching (PSM), and second, we carried out a cross-lagged longitudinal analysis.
PSM and Cross-Sectional Analysis of the Average Treatment Effect of the Treated
PSM is a technique used to receive causal estimates in settings where “treatment” has been nonrandomly assigned [26]. One of the benefits of the PSM approach is that it simulates a randomized controlled trial where individuals are randomly attributed to two groups—a treatment and a control group. The random attribution to the two groups allows assuming that the sociodemographic characteristics are evenly distributed between the two groups. The method consists of creating a post hoc quasi-experimental design that matches individuals who are similar on observable characteristics but where some of them have received a treatment, while the others have not. By pairing them, their random attribution to either treatment or control group is simulated [27]. In a true experiment, where treatment is assigned randomly, the probability to receive treatment is 0.5 [26, 28]. If the treatment was not assigned randomly (as in the case of our observational data), the probability is not .5. By means of PSM, we identify the probability that an individual would have received treatment (as a function of observable covariates) and then compare the two groups.
Our treatment condition is SI. Although SI is not a treatment in the traditional sense, with the PSM method, any exposure can be modeled as treatment [29]. In the setting of the present analysis, the “treatment condition” is the exposure to high SI, and the “control condition” is the exposure to low SI. A formal description of this analytic approach is provided in Appendix A. The strength of the PSM method is that it allows the capturing of a potential self-selection effect into a specific SI level before our baseline measurement.
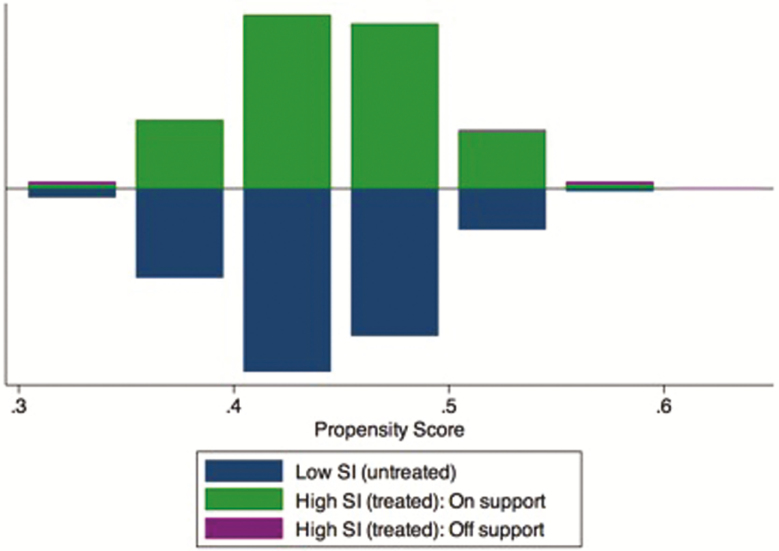
We adopted the nearest available match using caliper matching with a radius of 0.001. A first assumption central to the PSM method is common support. Common support considers that the individuals’ characteristics do not perfectly predict their attribution to the treatment. This condition enables individuals with the same characteristics to possibly be in either the treatment or the control group. Perfectly symmetrical histograms for the treatment and control groups indicate that the common support condition was met; the overlap between the two groups is large, and few cases are “off support” [30]. Our analysis reveals that our estimates are robust (Appendix Fig. A1).
