Abstract
Tumour lysis syndrome (TLS) is a life-threatening complication wherein massive tumour cell lysis results in severe metabolic abnormalities. TLS generally follows chemotherapy of rapidly proliferating haematological malignancies; spontaneous TLS and TLS from treatment of solid tumours are infrequently reported. We present a rare case of TLS following treatment of a large gastrointestinal stromal tumour (GIST) in a 63- year-old man. Imatinib was started for tumour size reduction prior to surgical intervention and in 5 days the patient developed metabolic derangements consistent with TLS. Imatinib was held and fluids, allopurinol and rasburicase were started. All metabolic abnormalities resolved in 3 days. Imatinib was restarted, and he eventually underwent surgical intervention. This is the second case demonstrating successful reinitiation of imatinib following TLS when treating GIST. We highlight the importance of risk factor assessment and need for pre-emptive therapy to prevent TLS when using tyrosine kinase inhibitor therapy.
Keywords: tyrosine kinase inhibitor, unwanted effects / adverse reactions, gastroenterology
Background
Tumour lysis syndrome (TLS) results from massive tumour cell lysis and generally follows chemotherapy or radiation treatment of malignancy. Rarely, TLS occurs spontaneously. This syndrome constitutes a medical emergency given the potential consequences related to severe metabolic disturbances, including acute kidney injury, hyperkalaemia, hyperphosphataemia, hyperuricaemia and hypocalcaemia. Cardiac dysrhythmias, seizures and even death are reported. The majority of cases of TLS are associated with treatment of rapidly proliferating tumours, particularly high-grade lymphomas and acute lymphoblastic leukaemia. Significantly fewer cases of TLS have been reported in association with solid tumours, either spontaneously or following treatment.1 A systematic review of reported TLS cases between 1977 and 2015 found only 120 reports of TLS occurring in patients with solid tumours.2 Twelve additional cases were reported by Myint et al in 2015.3 Of these, only two cases occurred in patients with metastatic gastrointestinal stromal tumours (GIST), both following treatment with tyrosine kinase inhibitors.4 5 We found three other cases of imatinib-induced TLS when treating GIST6–8 and present an additional case in this report. Historically, GISTs have been considered chemo-resistant tumours, but use of tyrosine kinase inhibitors has dramatically impacted the treatment of locally advanced and metastatic GIST, resulting in significantly improved outcomes.9 Given that imatinib is now mainstream therapy for GIST, recognition of risk factor and expectant management of TLS increases in importance.
Case presentation
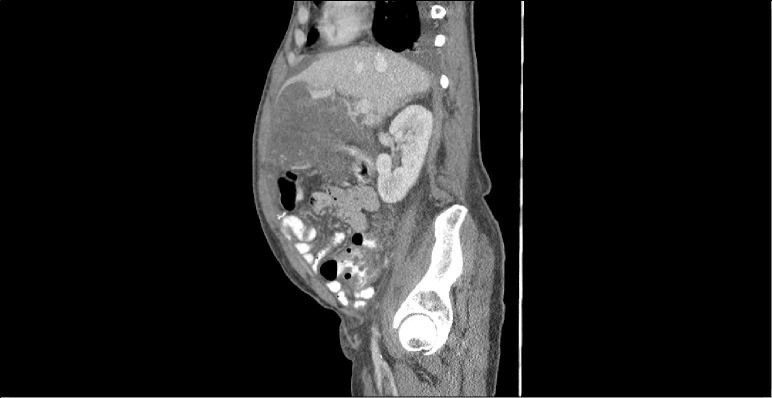
A 63-year-old man with chronic obstructive pulmonary disease was hospitalised with abdominal pain and shortness of breath. Abdominal CT revealed small pleural effusions, ascites and a large abdominal mass (30×16×30 cm) (figure 1). Biopsy with KIT staining was positive for GIST, and he was discharged with plans to start imatinib for tumour size reduction prior to surgical intervention. However, worsening dyspnoea and abdominal pain prompted readmission prior to imatinib initiation. Baseline laboratory evaluation included the following normal values: serum creatinine 69.83 μmol/L (normal 60–110 μmol/L), blood urea nitrogen (BUN) 7.86 mmol/L, potassium 4.9 mmol/L, calcium 2.27 mmol/L and phosphate 1.5 mmol/L (normal 1.12–1.45 mmol/L). Additional studies included lactic dehydrogenase (LDH) of 452 U/L (normal 100–250 U/L) and a uric acid level of 487.74 umol/L (normal 202.2–428.26 μmol/L) Oncology consultants evaluated the patient and thought symptoms were a result of his large tumour burden and started imatinib 400 mg daily. Five days later, he developed acute kidney injury (AKI), hyperkalaemia and hyperphosphataemia.
