People with multiple sclerosis reduced physical activity following periods of high fatigue and depressed mood. In contrast, higher than usual physical activity was followed by improved fatigue and mood.
Keywords: multiple sclerosis, pain, fatigue, depressed mood, cognitive function, physical activity
Abstract
Background
Symptom severity is negatively associated with physical activity in multiple sclerosis (MS). However, it is unclear how physical activity and symptoms correlate on a day-to-day basis in persons with MS.
Purpose
To determine the temporal within-person associations of pain, fatigue, depressed mood, and perceived cognitive function with physical activity in MS.
Methods
Ambulatory adults with MS (N = 107) completed 7 days of home monitoring. Continuous physical activity data (assessed via wrist-worn accelerometer) and concurrent ecological momentary assessment (5X/day) of pain, fatigue, depressed mood, and perceived cognitive function were collected. Data were analyzed using multilevel mixed modeling.
Results
Fatigue and depressed mood demonstrated bidirectional associations with physical activity, whereas pain and cognitive function did not. Higher than usual fatigue (B = −5.83, p = .001) and depressed mood (B = −4.12, p = .03) were followed by decreased physical activity. In contrast, higher than usual physical activity was associated with subsequent decline in fatigue (B = −0.001, p = .02) and depressed mood (B = −0.0007, p = .02); however, the association between physical activity and fatigue varied across the day.
Conclusions
Physical activity is dynamically related to fatigue and mood on a moment-to-moment basis in MS. Efforts to increase physical activity in MS must incorporate a focus on how symptoms affect and are affected by activity.
Introduction
Multiple Sclerosis (MS) is an autoimmune disease of the central nervous system that currently affects nearly 2.5 million worldwide [1]. Although MS is classically associated with motor impairment and gait disturbance, individuals with MS typically experience a constellation of symptoms, such as fatigue [2–7], poor cognitive function (e.g., impaired memory, executive functioning, verbal fluency, attention, and processing speed; [8–13]), chronic pain [14–17], and depression [18–20]. Because current treatments cannot reverse pre-existing or prevent future neurological damage, MS typically follows a course of increasing symptoms (e.g., pain, fatigue, depressed mood, and cognitive dysfunction) and disability over a lifetime. These symptoms often produce functional limitations in excess of those that are attributable to MS-related neurological damage [21]. As a result, discovering ways to achieve and maintain optimal functioning in the face of mounting symptoms is a paramount goal for clinicians and researchers [22, 23].
Individuals with MS are less physically active in terms of activities of daily living and recreation, compared with the general population and to persons with other chronic conditions [24–26]. This is unfortunate, given that participation in physical activity is associated with improved functional abilities and symptom burden [27], whereas low levels of physical activity have been linked to increased fall risk [28], apathy and depression [26], and reduced quality of life for persons with MS [29]. Consequently, there is a critical need to identify factors that contribute to activity, thereby protecting against the deleterious effects of a sedentary lifestyle.
A growing body of evidence suggests the role of pain, fatigue, mood, and cognitive function symptoms as important determinants of physical activity levels in MS [25, 30–34]. However, existing literature that has examined relationships between various/common symptoms and physical activity in MS is limited by cross-sectional design and aggregation of repeated measures data (e.g., averaging of real-time pain ratings or accelerometer data across the week) [27, 30–34]. Although examination of between-person associations has been important in establishing that those with greater symptom burden tend to be less active, we know much less about how common MS symptoms and activity are dynamically related within-person in daily life; for example, when pain is high, do people tend to respond by reducing activity (i.e., “slowing down”)? Or, does pain increase following a period of higher than usual physical activity?
A better understanding of the association between various symptoms and physical activity in MS has critical clinical implications, such as providing clues into the mechanism of underlying activity patterns and symptoms, and directing targeted behavioral interventions such as exercise, activity pacing, and energy conservation to improve activity levels and/or symptom severity. The aim of this study was to examine the relative associations of four common MS symptoms—pain, fatigue, depressed mood, and perceived cognitive function—to physical activity in the daily lives of ambulatory individuals with MS. Specifically, we aimed to examine the bidirectional temporal associations between self-reported symptoms and physical activity. Given the lack of MS-specific studies of this design, hypotheses were based on research in other chronic conditions [35]. We predicted that momentary increases in pain and fatigue would predict decreased physical activity in the subsequent time period, and that increased momentary physical activity would be related to increased subsequent pain and fatigue. Previous data (non-MS) regarding between- person findings support a positive effect of physical activity on cognitive functioning [36] and depression [26, 31]; thus, we expected negative temporal associations (both directions) between physical activity and the intensity of cognitive problems and depressed mood. In a set of supplementary analyses, we examined whether observed associations were static or changed across the day (i.e., does the association between pain and activity fluctuate from morning to night?).
Methods
Participants
We recruited 108 adults with clinical diagnosis of MS (all subtypes) who met the following inclusion criteria: (i) ≥18 years of age; (ii) able to speak/read English at 6th grade level; and (iii) able to ambulate with minimal assistance (use of cane/walker permitted). Exclusion criteria included the following: (i) MS exacerbation (“relapse”) within the past 30 days (per volunteer self-report; if positive or unsure of relapse within past month, volunteer could enroll after 30 days relapse-free); (ii) atypical sleep/wake pattern (e.g., shift work); (iii) diagnosis of rheumatologic disease or fibromyalgia; and (iv) change in disease-modifying therapy regimen during study.
Study Procedures
This observational study utilized baseline surveys, ecological momentary assessment (EMA) of symptoms, and physical activity (accelerometer) data. Previous papers highlighted the day-to-day variability in all four MS symptoms (pain, fatigue, depressed mood, and cognitive function [37]), covariation of MS symptoms [38], and association of symptoms to daily functioning and well-being [39]; nevertheless, this paper addresses one of the primary a priori aims of the study. Data collection was conducted at the University of Michigan (UM) between October 2014 and March 2016. Institutional Review Board approval was granted prior to initiation of study activities. Participants were recruited through physician referrals, flyers placed in UM medical clinics and community locations, in-person outreach at community events, electronic medical records, existing participant registries, and the UM human participants’ recruitment website. Research assistants screened participants by telephone and scheduled eligible volunteers for a baseline visit, in which they completed consent procedures and a survey battery (administered online via Qualtrics) and participated in training on use of the PRO-Diary (CamNTech, Cambridge, United Kingdom) wrist-worn monitor and the end-of-day online diaries.
