Abstract
Background
The aim of this study was to evaluate the outcomes of surgical resection in patients with radiographically noninvasive lung adenocarcinoma according to the surgical strategy.
Methods
A retrospective study was conducted of 128 patients who underwent pulmonary resection for ground-glass opacity (GGO)–dominant nodules measuring ≤2 cm with a consolidation/tumor ratio ≤0.25 based on computed tomography between 2008 and 2015. The 5-year disease-free survival (DFS) rate and 5-year overall survival (OS) rate were analyzed.
Results
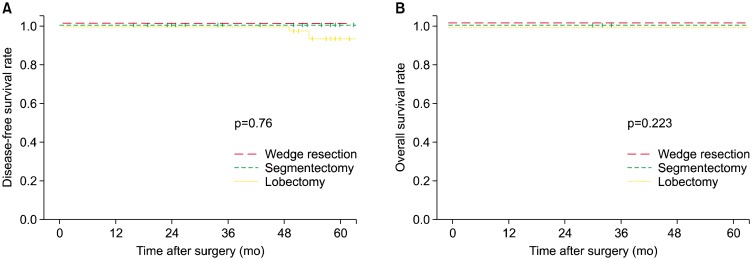
Among the 128 patients, wedge resection, segmentectomy, and lobectomy were performed in 40 (31.2%), 22 (17.2%), and 66 patients (51.6%), respectively. No significant differences were found among the groups in the mean size of tumors (p=0.119), the rate of pure-GGO nodules (p=0.814), the consolidation/tumor ratio (p=0.695), or the rate of invasive adenocarcinoma (p=0.378). Centrally located tumors were more common in the lobectomy group (21.2%) than in the wedge resection (0%) or segmentectomy (0%) groups (p=0.001). There were no significant differences in the 5-year DFS rate (100%, 100%, 92.7%, respectively; p=0.76) or 5-year OS rate (100%, 100%, 100%; p=0.223) among the wedge resection, segmentectomy, and lobectomy groups.
Conclusion
For radiographically noninvasive lung adenocarcinoma measuring ≤2 cm with a consolidation/tumor ratio ≤0.25, wedge resection and segmentectomy could be comparable surgical options to lobectomy.
Keywords: Adenocarcinoma; Nodules, solitary pulmonary; Lobectomy; Chest; Computed tomography
Introduction
In 1995, the Lung Cancer Study Group reported the only randomized trial comparing lobectomy and sublobar resection (SLR) for the treatment of T1N0 non-small-cell lung cancer (NSCLC). In this study, a tripling of the local recurrence rate among patients who underwent SLR was observed [1]. Other non-randomized studies demonstrated increased local recurrence in patients who underwent SLR compared to those who underwent lobectomy [2]. For these reasons, lobectomy has remained the treatment of choice for stage I NSCLC, with SLR reserved as a compromise operation for high-risk patients who are operative candidates but for whom an anatomic lobectomy is contraindicated. However, several recent studies demonstrated comparable recurrence and survival rates between lobectomy and SLR for stage IA lung cancers [3,4]. As a result of recent increases in the frequency of medical screening and advances in the detection of smaller tumors based on improvements in computed tomography (CT) resolution, ground-glass opacities (GGOs) are being diagnosed earlier and with smaller size than in the past. It has been demonstrated that GGOs are more likely to be preinvasive lesions and early adenocarcinomas, such as adenocarcinoma in situ, minimally invasive adenocarcinoma, or lepidic predominant invasive adenocarcinoma [5,6]. Part-solid GGOs have a solid component, which may represent invasive growth. These observations suggest that the degree of pathologic invasive growth in adenocarcinomas could be quantified according to the proportion of increased solid density in the CT appearance of the lesion [7]. Moreover, numerous studies have demonstrated equivalent overall survival (OS) and recurrence-free survival rates with SLR for stage IA NSCLC when compared to lobectomy in properly selected patients. However, the appropriate method of SLR is also a topic of debate.