Figure 1.
CT abdomen and pelvis shows dominant heterogeneously enhancing mass occupying nearly the entire upper abdomen measuring 29.8×16×29.8 cm; encases nearly the entire stomach, portions of the small bowel, left lobe of liver, spleen and pancreas.
Investigations
Serum creatinine peaked at 237.85 μmol/L, BUN was elevated at 25.3 mmol/L, potassium 7.5 mmol/L and phosphate 2.29 mmol/L with change in calcium to 1.97 mmol/L. Additionally, LDH increased to 565 U/L and uric acid rose to 666.18 μmol/L. Abdominal and pelvic CT showed pelvic ascites, but no evidence of obstruction, hydronephrosis or increased size of the GIST. There was normal return of urine following placement of a bladder catheter.
Differential diagnosis
In accordance with the Cairo-Bishop laboratory criteria,10 a diagnosis of TLS was made.
Treatment
The patient was transferred to the intensive care unit for close monitoring of cardiac rhythm and overall status. Aggressive fluid hydration was initiated, and calcium gluconate, sodium bicarbonate and intravenous insulin with dextrose were administered for hyperkalaemia. Imatinib was discontinued and allopurinol 300 mg daily was started. Additionally the patient was treated with intravenous rasburicase 2.5 mg, followed by a second dose of 7.5 mg.
Outcome and follow-up
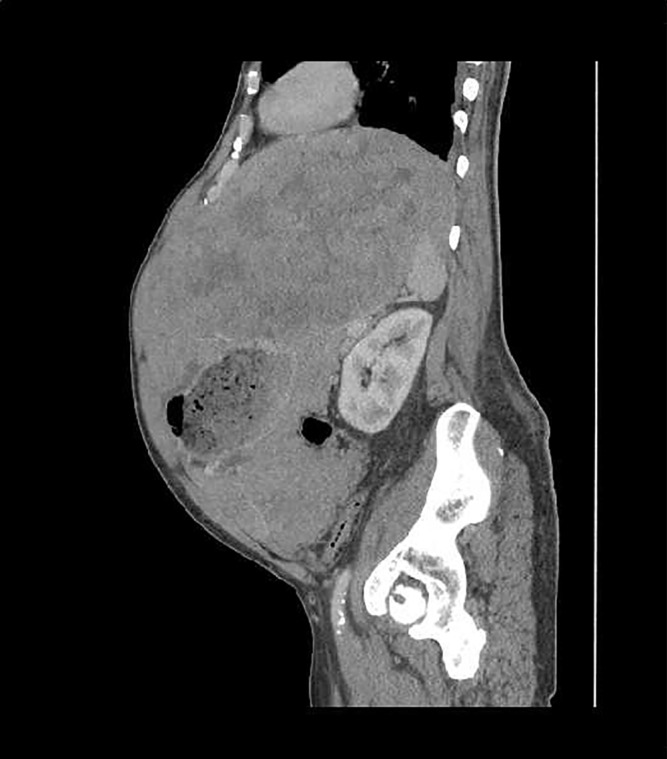
After 3 days, his metabolic derangements completely resolved. Because of his significant tumour burden, oncology consultants thought imatinib should be restarted with continued prophylaxis. Daily allopurinol 300 mg was continued. He tolerated reinitiation of imatinib 400 mg by mouth daily quite well and was subsequently discharged with plans for future surgical intervention. He was readmitted in 2 months with inability to eat and failure to thrive, deemed secondary to tumour bulk, and underwent successful surgical debulking. Imatinib 400 mg was restarted postoperatively along with allopurinol 300 mg daily. At 3 months postoperatively, CT of the abdomen and pelvis showed a mass-like process in the right upper quadrant and slightly left of midline measuring 9.2×11.2 cm, but decreased pelvic ascites and no evidence of lymphadenopathy (figures 2 and 3). Imatinib was continued, and at 6 months postoperatively repeat imaging showed a persistent stable mass with minimal to no change. However, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) had increased to 155 and 168 U/L. Evaluation included negative hepatitis studies, normal ceruloplasmin, negative antimitochondrial antibody, normal alpha antitrypsin and antinuclear antibody of <40 (normal). Imatinib was discontinued because of concerns about possible liver toxicity and at 3 months, liver enzymes had normalised. Imatinib was restarted at a reduced dose of 300 mg daily, and the patient has tolerated the medication well. His GIST remains stable.