The 7 day home monitoring period began on the day following the baseline visit. During this period, participants wore the PRO-Diary on their nondominant wrist continuously, except while bathing or swimming. Five times a day (upon waking, 11 am, 3 pm, 7 pm, and bedtime), participants were prompted to enter self-reported ratings of pain, fatigue, depressed mood, and perceived cognitive functioning. For the fixed-time ratings, participants were alerted to provide ratings with an audible alarm; participants initiated ratings at wake and bed times. After the home monitoring period was concluded, participants returned their PRO-Diary to the lab, where data were downloaded and cleaned. Participant compensation was based on the number of days completed.
Measures
Baseline measures
Participants completed surveys of demographic (e.g., age, sex) and clinical variables (MS subtype, year of diagnosis), which were confirmed through medical record review. Two short form measures from the Quality of Life in Neurological Disorders (Neuro-QoL) [40] measurement system were used to assess self-reported upper and lower extremity functioning at baseline. Eight items from the Neuro-QoL Upper Extremity Function (Fine Motor, ADL) and eight items from the Neuro-QoL Lower Extremity (Mobility) item bank were rated on a scale from 1 (unable to do) to 5 (without any difficulty). For each short form, items were summed and converted to a T-score metric with a mean = 50 and standard deviation = 10 (reference is general population). The Neuro-QoL physical functioning measures have been validated in MS [41]; internal consistency for both the upper extremity (α = 0.92) and lower extremity (α = 0.94) short forms was excellent.
Ecological momentary assessment
Pain intensity was measured with the item: “What is your level of pain right now?” rated on a scale from 0 = “no pain” to 10 = “worst pain imaginable.”
Fatigue intensity, defined for respondents as tiredness or weariness [42], was measured with the item: “What is your level of fatigue right now?” rated on a scale from 0 = “no fatigue” to 10 = “extremely severe fatigue.”
Depressed mood was measured with the item: “What is your level of depression right now?” rated on a scale from 0 = “not at all depressed” to 10 = “extremely depressed.”
Perceived Cognitive Function was measured with the item: “What is your level of cognitive functioning right now?” rated on a scale from 0 = “my thinking is sharp and quick” to 10= “my thinking is very difficult or slow.”
These measures demonstrated good construct validity in this dataset [37].
Accelerometer measures
In addition to the user interface for entering EMA symptom ratings, the PRO-Diary has a triaxial micro-electromechanical systems accelerometer allowing for collection of physical movement data. The PRO-Diary was programmed to record activity in 15 s epochs. Raw acceleration measurements were processed with on-board software to generate “activity counts” where higher activity counts related to more physical activity. At wake (i.e., when participants became fully awake, not necessarily the time they got out of bed) and bedtime (i.e., when participants turned out the light or intended to go to sleep, not necessarily the time they got into bed), participants entered the time they woke up/went to bed into the PRO-Diary, which helped in identifying periods of wake/sleep in the data. All accelerometer data went through extensive data cleaning using a standardized protocol to identify invalid data and to classify sleep or wake activity data.
Physical activity
Average activity counts per minute were aggregated between each EMA rating time point to provide an estimate of activity that preceded and followed each EMA rating. Means for average activity counts per minute were calculated for the following time frames: wake–11 am, 11 am–3 pm, 3 pm–7 pm, and 7 pm–bedtime. The PRO-Diary accelerometer has shown good construct validity for measuring differences in physical activity from sedentary to moderate level physical activity in individuals with and without mobility impairments [43].
Data Analyses
Preliminary data analyses
Descriptive statistics for key study variables were calculated and analyzed for proportion of missing data and normality. Zero-order correlational analyses (for continuous variables) and analyses of variance (ANOVA; for categorical variables) were used to examine bivariate associations between demographic, clinical, and study variables.
Primary data analyses
We used multilevel modeling (MLM) to test the primary study questions. Using MLM allowed us to simultaneously model between- and within-person variance, account for the auto-correlation of within-person observations, and retain as many data points as possible. Momentary predictor variables were centered [44] prior to running MLMs; deviation scores for EMA symptoms and for physical activity counts (average for each interval) were created by person-centering each symptom and physical activity score so that the centered value indicated the momentary change from each person’s own weekly symptom/activity average. We constructed an MLM with physical activity as the criterion, and entered all symptoms and relevant covariates into a single model to test the relative contribution of each symptom to later activity outcomes. We then constructed four MLMs, one for each symptom, and entered all relevant covariates and the activity counts variable to examine the contribution of physical activity to later symptom ratings. Covariates—age, MS subtype, upper/lower extremity physical functioning, and average/aggregated level of time-varying EMA/activity variables—were determined based on previous associations in the literature [45] and statistical recommendations [46]. Intercept was specified as a random effect in all models; in addition, time-varying predictors were evaluated for inclusion as random effects using Akaike Information Criteria (AIC) [47] and the Bayesian Information Criteria (BIC) [48] where smaller values indicate better model fit. To test whether symptom/activity associations were stable across the day, interaction terms (e.g., TimePointXPhysicalActivity) were entered as predictors; significant interaction terms were probed using simple slopes analysis. To determine effect size, we calculated the amount of shared variance (pseudo-R2) between momentary symptoms that showed significant associations [49]. In a set of supplementary analyses, all previously described models were run replacing physical activity with “percent immobile time,” a mobility statistic derived from the accelerometer that is calculated as the percentage of 15 s epochs in the given interval scored as immobile [total immobile time/(interval duration minus total invalid time (activity) × 100]; higher percent immobile scores indicated a larger proportion of inactive or sedentary time. Patterns of findings for percent immobile time were identical to those for physical activity (in expected, opposite directions). For simplicity, only analyses for physical activity are presented here. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Preliminary Results
One person withdrew from the study prior to the home monitoring; data from the remaining 107 participants were analyzed. Data distribution statistics (e.g., all skew < 1.35, all kurtosis < 3.59) and graphical representations indicated that all data were sufficiently normally distributed for parametric statistics [50]. Missing data rates were low, as noted in previous publications [37–39]; six hundred out of a possible 3,745 EMA data points (16.6%) were missing and 55 participants had ≤3 missing out of a possible 35 EMA data points (5 EMA per day for 7 days).
Most of the sample was female (n = 74; 69.2%), white (n = 88, black n = 10, Asian n = 5, other n = 4), with relapsing-remitting MS (RRMS) subtype (n = 78; progressive MS n = 29). Means and standard deviations for key study variables are at the bottom of Table 1. Weekly averages of EMA ratings of symptoms were on average modest; fatigue was the highest (3.42), but still in the mild range. Notably, there was a wide range of symptom severity, with some reporting a weekly average of no (0/10) symptoms and others reporting a high level of symptoms. Average physical activity for this sample (mean = 207.50 ± 79.23) is somewhat lower than what has been found in previous studies in samples of women with (mean = 317.80 ± 89.50) and without osteoarthritis (mean = 380.00 ± 106.10) [35], individuals with osteoarthritis and clinically significant fatigue (mean = 341.42 ± 100.68) [51], and back pain (mean = 228.21 ± 8.16) [52], but somewhat higher than a sample of individuals with spinal cord injury and chronic pain (mean = 175.99 ± 107.00) [53].