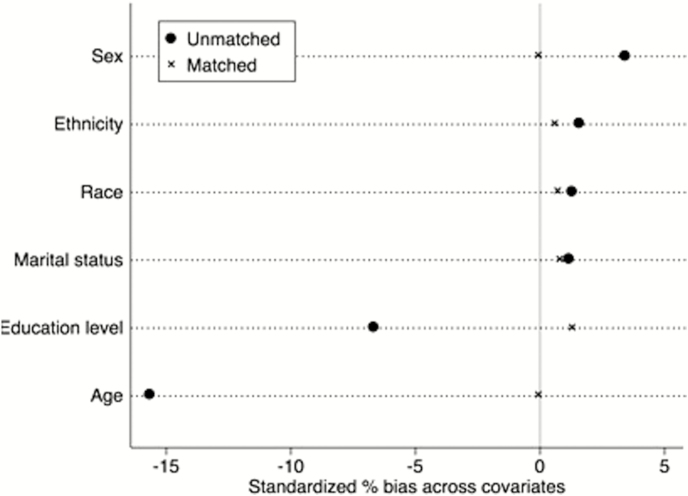
A second assumption underlying the PSM method is that the treatment condition is exogenous, and thus, there is unconfoundedness present [28, 31]. Hence, the receipt of treatment does not depend on the outcomes. Accordingly, systematic differences in outcomes between the treatment group and the control group are due to the treatment condition. This assumption is referred to as the conditional independence assumption (CIA). A limitation of this method is that it does not account for unobserved factors. We included the covariates of age, gender, race, ethnicity, education level, and marital status in our selection model. Other than the directly investigated PROMIS item banks of SI, PI, PF, and D, we excluded additional health-related variables. Appendix Fig. A2 presents the standardized bias in percentages between the means of the treatment and control group for the included covariates before and after matching. The figure demonstrates that the matching reduced bias and thus improved the CIA for all covariates.
Although PSM offers a strategy to address confounding in nonexperimental data, it has limitations. The main limitation is that it allows for confounding correction based on observable data (i.e., only variables in the database can be included in the selection model). As a consequence, nonobservable confounders are omitted, and their potentially biasing effect cannot be assessed [32, 33].
Cross-Lagged Longitudinal Analysis
Using the original dataset without matching including all observations, lagged multilevel model analysis was performed between SI and PI, and SI and PF. As propensity scores were estimated only for baseline assessments, t-scores for SI were used instead for lagged analysis, with average pain intensity, age, gender, race, ethnicity, education level, and marital status modeled as covariates. We included patients who completed at least two follow-up treatments within a 90-day time period for analysis. In using this approach, it was assumed that the lagged changes between time points (e.g., the change in scores from patients’ first assessment to their second assessment, compared with the change from their second to third assessment) were relatively comparable with one another across patients. We chose this analytic approach for two reasons. First, there was significant asynchrony in terms of data collection; as most patients did not come in for visits or complete CHOIR assessments at regular intervals, there was significant variability in terms of the duration of time between completed assessments. Second, it was assumed that there would be significant heterogeneity in terms of treatment exposure within the patient sample; given the multidisciplinary nature of the outpatient pain center, it was possible that patients would receive some combination of analgesic medications, treatment from physical or occupational therapy or psychology, or interventional pain procedures. However, this pattern of treatment exposure was likely to be highly variable between patients in terms of the scheduling and specific treatment exposure, suggesting that any changes in treatment would not have occurred in uniformly scheduled ways across patients. As a result, we chose to adopt an analytic approach that allowed for maximal inclusion of data within a relatively constrained time window. Mediation of depression was tested in our lagged models for any significant lagged direct effects (e.g., SI on PF or PI or vice versa), while controlling for scores in the endogenous variable at the previous time point. Our rationale for including depression as a potential mediator between SI and PI and SI and PF was based on previous studies, which have demonstrated that emotional distress may both arise from and disrupt social relationships [34].
Software
The propensity score was calculated using the program psmatch2 a package included in the statistical software STATA. Correlation analyses and computation of descriptive statistics were performed using SPSS (Windows version 20, Chicago, IL, USA). Cross-sectional path modeling and multilevel path modeling analyses were conducted using Mplus (Version 6.12, Los Angeles, CA: Muthén & Muthén).
Results
Patient Demographics and PSM
Between July 2014 and July 2016, 2,423 people with chronic noncancer pain completed the CHOIR surveys. Of these, we were able to compute propensity scores using baseline data for 211 patients. Separately, 312 patients had at least two follow-up treatments over the course of a 90-day time period and were considered for longitudinal analysis (n of observations across this participant subset in 90-day period = 794). Consequently, our study samples for the cross-sectional propensity score analysis and for the longitudinal analysis were 4,950 and 312 patients, respectively. In regards to gender, race, ethnicity, education level, and marital status, the sample identified as female (69.2%), Caucasian (67.3%), Hispanic/Latino (14.2%), with a median education level of an associate’s degree, and married (54.5%). Mean and SD (M ± SD) of PROMIS measures derived from the initial clinic visit (n = 211) are displayed in Table 1 and were SI = 48.41 ± 15.79, PI = 67.47 ± 5.99, PF = 32.49 ± 6.00, depression = 56.82 ± 12.68, and average pain intensity = 6.39 ± 1.75.