Figure 2.
CT abdomen and pelvis. Mass-like process at level of celiac axis in right upper quadrant and slightly left of midline measures 9.2×11.2 cm. Probable mass-like process to left aspect of greater curvature of the stomach extending into overlying peritoneum, surrounding the stomach and part of the spleen, measuring 11.5×15.5 cm. Overall, size of mass is smaller than in previous scans.
Figure 3.
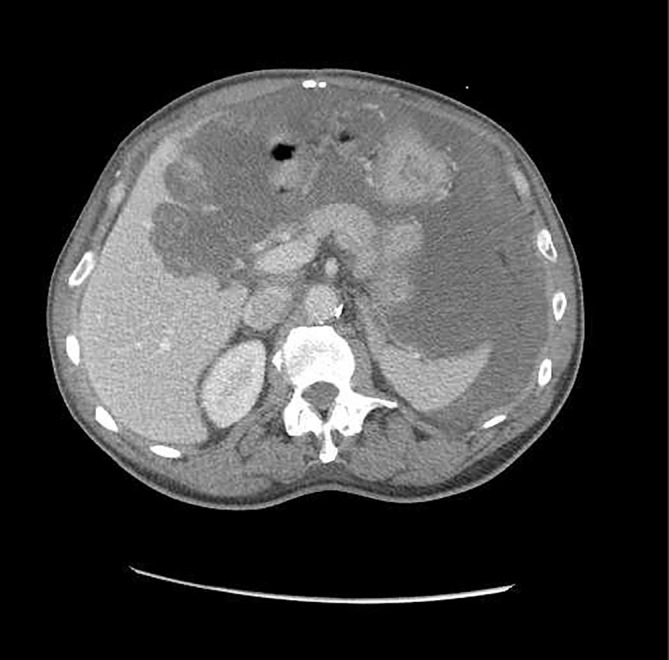
CT abdomen and pelvis. Abdominal mass persists but smaller compared with previous scans.
Discussion
GISTs are rare mesenchymal tumours of the gastrointestinal tract with an incidence of 10–20 cases per million individuals.11 12 Historically, surgical resection has been the mainstay of treatment, but reported 5-year actuarial survival rates were 54% with high disease recurrence for patients even with complete resection of gross primary tumours.9 13 Previously, GISTs were considered ‘chemo-resistant’ as response rates to chemotherapy approximated 5%–7%12; however in recent years, imatinib has emerged as revolutionary treatment for GIST, both as neoadjuvant and adjuvant therapy, and as first-line treatment for metastatic disease.9 12 The majority of GISTs have a c-kit proto-oncogene mutation which results in KIT receptor tyrosine kinase activation resulting in cell cycle activation, inhibition of apoptosis and unopposed cell growth.14 Imatinib targets the c-KIT tyrosine kinases and was first used to successfully treat metastatic advanced GIST in 2000.15 Multiple studies have since demonstrated high clinical benefit with use of imatinib to treat GIST. Early studies found that 80%–88% of patients achieved clinical benefit with imatinib including significant improvements in long-term survival rates for advanced or metastatic disease, reduced disease recurrence and improved relapse-free survival rates with adjuvant use.16 Further, imatinib use assists in ‘downstaging tumours and reducing surgical morbidities’ when used as neoadjuvant therapy.12
In general, imatinib is well-tolerated. Though side effects are common, they are generally mild and improve during the course of therapy. A systematic review of the safety profile of imatinib in chronic myelogenous leukaemia and GIST reported common side effects that included nausea and vomiting (52%–57%) and diarrhoea (45%–52%).16Oedema, skin rash, muscle cramps and arthralgias, and headache were other reported side effects as well as elevated hepatic transaminases and haematological side effects. The rate of serious toxicity was low. Serious toxicities reported included haematological abnormalities, particularly anaemia (7%–10%) and neutropenia (7% in patients with advanced GIST).16 Though cardiotoxicity has been noted as a serious side effect, newer studies suggest that cardiotoxicity in patients taking imatinib occurs at rates similar to the general population.17 When severe imatinib toxicity was noted, it most often occurred early in the course of therapy, and correlated with dose, disease stage and individual patient characteristics like advanced age and female gender.16 TLS as a potential toxicity of imatinib therapy is rarely reported. Our literature found only five other reports of TLS in patients treated for GIST4–8 (see table 1).