Table 1.
Correlations and descriptive statistics for demographic and key study variables (N = 107)
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Age (years) | – | ||||||||
| 2. MS duration (years) | 0.43** | – | |||||||
| 3. Paina | −0.01 | −0.02 | – | ||||||
| 4. Fatiguea | −0.11 | −0.03 | 0.70** | – | |||||
| 5. Depressed mooda | −0.16 | −0.11 | 0.39** | 0.48** | – | ||||
| 6. Cognitive functiona | 0.02 | 0.01 | 0.55** | 0.66** | 0.53** | – | |||
| 7. Upper extremity | −0.29** | −0.10 | −0.34** | −0.28** | −0.10 | −0.22* | – | ||
| 8. Lower extremity | −0.39** | −0.27** | −0.38** | −0.32** | −0.09 | −0.24* | 0.70** | – | |
| 9. Physical activity | −0.46** | −0.09 | 0.12 | 0.08 | 0.15 | 0.12 | 0.19 | 0.20* | – |
| Mean | 45.16 | 9.49 | 2.44 | 3.42 | 1.20 | 1.78 | 45.36 | 45.92 | 207.50 |
| SD | 11.73 | 8.36 | 1.74 | 1.94 | 1.62 | 1.76 | 9.72 | 9.95 | 79.23 |
| Min–Max | 23–67 | 0–44 | 0–6.73 | 0–8.10 | 0–8.37 | 0–8.25 | 12.80–53.80 | 19.20–58.60 | 57.32–503.28 |
MS Multiple sclerosis.
aPerson-averaged EMA ratings.
*p < .05; ** p < .01.
Correlations between aggregated EMA symptom variables were moderate to high (Table 1); notably, correlations between symptoms and physical activity were small and nonsignificant. Average symptoms levels were not significantly different by sex (all p > 0.14). Women had higher physical activity counts compared with men [220.98 average activity counts/minute ± 83.44 vs 177.27 average activity counts/minute ± 59.56; F(1, 106) = 7.36, p = .008]. Physical activity was related to MS subtype; individuals with RRMS had higher physical activity than individuals with progressive MS [216.94 average activity counts/minute ± 78.51 vs 182.09 average activity counts/minute ± 76.80; F(1, 106)=4.22, p = .04]. Compared with those with progressive MS, those with RRMS had significantly higher average fatigue [3.69 ± 1.95 vs 2.67 ± 1.74; F(1,106) = 6.17, p = .02], depressed mood [1.45 ± 1.78 vs 0.54 ± 0.77; F(1,106) = 7.04, p = .009], and perceived cognitive function [2.03 ± 1.92 vs 1.09 ± 0.91; F(1,106) = 6.40, p = .01]. Pain did not differ by MS subtype (F(1,106) = 1.17, p = .28).
Symptoms Predicting Later Physical Activity
In analyses that assumed stable associations across the day (i.e., no diurnal effects), momentary increases in fatigue predicted decreased physical activity in the next time period (Table 2). Similarly, momentary increases in depressed mood predicted decreased physical activity in the next time period. Together, fatigue and mood accounted for 3.6 per cent of between-person and 12.6 per cent of within-person variance in physical activity. Neither pain intensity nor cognitive problems significantly predicted later physical activity.
Table 2.
Results of multilevel models examining the association between preceding symptoms—pain, fatigue, depressed mood, and cognitive function—and subsequent physical activity, controlling for age, MS subtype, upper- and lower-extremity functioning, and average of EMA symptom ratings
| Fixed effects | |||
|---|---|---|---|
| Physical activity (average activity counts/minute) | |||
| B | SE | p | |
| Between-person predictor variables (time invariant) df = 96 | |||
| Intercept | 338.07 | 53.24 | <.0001 |
| Age | −3.15 | 0.75 | <.0001 |
| MS type | 8.86 | 18.18 | .63 |
| UE functioning | −0.26 | 0.80 | .75 |
| LE functioning | −0.03 | 0.98 | .98 |
| Avg. pain | 6.01 | 6.01 | .31 |
| Avg. fatigue | −3.04 | 6.76 | .65 |
| Avg. depressed | −0.16 | 4.94 | .97 |
| Avg. cognitive | 4.53 | 5.42 | .41 |
| Within-person predictor variables—Symptom ratings (preceding activity) df = 2,080 | |||
| Δ Pain | 0.32 | 2.20 | .89 |
| Δ Fatigue | −5.40 | 1.54 | .0005 |
| Δ Depressed | −5.92 | 2.03 | .004 |
| Δ Cognitive | 0.94 | 1.71 | .58 |
| Between-person R-squared (all symptoms combined) = 0.036 | |||
| Within-person R-squared (all symptoms combined) = 0.126 | |||
B unstandardized beta; SE standard error; Δ person-centered variable representing momentary deviation (change) from a person’s average.
An (AR1) autoregressive matrix was used to model the error variance; Intercept and momentary pain, fatigue, and cognitive function were included as random effects; MS type relapsing remitting MS subtype, progressive subtypes was reference category; LE lower extremity; UE upper extremity; Avg. person-average of EMA symptom ratings during home monitoring period.
Physical Activity Predicting Later Symptoms
In analyses that assumed stable associations across the day, changes in physical activity were only associated with subsequent fatigue and depressed mood, but not pain or cognitive symptoms (Table 3). Specifically, increases in physical activity predicted later decreases in fatigue and depressed mood. Physical activity accounted for 3.2%–9.0% of the between-person and 1.2%–11.3% of the within-person variance in symptom ratings.
Table 3.