The Japan Clinical Oncology Group (JCOG) conducted a prospective radiologic study of thin-section computed tomography (TSCT) to identify criteria that predict pathologic noninvasiveness in clinical IA lung cancer arising in the periphery of the lung (JCOG 0201), and defined radiological noninvasive adenocarcinoma as an adenocarcinoma measuring ≤2 cm with a consolidation/tumor (C/T) ratio ≤0.25, with high specificity [8]. Based on these criteria, we evaluated a cohort of patients who underwent resection for NSCLC measuring ≤2 cm and C/T ratio ≤0.25 to determine whether there was an association between the extent of surgical resection (wedge resection, segmentectomy, and lobectomy) and 5-year disease-free survival (DFS) rate, 5-year OS rate, and postoperative pulmonary function.
Methods
1) Patients and surgical procedures
Patients who underwent complete surgical resection for stage T1a NSCLC (tumor size ≤2 cm, C/T ratio ≤0.25) at our institution between January 2008 and December 2015 were included. Complete resection was defined as an absence of either macroscopic or microscopic residual cancer, especially in the resection margin. Patients with forced expiratory volume in 1 second (FEV1) <80%, suspected lymph node or distant metastasis at diagnosis, incomplete resection, other concomitant malignancies, or who received preoperative chemotherapy or radiotherapy were excluded. Finally, a total of 128 patients were enrolled in the study.
Patient demographics and clinical data were collected using a computerized patient record system. Clinical characteristics included age, sex, GGO characteristics, and C/T ratio. The preoperative health assessment included smoking status and FEV1. Operative data included the location and size of the tumor, histologic subtype, and lymph node involvement.
Surgical procedures included wedge resection, segmentectomy, and lobectomy. During the early study period, lobectomy was established as a standard treatment, so surgeons tended to perform lobectomy if possible. During the later study period, SLR including segmentectomy or wedge resection was more commonly performed, however, no definitive institutional policy was established. Surgical extent was chosen according to the surgeon’s own judgement based on the location, C/T ratio, and size of tumors.
Wedge resection, segmentectomy and lobectomy were compared for 5-year DFS rate, 5-year OS rate, and postoperative reduction of FEV1. We assessed the reduction of FEV1 after surgery based on each patient’s most recent pulmonary function test to determine the degree of postoperative loss of pulmonary function. The study was approved by the Institutional Review Board of University of Ulsan College of Medicine, Seoul, Korea (IRB approval no., 2018-0595) and was performed in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained from patients.
2) Definitions and follow-up
Noninvasive lung cancer was tentatively defined as a lung adenocarcinoma without nodal, vascular, or lymphatic invasion. The consolidation component was defined as an area of increased opacification that completely obscured the underlying vascular markings. A localized, nodular lesion characterized by a low-to-moderate increase in CT density that did not obscure lung structures, such as the pulmonary artery or vein and bronchus, was referred to as GGO. The extent of GGO was estimated by the tumor shadow disappearance rate (TDR) method [9]. The TDR was calculated using the following formula: TDR=(tumor size on mediastinal window)/(tumor size on lung window).
Recurrence was diagnosed by physical examination and diagnostic imaging. Histological or cytological confirmation of recurrence was made when clinically feasible. Local recurrence was defined as disease recurrence in adjacent anatomic sites, including the surgical margin, ipsilateral hemithorax, and mediastinum after surgical resection. Distant metastasis was defined as tumor recurrence in the contralateral lung or outside the hemithorax and mediastinum after surgical resection.
Tumors were classified by location as subpleural, peripheral, and central. The distance from the hilum to the visceral pleura was divided in half, and a lesion was classified as central if it was closer to the hilum, and peripheral if it was closer to the visceral pleura. Peripheral lesions in contact with the visceral pleura were classified as subpleural lesions.
Patients were staged according to the seventh edition of the American Joint Committee on Cancer guidelines [10]. Histologic subtypes of lung cancer were determined according to the World Health Organization classification [11]. Nodal status was classified as N0 if the short diameters of all mediastinal lymph nodes were less than 10 mm, no hilar lymph nodes were identified, and positron emission tomography, if done, showed no abnormal uptake. The median follow-up duration in the wedge resection, segmentectomy, and lobectomy groups was 35.9, 38, and 45 months, respectively (p=0.067).
