Abstract
This study determined the feasibility of the cuff technique for small-caliber vascular grafts in a rat model. A graft was implanted with the cuff technique or suture technique in a 1-cm segment of the abdominal aorta in 12 rats. The mean aortic clamp time was 29 minutes with the cuff technique and 44 minutes with the suture technique; the cuff technique was significantly shorter. Abdominal angiography at 1 week after implantation showed no significant stenosis in 9 rats, focal stenosis of the mid-portion of the graft in 1 rat with each technique, and total occlusion of the graft in 1 rat with the suture technique. We have successfully used the cuff technique for anastomosis for small-caliber vascular grafts in an animal model.
Keywords: Vascular grafts, Cuff technique, Animal model
Introduction
Anastomotic technique is an important factor that has been shown in animal experiments to affect the outcome of implanted grafts. Conventionally, end-to-end anastomosis under a microscope is widely used, but it has some disadvantages, such as being a lengthy procedure, as well as being prone to leakage and delayed bleeding. In contrast, the cuff technique was first used in vascular and bronchial anastomosis in lung transplantation in a rat model [1]. Although the cuff technique is simpler and requires a shorter clamping time than end-to-end anastomosis, this technique has not been used in anastomosis for a vascular graft. Therefore, this study was performed to determine the feasibility of the cuff technique for small-caliber vascular grafts in a rat model.
Methods
This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IACUC no., BA 1104-081-028-01).
1) Tissue-engineered small-caliber vascular grafts
To create tissue-engineered 2-mm vascular grafts, 3% (w/v) poly (d,l-lactide-co-glycolide) (PLGA, Resomer RG; molecular weight, 110,000; lactide/glycolide molar ratio, 85/15) and 7% (w/v) poly-ɛ-caprolactone (PCL) were first dissolved in methylene chloride. Then 2% (w/v) methoxy-polyethylene glycol and 1% (w/v) elastin were added with heparin at 0.05 wt% and 20 ng/mL of vascular endothelial growth factor. An agitator was run to dissolve them completely until the solution became clear. The scaffolds were then created from the solution using an electrospinning system (HSP-30kV-2; NanoNC, Seoul, Korea).
2) In vivo implantation
Twelve Sprague-Dawley rats, weighing about 400 g, were intubated using a 16G catheter after induction with ether and ketamine, and were operated on under inhalation anesthesia. A midline laparotomy incision was performed, and the infrarenal abdominal aorta was isolated by sharp dissection. After proximal and distal clamping, a 1-cm segment of the aorta, below the renal artery and above the aortoiliac bifurcation, was resected. The graft was implanted with a cuff technique or suture technique, under an operative microscope of ×25 magnification. For the cuff technique, the distal side of the aorta was passed through the small pieces of an 18G catheter (cuff), and then everted over the cuff and fixed with 5-0 black silk ties (Fig. 1). The artificial vessel was then implanted between both sides of the aorta, and was fixed with 5-0 black silk ties tightly enough for it not to loosen. For the suture technique, 6 or 8 interrupted sutures were placed using 10-0 nylon. Both procedures were performed by 2 thoracic surgeons who had sufficient experience in vascular anastomosis in a clinical setting. Vascular anastomosis was performed in 6 rats in each group. The abdominal cavity and skin were closed, and animals were allowed to recover from anesthesia and kept in separate cages. Postoperatively, aspirin (15 mg/kg) was administered daily.
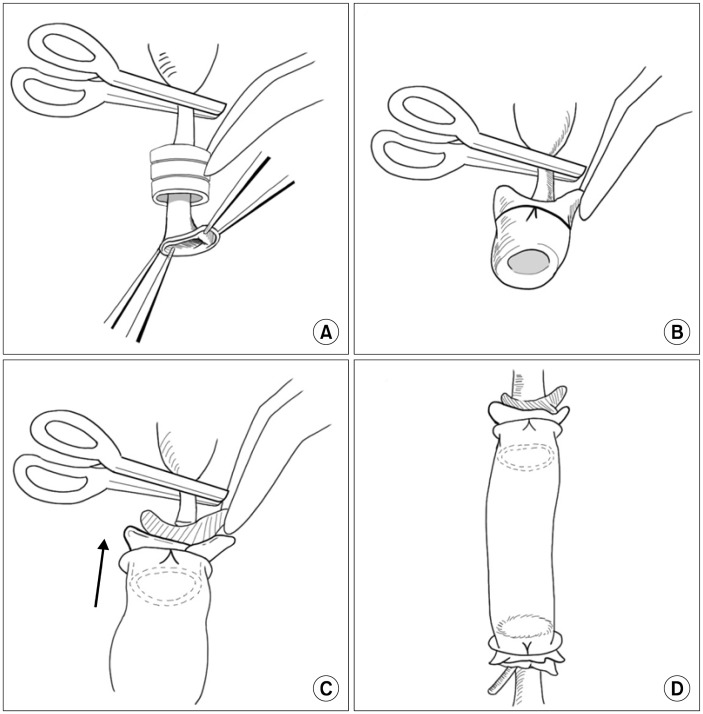
Fig. 1.
