Abstract
Objectives. To estimate trends in incidence, outcomes, and costs among hospital deliveries related to amphetamines and opioids.
Methods. We analyzed 2004-to-2015 data from the National Inpatient Sample, a nationally representative sample of hospital discharges in the United States compiled by the Healthcare Cost and Utilization Project, by using a repeated cross-sectional design. We estimated the incidence of hospital deliveries related to maternal amphetamine or opioid use with weighted logistic regression. We measured clinical outcomes and costs with weighted multivariable logistic regression and generalized linear models.
Results. Amphetamine- and opioid-related deliveries increased disproportionately across rural compared with urban counties in 3 of 4 census regions between 2008 to 2009 and 2014 to 2015. By 2014 to 2015, amphetamine use was identified among approximately 1% of deliveries in the rural West, which was higher than the opioid-use incidence in most regions. Compared with opioid-related and other hospital deliveries, amphetamine-related deliveries were associated with higher incidence of preeclampsia, preterm delivery, and severe maternal morbidity and mortality.
Conclusions. Increasing incidence of amphetamine and opioid use among delivering women and associated adverse gestational outcomes indicate that amphetamine and opioid use affecting birth represent worsening public health crises.
Opioid use in pregnancy has increased substantially over the past decade in the United States, particularly in rural counties.1–3 Opioid use during pregnancy is associated with adverse neonatal outcomes, including neonatal abstinence syndrome, which accounted for $3 billion in hospital costs between 2004 and 2014.3 Although the literature on neonatal morbidity associated with opioid use in pregnancy is substantial,4,5 less is known about other types of substance use during pregnancy. Among pregnant women, amphetamine use is the third-most-common reason for seeking substance use treatment after opioid and cannabis use,6 yet national and regional estimates of amphetamine use in pregnancy have not been characterized since 2004.7 Similar to opioid use, there is evidence that amphetamine-related stimulants are associated with adverse gestational outcomes, including a small increased risk for congenital cardiac malformations,8 preeclampsia,9,10 and preterm birth.9–11 These effects are hypothesized to result from impaired placental function because of the vasoconstrictive effects of stimulants.9
Increasing rates of substance use disorders may, in part, contribute to rising rates of severe maternal morbidity and mortality in the United States.12,13 Severe maternal morbidity and mortality is defined as a life-threatening diagnosis or the need to undergo a lifesaving procedure during a delivery hospitalization.14 Severe maternal morbidity is a proximate measure of maternal mortality and encompasses a broad range of serious maternal health complications that, without prevention and treatment, could lead to maternal death. To date, however, there are limited data on severe maternal morbidities that may be occurring among amphetamine- and opioid-related pregnancies.
We sought to provide updated national incidence data on maternal amphetamine and opioid use and determine whether incidence varied across US census regions or by rural compared with urban residence. Furthermore, we aimed to characterize clinical outcomes and delivery-related health care utilization and costs among deliveries related to amphetamine or opioid use. We hypothesized that amphetamine use in pregnancy has increased, paralleling trends identified in the general adult population.15 In addition, we hypothesized that deliveries related to amphetamine or opioid use are associated with increased incidence of several adverse gestational outcomes, including severe maternal morbidity and mortality and higher hospital costs, compared with deliveries not related to amphetamine or opioid use.
METHODS
We conducted a retrospective, repeat cross-sectional analysis using 2004–2015 data from the National Inpatient Sample, a nationally representative sample of hospital discharges in the United States compiled by the Healthcare Cost and Utilization Project (HCUP) of the Agency for Healthcare Research and Quality.16 All analyses comply with the methodological standards outlined for working with data from the National Inpatient Sample.17
Sample
The sample consisted of all hospital deliveries occurring between 2004 and 2015, which were identified by using a published algorithm that incorporates International Classification of Disease, 9th Edition, Clinical Modification (ICD-9-CM) diagnosis codes for hospital deliveries, diagnosis-related group delivery codes, and ICD-9-CM codes for select delivery-related procedures.18 We identified maternal amphetamine use disorders by using ICD-9-CM codes for amphetamine dependence or abuse and poisoning (304.40–304.43, 305.70–305.73, 969.72). Hospitalizations were defined as amphetamine-related if a diagnosis code for amphetamine dependence or abuse or poisoning by amphetamines was listed in any diagnosis field. Although diagnostic codes do not discriminate between methamphetamine and nonmedical use of prescription amphetamines, the most recent data available reveal that the majority (94%) of individuals with amphetamine use disorders seek care for methamphetamine-related use.19 Additional substance use categories, including opioid use, were defined by using ICD-9-CM coding schemes developed by HCUP.20
We described a number of characteristics in our sample, including age, payer, race/ethnicity, income quartile of patient’s zip code, hospital census region, and other types of co-occurring substance use. We defined location of residence as rural or urban by using the National Center for Health Statistics Classification and Urban Influence Codes.21 We grouped payment sources into public insurance (Medicaid and Medicare), private insurance, and uninsured or self-pay. Given that less than 0.6% of the delivery hospitalizations were funded by Medicare, public sources are referred to as Medicaid throughout the study. Mean number of chronic conditions were described as computed by HCUP by number of Clinical Classification Software categories identified in each record.22 We identified attention-deficit hyperactivity disorder, the most common diagnosis for which amphetamines are prescribed, by using ICD-9-CM codes 314.00 and 314.01.