Table 1.
Case reports of tumour lysis syndrome (TLS) and gastrointestinal stromal tumour (GIST)
| Case reports of TLS and GIST | Age Sex |
Tumour | Drug d ose | Pre treatment | Post treatment | Outcome |
| Saylor and Reid 20074 | 56 years Male |
Metastatic GIST to mesentery, omentum, liver and small bowel | Sunitinib 50 mg daily (previously non-compliant with imatinib) |
Creatinine 70.7 μmol/L Potassium reported as normal |
At 7 days:
Creatinine 123.8 μmol/L Potassium 5.9 mmol/L Phosphorus 2.261 mmol/L Uric acid 463.9 μmol/L |
Metabolic abnormalities improved with hydration; persistent bleeding from tumour fistula; death |
| Pinder et al 20075 | 81 years Male | Initial tumour completely resected 2002; 3 years later had recurrent large GIST involving mesentery and abdominal wall musculature measuring 20×11×25 cm |
Imatinib 400 mg daily |
Creatinine 139 μmol/L Urea 11.3 mmol/L Alkaline phosphatase 456 IU/L |
At 3 days:
Creatinine 270 μmol/L Urea 34.8 mmol/L Potassium 6.4 mmol/L Uric acid 574 μmol/L |
Death 11 days after initial imatinib dose |
| Karachiwala 20157 |
60 years Female |
Initial pelvic mass resected; large abdominal mass positive for GIST recurred postoperatively extending from pelvis to impinge on stomach and pancreas | Imatinib 400 mg daily: developed nausea and vomiting and dose reduced to 200 mg daily |
Creatinine 79.2 μmol/L Calcium 2.4 mmol/L Potassium 4.4 mmol/L |
At 8 days:
Creatinine 422 μmol/L (peaked 512 μmol/L) Urea 7.8 mmol/L (peaked 38.5 mmol/L) Potassium 4.4 mmol/L (peaked 5.0 mmol/L) Calcium 1.96 mmol/L Phosphorous 0.65 mmol/L |
Treated with rasburicase 6 mg daily and allopurinol 100 mg orally daily; hydrated; haemodialysis started; however, no improvement and patient died |
| Terada et al 20146 | 69 years Male |
Disseminated peritoneal GIST | Imatinib 400 mg daily (reduced to 300 mg following resolution of TLS) |
Creatinine 58.34 μmol/L Urea 8.57 mmol/L Uric acid 446.13 μmol/L Lactic dehydrogenase 420 |
Within 1 week:
Creatinine 199.83 μmol/L K 4.3 mmol/L Uric acid 642.38 μmol/L Phosphate 1.64 mmol/L |
Imatinib stopped; TLS improved with hydration and allopurinol; on follow-up tumour size increased and imatinib restarted at 300 mg/day; patient tolerated; dramatic improvement in tumour size |
| Jeng-Nian Yuan 20178 |
43 years Male |
Metastatic GIST involving liver, mesentery, omentum and peritoneum | Imatinib 400 mg daily |
Creatinine 106.1 μmol/L Urea 3.92 mmol/L Potassium 4.3 Uric acid 600.75 μmol/L Phosphate 1.13 mmol/L Calcium 1.26 mmol/L ALT 59 AST 74 |
Day 1 post imatinib:
Creatinine 291.79 μmol/L Urea 22.14 mmol/L Potassium 5.4 mmol/L Uric acid 1142.02 μmol/L Phosphate 1.35 mmol/L Calcium 0.81 mmol/L Day 2 post imatinib: Creatinine 512.84 μmol/L Urea 30 mmol/L Potassium 8.8 mmol/L Uric acid 1623.8 μmol/L Day 3 post imatinib: ALT 5250 AST 13 900 |
Patient became unconscious after first dose of imatinib and developed severe metabolic abnormalities and progressive liver failure He died 4 days after initial imatinib dose |
In 2004, Cairo and Bishop offered a new classification and grading system for TLS using criteria for laboratory definition and incorporating clinical signs/symptoms to define clinical TLS.10 Our patient met criteria for laboratory TLS and was diagnosed with AKI per Kidney Disease: Improving Global Outcomes guidelines,18 but did not meet the technical diagnosis of Grade 1 clinical TLS as proposed by Cairo and Bishop wherein serum creatinine is 1.5× ULN.10