3) Statistical analysis
Comparisons of the characteristics of the wedge resection, segmentectomy, and lobectomy groups were performed using the chi-square test for categorical variables (expressed as frequency and percentage) and 1-way analysis of variance for continuous variables (expressed as mean and standard deviation). A Kaplan-Meier curve was used to analyze OS and DFS rate in the 3 groups, and the statistical significance of differences was determined by the log-rank test. All statistical calculations were performed using the survival and ggplot2 packages of the R ver. 3.2.5 (The R Foundation for Statistical Computing, Vienna, Austria). All reported p-values were 2-sided, and p-values <0.05 were considered to indicate statistical significance.
Results
Among the 128 patients who met the inclusion criteria for the study, 40 (31.2%) underwent wedge resection, 22 (17.2%) underwent segmentectomy, and 66 underwent (51.6%) lobectomy. The clinical characteristics of the patients who underwent wedge resection, segmentectomy, or lobectomy are summarized in Table 1. Age, sex, smoking history, preoperative FEV1, histology, GGO characteristics, and the C/T ratio were distributed similarly in the 3 groups. The pathological characteristics of the 3 groups are shown in Table 2. Invasive adenocarcinoma was the most common histological subtype in all 3 groups, with 25 patients (62.5%), 13 patients (59.1%) and 41 patients (62.1), respectively, followed by minimally-invasive adenocarcinoma and adenocarcinoma in situ. However, there was no significant difference in the distribution of histological subtypes among the 3 groups (p=0.378). Patients who underwent wedge resection were significantly more likely to have undergone lymph node sampling, with 33 patients (82.5%), while patients who had segmentectomy or lobectomy were more likely to have undergone lymph node dissection at the time of surgery, with 18 patients (81.8%) and 64 (97%), respectively (p<0.001). There were no statistically significant differences in the mean size or size distribution of tumors in the 3 groups, as shown in Table 3 (p=0.119 and p=0.403, respectively). Centrally located tumors were more common in the lobectomy group (21.2%) than in the wedge resection (0%) or segmentectomy (0%) groups (p=0.001), as shown in Table 3.
Table 1.
Clinical characteristics of patients who underwent wedge resection, segmentectomy, or lobectomy
| Characteristic | Wedge resection (n=40) | Segmentectomy (n=22) | Lobectomy (n=66) | p-value |
|---|---|---|---|---|
| Age (yr) | 60.4±7.7 | 57.5±7.9 | 59.4±7.9 | 0.109 |
| Sex (male:female) | 23:17 | 9:13 | 29:37 | 0.489 |
| Smoking | 17 (42.5) | 6 (27.3) | 24 (36.4) | 0.491 |
| Preoperative forced expiratory volume in 1 second (%) | 91.3±12.7 | 87.6±12.3 | 92.7±13.6 | 0.246 |
| Ground glass opacity characteristics | 0.814 | |||
| Pure | 38 (95.0) | 18 (81.8) | 57 (86.4) | |
| Partially solid | 2 (5.0) | 4 (18.2) | 9 (13.6) | |
| Consolidation to tumor ratio (%) | 1.91±5.8 | 1.14±3.7 | 2.4±6.4 | 0.695 |
Values are presented as mean±standard deviation for continuous variables and number (%) for categorical variables.
Table 2.
Pathologic characteristics of patients who underwent wedge resection, segmentectomy, or lobectomy
| Characteristic | Wedge resection (n=40) | Segmentectomy (n=22) | Lobectomy (n=66) | p-value |
|---|---|---|---|---|
| Histology | 0.378 | |||
| Atypical adenomatous hyperplasia | 2 (5.0) | 0 | 0 | |
| Adenocarcinoma in situ | 4 (10.0) | 1 (4.5) | 4 (6.1) | |
| Minimally invasive adenocarcinoma | 9 (22.5) | 8 (36.4) | 21 (31.8) | |
| Invasive adenocarcinoma | 25 (62.5) | 13 (59.1) | 41 (62.1) | |
| Lymph node test | 0.000 | |||
| Not done | 6 (15.0) | 0 | 0 | |
| Sampling | 33 (82.5) | 4 (18.2) | 2 (3.3) | |
| Dissection | 1 (2.5) | 18 (81.8) | 64 (97.0) | |
| Nodal upstaging | 0 | 0 | 1 (1.6) | 1.000 |
Values are presented as number (%).