Outcome Measures
Incidence of amphetamine-related and opioid-related hospitalizations.
We estimated trends in the number of delivery hospitalizations with codes for maternal amphetamine and opioid use for each 2-year period.
Clinical outcomes, health care utilization, and health care costs.
We compared clinical outcomes across 3 populations: all deliveries with ICD-9-CM codes for maternal amphetamine use, all deliveries with ICD-9-CM codes for maternal opioid use, and all other hospital deliveries. Use of both substances was identified in approximately 0.03% (weighted n = 2255/total n = 7 611 192) of deliveries and were not analyzed separately because estimates were imprecise because of low frequency. Clinical outcomes of interest included preeclampsia, placental abruption, preterm delivery, cesarean delivery, and severe maternal morbidity and mortality. We abstracted variables for clinical conditions of interest with relevant ICD-9-CM codes: preeclampsia (642.4x, 642.5x, 642.6x, 642.7x), placental abruption (641.2x), preterm delivery (644.21), and cesarean delivery (740.x, 741.x, 742.x, 744.x, 749.9). Severe maternal morbidity and mortality were identified by using standardized diagnosis and procedure codes outlined by Centers for Disease Control and Prevention.23 The algorithm includes 25 indicators of end-organ dysfunction such as renal failure, shock, embolism, eclampsia, and mechanical ventilation.
Next, we compared rates of hospital transfer, length of stay, and per-capita hospital costs across the 3 groups. We used delivery-associated hospital charges to calculate cost with HCUP’s cost-to-charge ratio files.24 We inflation-adjusted cost estimates to 2015 dollars.25
Data Analysis
Characteristics and incidence of amphetamine-related and opioid-related hospitalizations.
We first obtained weighted frequencies to describe characteristics across each population of interest in the 2 most recent years of data. We then estimated the incidence of hospital deliveries complicated by amphetamine or opioid use with weighted logistic regression and predictive margins. Subgroup analyses were stratified first by rural compared with urban residence and then by census region with models that interacted rural versus urban residence with time.
Clinical outcomes, health care utilization, and health care costs.
We measured clinical outcomes of interest, including preeclampsia, placental abruption, preterm delivery at less than 37 weeks, cesarean delivery, and severe maternal morbidity and mortality by using weighted multivariable logistic regression with predictive margins across deliveries related to amphetamine use, opioid use, and other hospital deliveries. We then compared the incidence of the 10 most frequent morbidities in the overall sample across the 3 groups by using similar models. We pooled data across the 2 most recent years of available data for these analyses.
We then used generalized linear models with a γ distribution and log-link function and predictive margins to estimate mean length of stay and hospital costs given that these data were not normally distributed (Shapiro–Wilk test; P < .001 for each).26 We performed subgroup analyses by mode of delivery.
We adjusted all models for age, primary insurance payer, median household income for the patient’s zip code, rural compared with urban residence, and census region.
We conducted all analyses with Stata version 14.2 (StataCorp, College Station, TX). We pooled data into 2-year periods to increase the precision of our estimates. All results are weighted, unless otherwise noted, to allow for nationally representative inferences and to account for changes in the National Inpatient Sample sampling strategy in 2012. In 2015, ICD-9-CM diagnosis codes were only available for the first 3 quarters. We adjusted the survey weights in 2015 to generate annualized estimates from the first 3 quarters of data. The number of observations with missing values for the covariates included in our adjusted models was approximately 2% of all delivery hospitalizations, which was considered sufficient for analysis. We considered 2-sided P < .05 to be statistically significant.