The estimated mortality rate for TLS in the setting of solid tumours ranges between 33% and 47%.1 19 With TLS in GIST, death occurred in three of the five reported cases. Thus, a threefold approach of risk factor identification, employment of prevention strategies and aggressive management if TLS occurs is needed. If risks are identified, patients should be stratified into low, intermediate or high risk and preventive strategies should be implemented according to risk.10 20 21 Low-risk patients are considered those with non-haematological malignancies, tumours with low proliferative rates, treatment with low-intensity cyto-reductive therapy and normal uric acid, LDH and hydration status prior to onset of therapy. High-risk patients are those with highly proliferative tumours, high tumour burden (per pre-existing white blood cell counts and LDH levels), those patients with poor hydration and abnormal kidney function. In the previously reported cases of TLS in GIST, all patients had high tumour load with metastasis. Our patient had high tumour bulk plus a slightly elevated uric acid and elevated LDH prior to therapy.
Expert guidelines published in 2015 recommend that low-risk patients be closely monitored and adequately hydrated, while intermediate-risk and high-risk patients are hydrated and treated with allopurinol or rasburicase, respectively.20 Though older studies identified acidic urine as a risk for TLS, alkalinisation of the urine is no longer recommended given the risk of xanthine obstructive uropathy.21 Both allopurinol and rasburicase have been used for prevention and treatment of TLS; however, rasburicase is considered superior for treatment given its rapid onset of action and method of action. Rasburicase catalyses oxidation of uric acid to allantoin, a water-soluble compound, and is the preferred medication for treating patients with high-risk haematological malignancies and for patients with pretreatment elevated uric acid levels.20 21 In addition to allopurinol, rasburicase therapy and hydration, it is important to correct the underlying metabolic abnormalities of TLS. The patient should be closely monitored for seizures, cardiac arrhythmias and AKI, and treated accordingly. Additionally, drug discontinuation is recommended, at least until the metabolic disturbances are corrected.
Our case represents the second report of TLS in GIST wherein imatinib was successfully restarted once all metabolic abnormalities were resolved. This is important to note as studies have demonstrated that interruptions and/or discontinuation can result in rapid recurrence or worsening of GIST.14 Thus, appropriate risk factor assessment for TLS, appropriate prevention, detection and prompt treatment is important in management of patients with GIST who require tyrosine kinase inhibitor therapy.
Learning points.
Imatinib use for gastrointestinal stromal tumour (GIST) is associated with improved outcomes and prolonged survival.
Tumor lysis syndrome (TLS) is a rare complication of imatinib therapy of GIST.
Risk factors for TLS in GIST include dehydration, renal abnormalities, elevated pre-treatment uric acid and/or lactic dehydrogenase, and large tumour bulk.
Allopurinol and rasburicase are used for both prevention and treatment of TLS.
Even when TLS occurs with initial imatinib therapy, careful assessment and prophylaxis for TLS may allow reinstitution of imatinib for recurrent GIST.