Table 3.
Radiologic characteristics of patients who underwent wedge resection, segmentectomy, or lobectomy
| Characteristic | Wedge resection (n=40) | Segmentectomy (n=22) | Lobectomy (n=66) | p-value |
|---|---|---|---|---|
| Location (lobe) | 0.036 | |||
| Right upper lobe | 11 (27.5) | 6 (27.3) | 32 (48.5) | |
| Right middle lobe | 0 | 0 | 6 (9.1) | |
| Right lower lobe | 12 (30.0) | 4 (18.2) | 10 (15.2) | |
| Left upper lobe | 10 (25.0) | 7 (31.8) | 9 (13.6) | |
| Left lower lobe | 7 (17.5) | 5 (22.7) | 9 (13.6) | |
| Location | 0.001 | |||
| Central | 0 | 0 | 14 (21.2) | |
| Peripheral | 23 (57.5) | 15 (68.2) | 41 (62.1) | |
| Subpleural | 17 (42.5) | 7 (31.8) | 11 (16.7) | |
| Tumor size (mm) | ||||
| Mean | 10.7±4.3 | 11.2±3.3 | 12.3±3.9 | 0.119 |
| 0–10 | 19 (47.5) | 10 (45.5) | 26 (39.4) | 0.403 |
| 10–20 | 21 (52.5) | 12 (54.5) | 40 (60.6) | |
Values are presented as number (%) for categorical variables and as mean±standard deviation for continuous variables.
Fig. 1A shows that there were no significant differences in the 5-year DFS rate (100%, 100%, 92.7%, respectively; p=0.76) among the wedge resection, segmentectomy, and lobectomy groups. Tumor recurrence was observed in 1 patient in the wedge resection group. The patient underwent right lower lobe wedge resection and had a distant recurrence in the left upper lobe 85 months after the first operation. Tumor recurrence was observed in 2 patients in the lobectomy group. One patient underwent right upper lobectomy and had a distant recurrence in the left upper lobe 50 months after the first operation. The other patient underwent right middle lobectomy and had a regional recurrence 53 months after the first operation. Fig. 1B showed that there were no significant differences in the 5-year OS rate (100%, 100%, 100%, respectively; p=0.223) among the wedge resection, segmentectomy, and lobectomy groups. One death occurred in the wedge resection group. The patient was a 63-year-old woman who underwent right lower lobe wedge resection for a 13-mm GGO. She expired due to severe variceal bleeding due to underlying liver cirrhosis 65 months after the operation. No deaths occurred in the segmentectomy or lobectomy group during the follow-up period.
Fig. 1.
Kaplan-Meier curves after resection for radiographically noninvasive adenocarcinoma of the lung stratified by surgical extent. (A) Disease-free survival. (B) Overall survival.