RESULTS
The study sample consisted of an estimated 47 164 263 (unweighted n = 9 638 262) deliveries occurring nationally between 2004 and 2015. There were an estimated 82 254 (0.17%; 95% confidence interval [CI] = 0.16, 0.19) delivery hospitalizations that included 1 or more diagnoses identifying amphetamine use and 170 164 (0.36%; 95% CI = 0.34, 0.38) delivery hospitalizations that included 1 or more diagnoses identifying opioid use. Higher proportions of patients in both substance use groups had Medicaid as their primary payer, resided in rural counties, and lived in zip codes in the poorest national income quartile compared with other hospital deliveries (Table 1). Both substance use groups also had higher proportions of non-Hispanic White patients compared with other hospital deliveries, although non-Hispanic White mothers comprised a larger proportion of deliveries complicated by opioid (79.3%; 95% CI = 77.3, 81.1) compared with amphetamine use (56.4%; 95% CI = 54.1, 58.7). Comorbid tobacco use was notably common across both groups and identified among approximately half of deliveries complicated by amphetamine or opioid use (46.0%; 95% CI = 43.9, 48.0, and 55.2%; 95% CI = 53.8, 56.5, respectively) compared with 5.6% (95% CI = 5.4, 5.8) among other hospital deliveries. Similarly, cannabis, cocaine, alcohol, and sedative use were identified at significantly higher incidence among deliveries complicated by amphetamine use or opioid use compared with other hospital deliveries.
TABLE 1—
Characteristics of Delivering Women With Amphetamine and Opioid Use: National Inpatient Sample, United States, 2014–2015
| Characteristic | Amphetamine Use (n = 18 050) | Opioid Use (n = 50 011) | Other Hospital Deliveries (n = 7 545 380) |
| Age, y, weighted mean (95% CI) | 27.9 (27.7, 28.1) | 27.8 (27.7, 27.9) | 28.4 (28.3, 28.5) |
| Insurance payer, weighted % (95% CI) | |||
| Medicaid | 80.4 (78.7, 82.0) | 81.4 (80.2, 82.4) | 43.3 (42.5, 44.1) |
| Private | 10.8 (9.5, 12.3) | 14.7 (13.8, 15.6) | 51.3 (50.5, 52.2) |
| Uninsured | 8.8 (7.8, 9.9) | 4.0 (3.4, 4.7) | 5.4 (5.2, 5.7) |
| Income: bottom quartile,a weighted % (95% CI) | 39.3 (36.8, 41.8) | 37.1 (35.4, 38.9) | 28.3 (27.5, 29.2) |
| Residence: rural, weighted % (95% CI) | 21.5 (19.7, 23.5) | 21.7 (20.1, 23.4) | 13.3 (12.7, 13.9) |
| Hospital region, weighted % (95% CI) | |||
| Northeast | 1.2 (0.91, 1.5) | 24.1 (21.7, 26.7) | 16.0 (14.9, 17.2) |
| Midwest | 15.0 (13.3, 16.9) | 22.0 (19.7, 24.5) | 21.3 (20.1, 22.5) |
| South | 22.0 (20.1, 24.0) | 37.8 (35.0, 40.7) | 38.7 (37.1, 40.3) |
| West | 61.9 (59.2, 64.5) | 16.0 (14.2, 18.0) | 24.1 (22.8, 25.4) |
| Race/ethnicity, weighted % (95% CI) | |||
| Non-Hispanic White | 56.4 (54.1, 58.7) | 79.3 (77.3, 81.1) | 49.7 (48.8, 50.7) |
| Non-Hispanic Black | 6.0 (5.1, 6.9) | 5.0 (4.5, 5.7) | 13.7 (13.1, 14.2) |
| Hispanic | 20.1 (18.2, 22.1) | 6.2 (5.3, 7.3) | 19.5 (18.6, 20.3) |
| Asian or Pacific Islander | 2.3 (1.7, 3.2) | 0.51 (0.31, 0.86) | 5.5 (5.1, 5.9) |
| Native American | 4.3 (3.4, 5.4) | 1.4 (1.1, 1.8) | 0.66 (0.60, 0.73) |
| Other | 2.3 (1.7, 3.0) | 1.6 (1.3, 2.0) | 4.4 (4.1, 4.6) |
| Missing | 8.7 (7.2, 10.5) | 6.0 (4.6, 7.9) | 6.7 (6.0, 7.4) |
| No. of chronic conditions, weighted mean (95% CI) | 2.1 (2.1, 2.2) | 2.2 (2.1, 2.2) | 0.64 (0.62, 0.65) |
| Attention-deficit hyperactivity disorder, weighted % (95% CI) | 1.3 (0.95, 1.8) | 1.9 (1.6, 2.2) | 0.29 (0.28, 0.31) |
| Other substance use, weighted % (95% CI) | |||
| Tobacco | 46.0 (43.9, 48.0) | 55.2 (53.8, 56.5) | 5.1 (5.0, 5.3) |
| Cannabis | 26.4 (24.8, 28.1) | 10.4 (9.6, 11.1) | 0.87 (0.83, 0.91) |
| Opioids | 12.6 (11.4, 13.9) | NA | NA |
| Amphetamines | NA | 4.5 (4.1, 5.0) | NA |
| Cocaine | 4.2 (3.5, 5.1) | 5.2 (4.7, 5.8) | 0.11 (0.10, 0.12) |
| Alcohol | 5.1 (4.3, 5.9) | 1.9 (1.6, 2.2) | 0.10 (0.095, 0.11) |
| Sedatives | 2.1 (1.6, 2.7) | 2.4 (2.1, 2.7) | 0.017 (0.015, 0.019) |
Note. CI = confidence interval; NA = not applicable. The sample size was n = 7 611 192.