Footnotes
Contributors: All authors contributed to the development of this manuscript. JO and GK were primarily responsible for literature search, case presentation, differential diagnosis and review of images and pathology. JO and KJ were primarily responsible for literature search and writing of the abstract, background and discussion.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Caravaca-Fontán F, Martínez-Sáez O, Pampa-Saico S, et al. Tumor lysis syndrome in solid tumors: Clinical characteristics and prognosis. Med Clin 2017;148:121–4. 10.1016/j.medcli.2016.10.040 [DOI] [PubMed] [Google Scholar]
- 2. Mirrakhimov AE, Ali AM, Khan M, et al. Tumor lysis syndrome in solid tumors: an up to date review of the literature. Rare Tumors 2014;6:68–76. 10.4081/rt.2014.5389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Myint ZW, Verla-Tebit E, Cho BB, et al. Tumor lysis syndrome in a patient with metastatic non-small cell lung cancer: case report and literature review. Cancer Treat Commun 2015;4:10–14. 10.1016/j.ctrc.2015.03.002 [DOI] [Google Scholar]
- 4. Saylor PJ, Reid TR. Tumor lysis syndrome after treatment of a gastrointestinal stromal tumor with the oral tyrosine kinase inhibitor sunitinib. J Clin Oncol 2007;25:3544–6. 10.1200/JCO.2007.12.0790 [DOI] [PubMed] [Google Scholar]
- 5. Pinder EM, Atwal GS, Ayantunde AA, et al. Tumour Lysis Syndrome Occurring in a Patient with Metastatic Gastrointestinal Stromal Tumour Treated with Glivec (Imatinib Mesylate, Gleevec, STI571). Sarcoma 2007;2007:1–5. 10.1155/2007/82012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Terada S, Matsuyama Y, Onoda K, et al. Successful re-administration of imatinib without recurrence of tumor lysis syndrome in a patient with peritoneal gastrointestinal stromal tumor. Japanese J of Gastroenterology 2014;111:1782–8. [PubMed] [Google Scholar]
- 7. Karachiwala H, Colwell B. Imatinib Induced Tumor Lysis Syndrome in a Patient with Gastrointestinal Stromal Tumor. DMJ 2015;41:20–2. [Google Scholar]
- 8. Jeng-Nian Y, Li AFY C-YC. Tumor Lysis Syndrome in Gastrointestinal Stromal Tumor Treated with the Oral Tyrosine Kinase Inhibitor Imatinib: A Case Report. J Gastroenterol Res 2017;1:47–50. [Google Scholar]
- 9. Platoff RM, Morano WF, Marconcini L, et al. Recurrent Gastrointestinal Stromal Tumors in the Imatinib Mesylate Era: Treatment Strategies for an Incurable Disease. Case Rep Oncol Med 2017;2017:1–8. 10.1155/2017/8349090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol 2004;127:3–11. 10.1111/j.1365-2141.2004.05094.x [DOI] [PubMed] [Google Scholar]
- 11. Din OS, Woll PJ. Treatment of gastrointestinal stromal tumor: focus on imatinib mesylate. Ther Clin Risk Manag 2008;4:149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feng X, Morris D. Associate Professor, Tom Baker Cancer Center, Cancer Control, Alberta Health Services, Calgary, Alberta, Canada. Systemic Treatment for Gastrointestinal Stromal Tumor—A State of Art. Oncology & Hematology Review 2014;10:110–22. [Google Scholar]
- 13. DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ksienski D. Imatinib mesylate: past successes and future challenges in the treatment of gastrointestinal stromal tumors. Clin Med Insights Oncol 2011;5:CMO.S4259–379. 10.4137/CMO.S4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 2001;344:1052–6. 10.1056/NEJM200104053441404 [DOI] [PubMed] [Google Scholar]
- 16. Thanopoulou E, Judson I. The safety profile of imatinib in CML and GIST: long-term considerations. Arch Toxicol 2012;86:1–12. 10.1007/s00204-011-0729-7 [DOI] [PubMed] [Google Scholar]
- 17. Trent JC, Patel SS, Zhang J, et al. Rare incidence of congestive heart failure in gastrointestinal stromal tumor and other sarcoma patients receiving imatinib mesylate. Cancer 2010;116:184–92. 10.1002/cncr.24683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. KDIGO Clinical Practice Guideline for Acute Kidney Injury. https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf (Accessed May 2017).
- 19. Baeksgaard L, Sørensen JB. Acute tumor lysis syndrome in solid tumors–a case report and review of the literature. Cancer Chemother Pharmacol 2003;51:187–92. 10.1007/s00280-002-0556-x [DOI] [PubMed] [Google Scholar]
- 20. Sarno J. Prevention and management of tumor lysis syndrome in adults with malignancy. J Adv Pract Oncol 20132013;4:101–6. [PMC free article] [PubMed] [Google Scholar]
- 21. Jones GL, Will A, Jackson GH, et al. Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br J Haematol 2015;169:661–71. 10.1111/bjh.13403 [DOI] [PubMed] [Google Scholar]