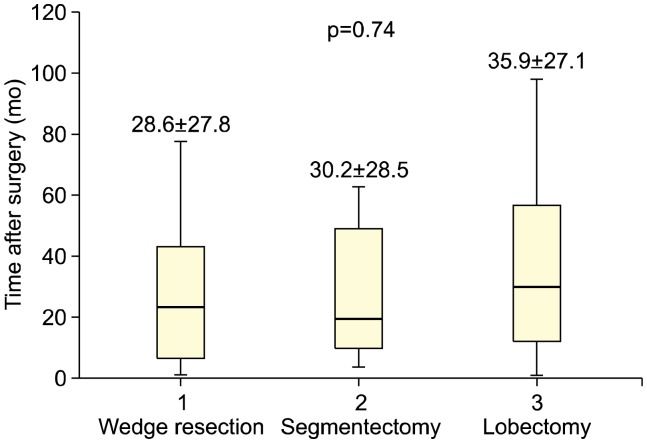
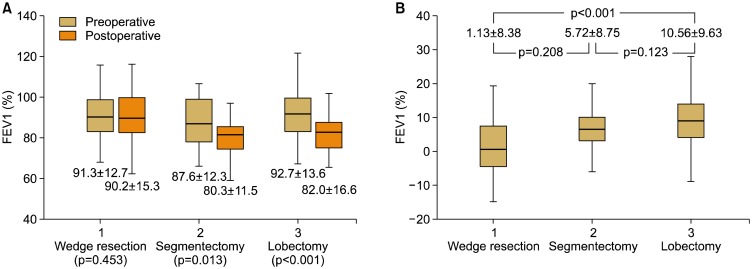
There were no preoperative differences in pulmonary function tests among the 3 groups (FEV1: 91.3%± 12.7% in the wedge resection group, 87.6%±12.3% in the segmentectomy group, and 92.7%±13.6% in the lobectomy group; p=0.246). The mean duration until the most recent pulmonary function test after surgery was not significantly different among the groups (wedge resection, 28.6±28.8 months; segmentectomy, 30.2±28.5 months; lobectomy, 35.9±27.1 months, respectively; p=0.74) (Fig. 2). In the segmentectomy and lobectomy groups, there was a statistically significant reduction in the postoperative FEV1 (from 87.6%±12.3% to 80.3%±11.5%, p=0.013 in the segmentectomy group and from 92.7%±13.6% to 82.0%±16.6%, p<0.001 in the lobectomy group), whereas there was no statistically significant reduction in the wedge resection group (from 91.3%±12.7% to 90.2%±15.3%, p=0.453) (Fig. 3A). The reduction in the postoperative FEV1 was the smallest in the wedge resection group (1.13%±8.38%), followed by the segmentectomy (5.72%±8.76%) and lobectomy groups (10.56%±9.63%) (p<0.001) (Fig. 3B). The postoperative reduction in FEV1 in the wedge resection group was significantly less than that in the lobectomy group (p<0.001). whereas the postoperative reduction in FEV1 in the segmentectomy group was not significantly less than that in the lobectomy group (p=0.123).
Fig. 2.
Interval (months) between surgery and the latest pulmonary function test stratified by surgical extent. Values are presented as mean±standard deviation.
Fig. 3.
Comparisons of pulmonary function tests stratified by surgical extent. Values are presented as mean±standard deviation. (A) Preoperative and postoperative FEV1 (%) stratified by surgical extent. (B) Reduction of FEV1 after surgery. FEV1, forced expiratory volume in 1 second.
Discussion
Based on a report by the Lung Cancer Study Group, anatomic lobectomy has been the standard surgical option for resection of stage IA NSCLC [1]. Lobectomy has advantages of better parenchymal margins, decreased local recurrence, better lymphatic clearance or sampling, and improved OS and DFS rates. In contrast, the potential benefits of SLR include lung parenchyma sparing and decreased perioperative morbidity and mortality, particularly in patients with poor pulmonary reserve. The evidence supporting the use of SLR for stage IA lung cancer is well-founded, and numerous contemporary studies have demonstrated equivalent OS and DFS rates for SLR when compared to lobectomy in properly selected patients [3,4]. Additionally, the intentional choice of SLR in patients otherwise eligible for lobectomy and whether preoperative CT can adequately differentiate appropriate candidates for SLR have been debated.
To identify the radiologic criteria that predict pathologic noninvasiveness in clinical stage IA adenocarcinoma located in the periphery of the lung, a prospective radiologic study utilizing TSCT was performed and defined radiological noninvasive lung adenocarcinoma as an adenocarcinoma measuring ≤2 cm with a C/T ratio ≤0.25, with high specificity. Subsequently, the preoperative radiologic criteria for noninvasive adenocarcinoma were evaluated according to both pathologic and prognostic results, and the correlation between radiologic findings and pathologic results was published [8]. Using the preoperative radiological categories presented above, comparative confirmation of the oncologic results according to the surgical method thus became necessary. The aim of the present study was to compare the surgical outcomes and degree of pulmonary function reduction in patients who underwent wedge resection, segmentectomy, and lobectomy, based on the predicted presence of noninvasive adenocarcinoma on preoperative imaging (tumor size ≤2 cm, with a C/T ratio ≤0.25). An important consideration was unexpected N1 or N2 disease, with a reported incidence of 4%–7%, according to Altorki et al. [12]. In this group of patients, only 1 patient (1.6%) with nodal upstaging was reported in the lobectomy group, and there were no cases of nodal upstaging in the wedge resection and segmentectomy groups. The addition of lymph node sampling to SLR is associated with improved survival, but inappropriate sampling may lead to inaccurate staging, which may influence the survival results [13]. Lymph node sampling may itself be therapeutic and can be a predictor of adequate resection to ensure appropriate margins. In our study, most patients underwent lymph node sampling or dissection, and only 6 patients (15%) in the wedge resection group did not undergo either nodal sampling or dissection (Table 2).