Represents patients living in a zip code with a median household income in the bottom national income quartile.
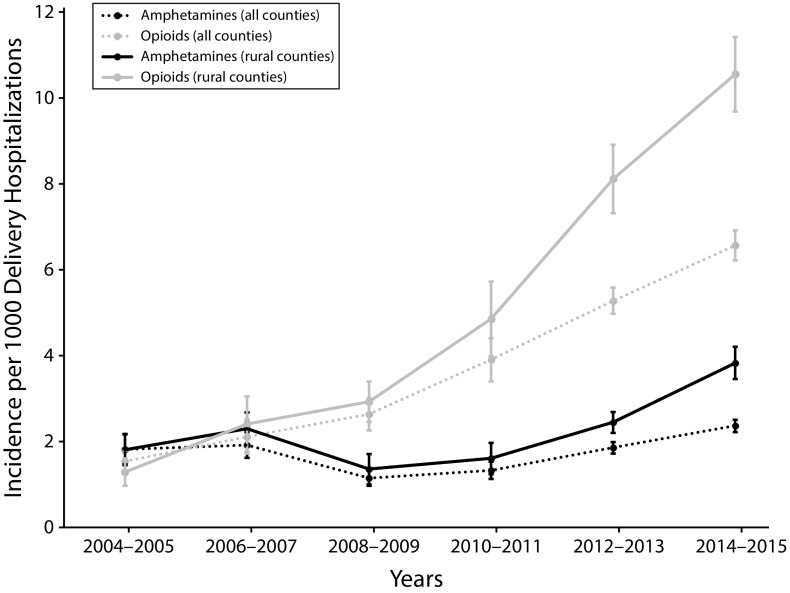
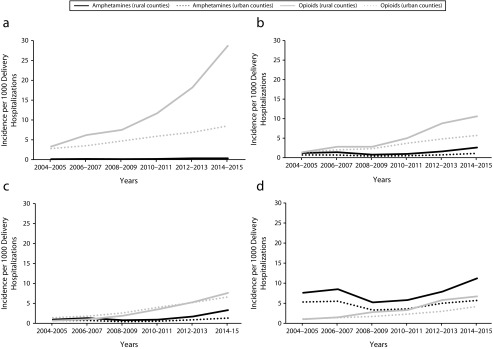
Amphetamine-related deliveries reached a nadir in 2008 to 2009 (1.2; 95% CI = 1.0, 1.3) per 1000 hospital deliveries) and then increased to its highest level of 2.4 (95% CI = 2.2, 2.5) per 1000 hospital deliveries by 2014 to 2015 (Figure 1). Opioid-related deliveries increased from 1.5 (95% CI = 1.3, 1.8) to 6.5 (95% CI = 6.2, 6.9) per 1000 delivery hospitalizations between 2004 to 2005 and 2014 to 2015. Maternal amphetamine use disproportionately affected rural compared with urban counties in the West, Midwest, and South between 2008 to 2009 and 2014 to 2015 (Figure 2). The highest incidence of amphetamine-related deliveries in 2014 to 2015 was in the rural West (11.2 [95% CI = 9.5, 13.0] per 1000 hospital deliveries). Opioid-related deliveries were identified at higher rates in rural compared with urban counties in 3 of the 4 census regions between 2004 to 2005 and 2014 to 2015: Northeast, South, and West. By 2014 to 2015, the highest incidence of opioid-related deliveries was in the rural Northeast (28.7 [95% CI = 24.9, 32.5] per 1000 hospital deliveries).