Results of multilevel models examining the associations between preceding physical activity and subsequent symptoms—pain, fatigue, depressed mood, and perceived cognitive function (controlling for age, MS subtype, and upper- and lower-extremity functioning)
| Fixed effects | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pain | Fatigue | Depressed mood | Cognitive function | |||||||||
| B | SE | p | B | SE | p | B | SE | p | B | SE | p | |
| Between-person predictor variables (time invariant) df = 98 | ||||||||||||
| Intercept | 2.34 | 1.36 | .09 | 4.42 | 1.63 | .008 | 0.94 | 1.14 | .41 | −0.02 | 1.55 | .99 |
| Age | 0.01 | 0.02 | .50 | −0.005 | 0.02 | .77 | −0.009 | 0.02 | .55 | 0.03 | 0.02 | .20 |
| MS type | 0.81 | 0.41 | .05 | 1.24 | 0.45 | .007 | 1.08 | 0.28 | .002 | 1.32 | 0.36 | .0004 |
| UE functioning | 0.002 | 0.01 | .85 | 0.02 | 0.01 | .09 | 0.006 | 0.02 | .71 | −0.01 | 0.01 | .42 |
| LE functioning | −0.05 | 0.01 | .001 | −0.07 | 0.02 | .001 | −0.02 | 0.02 | .33 | −0.02 | 0.02 | .31 |
| Avg. physical activity | 0.006 | 0.002 | .005 | −0.002 | 0.002 | .44 | 0.003 | 0.002 | .18 | 0.004 | 0.003 | .11 |
| Within-person predictor variables—physical activity (preceding symptom) df = 2,080 | ||||||||||||
| Δ Physical activity | 0.0007 | 0.0004 | .09 | −0.002 | 0.0006 | .02 | −0.0007 | 0.0003 | .02 | −0.00003 | 0.0004 | .93 |
| Between- person R-squared | 0.032 | 0.037 | 0.090 | 0.090 | ||||||||
| Within-person R-squared |
0.012 | 0.113 | 0.079 | 0.014 | ||||||||
B unstandardized beta; SE standard error; Δ Person-centered variable representing momentary deviation (change) from a person’s average.
An (AR1) autoregressive matrix was used to model the error variance; Intercept and centered momentary physical activity were included as random effects; MS type relapsing remitting MS subtype, progressive subtypes was reference category; Avg. person-average of EMA symptom ratings during home monitoring period.
Changes in Symptom/Activity Associations Across the Day
In analyses that explored diurnal fluctuations across the day, associations between preceding symptoms predicting later physical activity (Table 2) were consistent across the day, as evidenced by nonsignificant interaction terms for all four symptoms (all p > 0.52).
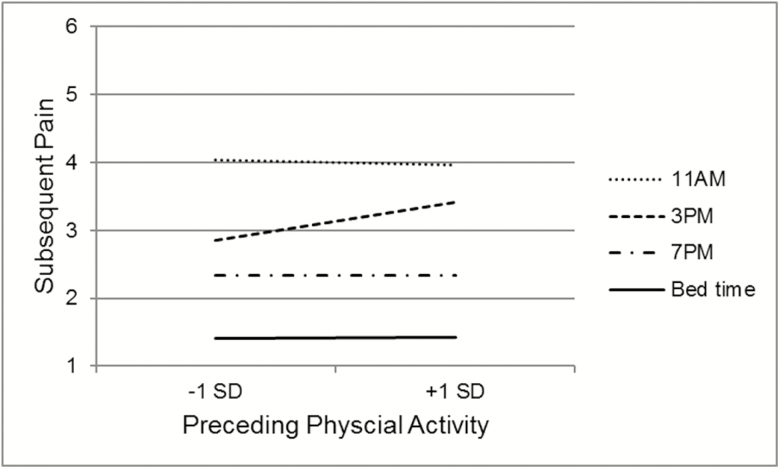
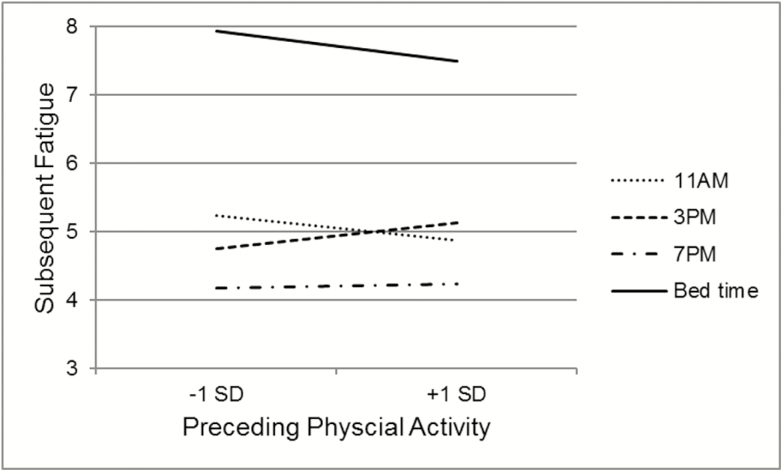
The associations between preceding physical activity and later symptoms (Table 3) did differ across the day for pain (Time Point × Physical Activity B = 0.001, SE = 0.0004, p = .01) and fatigue (Time Point × Time Point × Physical Activity B = −0.0016, SE = 0.0004, p < .001). As shown in Figure 1, there was no significant association between physical activity and subsequent pain for all time points, except for the 3 pm pain rating; higher than usual physical activity between 11 am and 3 pm was related to increased pain at 3 pm (B = 0.003, SE = 0.0007, p < .0001). As shown in Figure 2, the negative association between physical activity and subsequent fatigue was only observed at 11 am (B = −0.002, SE = 0.0009, p = .02) and bed time (B = 0.003, SE = 0.0007, p < .0001); midday, the association is the opposite, with periods of higher than usual physical activity followed by higher fatigue ratings (3 pm, B = 0.002, SE = 0.001, p = .02).
Fig. 1.
Simple slopes of the temporal association between changes in physical activity and subsequent pain rating. *Indicates that slope is significantly different from zero.
Fig. 2.
Simple slopes of the temporal association between changes in physical activity and subsequent fatigue rating. *Indicates that slope is significantly different from zero.
Discussion
To the best of our knowledge, this is the first study to capture the relative within-person association between four common chronic symptoms—pain, fatigue, perceived cognitive function, and depressed mood—and physical activity in the daily lives of persons with MS. We found that both fatigue and depressed mood demonstrated bidirectional associations with physical activity. When participants perceived an increase in fatigue or depressed mood, they tended to respond by slowing down or reducing physical activity; these associations were stable and showed no diurnal fluctuations. These data provide independent support for the emerging consensus that increased symptom burden may partially explain the reduction in physical activity in those with MS [33]. Partially consistent with expectations, when our participants were more physically active than usual, they experienced subsequent reductions in perceived fatigue and depressed mood; however, the direction of the association varied throughout the day for fatigue. Specifically, the findings for general daily associations (that assume diurnal stability) are driven by negative associations between activity and fatigue in the morning and bedtime, whereas midday, there is a positive association between activity and later fatigue (consistent with expectations). Collectively, these findings suggest that individuals with MS could experience overall reductions in fatigue and depressed mood through increases in physical activity; however, encouraging physical activity as a means to address these symptoms may present challenges in the setting of higher depressed mood or fatigue. Ultimately, interventions that aim to increase physical activity need to be informed by the role that fatigue, mood, and time of day play in physical activity in MS.
