Abstract
Non-structural carbohydrates (NSCs), the stored products of photosynthesis, building blocks for growth and fuel for respiration, are central to plant metabolism, but their measurement is challenging. Differences in methods and procedures among laboratories can cause results to vary widely, limiting our ability to integrate and generalize patterns in plant carbon balance among studies. A recent assessment found that NSC concentrations measured for a common set of samples can vary by an order of magnitude, but sources for this variability were unclear. We measured a common set of nine plant material types, and two synthetic samples with known NSC concentrations, using a common protocol for sugar extraction and starch digestion, and three different sugar quantification methods (ion chromatography, enzyme, acid) in six laboratories. We also tested how sample handling, extraction solvent and centralizing parts of the procedure in one laboratory affected results. Non-structural carbohydrate concentrations measured for synthetic samples were within about 11.5% of known values for all three methods. However, differences among quantification methods were the largest source of variation in NSC measurements for natural plant samples because the three methods quantify different NSCs. The enzyme method quantified only glucose, fructose and sucrose, with ion chromatography we additionally quantified galactose, while the acid method quantified a large range of mono- and oligosaccharides. For some natural samples, sugars quantified with the acid method were two to five times higher than with other methods, demonstrating that trees allocate carbon to a range of sugar molecules. Sample handling had little effect on measurements, while ethanol sugar extraction improved accuracy over water extraction. Our results demonstrate that reasonable accuracy of NSC measurements can be achieved when different methods are used, as long as protocols are robust and standardized. Thus, we provide detailed protocols for the extraction, digestion and quantification of NSCs in plant samples, which should improve the comparability of NSC measurements among laboratories.
Keywords: detailed NSC measurement protocols, enzyme method, HPAE-PAD ion chromatography, HPLC, non-structural carbohydrates, phenol-sulfuric acid method, plant sample handling
Introduction
Non-structural carbohydrates (NSCs) play a central role in plant metabolism. As the primary products of photosynthesis, they carry both energy and carbon for plant biosynthesis, and are involved in almost all critical plant physiological processes (Gleixner et al. 1993, Hartmann and Trumbore 2016). New attention has been given to the storage role of NSCs in plant physiological processes due to increased awareness of their potentially critical but underappreciated role in plant responses to environmental changes (Asner and Martin 2016, Aubin et al. 2016). Recent debates regarding the role of NSCs in both carbon- and hydraulic-related mechanisms of drought-induced tree mortality (McDowell et al. 2008, 2011, Sala et al. 2010) have given rise to an increasing body of comparative studies that explore the role of NSC reserves in carbon starvation, turgor maintenance, xylem-embolism prevention and rehydration (Anderegg et al. 2016, Nardini et al. 2016, Savi et al. 2016, Wagner et al. 2016, Yoshimura et al. 2016, Adams et al. 2017). The development of new hypotheses and studies clearly indicates that this is a growing field of inquiry and that our understanding of the role of NSCs in plants during periods of environmental stress is still insufficient (Dietze et al. 2014, Lintunen et al. 2016, Martínez-Vilalta et al. 2016, Nardini et al. 2016).
Exploring the role of NSCs in plant functioning has been challenged by a lack of consistency in NSC measurements among methods and laboratories. In the first broad and ambitious inter-laboratory comparison (29 participating laboratories), measurements of NSC concentrations in a standard set of plant samples varied 8–13 times (Quentin et al. 2015). Therefore, it is questionable whether absolute values of NSC can be meaningfully compared among laboratories or across studies when carried out by different research groups using different analytical approaches. To address this issue, comparisons of NSCs among methods and laboratories could be limited to relative effects between measurements made in the same laboratory (e.g., Adams et al. 2017), though this approach still may have drawbacks and require detailed method descriptions (Germino 2015, Martínez-Vilalta et al. 2016). Although the study by Quentin et al. (2015) was not designed to partition the contributions of different factors that could influence NSC measurements (i.e., plant material type, sample handling, extraction protocols, lab practices and quantification methods), their results clearly indicated that some or all of these factors need further examination and could be the main targets for protocol improvements. In light of these issues, an urgent need has been identified to explore sources of variability in NSC measurements and to develop standard protocols (Germino 2015, Quentin et al. 2015).
While plant NSCs are made up of numerous compounds, the dominant forms are water-soluble, low-molecular-weight carbohydrates such as mono- and oligosaccharides, and starch, which is the dominant polysaccharide storage form of NSCs; fructans are also important NSCs in a range of plant taxa (Mooney 1972, Chapin et al. 1990). Many methods and protocols currently used for measuring NSCs differ widely in their ability to extract and quantify these different compounds. While the precision (i.e., reproducibility) of NSC measurements within a given laboratory can be high, the accuracy of NSC measurements becomes more important when comparing values among different laboratories and species. Both measures play a role in the overall quality of a method. Increasing the number of measurements taken on a sample can produce a more robust average of NSC concentrations; however, since the number of samples that can be analyzed is often a limitation in NSC analyses, reducing the errors related to analytical procedures is the primary step for more accurate and consistent NSC measurements within and across laboratories.
A variety of extraction procedures and quantification methods are currently used in plant NSC measurements. Water-soluble sugars are extracted with different solvents, such as water (Wong 1990, Adams et al. 2013), ethanol (Hendrix 1993) or various mixtures (Dickson 1979, Kanabus et al. 1986). Extraction protocols also differ in their approaches such as sequential or separate extractions, percolation, ultrasonic treatment, and different incubation temperatures and periods (Hansen and Møller 1975, Bhandari et al. 2008, Quentin et al. 2015). Similarly, there is a range of quantification methods for carbohydrates that vary greatly in sample throughput, analytical costs and the specificity of NSC detection. Generally, NSC quantification methods fit into three categories of analyses. First, the ion chromatographic (IC) methods, which have a long runtime, low sample throughput, and high equipment and medium consumable costs. However, the advantage of these methods is the ability to separate and quantify a wide range of mono- and oligosaccharides as well as sugar alcohols in mixtures. High-performance liquid chromatography, an IC method, can be used with varying detectors like light scattering detectors (Dvořačkova et al. 2014) and mass spectrometers (Hammad et al. 2009). Another widely used method is high-performance anion exchange with pulsed amperometric detection (HPAE-PAD), which combines ion chromatography with a sensitive electrochemical detector (Raessler et al. 2008, 2010). Second, selective enzymatic methods, which have a medium to high sample throughput, low equipment but high consumable costs, and are mainly used for the quantification of simple sugars (e.g., sucrose, fructose and glucose) only. Here the sugars are enzymatically converted to gluconate-6-P and linked to the simultaneous reduction of NAD+ to NADH, which can be monitored at 340 nm with UV–Vis spectrometers (e.g., Wong 1990, Hoch et al. 2002, Dickman et al. 2015). Third, the acid methods have a high sample throughput, low equipment and consumable cost, but unselectively oxidize all water-soluble sugars and storage NSCs, glycoproteins and glycolipids when using concentrated acids. In addition, structural glycans such as cellulose and hemicelluloses may also be partially hydrolyzed by acids (Chow and Landhäusser 2004, Richter et al. 2009). The concentration of the oxidized products is then measured colorimetrically by adding color-producing reagents such as phenol, anthrone, orcinol or resorcinol (Masuko et al. 2005, Quentin et al. 2015). Among those, the phenol-sulfuric acid method has been found to be more suitable than other acid methods (Chow and Landhäusser 2004, Masuko et al. 2005). The great variability in the extraction and quantification techniques complicates the interpretation of NSC data (Quentin et al. 2015) and forces great caution when comparing results from different studies using different methodologies (Martínez-Vilalta et al. 2016).
The handling and processing of plant samples prior to NSC analysis is another factor influencing the precision and accuracy of NSC quantification. Several procedures are commonly used for sample preparation (Wanek et al. 2001, Richter et al. 2009, Quentin et al. 2015). Some protocols recommend measures for sample handling that are often difficult to achieve, particularly when sampling in remote locations. It has been widely suggested that samples collected in the field need to be stored in coolers containing ice or dry ice and upon return to the lab or field station are either shock-frozen in liquid nitrogen or microwaved to stop enzymatic activity or are immediately dried using drying ovens or freeze-drying (Popp et al. 1996, Dickman et al. 2015, Quentin et al. 2015). In addition to the substantial logistical constraints of some of these sampling measures, differences in sample processing procedures could have an impact on plant sample quality and provide another source of variation in NSC measurements. Here, we compare different plant sample handling, NSC extraction and measurement protocols for the quantification of NSCs across six well-established laboratories, all of which have a history of using one of the three NSC quantification methods, the IC, enzyme or acid method. Our objectives were to:
Explore the impact of sample handling, lab procedures and quantification methods on the accuracy and precision of NSC measurements.
Develop detailed protocols for sugar extraction, starch hydrolysis and three common NSC quantification methods, to promote greater inter-lab consistency of NSC quantification.