FIGURE 1—
National Trends in Amphetamine and Opioid Use Among Delivering Women: National Inpatient Sample, United States, 2004–2015
Note. The sample size was n = 47 164 263. All data are survey-weighted and represented as rate per 1000 delivery hospitalizations. Whiskers indicate 95% confidence intervals.
FIGURE 2—
National Trends in Amphetamine and Opioid Use Among Delivering Women by Rural Versus Urban Residence and Census Region (a) Northeast, (b) South, (c) Midwest, and (d) West: National Inpatient Sample, United States, 2004–2015
Note. The sample size was n = 47 164 263. Amphetamine-related delivery hospitalizations in the rural Northeast are not reported per Healthcare Cost and Utilization Project guidelines (≤ 10 unweighted cases for each timepoint).
Antenatal diagnoses of preeclampsia and placental abruption were identified among higher proportions of amphetamine-related compared with opioid-related and other hospital deliveries (Table 2). Among amphetamine-related deliveries, the incidence of preeclampsia was approximately 2 times higher than opioid-related and other hospital deliveries. Compared with opioid-related and other deliveries, amphetamine-related deliveries were also associated with higher incidence of preterm delivery (amphetamine: 16.7% [95% CI = 15.3, 18.0]; opioid: 12.6 [95% CI = 11.9, 13.4]; other hospital deliveries: 5.8% [95% CI = 5.7, 5.9]) and severe maternal morbidity and mortality (amphetamine: 3.8% [95% CI = 3.1, 4.4]; opioid: 2.4% [95% CI = 2.1, 2.7]; other hospital deliveries: 1.6% [95% CI = 1.6, 1.7]).
TABLE 2—
Adjusted Health Outcomes, Health Care Utilization, and Expenditures Among Hospital Deliveries Complicated by Amphetamine and Opioid Use: National Inpatient Sample, United States, 2014–2015
| Variables | Amphetamine Use (n = 18 050) | Opioid Use (n = 50 011) | Other Hospital Deliveries (n = 7 545 380) |
| Antenatal diagnoses, weighted % (95% CI) | |||
| Preeclampsia | 9.3 (8.2, 10.4) | 4.4 (4.0, 4.9) | 4.8 (4.7, 4.8) |
| Placental abruption | 4.3 (3.6, 5.0) | 3.1 (2.8, 3.5) | 1.0 (1.0, 1.1) |
| Clinical outcomes, weighted % (95% CI) | |||
| Preterm delivery (< 37 wk) | 16.7 (15.3, 18.0) | 12.6 (11.9, 13.4) | 5.8 (5.7, 5.9) |
| Cesarean delivery | 37.4 (35.6, 39.3) | 34.5 (33.5, 35.6) | 32.6 (32.3, 32.8) |
| Severe maternal morbidity or mortality | 3.8 (3.1, 4.4) | 2.4 (2.1, 2.7) | 1.6 (1.6, 1.7) |
| Health care utilization: hospital transfer, weighted % (95% CI) | 1.1 (0.7, 1.4) | 0.63 (0.47, 0.81) | 0.14 (0.12, 0.16) |
| Length of stay, days, mean (95% CI) | |||
| All deliveries | 2.9 (2.9, 3.0) | 3.0 (2.9, 3.1) | 2.6 (2.6, 2.7) |
| Vaginal deliveries | 2.4 (2.3, 2.5) | 2.5 (2.5, 2.6) | 2.2 (2.2, 2.2) |
| Cesarean deliveries | 4.0 (3.8, 4.1) | 4.3 (4.1, 4.4) | 3.6 (3.5, 3.6) |
| Cost per delivery hospitalization, US $, mean (95% CI)a | |||
| All deliveries | 5700 (5500, 5900) | 5400 (5200, 5500) | 4600 (4600, 4700) |
| Vaginal deliveries | 4100 (4000, 4200) | 4300 (4100, 4500) | 3500 (3500, 3600) |
| Cesarean deliveries | 7300 (7100, 7600) | 7500 (7200, 7800) | 6100 (6000, 6200) |
Note. CI = confidence interval. The sample size was n = 7 611 192. All proportions are survey-weighted and represented as rate per 100 delivery hospitalizations (95% CI) unless otherwise noted. Adjusted for age, payer, income, rural compared with urban residence, and hospital region.
Costs are inflation-adjusted to 2015 US dollars.
With the exception of heart failure among opioid-related deliveries, each of the 10 specific severe maternal morbidities we examined occurred more frequently among amphetamine- and opioid-related deliveries compared with other hospital deliveries (Table A, available as a supplement to the online version of this article at http://www.ajph.org). Compared with opioid-related deliveries, amphetamine-related deliveries were associated with higher incidence of blood transfusion, heart failure or arrest during a surgery or procedure, and eclampsia. Adult respiratory distress syndrome was the third-most-common morbidity among amphetamine- and opioid-related deliveries and the sixth-most-common morbidity among other hospital deliveries.