Schematic diagram of cuff technique. (A) Insertion of abdominal aorta through the cuff. (B) Eversion of aorta and fastening of everted aorta over cuff with a black silk circumferential ligature. (C) Insertion of abdominal aorta into the artificial graft. (D) Fastening of artificial graft to the abdominal aorta and completion of anastomosis.
3) Follow-ups and angiography
The time of anastomosis was measured, and immediate complications such as bleeding and death were recorded. At the end of the study period, all rats were anesthetized by ketamine and Rompun to receive left carotid artery cannulation with a 24G catheter. Digital subtraction angiography (General Electric Cardiac Series, 9800; Salt Lake City, UT, USA) was performed in vivo, followed by explantation of the implanted graft, including the proximal and distal native aorta.
Results
1) In vivo results
The small-caliber vascular grafts were easily implanted into the abdominal aorta using the cuff technique. The mean aortic clamp time was 29 minutes (range, 15–35 minutes) with the cuff technique and 44 minutes (range, 30–60 minutes) with the suture technique; the cuff technique was significantly shorter. No postoperative complications, such as massive bleeding or paralysis of the lower legs, were observed with either technique.
2) Angiographic examination
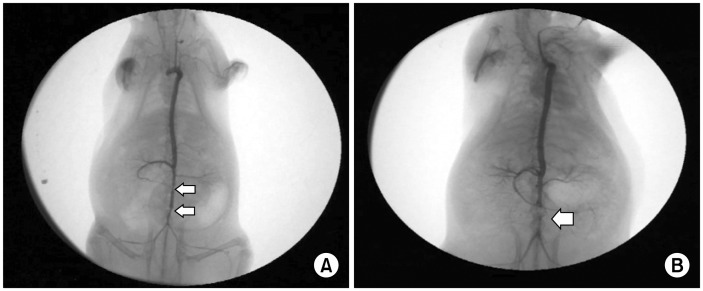
Abdominal angiography showed no significant stenosis in 9 rats (5 with the cuff and 4 with the suture method) at the 1-week follow-up (Fig. 2A), and the diameter of the mid-portion of the graft was larger than that of the proximal and distal ends. Focal stenosis of the mid-portion of the graft was identified in 2 rats (1 in each group), and total occlusion of the graft was found in 1 rat that underwent the suture technique at the 1-week follow-up (Fig. 2B).
Fig. 2.
Angiographic findings 1 week after implantation. (A) No stenosis: 2 arrows indicate proximal and distal anastomosis. The diameter of the mid-portion of the graft is larger than that of the proximal and distal ends. (B) Total occlusion: arrow indicates aneurysmal change of the midportion.
3) Histopathologic examination
Grossly, the implanted scaffold maintained its own shape; however, a microscopic examination showed that only a portion of the scaffold had degraded and a lining of endothelial cells was found over the inner surface of that portion of the graft.
Discussion
Commercially available artificial vascular grafts are currently limited to medium- and large-caliber vascular grafts; small-caliber vascular grafts, with a diameter of less than 2 mm, are in development. This may be the case because the technique involved in the application of small-caliber vascular grafts is much more difficult than that of large-caliber grafts. In a cost-benefit analysis, the disadvantages of using artificial vascular grafts are not offset by their advantages when this difficulty is taken into account for small-caliber grafts. Electrospun nanofiber scaffolds, made of PLGA and PCL, may serve as temporary grafts for tissue engineering applications.
For anastomosis, we performed the cuff technique, which has been used for anastomosis in lung transplantation in animal models [1]. The advantage of this method is its relative ease and the security of the anastomosis, as well as a dramatic reduction in clamping time. Therefore, the mean clamping time of this study was only 36 minutes, which is shorter than other studies, which have reported 67–72 minutes [2]. However, one of the disadvantages of the cuff technique is that this method may reduce the internal diameter due to stenosis from cuff insertion. However, Wu et al. [3] showed that the cuff method provided a simple, rapid, and reliable technique for vascular anastomosis, that the endothelium was continuous across both anastomosis sites, and that the external presence of the cuff may help to prevent post-transplant stenosis.
In conclusion, we have successfully used the cuff technique for anastomosis for tissue-engineered small-caliber vascular grafts in an animal model, and it would be useful to experiment with this technique for vascular anastomosis using very small-caliber vascular grafts.
Acknowledgments
This work was supported by a grant of the Seoul National University Bundang Hospital Research Fund (grant no., 02-2011-019).
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Guo H, Nie J, Fan K, et al. Improvements of surgical techniques in a rat model of an orthotopic single lung transplant. Eur J Med Res. 2013;18:1. doi: 10.1186/2047-783X-18-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pektok E, Nottelet B, Tille JC, et al. Degradation and healing characteristics of small-diameter poly(epsilon-caprolactone) vascular grafts in the rat systemic arterial circulation. Circulation. 2008;118:2563–70. doi: 10.1161/CIRCULATIONAHA.108.795732. [DOI] [PubMed] [Google Scholar]
- 3.Wu J, Ge X, Fahy GM. Ultrarapid nonsuture mated cuff technique for renal transplantation in rabbits. Microsurgery. 2003;23:369–73. doi: 10.1002/micr.10145. [DOI] [PubMed] [Google Scholar]