Materials and methods
Study design
In this study, we performed three separate experiments. In Experiment 1, we investigated the effect of storage temperature and timing prior to drying, as well as the drying method (freeze-drying vs oven-drying, preceded or not by microwaving) on NSC concentrations. In Experiment 2, we assessed the robustness of NSC measurements for common samples when both extraction and quantification were performed by different laboratories using two solvents (water vs ethanol) for soluble sugar extraction and three quantification (IC, enzyme, acid) methods. Specifically, we used HPAE-PAD (IC method), invertase, hexokinase and dehydrogenase (enzyme method), and phenol and sulfuric acid (acid method) to quantify sugars. In Experiment 3, we investigated the robustness of methods among labs and within NSC quantification by extracting sugars in all six laboratories, which were subsequently analyzed in one laboratory, and vice versa, where all six laboratories analyzed samples that had been extracted in one laboratory.
Synthetic and plant sample materials
We constructed two different synthetic samples (s1 and s2) with known proportions of soluble sugar and starch by combining compounds that are commonly found in plant tissues (see Table S1 available as Supplementary Data at Tree Physiology Online). To ensure that the cellulose, lignin and starch were free of sugars, they were washed with deionized water at 4 °C, with the supernatants being discarded after centrifugation, and the remaining pellet freeze-dried. Pectin and xylan were not washed since they are water-soluble. Nine different plant materials types (different tissues and organs) collected from aspen (Populus tremuloides Michx.), lodgepole pine (Pinus contorta Loudon) and plum (Prunus domestica ssp. domestica L.) near Edmonton, AB, Canada (see Table S2 available as Supplementary Data at Tree Physiology Online) were used. All plant materials used in the experiments were collected fresh and, apart from the plant materials used in Experiment 1 (see details below), all other materials were oven-dried within 5 min of collection by heating samples to 100 °C for 1 h to deactivate enzymes, followed by drying the samples at 70 °C for 3 days. Synthetic and plant material samples were homogenized to a fine powder using a ball mill (TissueLyser II, by Qiagen Inc., Mississauga, ON, Canada) at 30 Hz for 30 s with a 20-ml milling jar and a 20 mm stainless steel grinding ball. Samples were stored in airtight glass bottles in the dark at room temperature.
Soluble sugar extraction
All laboratories used the same extraction protocol and the details of the protocol are provided in the Supplementary Data section (Protocol S1 available as Supplementary Data at Tree Physiology Online). Apart from Experiment 2, where we compared water and ethanol as solvent for sugar extraction, all other experiments used ethanol as a solvent. For ethanol extraction, samples were boiled in 80% ethanol for 10 min at 90 °C (Protocol S1 available as Supplementary Data at Tree Physiology Online). The supernatant was used for sugar quantification with either the IC (HPAE-PAD), enzyme or acid (phenol-sulfuric acid) method (Protocols S3, S4 and S5, respectively, available as Supplementary Data at Tree Physiology Online). The remaining pellet was washed and dried at 60 °C to remove residual ethanol and was subsequently used for starch digestion and quantification (see below).
Starch digestion
We used the same starch digestion method in all laboratories, converting starch into soluble oligosaccharides and then to glucose. Details of this two-step method are provided in Protocol S2 available as Supplementary Data at Tree Physiology Online. In the first step the starch in the pellet was broken down into water-soluble glucans using α-amylase from Bacillus licheniformis (Sigma cat. no. A4551) at 85 °C for 2 h. After separating the solids by centrifuge at 13,000g for 1 min, the glucans contained in the supernatant were hydrolyzed into glucose using amyloglucosidase from Aspergillus niger (Sigma cat. no. ROAMYGLL) at 55 °C for 2 h. This two-step procedure avoids the unspecific conversion of non-starch polysaccharides into glucose by amyloglucosidase (Denison et al. 1990). The resulting glucose hydrolysate was then quantified using one of three glucose determination methods: IC (HPAE-PAD), or enzymatically using hexokinase or peroxidase-glucose oxidase (PGO) (Protocols S3 to S5 available as Supplementary Data at Tree Physiology Online). Results obtained were in glucose equivalents and multiplied by 0.9 to provide the starch concentration (Sullivan 1935).
Experiment 1. Sample handling
We tested the effects of plant material, field storage temperature, time prior to drying, drying method and microwaving on NSC concentrations. Three types of plant materials were used in this experiment: aspen leaves, 2-year-old aspen twigs (2–3 mm diameter) and white spruce needles. All analyses were performed in one laboratory (Edmonton, Alberta).
Large bulk collections were made for each plant material type. After homogenization (thorough mixing) of each material type, we took 11 samples from each material type (~50 g each) and assigned them to one of three treatment categories: (i) one sample, which was lyophilized (freeze-dried) immediately for 3 days; (ii) five samples, which were microwaved immediately at 600 W (1200 W microwave oven set at a power level of 5/10) for 90 s to deactivate enzymes; and (iii) the remaining five samples were not microwaved. We paired a microwaved with a not-microwaved sample. Each pair was randomly assigned to one of five storage treatments: (i) no storage (i.e., oven-dried right away, see below); (ii) 8 h storage in an incubator at 20 °C; (iii) 8 h storage in a refrigerator at 4 °C; (iv) 24 h storage at 4 °C; and (v) 48 h storage at 4 °C. After the completion of each storage treatment, the samples were immediately heated to 100 °C for 1 h, followed by drying at 70 °C for 3 days. All samples were taken and analyzed in triplicate. After drying, all samples were ball-milled to a fine powder (see above) and kept in sealed glass vials until soluble sugar and starch analysis. We extracted sugars with hot ethanol and quantified them using the phenol-sulfuric acid method (Protocols S1 and S5 available as Supplementary Data at Tree Physiology Online). Starch was digested with enzymes and quantified with PGO (Protocols S2 and S5 available as Supplementary Data at Tree Physiology Online). The sugar extracts were also analyzed using the HPAE-PAD method (Protocol S3 available as Supplementary Data at Tree Physiology Online) to explore whether the handling treatments affected the proportions of sucrose, glucose, fructose and galactose.
Experiment 2. NSC extraction and quantification
The objective of the second experiment was to assess the variability of NSC measurements from common samples when performed by six different laboratories using the same extraction procedure, but one of three different quantification methods (IC, enzyme and acid). To test differences in NSC concentrations between two extraction solvents (ethanol and water) we used our two constructed synthetic samples (s1 and s2). Ethanol extraction and subsequent starch digestion procedures followed the protocols described in Protocols S1 and S2 available as Supplementary Data at Tree Physiology Online. For extracting sugars with water, we replaced the hot ethanol described in Protocol S1 with hot water (90 °C). However, starch is also soluble in water and its solubility depends on the proportion of its components (i.e., amylose and amylopectin) and temperature (Green et al. 1975, Lineback 1986, Ramesh et al. 1999, Ratnayake and Jackson 2006). In a separate study we found that starch gelatinized and partially went into solution when extracted with hot water (90 °C, 56% solubilized) or even cold water (4 °C, 0.4%); however, when ethanol ≥80% was used no starch was gelatinized (i.e., up to 2% starch solubilized in 60% ethanol) (data not shown). Since we wanted to quantify sugars and starch separately, we had to perform two separate extractions for each sample when using water as a solvent (i.e., subsamples s1.1 and s1.2 for sample s1; subsamples s2.1 and s2.2 for sample s2). After extraction and centrifuging of the first set of subsamples (s1.1 and s2.1), we measured sugar concentrations in the supernatant using the three quantification methods (Protocols S3–S5 available as Supplementary Data at Tree Physiology Online). The second set of subsamples (s1.2 and s2.2), however, was not centrifuged and separated after the water extraction and we used the starch digestion protocol to quantify the total NSC concentration (i.e., sum of soluble sugars and the glucose hydrolysate from the starch digestion) (Protocol S2 available as Supplementary Data at Tree Physiology Online). To determine the starch concentration, we subtracted the sugar concentration determined in the first subsample from the total NSC concentration of the second subsample and multiplied that value by 0.9.
To determine differences in the quantification of NSC concentration among different methods and material types including the synthetic samples, we compared the three different NSC quantification methods (IC, enzyme and acid) on a range of different plant materials (see Table S2 available as Supplementary Data at Tree Physiology Online) using the same protocol for extracting sugars (80% ethanol) and digesting starch (Protocols S1 and S2 available as Supplementary Data at Tree Physiology Online).
Experiment 3. Robustness of method
This experiment had two coincident parts, which evaluated the robustness and contribution of lab procedures and methods and their impact on the measurement of NSC concentrations. In the first part, we extracted sugars from common plant samples using the same extraction protocol independently in all six laboratories, but then we analyzed the extracts and pellets in one laboratory using the acid method. In the second part, we extracted samples in one laboratory and then we measured NSC concentrations in all six laboratories independently using the three different quantification methods.
For the first part, we extracted soluble sugars from three samples of two plant material types (aspen and pine root; see Table S2 available as Supplementary Data at Tree Physiology Online) in all six laboratories. After the extraction of each sample, 1.5 ml of each extract was dried in a 2 ml screw-cap vial at 60 °C for 16 h, while the remaining pellet of each sample was dried at 60 °C for 4 h. Both, the dried extracts and pellets (six samples from each laboratory) were sent to Edmonton, where we measured sugar and starch concentrations.