Amphetamine and opioid-related deliveries were also associated with greater mean costs compared with other deliveries. These associations were consistent across mode of delivery (vaginal compared with cesarean). Costs were not significantly different between amphetamine- and opioid-related deliveries.
DISCUSSION
Both amphetamine- and opioid-related deliveries are increasingly common among women in the United States. The trends in delivery hospitalizations related to amphetamines identified in this study correspond with national data that suggest declining use among the general population in the mid-2000s followed by increasing incidence starting in 2008 to 2009.15 Similar to trends in the general population, the incidence of amphetamine-related delivery hospitalizations was the highest in the West and in rural counties across most census regions. Our findings reveal that by 2014 to 2015, maternal opioid use complicated nearly 3% of all delivery hospitalizations in the rural Northeast. Amphetamine- and opioid-related deliveries were both associated with worse outcomes and higher costs compared with other deliveries. Compared with opioid-related deliveries, amphetamine-related deliveries were associated with higher levels of preterm delivery, composite severe maternal morbidity and mortality, and several individual indicators of severe maternal morbidity. In addition, we corroborate previous evidence of increased incidence of preeclampsia and placental abruption among amphetamine-related deliveries. These data highlight maternal amphetamine use as an emerging public health concern and deepen our understanding of the impact on maternal health of both amphetamines and opioids.
Previous studies described increased odds of several in-hospital maternal morbidities and mortality among delivering women with opioid use disorders.12 Maternal mortality review committees have also recently described rising rates of maternal deaths from opioid use disorder.27,28 To our knowledge, no previous studies have reported the association between maternal diagnoses of amphetamine-related, in-hospital, severe maternal morbidity and mortality. Furthermore, we found the risk of severe maternal morbidity and mortality occurring among mothers with amphetamine use was 1.6 times the rate of severe maternal morbidity and mortality occurring among mothers with opioid use. These data underscore the need for the maternal health community to direct attention not only toward opioids but also toward the use of amphetamines in the perinatal period.
There are several possible explanations for the adverse outcomes we identified among amphetamine- and opioid-related deliveries. First, outcomes could be related to the direct effects of amphetamines or opioids. For example, the vasoconstrictive effects of amphetamines are thought to impair placental function, resulting in higher risk for complications related to placental function such as preeclampsia and placental abruption.9 Second, substance use is associated with later prenatal care and fewer prenatal appointments, which reduces the potential health benefits of such care.29,30 Third, unplanned pregnancy, comorbid health conditions, or comorbid use of other medications or substances could contribute to the higher levels of morbidity identified in the present study. Finally, stressors associated with substance use disorders—particularly untreated substance use disorders—such as high-risk sexual behaviors, physical and sexual abuse, and unstable home environments, may also have an impact on pregnancy outcomes.
Regardless, early universal screening, brief intervention (engaging the patient in a short conversation, providing feedback and advice), and referral to treatment is effective in improving maternal and infant outcomes among pregnant women with substance use.29 There are some challenges, however, in implementing this model. First, access to treatment for pregnant women with addiction is often inadequate, particularly in rural areas.31,32 A recent wave of legislation penalizing pregnant women for substance use during pregnancy, which experts cite as ineffective and detrimental to maternal and child health,29,33 has had a disproportionate impact on women in rural areas,34 where the burdens of amphetamine and opioid use are the highest. Although cessation of methamphetamine use before delivery has been associated with improved birth outcomes, including gestational age at birth,35 evidence-based pharmacologic treatment options for amphetamine use are lacking. Understanding how to best promote cessation among amphetamine-using women should be a priority for policy and clinical initiatives.
Limitations
These findings should be interpreted with consideration of the limitations of our data source and study design. Despite recommendations for universal screening, not all pregnant patients are screened for substance use, and patients who are screened may be reluctant to disclose substance use. In addition, identification of these conditions is based on diagnosis codes, which may underrepresent true incidence. As such, our point estimates are likely to be conservative. We are also unable to distinguish between categories of amphetamine use, such as methamphetamine-specific use, because of limitations in ICD-9-CM coding. Given the retrospective nature of the study, we were unable to account for the dose–response relationship between amphetamine or opioid exposure and perinatal outcomes or the temporal relationship between last use and delivery. Lastly, within categories of substance use, we were unable to assess differences in outcomes among treated and untreated substance use disorders.