In contrast with expectations, pain showed no overall temporal association with physical activity in this sample, with the exception of a single positive midday association between higher than usual physical activity and later pain intensity. The null association between pain and physical activity at all other time points rendered the “whole day” analyses nonsignificant, despite the isolated midday pain/activity association. These within-person findings are inconsistent with previous cross-sectional findings that people with MS who are more physically active have lower pain levels [54]. There are no known previous studies that have examined within-person associations with how physical activity relates to later experience of pain. Although this association is only seen midday in this sample, it may be a rather salient association for participants who perceive that, generally speaking, they experience more pain after they are physically active.
Perceived cognitive function had no relationship with physical activity levels in this study, at either the between- or within-person level. Cross-sectional work has shown a negative association between subjective cognitive problems and physical activity (measured objectively) in MS [55]. Among aspects of cognitive functioning, processing speed has shown the most robust association with physical activity [42] and is sensitive to improvements following physical activity intervention [36, 55]. It is possible that the methods used to assess cognitive symptoms in this study (self-rated perception of speed and difficulty of thinking) may have lacked the sensitivity to detect an association with physical activity. Furthermore, physical (cardiovascular) fitness is thought to be a primary mechanism linking physical activity to brain health and cognitive functioning in the general population and those with neurological conditions [56–58]. Because this study did not examine vigorous physical activity (e.g., exercise) specifically, our measure of physical activity may not adequately reflect physical fitness, which is likely to show a more robust association with cognitive function compared with physical activity [58]. Future studies could employ short, objective cognitive performance tests that could be administered in real time, concurrent with measures of various levels of activity intensity to better explore between- and within-person associations between physical activity and cognitive functioning in MS.
A comprehensive approach to understanding and treating symptoms in MS recognizes that symptoms are often interrelated [38] (or “cluster” together [59]) and are associated with physical activity [30, 31, 34], as both an antecedent and a consequence. From this perspective, a multimodal approach to treating symptoms, including both medical and behavioral approaches, holds the greatest potential to be effective for both symptom management and optimization of physical functioning [60]. Activity modification treatment strategies are commonly used across disciplines and can take two main forms. Activity pacing is the regulation of activity level or rate in an effort to improve function and/or achieve personal goals [61]. In contrast, energy conservation is the regulation of activity in an effort to keep symptoms at a manageable level [61]. While both activity pacing and energy conservation have not demonstrated an impact on chronic pain severity in either MS or other populations [51, 61–63], energy conservation interventions have demonstrated positive effects on fatigue and fatigue impact in MS [64–69], with some exceptions [70]. Consistent with this notion, Murphy and Clauw predicted that activity modification interventions could have a greater effect on fatigue than on pain, particularly in populations with significant fatigue burden, such as MS [71]. Although work to develop and test exercise-based interventions for the treatment of symptoms in MS is relatively new, there is promising evidence that exercise of varying types (e.g., yoga, bicycling [72], and resistance training [73]) is effective in reducing fatigue [73–75] and depressed mood [73, 76–78]. In addition, cognitive behavioral strategies, that may incorporate aforementioned strategies of activity modification and exercise in combination with strategies to improve sleep, stress, emotional regulation, and social relationships, have demonstrated positive effects on fatigue [79, 80], as well as pain and depressed mood in MS [80].
Findings from the current study hold a number of implications for behavioral interventions meant to improve either activity or symptom severity. While modifying activity through pacing or energy conservation is often thought to keep symptoms at a manageable level by preventing people from “overdoing it,” these data suggest that higher than average levels of physical activity are actually related to improved fatigue and depressed mood. Findings that fatigue and depressed mood show bidirectional associations with physical activity suggest that interventions that are effective at improving either fatigue, depressed mood, or activity could have multiple downstream positive effects on the other outcomes. These findings also suggest the need for flexibility in any approach to managing symptoms that may need to change across the day as people’s activity and symptom levels fluctuate diurnally. For instance, traditional pacing or energy conservation strategies that serve to prevent activity peaks may be helpful midday, but unhelpful in the morning or evening in terms of fatigue severity. Given that these findings warrant replication in a larger and more diverse sample of individuals with MS, and these ideas need to be tested in the context of a clinical trial, firm clinical recommendations are not possible. However, the findings do highlight the need to think critically and carefully about how activity and symptoms may interact dynamically over the course of a day and to consider the possibility of individual differences in such associations.
Study Limitations
In a previous examination of these data, we showed that the EMA ratings suggest lower symptom severity compared with ratings on standardized recall surveys. Specifically, this sample’s scores were comparable with other MS study samples on the Brief Pain Inventory [63, 81–83], Patient Health Questionnaire-9 [84], Perceived Deficits Questionnaire [85], and Fatigue Severity Scale [86] (see Ref. [37] for full details and discussion). However, the generally mild symptoms in the sample limit generalizability of the findings to those with MS and greater symptoms burden. In addition, type of physical activity (e.g., recreation, housework, and physical therapy) was not captured, although type of activity may have implications for symptom experience. As person-level changes in physical activity do not necessarily capture exercise behavior, these findings cannot be used to make any conclusions about the association of exercise and symptoms. These data may however contribute to our understanding of the benefits of physical activity that occurs in the context of activities of daily living, or of “lifestyle physical activity,” the accumulation of activity in the course of daily activities [87]. A potential fruitful line of inquiry would be to evaluate the impact variable intensity exercises interventions have on pain, fatigue, cognition, and mood for those with MS. Future studies should also determine differences in the relationship among physical activity and symptoms across the MS disability spectrum and when compared with healthy matched controls.
The magnitude of associations found in these analyses was relatively small, especially in analyses in which physical activity predicted subsequent symptoms. While the effect sizes of the momentary associations were small in absolute terms, these results must be evaluated in terms of their relevance in the day-to-day lives of those who live with MS [88]. Indeed, much like each “at bat” contributes to the batting average of a baseball player, accumulation of small, momentary associations over weeks, months, and years can have much larger cumulative effects [88].
Conclusion
This study builds on previous cross-sectional research to show that symptoms and physical activity are dynamically and temporally related to a moment-to-moment basis in MS. Findings provide support for interventions that favor a multimodal approach for the improvement of physical activity or symptom burden, which considers the natural association between physical activity, pain, fatigue, and mood in the daily lives of people with MS.