Table 1.
Mean (M) and standard deviation (SD) of PROMIS® measures of social isolation, pain interference, physical function, depression, and pain intensity
| M ± SD | |
|---|---|
| Social isolation | 48.41 ± 15.79 |
| Pain interference | 67.47 ± 5.99 |
| Physical function | 32.49 ± 6.00 |
| Depression | 56.82 ± 12.68 |
| Pain intensity, average | 6.39 ± 1.75 |
Estimates based on initial clinic visit responses from 211 patients. PROMIS Patient Reported Outcome Measurement Information System.
Aim 1: The Dynamic Effects of SI on Physical Health
Our primary hypothesis was that people living with persistent pain who reported higher levels of SI at the initiation of treatment would have lower levels of physical health (PI and PF) over the course of short-term care. The analysis of the average treatment effect of the treated indicated that patients with higher SI scores reported significantly higher levels of PI and significantly lower levels of PF (Table 2).
Table 2.
The effects of low (1–49 points) and high (50–100 points) social isolation (SI) levels on pain interference (PI) and physical function (PF)
| Low SI | High SI | Difference | |
|---|---|---|---|
| PI | 61.92 | 66.39 | 4.47*** |
| PF | 39.55 | 35.21 | −4.33*** |
| n | 2767 | 2183 |
Two sample t-test for statistical significance.
Covariates included are age, gender, race, ethnicity, education level, and marital status.
*p < .1, **p < .05, ***p <.01.
The first row in Table 2 shows that individuals in the high SI subgroup (i.e., poorer social health) have a statistically significant 4.47 points higher level of PI (i.e., poorer physical health) than individuals in the low SI subgroup. The second row shows that individuals in the high SI subgroup have a statistically significant 4.33 points lower level of PF (i.e., poorer physical health) than individuals in the low SI subgroup. Descriptive statistics for patients at each time point within the 90-day window can be found in Table 3.
Table 3.
Descriptive statistics for longitudinal analysis
| Time 1 (n = 312) | Time 2 (n = 312) | Time 3 (n = 136) | Time 4 (n = 27) | Time 5 (n = 7) | |
|---|---|---|---|---|---|
| Depression | 56.1 (12.4) | 55.1 (13.1) | 56.1 (13.8) | 52.4 (14.0) | 53.1 (18.0) |
| Pain interference | 67.1 (6.2) | 66.7 (6.7) | 66.7 (6.6) | 66.1 (7.5) | 69.1 (5.4) |
| Physical function | 32.4 (6.7) | 32.5 (6.8) | 31.8 (6.7) | 33.9 (9.9) | 29.6 (3.1) |
| Social isolation | 47.8 (15.8) | 47.9 (16.3) | 49.6 (17.1) | 49.8 (17.8) | 50.7 (18.7) |
| Pain intensity | 6.2 (1.9) | 5.9 (2.0) | 5.9 (2.0) | 5.8 (2.0) | 6.0 (1.4) |
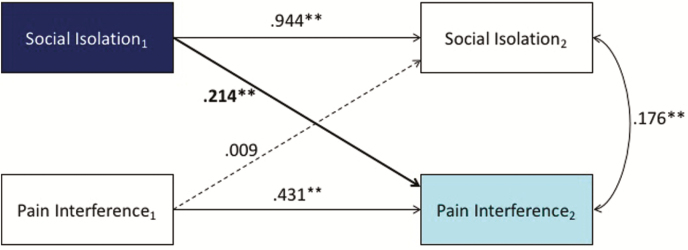
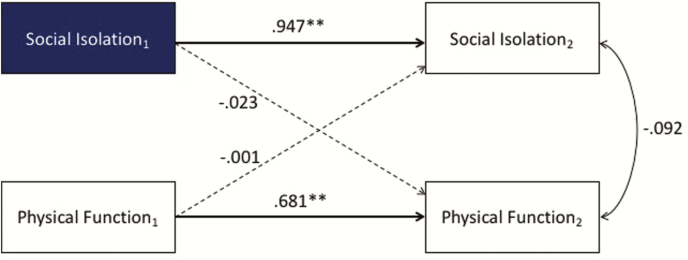
Cross-lagged longitudinal analysis showed that SI predicted PI at the next time point, above and beyond the effects of pain and covariates, but not vice versa (Fig. 1). However, there were no cross-lagged associations between PF and SI when covariates were included (Fig. 2). Results were modeled with average pain intensity, age, gender, race, ethnicity, education level, and marital status as covariates. All coefficients were standardized (**p < .01, *p < .05).