Public Health Implications
Although the opioid epidemic continues to escalate, policymakers and providers can leverage our early identification of rising amphetamine use during pregnancy to stem an additional drug epidemic. Similar to the opioid epidemic, a thoughtful response to amphetamine use will require a public health approach.33 Key pillars in the fight against opioids have included efforts to reduce unintended pregnancy, engage practitioners in universal screening, increase access to evidence-based care, and provide education and the opportunity for informed consent with respect to maternal drug testing and reporting practices. Policymakers should prioritize a response to substance use that builds adequate treatment capacity to address current substance use epidemics and can be adapted for future epidemics that may involve other substances. Bold action is needed to improve the health of mothers and children in the United States.
ACKNOWLEDGMENTS
These analyses would not be possible without the data collection efforts of Healthcare Cost and Utilization Project’s data partners: https://www.hcup-us.ahrq.gov/db/hcupdatapartners.jsp.
CONFLICTS OF INTEREST
V. K. Dalton is a paid, expert witness for Bayer. The other authors have no conflicts of interest to report.
HUMAN PARTICIPANT PROTECTION
Institutional review board approval was not needed because the study used de-identified, publicly available data.
Footnotes
See also Patrick, p. 22.
REFERENCES
- 1.Patrick SW, Schumacher R, Benneyworth B, Krans E, McAllister J, Davis M. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA. 2012;307(18):1934–1940. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 2.Villapiano NL, Winkleman T, Kozhimannil K, Davis M, Patrick S. Rural and urban differences in neonatal abstinence syndrome and maternal opioid use, 2004 to 2013. JAMA Pediatr. 2017;171(2):194–196. doi: 10.1001/jamapediatrics.2016.3750. [DOI] [PubMed] [Google Scholar]
- 3.Winkelman TNA, Villapiano N, Kozhimannil KB, Davis MM, Patrick SW. Incidence and costs of neonatal abstinence syndrome among infants with Medicaid: 2004–2014. Pediatrics. 2018;141(4):e20173520. doi: 10.1542/peds.2017-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patrick SW, Dudley J, Martin PR et al. Prescription opioid epidemic and infant outcomes. Pediatrics. 2015;135(5):842–850. doi: 10.1542/peds.2014-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy UM, Davis JM, Ren Z, Greene MF. Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130(1):10–28. doi: 10.1097/AOG.0000000000002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Department of Health and Human Services. Treatment Episode Data Set (TEDS): 2002–2012. National admissions to substance abuse treatment services. HHS publication no. SMA 14-4850. BHSIS, Series S-71. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013.
- 7.Cox S, Posner SF, Kourtis AP, Jamieson DJ. Hospitalizations with amphetamine abuse among pregnant women. Obstet Gynecol. 2008;111(2 pt 1):341–347. doi: 10.1097/01.AOG.000300377.82722.ad. [DOI] [PubMed] [Google Scholar]
- 8.Huybrechts KF, Bröms G, Christensen LB et al. Association between methylphenidate and amphetamine use in pregnancy and risk of congenital malformations: a cohort study from the international pregnancy safety study consortium. JAMA Psychiatry. 2018;75(2):167–175. doi: 10.1001/jamapsychiatry.2017.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen JM, Hernández-Díaz S, Bateman BT et al. Placental complications associated with psychostimulant use in pregnancy. Obstet Gynecol. 2017;130(6):1192–1201. doi: 10.1097/AOG.0000000000002362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrade C. Adverse gestational outcomes associated with attention-deficit/hyperactivity disorder medication exposure during pregnancy. J Clin Psychiatry. 2018;79(1):18f12136. doi: 10.4088/JCP.18f12136. [DOI] [PubMed] [Google Scholar]
- 11.Ladhani NN, Shah PS, Murphy KE. Prenatal amphetamine exposure and birth outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011;205(3):219.e1–219.e7. doi: 10.1016/j.ajog.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Maeda A, Bateman BT, Clancy CR, Creanga AA, Leffert LR. Opioid abuse and dependence during pregnancy: temporal trends and obstetrical outcomes. Anesthesiology. 2014;121(6):1158–1165. doi: 10.1097/ALN.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 13.Admon LK, Winkelman TNA, Moniz MH, Davis MM, Heisler MDV. Disparities in chronic conditions among women hospitalized for delivery, United States 2005–2014. Obstet Gynecol. 2017;130(6):1319–1326. doi: 10.1097/AOG.0000000000002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120(5):1029–1036. doi: 10.1097/aog.0b013e31826d60c5. [DOI] [PubMed] [Google Scholar]
- 15.Maxwell JC, Brecht M-L. Methamphetamine: here we go again? Addict Behav. 2011;36(12):1168–1173. doi: 10.1016/j.addbeh.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality. National Inpatient Sample. 2018. Available at: https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed June 28, 2018.