For the second part, we extracted soluble sugars and isolated starch pellets from two plant material types (aspen fine roots and pine fine roots) (see Table S2 available as Supplementary Data at Tree Physiology Online) using hot ethanol. To produce enough material for the six laboratories, we extracted 10 batches of the same sample material, which we combined, homogenized and dried. Once distributed to each lab, the dried extracts were reconstituted with 1.5 ml of deionized water, mixed, and heated to 90 °C in a water bath for 5 min. After being cooled to room temperature, the extracts were analyzed for sugar concentrations using the three different quantification methods specific to each laboratory used in Experiment 2. From the homogenized and dried pellet material, each lab selected three replicates each (10 mg) and measured starch concentration using the starch digestion procedure described in Protocol S2 available as Supplementary Data at Tree Physiology Online.
Data analysis
To determine the effect of sample preparation and storage on NSC concentrations (Experiment 1), we used three separate analyses: (i) the effect of sample handling (freeze dried, microwaved, not microwaved) with no time in storage; (ii) the interaction of microwaving and storage at 4 °C or 20 °C for 8 h; and (iii) the interaction of microwaving and timing of storage (run immediately, 8 h at 4 °C, 24 h at 4 °C, 48 h at 4 °C). Prior to analysis we used a Levene’s test for unequal variance to determine if data were heteroscedastic or homoscedastic. If data were homoscedastic, we used ANOVA to analyze the data with handling treatment, storage temperature and storage timing as fixed factors, depending on the analysis. If significant main effects or interactions were found, we used Fisher’s LSD post hoc to identify specific differences. However, if a Levene’s test indicated that data were heteroscedastic (P < 0.05), we analyzed data with the non-parametric Kruskal–Wallis test, using a Dunn’s test for post hoc analysis. These analyses were conducted separately for sugar, starch and total NSC concentration of each plant material. All statistical analyses in this study were performed using SPSS 23 (IBM, Armonk, NY, USA) with an alpha of 0.05 used to indicate significant effects. A summary of Levene’s test results and the summary statistical tables for all analyses in this study are available in the Supplementary Statistical Data available as Supplementary Data at Tree Physiology Online.
For Experiment 2 (the effect of sugar quantification method on NSC results) we assessed the measurement accuracy for the synthetic samples (s1 and s2), by comparing measured results for each sugar quantification method to the expected (known) concentration of each synthetic sample using a one-sample t-test. We also calculated the difference between the measurement and the expected values for each of the synthetic samples as a percentage of the measurement of sugar, starch and total NSC, respectively. For synthetic sample s1, the expected concentrations were adjusted for each method for sugar and total NSC, as s1 contained sugars other than glucose, fructose and sucrose that were only detectable by some methods (i.e., enzyme, none; IC, galactose only; and acid, galactose, maltose, melibiose and raffinose; Table S1 available as Supplementary Data at Tree Physiology Online). We also analyzed the effect of extraction solvent (ethanol vs water) on the measurement of the synthetic samples. We used one-sample t-tests to assess whether results for each combination of solvent and quantification method were significantly different from the expected (known) concentrations. For comparison of NSC concentrations in samples collected from trees among quantification methods (IC, enzymatic and acid), we first conducted a Levene’s test for unequal variance separately for each tissue and each NSC measurement (sugar, starch, total NSC). Analogous to the statistical methods described above for Experiment 1, we then used a mixed effects ANOVA with a post hoc Fisher’s LSD test to analyze homoscedastic data, and if data were heteroscedastic we used Kruskal–Wallis with a post hoc Dunn’s test. The mixed effects ANOVA had quantification method as a fixed factor, and laboratory as a random factor nested within method. For the Kruskal–Wallis analysis we used quantification method as the main effect, and did not include a nested factor for laboratory as this analysis is limited to a one factor approach. We performed the data analyses separately for each plant sample type for sugar, starch and total NSC concentrations.
For Experiment 3, we analyzed data for differences between independent and centralized extraction and also between independent and centralized quantification of sugars, starch and NSC concentrations. Repeating our statistical approach for Experiments 1 and 2, we first assessed data with a Levene’s test to determine whether to proceed with a subsequent mixed effects ANOVA or a non-parametric Kruskal–Wallis or Mann–Whitney test. To analyze the effect of extraction (independent vs centralized), this factor was used as a fixed factor in ANOVA, and the equivalent main effect in the Mann–Whitney analysis (equivalent to Kruskal–Wallis for the comparison of only two means). Additionally, for the mixed effect ANOVA, we added laboratory as a random factor. To assess quantification effects, we used either one-way ANOVA or Kruskal–Wallis with laboratory of extraction as a fixed effect.
Results
Experiment 1. Sample handling
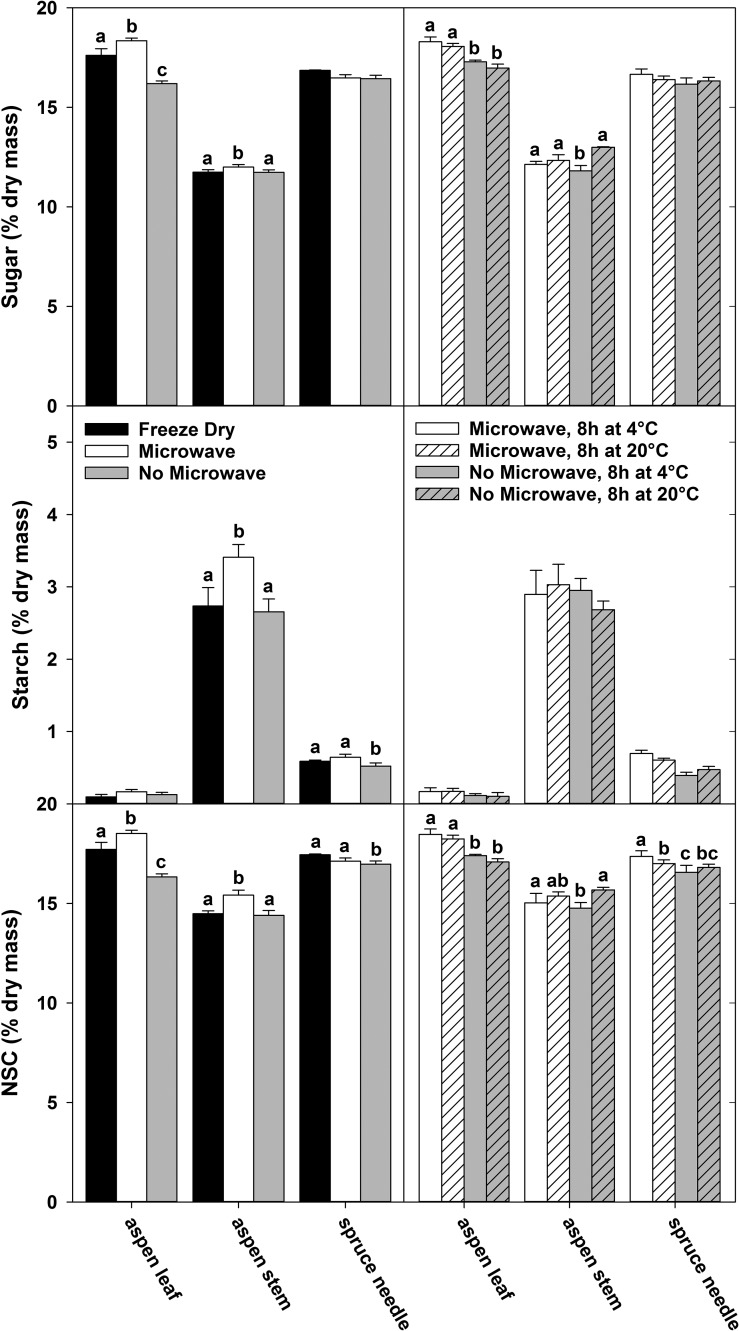
Overall, statistically significant differences in sugar and starch concentrations among the various sample handling treatments within a plant material type were small relative to the measurement mean (Figure 1, left panels). However, the differences among plant material types were much greater than the effects of sample handling. Aspen leaves showed the greatest response to the treatments, but only in the concentration of sugars (Figure 1). Microwaving followed by drying produced the highest sugar concentrations in aspen leaves, followed by the immediately freeze-dried sample. There was a slight but significant effect on the sugar concentrations in aspen stems (P < 0.05), but no effect on sugar in spruce needles (P > 0.05, Figure 1). Also in spruce needles, not microwaving the samples prior to drying resulted in a small reduction of starch concentration compared with microwaved or immediately freeze-dried samples (P < 0.05), while microwaving of the aspen stem material prior to oven drying resulted in slightly higher starch concentrations compared with immediately freeze-drying or not microwaving (P < 0.05, Figure 1). The largest difference between microwaved and not microwaved samples we observed within a plant material type was 2.2% of dry mass for aspen leaf NSC, a difference of less than 12% in concentrations.