Fig. 1.
Cross-lagged longitudinal analysis showed that social isolation (SI) predicted pain interference (PI), but not vice versa. Asterisks indicate statistically significant levels.
Fig. 2.
Cross-lagged longitudinal analysis showed that social isolation (SI) did not predict physical function (PF). Asterisks indicate statistically significant levels.
Aim 2: Processes of SI and Physical Health
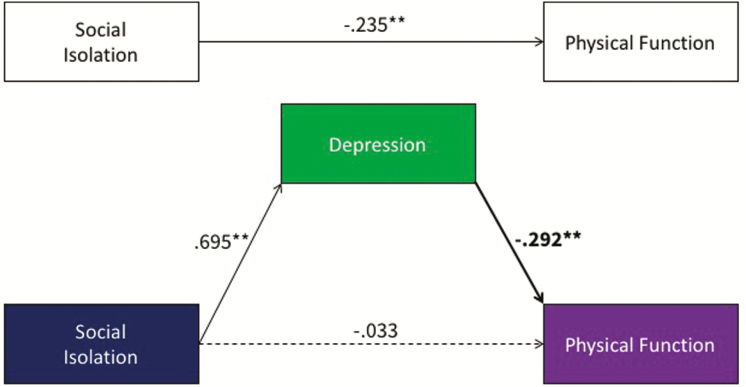
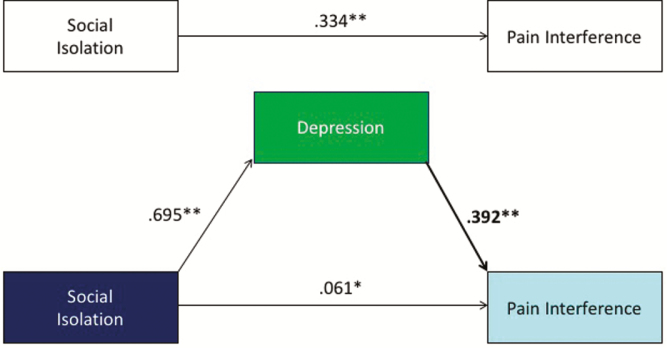
Our secondary hypothesis was that depression would mediate the relationship between SI and physical health. Cross-sectional mediation modeling showed that depression partially mediated the relationship between SI (for the PSM sample) and PF ([−0.256, −0.126] Fig. 3) and partially mediated the relationship between SI and PI ([0.152, 0.272] Fig. 4). As the effect of SI on PI was the only significant cross-lagged effect noted, we tested depressive symptoms as a potential mediator of this effect in the lagged models. There was a significant lagged effect of SI on depression scores, such that higher SI scores predicted higher depression scores at the next time point (β = 0.260, p < .001), but depression scores did not predict later scores on PI, above and beyond other variables in the model (β = −0.020, p = .75). Accordingly, depressive symptoms were not found to be a significant mediator of the effects of SI on PI in the longitudinal models ([−0.037, 0.027]).
Fig. 3.
Cross-sectional mediation modeling showed that depression partially mediated the relationship between social isolation (SI) and physical function (PF). Asterisks indicate statistically significant levels.
Fig. 4.
Cross-sectional mediation modeling showed that depression partially mediated the relationship between social isolation (SI) and pain interference (PI). Asterisks indicate statistically significant levels.