In the present study, 1 patient in the wedge resection group and 2 patients in the lobectomy group experienced recurrence after surgery. In fact, these 3 patients may have had metachronous lung cancer. Lung cancers are considered metachronous if the histology is different or if the histology is the same and there is a ≥4-year interval between the cancers with no evidence of systemic metastasis [14]. However, it is difficult to distinguish between recurrent and metachronous lung cancer if the histology is the same in a different lobe or contralateral lung. Moreover, no statistically significant differences in the postoperative results were found among the 3 groups, even though 3 patients were classified as having tumor recurrence rather than metachronous lung cancer.
Reduction of pulmonary function was correlated with the extent of resection, as manifested by a decrease in FEV1. According to this result, wedge resection offers a significant advantage compared to lobectomy in terms of its preservation of pulmonary function. Interestingly, there was no statistically significant difference in the reduction in postoperative FEV1 between the segmentectomy and lobectomy groups, although the reduction in postoperative FEV1 in the segmentectomy group (5.72%±8.76%) was lower than that in the lobectomy group (10.56%± 9.63%) in the present study. These results correspond with the results of earlier retrospective studies reporting that wedge resection best preserves pulmonary function with similar spirometry changes to those observed after mediastinal procedures without lung resection and that segmentectomy may help minimize loss of forced vital capacity but not FEV1 or diffusing capacity of the lungs for carbon monoxide, compared with lobectomy [15]. Although a variety of different results and explanations have been presented [16–20], the functional benefits of segmentectomy remain ill defined.
The present study had limitations stemming from the fact that it was a retrospective review with a small sample size, which may have been affected by selection bias. A solid component may be seen as a GGO with a partial volume effect on CT without thin sections, and the National Comprehensive Cancer Network therefore recommends that TSCT (slice thickness <1.5 mm) be used to measure GGOs. Because of the limitations of retrospective studies, patients who underwent CT prior to the general use of TSCT were included and the images could not be corrected. Moreover, decisions about surgical extent and the follow-up period for pulmonary function testing after surgery were not defined in a protocol and therefore varied. A prospective, randomized study with a large group and long-term follow-up is necessary to obtain more conclusive results.
In conclusion, for radiographically noninvasive adenocarcinoma measuring ≤2 cm with a C/T ratio ≤0.25, wedge resection and segmentectomy could be comparable surgical options to lobectomy, and wedge resection can provide better postoperative pulmonary function preservation than lobectomy. Future studies will be required to investigate the differences in oncologic outcomes and postoperative pulmonary function preservation among wedge resection, segmentectomy, and lobectomy.
Acknowledgments
This study was supported by a Grant of the Samsung Vein Clinic Network (Daejeon, Anyang, Cheongju, Cheonan; Fund no. KTCS04-112).
Footnotes
This study was presented at the 49th Annual Meeting of the Korean Society for Thoracic and Cardiovascular Surgery.