Figure 1.
The effect of sample handling and storage on the concentrations of sugar, starch and total NSC for three plant materials. Samples were freeze-dried, microwaved prior to oven drying or placed in a drying oven without microwaving, but not stored prior to drying (left panels). A comparison was performed of samples that were either microwaved or not microwaved, then stored at 4 or 20 °C for 8 h before oven drying (right panels). Significant differences among treatments for each sample material in each panel are shown with letters (P < 0.05). All data shown here were measured with the phenol-sulfuric acid method, and means of three replicates are shown for each bar. Error bars are one standard deviation.
Storing samples at room temperature (20 °C) compared with refrigeration for 8 h had almost no effect on the sugar and starch concentrations in samples. Only a slight increase (0.27% dry mass) was found for soluble sugars in aspen stem, and only in the absence of microwaving (P < 0.05). If samples were microwaved, then storage temperature had no effect at all (P > 0.05, Figure 1, right panels). Analyzing the same samples using IC confirmed that the length of storage and the exposure to higher storage temperature did not affect the ratio of sucrose, glucose, fructose and galactose (data not shown). Storage of up to 48 h at 4 °C had little effect on sugar, starch and NSC concentrations, whether samples were microwaved or not prior to storage. Slight but significant differences (P < 0.05) were observed between storage times, but generally lacked a clear temporal pattern and were dependent on plant material type and whether samples were microwaved or not (see Figure S1 available as Supplementary Data at Tree Physiology Online).
Experiment 2. NSC extraction and quantification
Extraction and quantification of NSC in samples with known sugar and starch concentrations (s1 and s2)
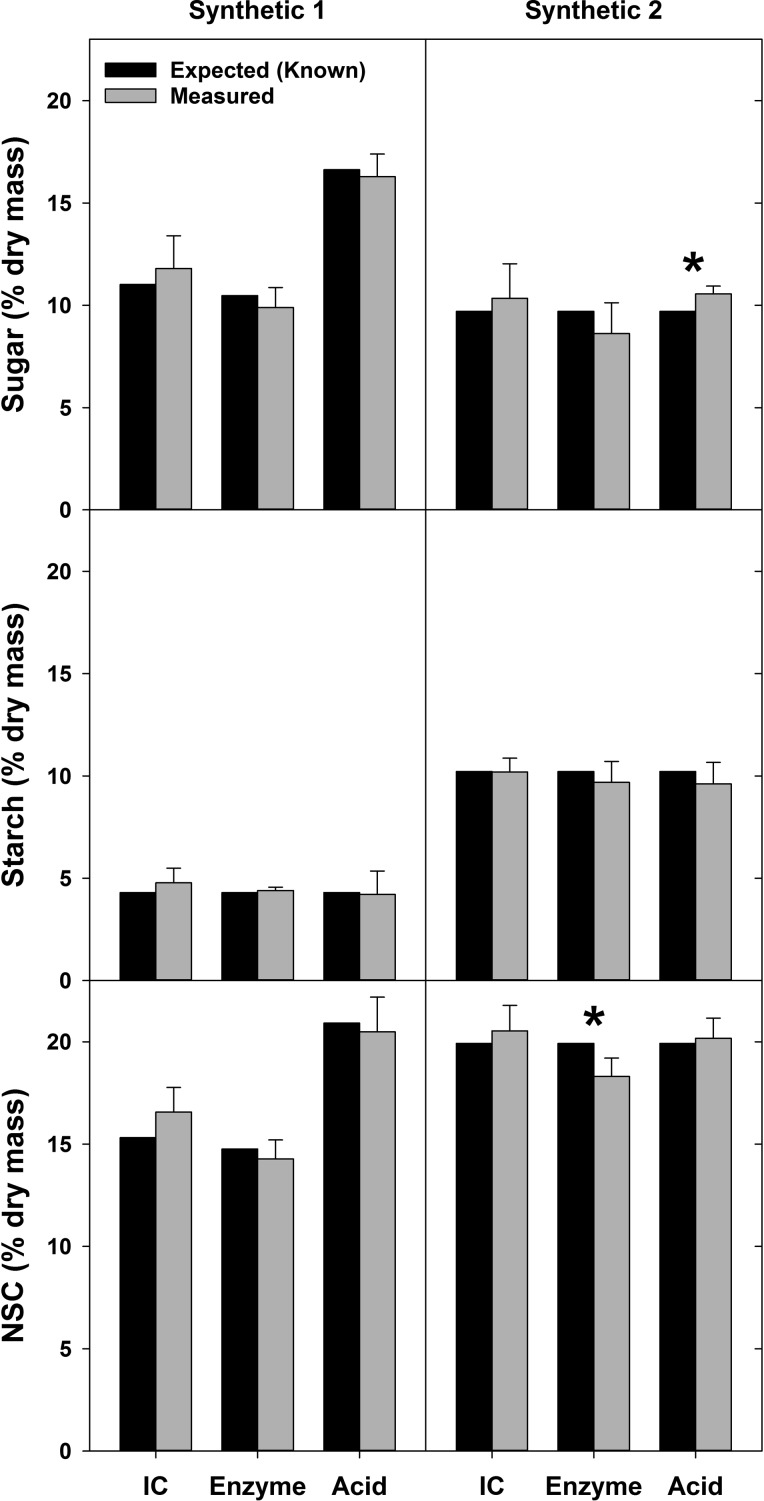
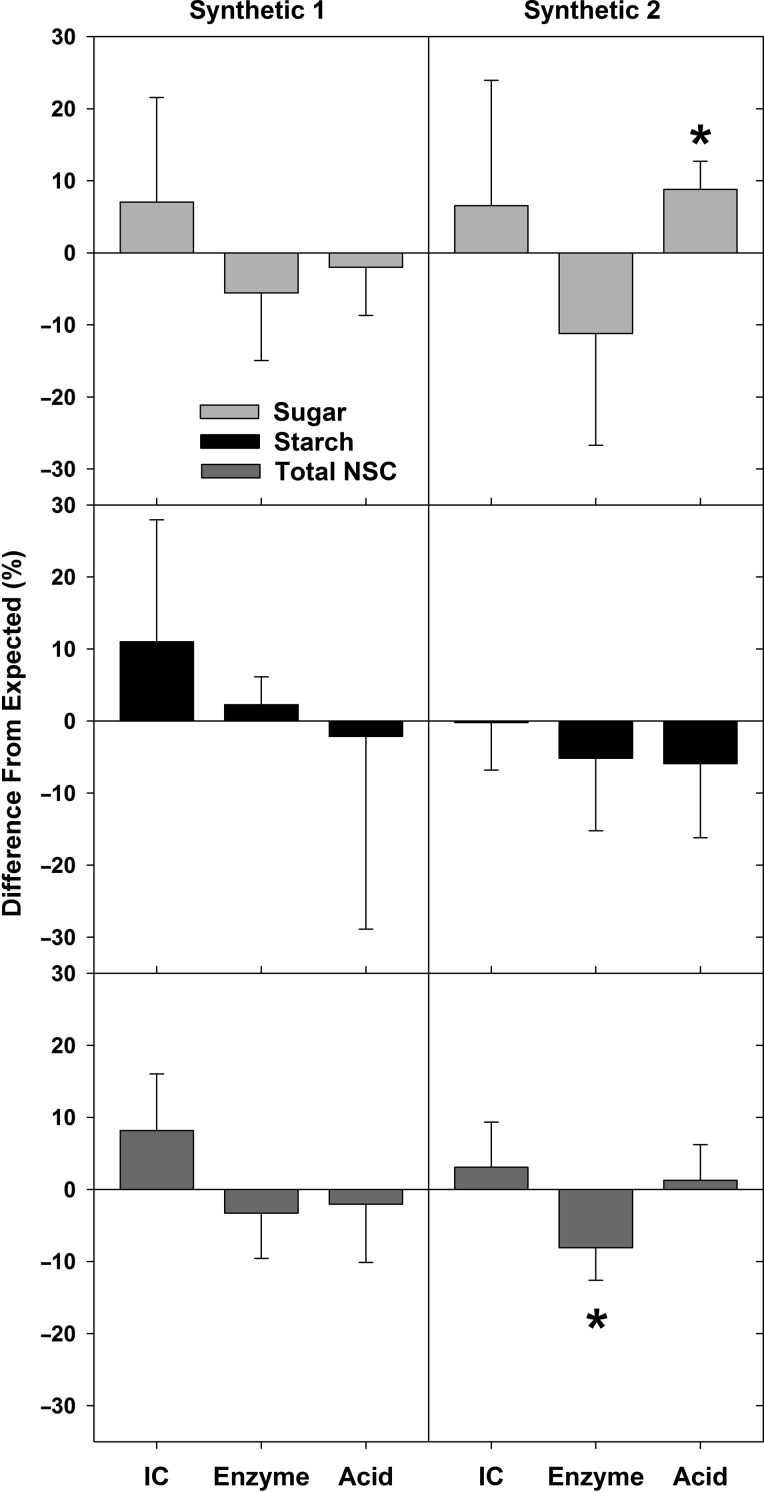
When comparing NSC measurements of the synthetic samples s1 and s2 using the three different quantification methods with ethanol sugar extraction, we found that the measured mean sugar, starch and total NSC concentrations were within 1.7% of the expected (known) values (Figure 2). For 15 of the 18 means derived from the separate extraction and quantification in each lab (i.e., three subsamples, six laboratories, two synthetic samples) the expected values were within one standard deviation of the measured means (accuracy). Analysis with one-sample t-tests found significant differences between measurements and known concentrations in only two cases, for sample s2 sugar with the acid method and sample s2 total NSC using the enzyme method (Figure 2). When the same data were quantified as a percentage of the difference between the measured and the expected concentrations of sugar, starch or NSC in the synthetic samples, the method-specific means for each NSC constituent and sample were less than 11.5% different from the expected value (Figure 3). We found that the measurement means that were furthest from the expected values were the soluble sugar measurement for sample s2 when using the enzyme method (–11.2%) and for starch concentration of sample s1 when measured with IC (+11.0%). The measurements closest to the expected values were for starch concentration of sample s2 measured with IC (–0.2%) and the total NSC concentration in sample s2 quantified with the acid method (1.3%) (Figure 3).