Discussion
Our study provides further characterization of the causal relationship between SI and physical health. Social health/situation and its role in understanding how these factors influence the physical and mental health environment have been an underappreciated domain in the fields of rehabilitation science and pain medicine [17]. Moreover, as the causality between these factors may be bidirectional, nonexperimental studies often do not allow for identifying causal mechanisms. Our findings provide further evidence that an individual’s social health/situation can inform the status of an individual’s physical health, using an approach that allows for causal inference. Notably, the findings from our PSM analysis suggest that individuals with high levels of SI have much higher levels of PI and lower levels of PF than individuals with low levels of SI. The effect of SI is slightly stronger on PI than on PF. Our longitudinal analysis indicates that feelings of SI predicted higher ratings of pain-related interference (but not PF ratings) at a later time point, while neither PI nor PF was found to predict later ratings of SI. Whereas PF might be considered more of a measure of discrete physical abilities, our conceptualization of PI implicates it as a more subjective measure related to appraisal of pain as a hindrance to engagement with physical, cognitive, emotional, and recreational activities. Given our previous work demonstrating differential pathways between PI and PF [35], it was expected that SI would have a stronger impact on PI relative to PF, which may be due to a tendency to appraise pain and one’s social environment in a negative fashion (as in depression). Interestingly, our longitudinal results did not implicate depressive symptoms as a significant mediator of the effects of SI on PI, suggesting that SI should be considered as a parallel influence on PI. Overall, our findings support the relative importance of SI as a factor in pain-related appraisal and coping and demonstrate that a comprehensive assessment of the individual’s social context can provide a more comprehensive understanding of the differential trajectories for a person living with pain.
Our findings are consistent with other studies related to social and physical health in people living with chronic pain and also identify areas to integrate in future investigations. Evers et al. [5] have shown that the quality of social support obtained from one’s social network, along with the level of passive coping strategies, predicted long-term pain, and disability. Although our study did not investigate the role of social support, the effects of SI may be interpreted along the same lines; those patients reporting greater feelings of isolation, loneliness, or disconnection from others appeared to view their pain as a more problematic experience than those with relatively lower levels of SI. Notably, as neither PI nor PF was found to predict ratings of SI at a later time, our findings suggest either a greater degree of stability in feelings of SI (that does not track closely with pain-related impairments) or a need for targeted psychosocial interventions designed to improve interpersonal functioning and the enjoyment and meaningfulness of social interactions.
Similarly, Cheatle et al. [36] have shown that the level of social withdrawal had implications beyond PI and PF status and was predictive of suicidal ideation for people with chronic pain. Although our study did not articulate the effects that passive coping strategies (such as prolonged patterns of behavioral or social avoidance due to pain) may have played in the outcomes of our patients, it is feasible that a passive approach to pain may have reinforced the high levels of pain-related physical and psychosocial problems noted in our sample. Our results, and those of other researchers, highlight the importance of consideration of the biopsychosocial model [37], not only in understanding the prediction and alleviation of pain but also in physical and mental health outcomes.
Collectively, these studies are in line with the theory that social context significantly affects the processing and experience of pain. Eisenberger [38] provides evidence from multiple studies that demonstrate the similarities in neurobiological substrates of the painful sensations associated with social disconnection and physical pain. From a positive psychology perspective, our results suggest that having a robust social network provides a “pain-buffering” effect. The Institute of Medicine Report on Relieving Pain in America [39] highlights that pain has significant social consequences, and our data complement this model by providing evidence of a more intricate relationship in that the degree of SI also has an effect on the perception of pain and physical consequences. A deeper understanding of the processes that SI and a person’s environment can have on the influence of their health, and adaptation to pain is required. Furthermore, the potential influencing factors of SI should also be considered. For example, people with chronic pain can feel stigmatized by family, friends, health professionals, and the general public [40], which could contribute toward and further perpetuate SI.
Limitations and Future Directions
Some limitations of performing a longitudinal assessment of prospective observational data and clinical setting include reduced control over data collection and availability, as well as potential variability in the degree of pain interventions received by each patient (low internal validity). However, the strengths consist of broad inclusion criteria and a diverse environment (high external validity). The heterogeneity of a bundled treatment delivered within a tertiary referral center does not allow for interpretation of which treatment(s) were more effective than others, nor could we control for the co-occurring effects of pain medication, psychotherapy or physical therapy services, or interventional procedures. The survey time points between health care visits were not consistent between patients due to the nature of the clinical environment, as regular follow-up may not have been warranted, available, and/or desired by some patients. As a result of these factors, there was a large proportion of our patient sample that was unsuitable for inclusion in the longitudinal analysis due to a lack of follow-up time points. As our analysis focused on the use of data captured at clinic visits and not collected prospectively, our sample may reflect tendencies among a group of patients more inclined toward regular follow-ups in a tertiary care pain clinic, which may be due to increased pain, distress, or to other factors not captured by our model. Consequently, more structured prospective longitudinal studies of these factors appear indicated. Moreover, the results from the PSM analysis have to be read with caution as the CIA is not entirely fulfilled and the attribution to treatment and control groups thus not completely random.