Acknowledgements
Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under award number R03NR014515; PI: Kratz. Dr. Kratz was supported during manuscript preparation by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (award number 1K01AR064275). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no conflicts of interest to declare.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards Authors Anna L. Kratz, Nora E. Fritz, Tiffany J. Braley, Eric L. Scott, Emily Foxen-Craft, and Susan L. Murphy declare that they have no conflict of interest.
Primary data These findings were presented at the 2018 Society of Behavioral Medicine annual meeting.
Authors’ Contributions All authors contributed to the conceptualization and design of the study. ALK analyzed the data and all authors assisted in refining the analytical strategy and interpreting the results. ALK wrote the first draft of the paper and all other authors edited the manuscript and approved the final version.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the IRBMED at the University of Michigan prior to study initiation.
Informed Consent Informed consent was obtained from all individuals who participated in this study.
References
- 1. National Multiple Sclerosis Society 2012. Available at http://www.nationalmssociety.org/index.aspx. Accessibility verified October 14, 2017.
- 2. Kos D, Kerckhofs E, Nagels G, D’hooghe MB, Ilsbroukx S. Origin of fatigue in multiple sclerosis: Review of the literature. Neurorehabil Neural Repair. 2008;22:91–100. [DOI] [PubMed] [Google Scholar]
- 3. Krupp LB. Fatigue in multiple sclerosis: Definition, pathophysiology and treatment. CNS Drugs. 2003;17:225–234. [DOI] [PubMed] [Google Scholar]
- 4. Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Arch Neurol. 1988;45:435–437. [DOI] [PubMed] [Google Scholar]
- 5. Krupp LB, Christodoulou C. Fatigue in multiple sclerosis. Curr Neurol Neurosci Rep. 2001;1:294–298. [DOI] [PubMed] [Google Scholar]
- 6. Krupp LB, Serafin DJ, Christodoulou C. Multiple sclerosis-associated fatigue. Expert Rev Neurother. 2010;10:1437–1447. [DOI] [PubMed] [Google Scholar]
- 7. MacAllister WS, Krupp LB. Multiple sclerosis-related fatigue. Phys Med Rehabil Clin N Am. 2005;16:483–502. [DOI] [PubMed] [Google Scholar]
- 8. Amato MP, Ponziani G, Siracusa G, Sorbi S. Cognitive dysfunction in early-onset multiple sclerosis: A reappraisal after 10 years. Arch Neurol. 2001;58:1602–1606. [DOI] [PubMed] [Google Scholar]
- 9. Bagert B, Camplair P, Bourdette D. Cognitive dysfunction in multiple sclerosis: Natural history, pathophysiology and management. CNS Drugs. 2002;16:445–455. [DOI] [PubMed] [Google Scholar]
- 10. Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–1151. [DOI] [PubMed] [Google Scholar]
- 11. Huijbregts SC, Kalkers NF, de Sonneville LM, de Groot V, Reuling IE, Polman CH. Differences in cognitive impairment of relapsing remitting, secondary, and primary progressive MS. Neurology. 2004;63:335–339. [DOI] [PubMed] [Google Scholar]
- 12. Kesselring J, Klement U. Cognitive and affective disturbances in multiple sclerosis. J Neurol. 2001;248:180–183. [DOI] [PubMed] [Google Scholar]
- 13. Lazeron RH, Boringa JB, Schouten M, et al. Brain atrophy and lesion load as explaining parameters for cognitive impairment in multiple sclerosis. Mult Scler. 2005;11:524–531. [DOI] [PubMed] [Google Scholar]
- 14. Ehde DM, Gibbons LE, Chwastiak L, Bombardier CH, Sullivan MD, Kraft GH. Chronic pain in a large community sample of persons with multiple sclerosis. Mult Scler. 2003;9:605–611. [DOI] [PubMed] [Google Scholar]
- 15. Ehde DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH. The scope and nature of pain in persons with multiple sclerosis. Mult Scler. 2006;12:629–638. [DOI] [PubMed] [Google Scholar]
- 16. Ehde DM, Osborne TL, Jensen MP. Chronic pain in persons with multiple sclerosis. Phys Med Rehabil Clin N Am. 2005;16:503–512. [DOI] [PubMed] [Google Scholar]
- 17. Hirsh AT, Turner AP, Ehde DM, Haselkorn JK. Prevalence and impact of pain in multiple sclerosis: Physical and psychologic contributors. Arch Phys Med Rehabil. 2009;90:646–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Minden SL, Orav J, Reich P. Depression in multiple sclerosis. Gen Hosp Psychiatry. 1987;9:426–434. [DOI] [PubMed] [Google Scholar]
- 19. Patten SB, Beck CA, Williams JV, Barbui C, Metz LM. Major depression in multiple sclerosis: A population-based perspective. Neurology. 2003;61:1524–1527. [DOI] [PubMed] [Google Scholar]
- 20. Sadovnick AD, Remick RA, Allen J, et al. Depression and multiple sclerosis. Neurology. 1996;46:628–632. [DOI] [PubMed] [Google Scholar]
- 21. Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci. 1994;21:9–14. [PubMed] [Google Scholar]
- 22. Kraft GH, Johnson KL, Yorkston K, et al. Setting the agenda for multiple sclerosis rehabilitation research. Mult Scler. 2008;14:1292–1297. [DOI] [PubMed] [Google Scholar]
- 23. Joy JE, Johnston RB.. Multiple Sclerosis: Current Status and Strategies for the Future. Washington, DC: The National Academies; 2001. [PubMed] [Google Scholar]
- 24. Klaren RE, Motl RW, Dlugonski D, Sandroff BM, Pilutti LA. Objectively quantified physical activity in persons with multiple sclerosis. Arch Phys Med Rehabil. 2013;94:2342–2348. [DOI] [PubMed] [Google Scholar]
- 25. Motl RW, Arnett PA, Smith MM, Barwick FH, Ahlstrom B, Stover EJ. Worsening of symptoms is associated with lower physical activity levels in individuals with multiple sclerosis. Mult Scler. 2008;14:140–142. [DOI] [PubMed] [Google Scholar]
- 26. Vanner EA, Block P, Christodoulou CC, Horowitz BP, Krupp LB. Pilot study exploring quality of life and barriers to leisure-time physical activity in persons with moderate to severe multiple sclerosis. Disabil Health J. 2008;1:58–65. [DOI] [PubMed] [Google Scholar]
- 27. Rietberg MB, van Wegen EE, Uitdehaag BM, Kwakkel G. The association between perceived fatigue and actual level of physical activity in multiple sclerosis. Mult Scler. 2011;17:1231–1237. [DOI] [PubMed] [Google Scholar]