Figure 2.
Concentrations of sugar, starch and total NSC for two synthetic samples (s1 and s2). Expected values (i.e., the known concentrations of sugars and starch) and measurement means (actual) are shown for three NSC quantification methods: IC, enzyme and acid. Significant differences between measured results and expected values (P < 0.05) from a one-sample t-test are indicated with an asterisk. Expected values for sugar and total NSC differ among quantification methods for sample s1 as they show only the portion of sugars specifically measured by the corresponding methods (see Table S1 available as Supplementary Data at Tree Physiology Online). The expected value of sugars did not differ for s2, because this sample was constructed with sugars that all three quantification methods can detect (see Table S1 available as Supplementary Data at Tree Physiology Online). Expected values of starch for s1 and s2 are the same for all three quantification methods. Error bars above means are one standard deviation.
Figure 3.
Mean differences between measured values and expected (known) concentrations of sugar, starch and total NSC for the two constructed synthetic samples (s1 and s2). Differences are quantified as percent of the expected values (Figure 2) for each carbohydrate component of three extraction methods: IC, enzyme and acid. Significant differences from expected values (P < 0.05) are indicated with an asterisk, as determined from a one-sample t-test of measured concentrations and expected concentrations (same analysis as Figure 2). Error bars are one standard deviation.
In our comparison of the effect of sugar extraction solvent on measurement results we found that water extraction was more likely to produce results significantly different from known concentrations than ethanol extraction, as assessed with one-sample t-tests (see Tables S3–S5 available as Supplementary Data at Tree Physiology Online). As noted above, results for ethanol extractions were significantly different from expected values in two cases, whereas for water extractions results were significantly different from known concentrations in eight cases. Overall, we found a trend towards greater deviation from zero in the percent difference from the expected values with water extraction than with ethanol extraction. For the acid method, the difference between ethanol and water as extraction solvent was particularly striking. Water extraction resulted in a slightly lower total NSC than expected (see Table S5 available as Supplementary Data at Tree Physiology Online). However, this difference was driven by sugar concentrations that were two to five times higher (see Table S3 available as Supplementary Data at Tree Physiology Online) and by starch concentrations that were five to nine times lower than expected (see Table S4 available as Supplementary Data at Tree Physiology Online).
Quantification of NSC in plant samples with unknown sugar and starch concentrations
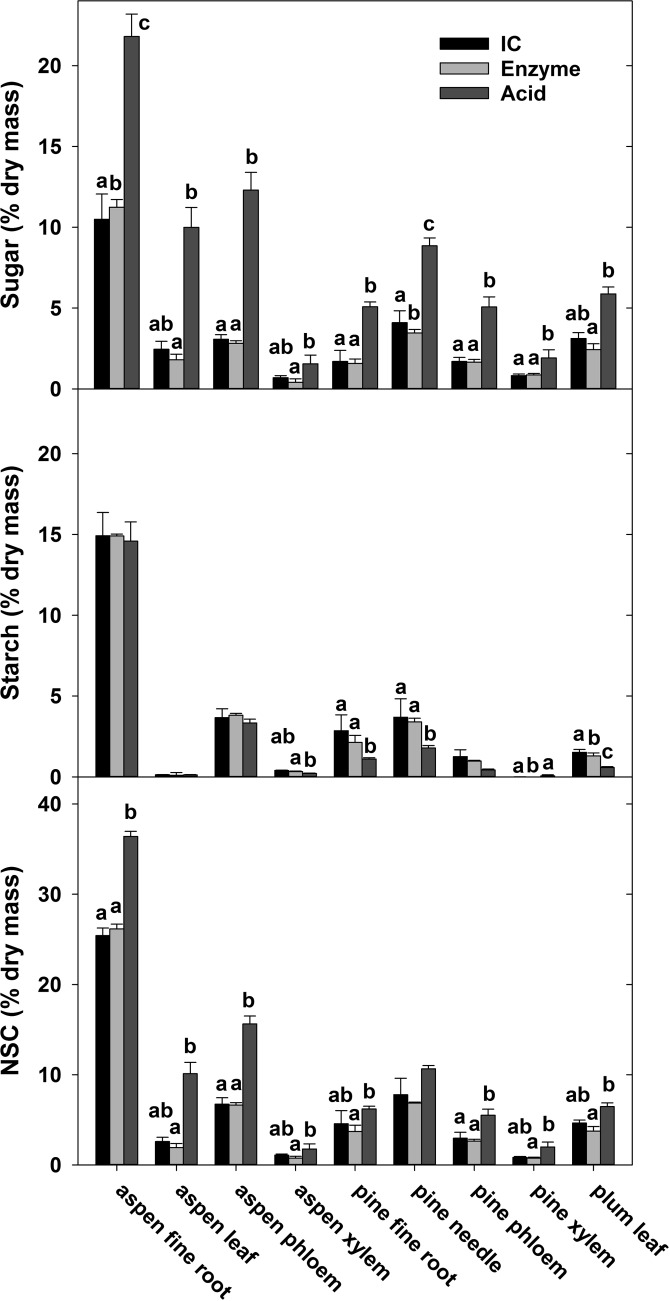
For independent lab NSC measurement of plant materials, we used only the ethanol extraction. For all samples we found a significant effect of sugar quantification method for at least one NSC component we assessed (i.e., sugar, starch and total NSC concentrations) in each sample (P < 0.05, Figure 4). For every plant sample type, sugar concentrations were significantly higher when sugars were quantified with the acid method compared with the enzyme method (P < 0.05), and sugars were often higher with the acid method than the IC method, in six out of nine samples. In some cases, this difference was substantial, particularly for aspen leaf and aspen phloem (Figure 4). We found that sugar concentrations measured with the acid method were between two to five times higher than sugars measured with the enzyme method (Figure 4). These large differences in soluble sugar measurements using the acid method are likely due to the inclusion of oligosaccharides and other compounds with glycosidic bonds in the quantification, which are not targeted by the other two quantification methods (P < 0.05, Figure 4). Differences in starch concentrations among methods were smaller, although lower starch concentrations with the acid method than with the enzyme method were found for four plant material types. Overall, the differences in total NSC concentration we found among the quantification methods for the different plant material types were largely a reflection of the different sugar types analyzed by the different methods.
Figure 4.
Concentrations of sugar, starch and total NSC in nine plant material types from three different tree species measured with three quantification methods (IC, enzyme and acid). Letters indicate significant differences among quantification methods within each material type for each carbohydrate component (P < 0.05). Error bars are one standard deviation.