In regards to future directions, it would be valuable for future studies to examine other potential mediators between SI and PI, such as pain self-efficacy, pain acceptance, and pain-related fear of movement. From a treatment standpoint, amelioration of SI in patients with chronic pain could involve motivational interviewing and group-based participatory training in mindfulness, loving-kindness, and compassion cultivation [41] in an effort to increase social engagement. Preliminary evidence suggests that a Mindfulness-Based Stress Reduction program was effective in reducing loneliness in older adults [42]. Furthermore, family hardiness and family social support should be a consideration in reducing the impact of SI, as it has been shown to decrease disability in people living with fibromyalgia [43].
Conclusions
The impact of pain is reduced in individuals who perceive a greater sense of inclusion from and engagement with others. Our study contributes to the fields of pain medicine and rehabilitation science by providing preliminary details on the degree to which an individual’s level of social embeddedness may influence the level of their physical and mental health. The finding that SI significantly affects individuals’ PI, particularly given the lack of effects of PI or PF on SI, would suggest that individuals’ level of social connection and engagement should be considered in the assessment and treatment plan. This study enhances the understanding of how social factors affect pain and have implications for how the effectiveness of therapeutic interventions may be improved.
Acknowledgments
N. V. Karayannis and J. A. Sturgeon were funded under the NIH T32 Program Award—Fellowship in Interdisciplinary Research Training in Pain and Substance Use Disorders [3T32DA035165]. I. Baumann was supported by a research visit grant from the Zurich University of Applied Sciences. S. C. Mackey is funded under the NIH (K24DA029262, R01AT008561, and P01AT006651) and the Redlich Pain Endowment. We are grateful for Ms. Jennifer Drew, MSW, for her review of the manuscript.
Appendix A
Formal Description of the Propensity Score Method
We estimate the average treatment effect on the treated (ATT) that is defined as the difference between expected outcome values with and without treatment for those who actually received a treatment [33] It is given by β = E (Y(1)−Y(0) | T = 1), whereby Y(0) is the outcome without treatment, Y(1) the outcome with treatment, and T the treatment.
Fig. A1.
Propensity score histogram by treatment status and common support. The common support condition requires that sociodemographic characteristics observed in the treatment group (high SI) can also be observed among the control group (low SI). This figure demonstrates that almost all treatment observations are “on support,” that is, they were matched to control observations. Only 16 treatment observations were excluded from the analysis because it was not possible to match them to control observations (“off support”).
Fig. A2.
Test of the conditional independence assumption (CIA). Bias in percentages between the means of the treatment and control group for the included covariates before and after matching. The figure demonstrates that the matching reduced bias and thus improved the CIA for all covariates.
Compliance with Ethical Standards
Authors’ ContributionN. V. Karayannis and I. Baumann conceived of the study. N. V. Karayannis, I. Baumann, and J. A. Sturgeon refined the protocol. I. Baumann and J. A. Sturgeon developed plans for the statistical analyses. N. V. Karayannis drafted the manuscript. All authors read and approved the final manuscript.
Ethical ApprovalStudy procedures, which involved exclusively retrospective review of clinical data, were approved by the Institutional Review Board at the Stanford University School of Medicine.
Informed ConsentPeople seeking treatment at the Stanford Pain Management Center provided informed consent and enrolled in CHOIR as part of their routine clinical care.
Authors’ Statement of Conflict of Interest and Adherence to Ethical StandardsThe Authors Nicholas V. Karayannis, Isabel Baumann, John A. Sturgeon, Markus Melloh, and Sean C. Mackey declare that they have no conflict of interest.