- 28. Sebastião E, Learmonth YC, Motl RW. Lower physical activity in persons with multiple sclerosis at increased fall risk: a cross-sectional study. Am J Phys Med Rehabil. 2017;96:357–361. [DOI] [PubMed] [Google Scholar]
- 29. Stroud NM, Minahan CL. The impact of regular physical activity on fatigue, depression and quality of life in persons with multiple sclerosis. Health Qual Life Outcomes. 2009;7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Motl RW, McAuley E. Symptom cluster as a predictor of physical activity in multiple sclerosis: Preliminary evidence. J Pain Symptom Manage. 2009;38:270–280. [DOI] [PubMed] [Google Scholar]
- 31. Motl RW, McAuley E, Wynn D, Suh Y, Weikert M, Dlugonski D. Symptoms and physical activity among adults with relapsing-remitting multiple sclerosis. J Nerv Ment Dis. 2010;198:213–219. [DOI] [PubMed] [Google Scholar]
- 32. Motl RW, Snook EM, McAuley E, Gliottoni RC. Symptoms, self-efficacy, and physical activity among individuals with multiple sclerosis. Res Nurs Health. 2006;29:597–606. [DOI] [PubMed] [Google Scholar]
- 33. Motl RW, Snook EM, Schapiro RT. Symptoms and physical activity behavior in individuals with multiple sclerosis. Res Nurs Health. 2008;31:466–475. [DOI] [PubMed] [Google Scholar]
- 34. Motl RW, Weikert M, Suh Y, Dlugonski D. Symptom cluster and physical activity in relapsing-remitting multiple sclerosis. Res Nurs Health. 2010;33:398–412. [DOI] [PubMed] [Google Scholar]
- 35. Murphy SL, Smith DM, Clauw DJ, Alexander NB. The impact of momentary pain and fatigue on physical activity in women with osteoarthritis. Arthritis Rheum. 2008;59:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sandroff BM, Klaren RE, Pilutti LA, Dlugonski D, Benedict RH, Motl RW. Randomized controlled trial of physical activity, cognition, and walking in multiple sclerosis. J Neurol. 2014;261:363–372. [DOI] [PubMed] [Google Scholar]
- 37. Kratz AL, Murphy SL, Braley TJ. Ecological momentary assessment of pain, fatigue, depressive, and cognitive symptoms reveals significant daily variability in multiple sclerosis. Arch Phys Med Rehabil. 2017;98:2142–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kratz AL, Murphy SL, Braley TJ. Pain, fatigue, and cognitive symptoms are temporally associated within but not across days in multiple sclerosis. Arch Phys Med Rehabil. 2017;98:2151–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kratz AL, Braley TJ, Foxen-Craft E, Scott E, Murphy JF 3rd, Murphy SL. How do pain, fatigue, depressive, and cognitive symptoms relate to well-being and social and physical functioning in the daily lives of individuals with multiple sclerosis?Arch Phys Med Rehabil. 2017;98:2160–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cella D, Lai JS, Nowinski CJ, et al. Neuro-QOL: Brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012;78:1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miller DM, Bethoux F, Victorson D, et al. Validating Neuro-QoL short forms and targeted scales with people who have multiple sclerosis. Mult Scler. 2016;22:830–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Motl RW, Gappmaier E, Nelson K, Benedict RH. Physical activity and cognitive function in multiple sclerosis. J Sport Exerc Psychol. 2011;33:734–741. [DOI] [PubMed] [Google Scholar]
- 43. Murphy SL, Kratz AL, Zynda AJ. Properties of wrist-worn accelerometers in individuals with spinal cord injury. Am J Occcup Ther. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: A new look at an old issue. Psychol Methods. 2007;12:121–138. [DOI] [PubMed] [Google Scholar]
- 45. Streber R, Peters S, Pfeifer K. Systematic review of correlates and determinants of physical activity in persons with multiple sclerosis. Arch Phys Med Rehabil. 2016;97:633–645.e29. [DOI] [PubMed] [Google Scholar]
- 46. Hoffman L, Stawski RS. Persons as contexts: Evaluating between-person and within-person effects in longitudinal analysis. Res Hum Dev. 2009;6:97–120. [Google Scholar]
- 47. Bozdogan H. Model selection and akaike information criterion (Aic) - the general-theory and its analytical extensions. Psychometrika. 1987;52:345–370. [Google Scholar]
- 48. Akaike H. Likelihood of a model and information criteria. J Econometrics. 1981;16:3–14. [Google Scholar]
- 49. Singer JD, Willett JB.. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 50. West SG, Finch JF, Curran PJ.. Structural Equation Models With Non-Normal Variables: Problems and Remedies. Newbury Park, PA: Sage; 1995. [Google Scholar]
- 51. Murphy SL, Kratz AL, Kidwell K, Lyden AK, Geisser ME, Williams DA. Brief time-based activity pacing instruction as a singular behavioral intervention was not effective in participants with symptomatic osteoarthritis. Pain. 2016;157:1563–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alschuler KN, Hoodin F, Murphy SL, Geisser ME. Ambulatory monitoring as a measure of disability in chronic low back pain populations. Clin J Pain. 2011;27:707–715. [DOI] [PubMed] [Google Scholar]
- 53. Kratz AL, Ehde DM, Bombardier CH, Kalpakjian CZ, Hanks RA. Pain acceptance decouples the momentary associations between pain, pain interference, and physical activity in the daily lives of people with chronic pain and spinal cord injury. J Pain. 2017;18:319–331. [DOI] [PubMed] [Google Scholar]
- 54. Motl RW, McAuley E, Snook EM, Gliottoni RC. Physical activity and quality of life in multiple sclerosis: Intermediary roles of disability, fatigue, mood, pain, self-efficacy and social support. Psychol Health Med. 2009;14:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Prakash A, Snook E, Kramer AF, Motl R. Correlation of physical activity with perceived cognitive deficits in relapsing-remitting multiple sclerosis. Int J MS Care. 2010;12:1–5. [Google Scholar]
- 56. Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci. 2003;14:125–130. [DOI] [PubMed] [Google Scholar]