Experiment 3. Robustness of method
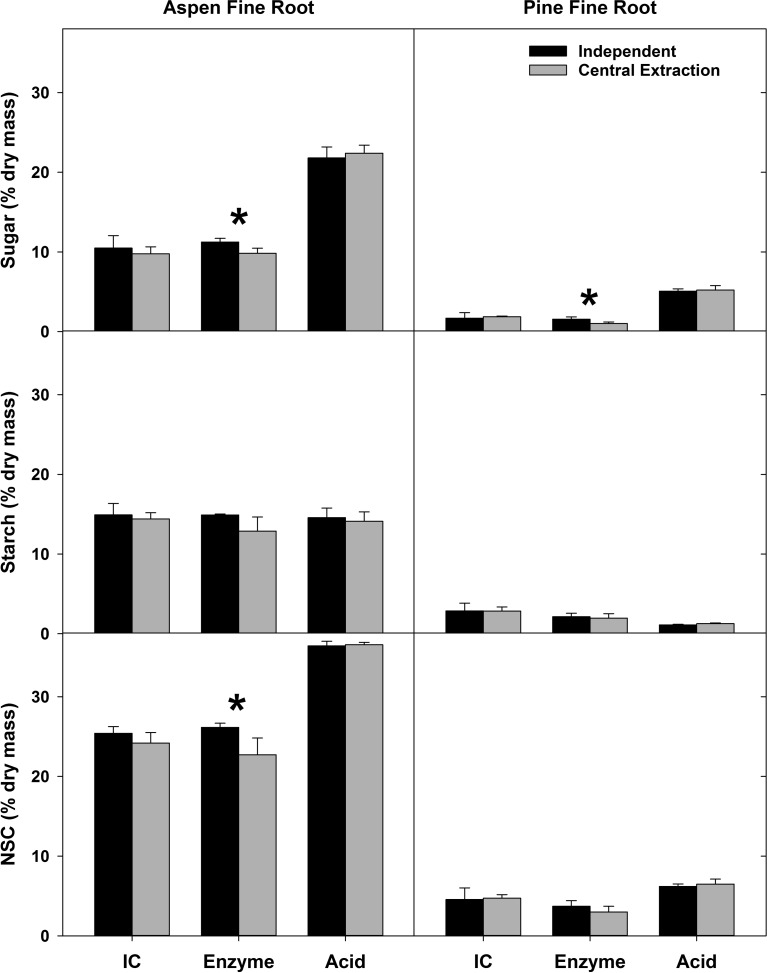
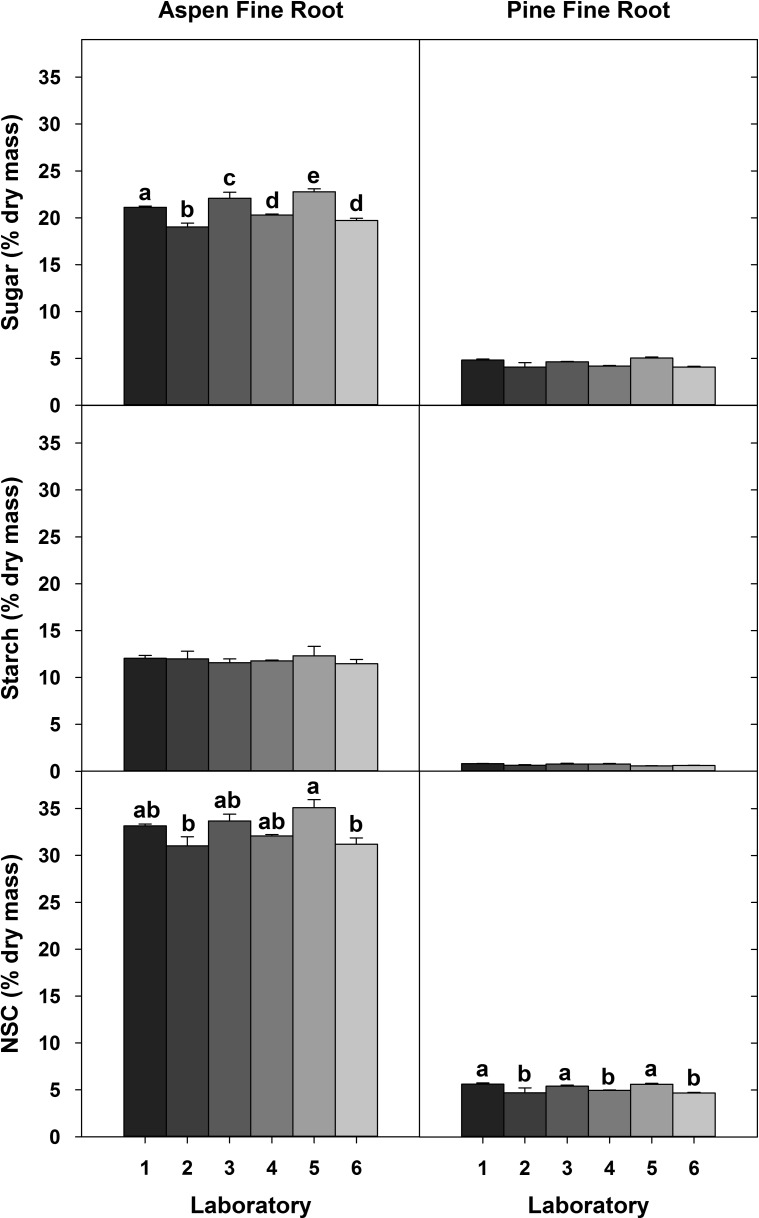
To explore whether the variation observed in sugar measurements can be attributed to variation among laboratories during the extraction or quantification process, i.e., the robustness of the method, we analyzed common samples with either a central extraction or central analysis. Despite no differences in the starch concentrations when extracted in different laboratories, significant differences in sugar measurements persisted. However, these differences were less pronounced for samples after a central extraction (Figure 5) than for the central quantification of NSCs (Figure 6).
Figure 5.
Concentrations of sugar, starch, and total NSC measured in two plant materials. ‘Independent’ refers to samples that were extracted and quantified in six individual labs using one of three quantification methods (IC, enzyme or acid); ‘Central extraction’ refers to samples that were centrally extracted in one lab, but quantified in six individual labs using the same quantification method (IC, enzyme or acid) which that lab used for independent samples. Asterisks indicate significant differences between independent and central extractions within each material type for each carbohydrate component (P < 0.05). Error bars are one standard deviation.
Figure 6.
Concentrations of sugar, starch and total NSC in plant samples that were extracted in six laboratories, but quantified centrally in one lab using the acid method. Letters indicate significant differences among labs for each plant material type for each carbohydrate pool (P < 0.05). Error bars are one standard deviation.
Discussion
Our study demonstrates that precise and accurate NSC measurements are possible using ion chromatography, enzyme or acid methods when applying a rigorous standard methodology for extraction and quantification of sugars and starch. Neither a central extraction of common samples nor a quantification of NSCs in a single laboratory improved upon the measurement variability that we found when we analyzed samples independently. This indicates that the measurement variability can be driven by both differences in quantification methods and deviations in protocols. For synthetic samples with known concentrations, our measurement variability was less than 11.5% of the expected value. This outcome is considerably different from Quentin et al. (2015), who found that NSC measurements of the same samples varied by orders of magnitude among laboratories. Our research suggests that the majority of variation in NSC measurements found by Quentin et al. (2015) is likely attributed to differences in quantification method, their specificity of NSC detection and that no common protocol was used for sugar extraction and starch digestion. We demonstrated that following a standard protocol, and being diligent while executing protocols, reduced the large variability in NSC concentrations that was reported by Quentin et al. (2015) to an acceptable level. We have developed standard methodologies and protocols that are user friendly, provide a range of options for NSC quantification, and are accessible to a wide range of laboratories. For that, we provide very detailed step-by-step descriptions of the different procedures and quantification methods and their associated calculations (see Protocols S1–S6 available as Supplementary Data at Tree Physiology Online) that will lead to NSC measurements of greater precision and accuracy and will enable much more confident comparisons among laboratories.
NSC measurements among methods and laboratories
Our study showed that the accuracy of measurements, when using different quantification methods, is high (i.e., the measurements within each quantification method were close to the expected values in the synthetic samples and the precision or reproducibility of each measurement was acceptable). However, because the three different quantification methods we compared measure different pools of NSCs, the greatest variability in NSC measurements we observed was in soluble sugar concentrations of natural plant samples (Figure 4). For example, we observed a doubling in sugar concentration in aspen fine roots when comparing the different quantification methods. Clearly, the sugar concentrations reflected the size of these different NSC pools, which can vary depending on the sample material and the species (see the differences in NSC measurements in the same plant material among quantification methods, Figure 4). For the nine plant materials we measured, all had greater total soluble sugar values when measured with the acid method than with the enzyme method, indicating that compounds other than glucose, fructose and sucrose were quantified in the extracts. These compounds can be oligosaccharides and other glucans, which were quantified by the acid method, but not by the enzyme method (see Materials and methods). However, as the analysis of our synthetic samples shows, we can rule out a partial hydrolysis of cellulose or hemicellulose with the phenol-sulfuric acid method described here (Chow and Landhäusser 2004). Regardless, our study shows that the choice of quantification method was by far the largest contributor to NSC measurement variability, while the results in Quentin et al. (2015) indicate that the quantification methods accounted only for 12% and 4% of the variability among laboratories for sugar and NSC, respectively. This indicates that the individual laboratories that participated in the Quentin et al. (2015) study had highly variable analytical protocols, even when using the same quantification method. In our study, however, we adopted standardized protocols for soluble sugar extraction and starch digestion, as well as standard procedures for each quantification method (following the recommendations of Quentin et al. 2015). Our results demonstrate that when using common analytical protocols, the differences among quantification methods become the dominant driver of variation in NSC measurements, as should be expected. This has significant implications for comparison of NSC results among studies: even if accurate results can be produced through standardizing protocols, the differences in sugar quantification methods will continue to preclude direct comparison of total sugars and NSC among studies that use these different quantification methods.