References
- 1. World Health Organization (WHO) The World Health Report 2007 – A Safer Future: Global Public Health Security in the 21st century. Geneva:WHO;2007. Available at http://www.who.int/whr/2007/en/index.html [Google Scholar]
- 2. Aslund C, Starrin B, Nilsson KW. Social capital in relation to depression, musculoskeletal pain, and psychosomatic symptoms: A cross-sectional study of a large population-based cohort of Swedish adolescents. BMC Public Health. 2010; 10: 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeVries AC, Glasper ER, Detillion CE. Social modulation of stress responses. Physiol Behav. 2003; 79: 399–407. [DOI] [PubMed] [Google Scholar]
- 4. Eisenberger NI, Jarcho JM, Lieberman MD, Naliboff BD. An experimental study of shared sensitivity to physical pain and social rejection. Pain. 2006; 126: 132–138. [DOI] [PubMed] [Google Scholar]
- 5. Evers AW, Kraaimaat FW, Geenen R, Jacobs JW, Bijlsma JW. Pain coping and social support as predictors of long-term functional disability and pain in early rheumatoid arthritis. Behav Res Ther. 2003; 41: 1295–1310. [DOI] [PubMed] [Google Scholar]
- 6. Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Associations between psychosocial factors and pain intensity, physical functioning, and psychological functioning in patients with chronic pain: A cross-cultural comparison. Clin J Pain. 2014; 30: 713–723. [DOI] [PubMed] [Google Scholar]
- 7. Melloh M, Elfering A, Käser A et al. Depression impacts the course of recovery in patients with acute low-back pain. Behav Med. 2013; 39: 80–89. [DOI] [PubMed] [Google Scholar]
- 8. Melloh M, Elfering A, Stanton TR et al. Who is likely to develop persistent low back pain? A longitudinal analysis of prognostic occupational factors. Work. 2013; 46: 297–311. [DOI] [PubMed] [Google Scholar]
- 9. Elfering A, Käser A, Melloh M. Relationship between depressive symptoms and acute low back pain at first medical consultation, three and six weeks of primary care. Psychol Health Med. 2014; 19: 235–246. [DOI] [PubMed] [Google Scholar]
- 10. Substantial qualitative and quantitative evidence supports the validity of PROMIS measures Available at http://www.healthmeasures.net/explore-measurement-systems/promis/measure-development-research/validation.
- 11. Cella D, Gershon R, Lai JS, Choi S. The future of outcomes measurement: Item banking, tailored short-forms, and computerized adaptive assessment. Qual Life Res. 2007; 16 (Suppl 1):133–141. [DOI] [PubMed] [Google Scholar]
- 12. Gershon R, Rothrock NE, Hanrahan RT, Jansky LJ, Harniss M, Riley W. The development of a clinical outcomes survey research application: Assessment Center. Qual Life Res. 2010; 19: 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fries JF, Cella D, Rose M, Krishnan E, Bruce B. Progress in assessing physical function in arthritis: PROMIS short forms and computerized adaptive testing. J Rheumatol. 2009; 36: 2061–2066. [DOI] [PubMed] [Google Scholar]
- 14. Kao MCJ, Cook K, Olson G, Pacht T, Darnall BD, Weber SC, Mackey SC. SNAPL-CAT: Catalyzing the rate-limiting step of big data psychometrics with item-response theory and advanced computerized adaptive testing (poster presentation). American Medical Informatics Associations (AMIA) 2014 Joint Summits on Translational Science. San Francisco, CA; 2014. [Google Scholar]
- 15. Cella D, Yount S, Rothrock N et al. ; PROMIS Cooperative Group. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007; 45:S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. PROMIS social isolation scoring manual: A brief guide to the PROMIS social isolation instruments Available at http://www.healthmeasures.net/administrator/components/com_instruments/uploads/15-09-01_16-44-48_PROMISSocialIsolationScoringManual.pdf
- 17. Hahn EA, DeWalt DA, Bode RK et al. ; PROMIS Cooperative Group. New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychol. 2014; 33: 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amtmann D, Cook KF, Jensen MP et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010; 150: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rothrock NE, Hays RD, Spritzer K, Yount SE, Riley W, Cella D. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS). J Clin Epidemiol. 2010; 63: 1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choi SW, Podrabsky T, McKinney N, Schalet BD, Cook KF, Cella D.. PROSetta Stone® Analysis Report: A Rosetta Stone for Patient Reported Outcomes. Chicago, IL: Department of Medical Social Sciences, Feinberg School of Medicine, Northwestern University; 2012. [Google Scholar]