- 58. Voss MW, Weng TB, Burzynska AZ, et al. Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. Neuroimage. 2016;131:113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Motl RW, McAuley E. Symptom cluster and quality of life: Preliminary evidence in multiple sclerosis. J Neurosci Nurs. 2010;42:212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Crayton H, Heyman RA, Rossman HS. A multimodal approach to managing the symptoms of multiple sclerosis. Neurology. 2004;63:S12–S18. [DOI] [PubMed] [Google Scholar]
- 61. Nielson WR, Jensen MP, Karsdorp PA, Vlaeyen JW. Activity pacing in chronic pain: Concepts, evidence, and future directions. Clin J Pain. 2013;29:461–468. [DOI] [PubMed] [Google Scholar]
- 62. Murphy SL, Lyden AK, Smith DM, Dong Q, Koliba JF. Effects of a tailored activity pacing intervention on pain and fatigue for adults with osteoarthritis. Am J Occup Ther. 2010;64:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Osborne TL, Jensen MP, Ehde DM, Hanley MA, Kraft G. Psychosocial factors associated with pain intensity, pain-related interference, and psychological functioning in persons with multiple sclerosis and pain. Pain. 2007;127:52–62. [DOI] [PubMed] [Google Scholar]
- 64. Lamb AL, Finlayson M, Mathiowetz V, Chen HY. The outcomes of using self-study modules in energy conservation education for people with multiple sclerosis. Clin Rehabil. 2005;19:475–481. [DOI] [PubMed] [Google Scholar]
- 65. Mathiowetz V, Matuska KM, Murphy ME. Efficacy of an energy conservation course for persons with multiple sclerosis. Arch Phys Med Rehabil. 2001;82:449–456. [DOI] [PubMed] [Google Scholar]
- 66. Mathiowetz VG, Finlayson ML, Matuska KM, Chen HY, Luo P. Randomized controlled trial of an energy conservation course for persons with multiple sclerosis. Mult Scler. 2005;11:592–601. [DOI] [PubMed] [Google Scholar]
- 67. Mathiowetz VG, Matuska KM, Finlayson M. Randomized clinical trial of an energy conservation course for persons with MS. Mult Scler J. 2005;11:121–122. [DOI] [PubMed] [Google Scholar]
- 68. Vanage SM, Gilbertson KK, Mathiowetz V. Effects of an energy conservation course on fatigue impact for persons with progressive multiple sclerosis. Am J Occup Ther. 2003;57:315–323. [DOI] [PubMed] [Google Scholar]
- 69. Blikman LJ, Huisstede BM, Kooijmans H, Stam HJ, Bussmann JB, van Meeteren J. Effectiveness of energy conservation treatment in reducing fatigue in multiple sclerosis: A systematic review and meta-analysis. Arch Phys Med Rehabil. 2013;94:1360–1376. [DOI] [PubMed] [Google Scholar]
- 70. Blikman LJ, van Meeteren J, Twisk JW, et al. ; TREFAMS-ACE study group Effectiveness of energy conservation management on fatigue and participation in multiple sclerosis: A randomized controlled trial. Mult Scler. 2017;23:1527–1541. [DOI] [PubMed] [Google Scholar]
- 71. Murphy SL, Clauw DJ. Activity pacing: What are we measuring and how does that relate to intervention?Pain. 2010;149:582–583. [DOI] [PubMed] [Google Scholar]
- 72. Oken BS, Kishiyama S, Zajdel D, et al. Randomized controlled trial of yoga and exercise in multiple sclerosis. Neurology. 2004;62:2058–2064. [DOI] [PubMed] [Google Scholar]
- 73. Dalgas U, Stenager E, Jakobsen J, et al. Fatigue, mood and quality of life improve in MS patients after progressive resistance training. Mult Scler. 2010;16:480–490. [DOI] [PubMed] [Google Scholar]
- 74. Mostert S, Kesselring J. Effects of a short-term exercise training program on aerobic fitness, fatigue, health perception and activity level of subjects with multiple sclerosis. Mult Scler. 2002;8:161–168. [DOI] [PubMed] [Google Scholar]
- 75. Pilutti LA, Greenlee TA, Motl RW, Nickrent MS, Petruzzello SJ. Effects of exercise training on fatigue in multiple sclerosis: A meta-analysis. Psychosom Med. 2013;75:575–580. [DOI] [PubMed] [Google Scholar]
- 76. Ensari I, Motl RW, Pilutti LA. Exercise training improves depressive symptoms in people with multiple sclerosis: Results of a meta-analysis. J Psychosom Res. 2014;76:465–471. [DOI] [PubMed] [Google Scholar]
- 77. Bombardier CH, Ehde DM, Gibbons LE, et al. Telephone-based physical activity counseling for major depression in people with multiple sclerosis. J Consult Clin Psychol. 2013;81:89–99. [DOI] [PubMed] [Google Scholar]
- 78. Cakt BD, Nacir B, Genç H, et al. Cycling progressive resistance training for people with multiple sclerosis: a randomized controlled study. Am J Phys Med Rehabil. 2010;89:446–457. [DOI] [PubMed] [Google Scholar]
- 79. van den Akker LE, Beckerman H, Collette EH, et al. ; TREFAMS-ACE Study Group Cognitive behavioral therapy positively affects fatigue in patients with multiple sclerosis: results of a randomized controlled trial. Mult Scler. 2017;23:1542–1553. [DOI] [PubMed] [Google Scholar]
- 80. Ehde DM, Elzea JL, Verrall AM, Gibbons LE, Smith AE, Amtmann D. Efficacy of a telephone-delivered self-management intervention for persons with multiple sclerosis: a randomized controlled trial with a one-year follow-up. Arch Phys Med Rehabil. 2015;96:1945–58.e2. [DOI] [PubMed] [Google Scholar]
- 81. Cleeland CS. Measurement of pain by subjective report. In: Chapman C. R. and Loeser J. D. (eds), Advances in Pain Research and Therapy (Vol. 12). New York, NY: Raven Press; 1989:391–403. [Google Scholar]
- 82. Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 83. Osborne TL, Raichle KA, Jensen MP, Ehde DM, Kraft G. The reliability and validity of pain interference measures in persons with multiple sclerosis. J Pain Symptom Manage. 2006;32:217–229. [DOI] [PubMed] [Google Scholar]
- 84. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sullivan JJL, Edgley K, Dehoux E. A survey of multiple sclerosis. Part 1: Perceived cognitive problems and compensatory strategy use. Can J Rehabil. 1990;4:99–105. [Google Scholar]
- 86. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. [DOI] [PubMed] [Google Scholar]
- 87. Motl RW. Lifestyle physical activity in persons with multiple sclerosis: The new kid on the MS block. Mult Scler. 2014;20:1025–1029. [DOI] [PubMed] [Google Scholar]
- 88. Abelson RP. A variance explanation paradox: when a little is a lot. Psychol Bull. 1985;97:129–133. [Google Scholar]