The effect of sugar extraction solvent on NSC
Our results clearly show that the solvent that is used to extract the water-soluble sugars can also significantly influence the measurement of NSC concentrations, but this outcome is very much dependent on the quantification method used. Using water to extract water-soluble sugars will also solubilize compounds other than sugars (i.e., pectin, xylan and even some partially degraded starch). Therefore, to accurately quantify sugar and starch concentrations separately, samples need to be analyzed twice (subsampled) when sugars are extracted with water. In the first subsample, the sugar concentrations are quantified in the extract (supernatant) and in the second subsample, the total NSC concentration (the water-soluble components plus the glucose from the digested starch) is quantified (see Materials and methods for more detail). This, however, creates a dilemma for the acid method, where in addition to the soluble sugars other water-soluble components including some starch and xylans are hydrolyzed by the sulfuric acid, leading to a tremendous over-estimate of the sugar concentration in the supernatant. Furthermore, the acid used in the acid method reacts with the digestive enzymes in the sample and interferes with the colorimetric quantification of sugars in the second subsample. Therefore, PGO is being used in acid methods for NSC quantification, which only measures the free glucose in the supernatant and the glucose hydrolysate from the starch. Consequently, the estimation of the starch concentration via deduction of the total sugar estimate of the first subsample from the NSC estimate of the second subsample will be erroneous (see Tables S3–S5 available as Supplementary Data at Tree Physiology Online). Therefore, the acid method is simply not applicable for samples that have been extracted with water.
These additional compounds after water extraction pose much less of a problem for the two other quantification methods, as they target and measure only specific compounds. However, even with IC and enzyme methods water extraction should be viewed with caution, as standard deviations for sugar and starch concentrations tended to be greater and the offset from the expected values larger (see Tables S3–S5 available as Supplementary Data at Tree Physiology Online). We therefore recommend that 80% ethanol should be used over water as an extraction solvent, allowing for better comparisons among quantification methods. This is particularly essential when the role of sugars, such as glucose, fructose and sucrose, and their relation to storage polysaccharides, such as starch in the functioning of NSC in plants, is the target of research. However, recent research has shown that more complex sugars and other compounds might also play a significant role in NSC reserve dynamics (Hoch 2007, Fischer et al. 2015). Overall, our results clearly indicate that it is of fundamental importance that (i) the users understand the different outcomes of the NSC extraction and quantification methods they are using and (ii) the proper method for extraction and detection is selected based on the research questions and objectives of the study.
The effect of sample preparation and storage on NSC
Surprisingly, the handling of samples during collection and prior to drying had much less of an effect on the variation in NSC concentrations than was previously thought (Quentin et al. 2015). We found that the greatest difference (13.5% between microwaved and not-microwaved for aspen leaf sugars) among our handling treatments, was much less than the variability that can be attributed to the effect of quantification method (82% for aspen leaf sugars between enzyme and acid methods). Stopping the enzymatic activity of samples through microwaving early in the handling process had the largest effect on NSC concentrations in samples compared with the timing or the need for refrigeration during storage prior to drying. However, the results were dependent on the sample type (i.e., aspen leaves and stems were more sensitive to the microwaving treatment than spruce needles). Our results indicate that with the materials we tested, not microwaving samples, or even storing samples without refrigeration (20 °C) for 8 h, had relatively little effect on the measured sugar and starch concentrations and their proportions. These results should be representative for most samples collected from woody species and meaningful comparisons among laboratories should be possible, as long as standard protocols and quantification methods are being used. Although not tested in our study, consistency in sample collection rather than sample handling may be of greater concern for reducing NSC measurement variability. Most perennial plants have temporal and spatial fluctuations of NSCs in their tissues, which are driven by the physiology and phenology of the plant (Landhäusser and Lieffers 2003, Martínez-Vilalta et al. 2016, Wiley et al. 2016). For example, samples should be collected at similar phenological stages of plants rather than artificial time intervals, and in the case of leaf material, they should be collected at similar times of the day (Blumenröther et al. 2007, Landhäusser 2011). While consistent and careful sample handling including cold storage during field work and timely sample processing (<8 h between sample collection and processing) is certainly advisable, researchers interested in measuring NSCs should be much more concerned with the sampling design, the analytical protocols and particularly, the choice of quantification method. To assist future comparative studies, a detailed description and information of the plant, its location and phenological stage, and the material and where it was collected on a plant would be very helpful to explore NSC dynamics in plants meaningfully.
Conclusions
Our results strongly suggest that strict procedural protocols for the extraction of soluble sugars, the digestion of starch and the quantification of NSCs are crucial for improving the comparability of NSC measurements in this field of science (Table 1). It is pertinent that researchers use the appropriate extraction and quantification method that fits their objectives and questions, while being aware of the different sugar pools they quantify. Maintaining a measurement variability of 10% appears very achievable; however, continuous quality control during the execution of NSC measurement protocols using standards made up of constructed synthetic and natural plant samples is necessary. Unlike for many other analytical techniques, no certified reference material that includes multiple NSC components (i.e., monosaccharides, disaccharides, oligosaccharides and starch) is available commercially, so researchers must construct their own (see Table S1 available as Supplementary Data at Tree Physiology Online). We recommend that researchers report the accuracy and precision of measurements of their standards in their publications to demonstrate quality control and allow for better comparability among studies. To continue to improve comparability, detailed information on the plant sample types, the collection time (e.g., diurnal cycles, phenology), and the methodology used is of great importance. Comparable and standard methodologies and laboratory procedures will be crucial for assessing the impact and validity of future research in plant carbon reserve dynamics, particularly when considering comparing NSCs across studies, laboratories and/or species.
Table 1.
Key findings and recommendations for NSC measurement and data analysis for each of three experiments in this study
| Exp. | Key finding | Recommendation |
|---|---|---|
| 1 | Sample handling and storage had a small effect on NSC results. | Consistent sample handling and storage in a study. Quantification method matters more than sample handling and storage protocol. |
| Microwaving was as effective, or more so, than rapid freezing. | Stopping enzymatic processes potentially degrading NSC is essential, but varies by tissue type. | |
| Differences in sample handling and storage in the range of conditions studied are unlikely to affect comparison of results among studies. | ||
| 2 | Reasonable accuracy (about ±10%) among laboratories following the same protocol. | Consistency in procedures among laboratories and use of internal plant tissue standards and standards with known quantities of NSC is critical for quality assurance. |
| Ethanol extractions were more accurate than water extractions. | Ethanol extraction (80% ethanol) should be standard practice in NSC measurement. | |
| Sugar quantification method had a strong influence on NSC results. | Be aware that different sugar quantification methods measure different sugar pools. | |
| Samples from different species and tissues can vary greatly in their constituent sugars. | NSC concentrations should not be directly compared across studies that differ in sugar quantification method. | |
| 3 | Centralized extraction or sugar quantification in a single laboratory had little effect on results. | Independent analysis in individual laboratories can produce reasonably accurate and comparable results, as long as standard protocols are carefully executed. |
Supplementary Material
Conflict of interest
None declared.
Funding
This research was supported by a Natural Sciences and Engineering Research Council of Canada grant to S.M.L.
Authors' Contributions
S.M.L., P.S.C. and H.D.A. conceived the study. All authors contributed equally to the design and planning of the final study. P.S.C., L.T.D., M.E.F., I.K., S.S., J.W. and B.W. analyzed samples and tested protocols. P.S.C., L.T.D., M.E.F., I.K., S.S., B.W., G.H., A.R. and S.M.L. developed detailed protocols and P.S.C. edited and compiled the supporting information; all authors contributed equally to the editing of the protocols. H.D.A. summarized and analyzed data. H.D.A. and S.M.L. wrote the initial manuscript draft, and all authors contributed equally to the editing of the manuscript.