- 17.Khera R, Angraal S, Couch T et al. Adherence to methodological standards in research using the National Inpatient Sample. JAMA. 2017;318(20):2011–2018. doi: 10.1001/jama.2017.17653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuklina EV, Whiteman MK, Hillis SD et al. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Matern Child Health J. 2008;12(4):469–477. doi: 10.1007/s10995-007-0256-6. [DOI] [PubMed] [Google Scholar]
- 19.Treatment Episode Data Set. 2004–2014 national admissions to substance abuse treatment services. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2016.
- 20.Heslin KC, Elixhauser A, Steiner CA. Hospitalizations involving mental and substance use disorders among adults. Statistical Brief 191. Rockville, MD: Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality; 2012. [PubMed]
- 21.National Center for Health Statistics, Centers for Disease Control and Prevention. NCHS urban–rural classification scheme for counties. Available at: https://www.cdc.gov/nchs/data_access/urban_rural.htm. Accessed April 2, 2018.
- 22. Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality. NCHRONIC: ICD-9-CM number of chronic conditions. September 2008. Available at: https://www.hcup-us.ahrq.gov/db/vars/nchronic/nisnote.jsp. Accessed April 2, 2018.
- 23.Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. 2017. Available at: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.html. Accessed June 28, 2018.
- 24. Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality. Cost-to-charge ratio files. 2018. Available at: https://www.hcup-us.ahrq.gov/db/state/costtocharge.jsp. Accessed June 28, 2018.
- 25.Bureau of Labor Statistics. Table 24. Historical Consumer Price Index for all urban consumers (CPI-U): U.S. city average, all items. Available at: https://www.bls.gov/cpi/tables/historical-cpi-u-201710.pdf. Accessed January 22, 2017.
- 26.Gregori D, Petrinco M, Bo S, Desideri A, Merletti F, Pagano E. Regression models for analyzing costs and their determinants in health care: an introductory review. Int J Qual Health Care. 2011;23(3):331–341. doi: 10.1093/intqhc/mzr010. [DOI] [PubMed] [Google Scholar]
- 27.Metz TD, Rovner P, Hoffman MC, Allshouse AA, Beckwith KM, Binswanger IA. Maternal deaths from suicide and overdose in Colorado, 2004–2012. Obstet Gynecol. 2016;128(6):1233–1240. doi: 10.1097/AOG.0000000000001695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Review to Action. Building US capacity to review and prevent maternal deaths. Report from nine maternal mortality review committees. Available at: http://reviewtoaction.org/Report_from_Nine_MMRCS. Accessed June 28, 2018.
- 29.American College of Obstetricians and Gynecologists. Committee opinion no. 711: Opioid use and opioid use disorder in pregnancy. Obstet Gynecol. 2017;130(2):e81–e94. doi: 10.1097/AOG.0000000000002235. [DOI] [PubMed] [Google Scholar]
- 30.El-Mohandes A, Herman AA, El-Khorazaty MN, Katta PS, White D, Grylack L. Prenatal care reduces the impact of illicit drug use on perinatal outcomes. J Perinatol. 2003;23(5):354–360. doi: 10.1038/sj.jp.7210933. [DOI] [PubMed] [Google Scholar]
- 31.Brown JD, Goodin AJ, Talbert JC. Rural and Appalachian disparities in neonatal abstinence syndrome incidence and access to opioid abuse treatment. J Rural Health. 2018;34(1):6–13. doi: 10.1111/jrh.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terplan M. Beyond the treatment box: perspectives on the federal response to opioid use, pregnancy, and neonatal abstinence syndrome. J Addict Med. 2017;11(3):176–177. doi: 10.1097/ADM.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 33.Patrick SW, Schiff DM. A public health response to opioid use in pregnancy. Pediatrics. 2017;139(3):e20164070. doi: 10.1542/peds.2016-4070. [DOI] [PubMed] [Google Scholar]
- 34.The Guttmacher Institute. Substance using during pregnancy. Available at: https://www.guttmacher.org/state-policy/explore/substance-use-during-pregnancy. Accessed June 28, 2018.
- 35.Wright TE, Schuetter R, Tellei J, Sauvage L. Methamphetamines and pregnancy outcomes. J Addict Med. 2015;9(2):111–117. doi: 10.1097/ADM.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]