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–22. doi: 10.1016/0003-4975(95)00537-U. [DOI] [PubMed] [Google Scholar]
- 2.Landreneau RJ, Sugarbaker DJ, Mack MJ, et al. Wedge resection versus lobectomy for stage I (T1 N0 M0) non-small-cell lung cancer. J Thorac Cardiovasc Surg. 1997;113:691–8. doi: 10.1016/S0022-5223(97)70226-5. [DOI] [PubMed] [Google Scholar]
- 3.Cao C, Gupta S, Chandrakumar D, Tian DH, Black D, Yan TD. Meta-analysis of intentional sublobar resections versus lobectomy for early stage non-small cell lung cancer. Ann Cardiothorac Surg. 2014;3:134–41. doi: 10.3978/j.issn.2225-319X.2014.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Namkoong M, Moon Y, Park JK. Lobectomy versus sublobar resection in non-lepidic small-sized non-small cell lung cancer. Korean J Thorac Cardiovasc Surg. 2017;50:415–23. doi: 10.5090/kjtcs.2017.50.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki K, Asamura H, Kusumoto M, Kondo H, Tsuchiya R. “Early” peripheral lung cancer: prognostic significance of ground glass opacity on thin-section computed tomographic scan. Ann Thorac Surg. 2002;74:1635–9. doi: 10.1016/S0003-4975(02)03895-X. [DOI] [PubMed] [Google Scholar]
- 6.Asamura H. Minimally invasive approach to early, peripheral adenocarcinoma with ground-glass opacity appearance. Ann Thorac Surg. 2008;85:S701–4. doi: 10.1016/j.athoracsur.2007.10.104. [DOI] [PubMed] [Google Scholar]
- 7.Kodama K, Higashiyama M, Yokouchi H, et al. Prognostic value of ground-glass opacity found in small lung adenocarcinoma on high-resolution CT scanning. Lung Cancer. 2001;33:17–25. doi: 10.1016/S0169-5002(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201) J Thorac Oncol. 2011;6:751–6. doi: 10.1097/JTO.0b013e31821038ab. [DOI] [PubMed] [Google Scholar]
- 9.Takamochi K, Nagai K, Yoshida J, et al. Pathologic N0 status in pulmonary adenocarcinoma is predictable by combining serum carcinoembryonic antigen level and computed tomographic findings. J Thorac Cardiovasc Surg. 2001;122:325–30. doi: 10.1067/mtc.2001.114355. [DOI] [PubMed] [Google Scholar]
- 10.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York (NY): Springer; 2010. [Google Scholar]
- 11.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–60. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 12.Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg. 2014;147:754–62. doi: 10.1016/j.jtcvs.2013.09.065. [DOI] [PubMed] [Google Scholar]
- 13.Cox ML, Yang CJ, Speicher PJ, et al. The role of extent of surgical resection and lymph node assessment for clinical stage I pulmonary lepidic adenocarcinoma: an analysis of 1991 patients. J Thorac Oncol. 2017;12:689–96. doi: 10.1016/j.jtho.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen KR, Meyers BF, Larner JM, Jones DR American College of Chest Physicians. Special treatment issues in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):290S–305S. doi: 10.1378/chest.07-1382. [DOI] [PubMed] [Google Scholar]
- 15.Gu Z, Wang H, Mao T, et al. Pulmonary function changes after different extent of pulmonary resection under video-assisted thoracic surgery. J Thorac Dis. 2018;10:2331–7. doi: 10.21037/jtd.2018.03.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keenan RJ, Landreneau RJ, Maley RH, Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg. 2004;78:228–33. doi: 10.1016/j.athoracsur.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Harada H, Okada M, Sakamoto T, Matsuoka H, Tsubota N. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg. 2005;80:2041–5. doi: 10.1016/j.athoracsur.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Nomori H, Cong Y, Sugimura H. Systemic and regional pulmonary function after segmentectomy. J Thorac Cardiovasc Surg. 2016;152:747–53. doi: 10.1016/j.jtcvs.2016.05.059. [DOI] [PubMed] [Google Scholar]
- 19.Nomori H, Shiraishi A, Cong Y, Sugimura H, Mishima S. Differences in postoperative changes in pulmonary functions following segmentectomy compared with lobectomy. Eur J Cardiothorac Surg. 2018;53:640–7. doi: 10.1093/ejcts/ezx357. [DOI] [PubMed] [Google Scholar]
- 20.Nomori H, Mori T, Ikeda K, Yoshimoto K, Iyama K, Suzuki M. Segmentectomy for selected cT1N0M0 non-small cell lung cancer: a prospective study at a single institute. J Thorac Cardiovasc Surg. 2012;144:87–93. doi: 10.1016/j.jtcvs.2012.03.034. [DOI] [PubMed] [Google Scholar]