References
- Adams HD, Germino MJ, Breshears DD, Barron-Gafford GA, Guardiola-Claramonte M, Zou CB, Huxman TE (2013) Nonstructural leaf carbohydrate dynamics of Pinus edulis during drought-induced tree mortality reveal role for carbon metabolism in mortality mechanism. New Phytol 197:1142–1151. [DOI] [PubMed] [Google Scholar]
- Adams HD, Zeppel MJB, Anderegg WRL et al. (2017) A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat Ecol Evol 1:1285–1291. [DOI] [PubMed] [Google Scholar]
- Anderegg WRL, Klein T, Bartlett M, Sack L, Pellegrini AFA, Choat B, Jansen S (2016) Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc Natl Acad Sci USA 113:5024–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asner GP, Martin RE (2016) Convergent elevation trends in canopy chemical traits of tropical forests. Glob Chang Biol 22:2216–2227. [DOI] [PubMed] [Google Scholar]
- Aubin I, Munson AD, Cardou F et al. (2016) Traits to stay, traits to move: a review of functional traits to assess sensitivity and adaptive capacity of temperate and boreal trees to climate change. Environ Rev 24:164–186. [Google Scholar]
- Bhandari P, Kumar N, Singh B, Kaul VK (2008) Simultaneous determination of sugars and picrosides in Picrorhiza species using ultrasonic extraction and high-performance liquid chromatography with evaporative light scattering detection. J Chromatogr A 1194:257–261. [DOI] [PubMed] [Google Scholar]
- Blumenröther MC, Löw M, Matyssek R, Oßwald W (2007) Flux-based response of sucrose and starch in leaves of adult beech trees (Fagus sylvatica L.) under chronic free-air O3 fumigation. Plant Biol 9:207–214. [DOI] [PubMed] [Google Scholar]
- Chapin FS, Schulze E, Mooney HA (1990) The ecology and economics of storage in plants. Annu Rev Ecol Syst 21:423–447. [Google Scholar]
- Chow PS, Landhäusser SM (2004) A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol 24:1129–1136. [DOI] [PubMed] [Google Scholar]
- Denison RF, Fedders JM, Tong CBS (1990) Amyloglucosidase hydrolysis can overestimate starch concentration of plants. Agron J 82:361–364. [Google Scholar]
- Dickman LT, Mcdowell NG, Sevanto S, Pangle RE, Pockman WT (2015) Carbohydrate dynamics and mortality in a piñon-juniper woodland under three future precipitation scenarios. Plant Cell Environ 38:729–739. [DOI] [PubMed] [Google Scholar]
- Dickson RE. (1979) Analytical procedures for the sequential extraction of 14C-labeled constituents from leaves, bark and wood of cottonwood plants. Physiol Plant 45:480–488. [Google Scholar]
- Dietze MC, Sala A, Carbone MS, Czimczik CI, Mantooth JA, Richardson AD, Vargas R (2014) Nonstructural carbon in woody plants. Annu Rev Plant Biol 65:667–687. [DOI] [PubMed] [Google Scholar]
- Dvořačkova E, Šnõblová M, Hrdlička P (2014) Carbohydrate analysis: from sample preparation to HPLC on different stationary phases coupled with evaporative light-scattering detection. J Sep Sci 37:323–337. [DOI] [PubMed] [Google Scholar]
- Fischer S, Hanf S, Frosch T, Gleixner G, Popp J, Trumbore S, Hartmann H (2015) Pinus sylvestris switches respiration substrates under shading but not during drought. New Phytol 207:542–550. [DOI] [PubMed] [Google Scholar]
- Germino MJ. (2015) A carbohydrate quandary. Tree Physiol 35:1141–1145. [DOI] [PubMed] [Google Scholar]
- Gleixner G, Danier HJ, Werner RA, Schmidt HL (1993) Correlations between the 13C content of primary and secondary plant products in different cell compartments and that in decomposing Basidiomycetes. Plant Physiol 102:1287–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MM, Blankenhorn G, Hart H (1975) Which starch fraction is water-soluble, amylose or amylopectin? J Chem Educ 52:729–730. [Google Scholar]
- Hammad LA, Saleh MM, Novotny MV, Mechref Y (2009) Multiple-reaction monitoring liquid chromatography mass spectrometry for monosaccharide compositional analysis of glycoproteins. J Am Soc Mass Spectrom 20:1224–1234. [DOI] [PubMed] [Google Scholar]
- Hansen J, Møller I (1975) Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal Biochem 68:87–94. [DOI] [PubMed] [Google Scholar]
- Hartmann H, Trumbore S (2016) Understanding the roles of nonstructural carbohydrates in forest trees – from what we can measure to what we want to know. New Phytol 211:386–403. [DOI] [PubMed] [Google Scholar]
- Hendrix DL. (1993) Rapid extraction and analysis of nonstructural carbohydrates in plant tissues. Crop Sci 33:1306–1311. [Google Scholar]
- Hoch G. (2007) Cell wall hemicelluloses as mobile carbon stores in non-reproductive plant tissues. Funct Ecol 21:823–834. [Google Scholar]
- Hoch G, Popp M, Körner C (2002) Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. Oikos 98:361–374. [Google Scholar]
- Kanabus J, Bressan RA, Carpita NC (1986) Carbon assimilation in carrot cells in liquid culture. Plant Physiol 82:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landhäusser SM. (2011) Aspen shoots are carbon autonomous during bud break. Trees 25:531–536. [Google Scholar]
- Landhäusser SM, Lieffers VJ (2003) Seasonal changes in carbohydrate reserves in mature northern Populus tremuloides clones. Trees 17:471–476. [Google Scholar]
- Lineback DR. (1986) Current concepts of starch structure and its impact on properties. J Jpn Soc Starch Sci 33:80–88. [Google Scholar]
- Lintunen A, Paljakka T, Jyske T et al. (2016) Osmolality and non-structural carbohydrate composition in the secondary phloem of trees across a latitudinal gradient in Europe. Front Plant Sci 7:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Vilalta J, Sala A, Asensio D, Galiano L, Hoch G, Palacio S, Piper FI, Lloret F (2016) Dynamics of non-structural carbohydrates in terrestrial plants: a global synthesis. Ecol Monogr 86:495–516. [Google Scholar]
- Masuko T, Minami A, Iwasaki N, Majima T, Nishimura S-I, Lee YC (2005) Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal Biochem 339:69–72. [DOI] [PubMed] [Google Scholar]
- McDowell N, Pockman WT, Allen CD et al. (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739. [DOI] [PubMed] [Google Scholar]
- McDowell NG, Beerling DJ, Breshears DD, Fisher RA, Raffa KF, Stitt M (2011) The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol Evol 26:523–532. [DOI] [PubMed] [Google Scholar]
- Mooney HA. (1972) The carbon balance of plants. Annu Rev 3:315–346. [Google Scholar]
- Nardini A, Casolo V, Dal Borgo A, Savi T, Stenni B, Bertoncin P, Zini L, McDowell NG (2016) Rooting depth, water relations and non-structural carbohydrate dynamics in three woody angiosperms differentially affected by an extreme summer drought. Plant Cell Environ 39:618–627. [DOI] [PubMed] [Google Scholar]
- Popp M, Lied W, Meyer AJ, Richter A, Schiller P, Schwitte H (1996) Sample preservation for determination of organic compounds: microwave versus freeze-drying. J Exp Bot 47:1469–1473. [Google Scholar]
- Quentin AG, Pinkard EA, Ryan MG et al. (2015) Non-structural carbohydrates in woody plants compared among laboratories. Tree Physiol 35:1146–1165. [DOI] [PubMed] [Google Scholar]
- Raessler M, Wissuwa B, Breul A, Unger W, Grimm T (2008) Determination of water-extractable nonstructural carbohydrates, including inulin, in grass samples with high-performance anion exchange chromatography and pulsed amperometric detection. J Agric Food Chem 56:7649–7654. [DOI] [PubMed] [Google Scholar]
- Raessler M, Wissuwa B, Breul A, Unger W, Grimm T (2010) Chromatographic analysis of major non-structural carbohydrates in several wood species – an analytical approach for higher accuracy of data. Anal Methods 2:532. [Google Scholar]
- Ramesh M, Ali SZ, Bhattacharya KR (1999) Starch components in hot-water soluble and insoluble fractions of rice flour. Starch/Stärke 51:308–310. [Google Scholar]
- Ratnayake WS, Jackson DS (2006) Gelatinization and solubility of corn starch during heating in excess water: new insights. J Agric Food Chem 54:3712–3716. [DOI] [PubMed] [Google Scholar]
- Richter A, Wanek W, Werner RA et al. (2009) Preparation of starch and soluble sugars of plant material for the analysis of carbon isotope composition: a comparison of methods. Rapid Commun Mass Spectrom 23:2476–2488. [DOI] [PubMed] [Google Scholar]
- Sala A, Piper F, Hoch G (2010) Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol 186:274–281. [DOI] [PubMed] [Google Scholar]
- Savi T, Casolo V, Luglio J, Bertuzzi S, Trifilo’ P, Lo Gullo MA, Nardini A (2016) Species-specific reversal of stem xylem embolism after a prolonged drought correlates to endpoint concentration of soluble sugars. Plant Physiol Biochem 106:198–207. [DOI] [PubMed] [Google Scholar]
- Sullivan JT. (1935) The estimation of starch. Ind Eng Chem Anal Ed 7:311–314. [Google Scholar]
- Wagner FH, Hérault B, Bonal D et al. (2016) Climate seasonality limits leaf carbon assimilation and wood productivity in tropical forests. Biogeosciences 13:2537–2562. [Google Scholar]
- Wanek W, Heintel S, Richter A (2001) Preparation of starch and other carbon fractions from higher plant leaves for stable carbon isotope analysis. Rapid Commun Mass Spectrom 15:1136–1140. [DOI] [PubMed] [Google Scholar]
- Wiley E, Rogers BJ, Hodgkinson R, Landhäusser SM (2016) Nonstructural carbohydrate dynamics of lodgepole pine dying from mountain pine beetle attack. New Phytol 209:550–562. [DOI] [PubMed] [Google Scholar]
- Wong S-C. (1990) Elevated atmospheric partial pressure of CO2 and plant growth. II. Non-structural carbohydrate content in cotton plants and its effect on growth parameters. Photosynth Res 23:171–180. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Saiki ST, Yazaki K, Ogasa MY, Shirai M, Nakano T, Yoshimura J, Ishida A (2016) The dynamics of carbon stored in xylem sapwood to drought-induced hydraulic stress in mature trees. Sci Rep 6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.