- 21. Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004; 20: 309–318. [DOI] [PubMed] [Google Scholar]
- 22. Cook KF, Schalet BD, Kallen MA, Rutsohn JP, Cella D. Establishing a common metric for self-reported pain: Linking BPI pain interference and SF-36 bodily pain subscale scores to the PROMIS pain interference metric. Qual Life Res. 2015; 24: 2305–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993; 31: 247–263. [DOI] [PubMed] [Google Scholar]
- 24. Hung M, Hon SD, Franklin JD et al. Psychometric properties of the PROMIS physical function item bank in patients with spinal disorders. Spine. 2014; 39: 158–163. [DOI] [PubMed] [Google Scholar]
- 25. Fries JF, Witter J, Rose M, Cella D, Khanna D, Morgan-DeWitt E. Item response theory, computerized adaptive testing, and PROMIS: Assessment of physical function. J Rheumatol. 2014; 41: 153–158. [DOI] [PubMed] [Google Scholar]
- 26. Antonakis J, Bendahan S, Jacquart P, Lalive R. On making causal claims: A review and recommendations. Leadersh Q. 2010; 21: 1086–1120. [Google Scholar]
- 27. Rosenbaum P. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985; 39: 33–38. [Google Scholar]
- 28. Rosenbaum P. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983; 70: 41–55. [Google Scholar]
- 29. Ima K. Causal inference with general treatment regimes. J Am Stat Assoc. 2004; 99: 854–866. [Google Scholar]
- 30. Dehejia RH, Wahba S. Causal effects in nonexperimental studies: Reevaluating the evaluation of training programs. J Am Stat Assoc. 1999; 94. [Google Scholar]
- 31. Imbens G. Nonparametric estimation of average treatment effects under exogeneity: A review. Rev Econ Stat. 2004; 86: 4–29. [Google Scholar]
- 32. Rosenbaum P. Observational Studies. Springer; 2002. [Google Scholar]
- 33. Caliendo M, Kopeinig S. Some practical guidance for the implementation of propensity score matching. J Econ Surv. 2008; 22: 31–72. [Google Scholar]
- 34. Sturgeon JA, Dixon EA, Darnall BD, Mackey SC. Contributions of physical function and satisfaction with social roles to emotional distress in chronic pain: A Collaborative Health Outcomes Information Registry (CHOIR) study. Pain. 2015; 156: 2627–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karayannis NV, Sturgeon JA, Chih-Kao M, Cooley C, Mackey SC. Pain interference and physical function demonstrate poor longitudinal association in people living with pain: A PROMIS investigation. Pain. 2017; 158: 1063–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cheatle MD, Wasser T, Foster C, Olugbodi A, Bryan J. Prevalence of suicidal ideation in patients with chronic non-cancer pain referred to a behaviorally based pain program. Pain Physician. 2014; 17: E359–E367. [PubMed] [Google Scholar]
- 37. Álvarez AS, Pagani M, Meucci P. The clinical application of the biopsychosocial model in mental health: A research critique. Am J Phys Med Rehabil. 2012; 91: S173–S180. [DOI] [PubMed] [Google Scholar]
- 38. Eisenberger NI. The pain of social disconnection: Examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 2012; 13: 421–434. [DOI] [PubMed] [Google Scholar]
- 39. IOM: Institute of Medicine Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC; 2011. [Google Scholar]
- 40. Holloway I, Sofaer-Bennett B, Walker J. The stigmatisation of people with chronic back pain. Disabil Rehabil. 2007; 29: 1456–1464. [DOI] [PubMed] [Google Scholar]
- 41. Hofmann SG, Grossman P, Hinton DE. Loving-kindness and compassion meditation: Potential for psychological interventions. Clin Psychol Rev. 2011; 31: 1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Creswell JD, Irwin MR, Burklund LJ et al. Mindfulness-based stress reduction training reduces loneliness and pro-inflammatory gene expression in older adults: A small randomized controlled trial. Brain Behav Immun. 2012; 26: 1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Preece JC, Sandberg JG. Family resilience and the management of fibromyalgia: Implications for family therapists. Contemp Fam Ther. 2005; 27: 559–576. [Google Scholar]