Abstract
A 63-year-old white man with a history of rheumatoid arthritis on adalimumab was admitted to the hospital for left arm swelling and erythema. On physical examination, the patient was afebrile and non-toxic appearing and there was tense oedema of the left forearm. Initial laboratory work was unremarkable except for elevated inflammatory markers. MRI of the arm showed non-specific findings of inflammation. The patient was started on empiric antibiotics but did not improve. Given the patient’s immunosuppression, early consideration was given to fungal or mycobacterial causes. Initial serum fungal studies were negative and the patient was taken for diagnostic local incision and biopsy of the left volar forearm. Grocott’s methenamine silver and periodic acid–Schiff staining revealed fungal organisms resembling Histoplasma and intraoperative fungal cultures grew Histoplasma capsulatum confirming the diagnosis. The patient was treated with a 6-month course of itraconazole with improvement in his condition and eventual complete resolution.
Keywords: infectious diseases, dermatology
Background
Cellulitis due to various microbes is a common presenting complaint in the hospital setting. While rare, in immunosuppressed patients, atypical organisms need to be considered in the differential diagnosis of cellulitis especially when there is lack of clinical response to antibacterial agents. Histoplasma capsulatum is a well-known cause of both isolated pulmonary and disseminated disease. Multiple case reports have documented Histoplasma in various non-pulmonary organ systems, however, these are usually associated with the widespread dissemination. Rarely patients can acquire a localised cutaneous infection from direct inoculation. When the diagnosis of a localised, non-pulmonary form of histoplasmosis is considered, there are minimal data to help guide diagnostic testing. Serum fungal studies alone are likely inadequately sensitive and specific in this setting and tissue biopsy should be performed with appropriate fungal staining and culture to enhance diagnostic yield. Primary cutaneous histoplasmosis (PCH) is rare in the literature and this case highlights the importance of early consideration for the condition especially in immunocompromised patients.
Case presentation
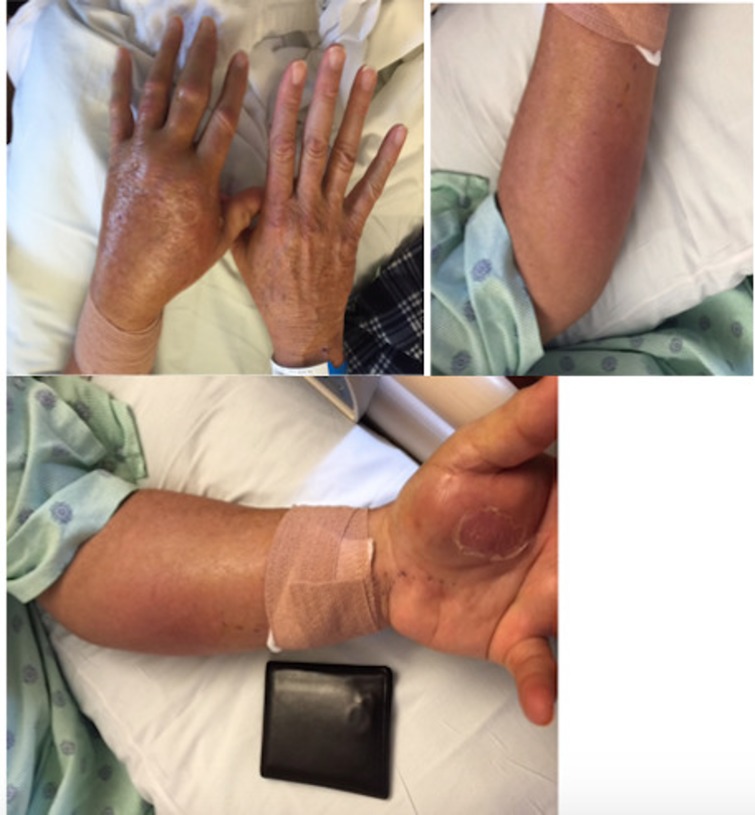
A 63-year-old white man with a history of rheumatoid arthritis on adalimumab was admitted to the hospital for evaluation and management of left arm swelling and erythema. He was treated in the outpatient setting with empiric antibiotics followed by a course of steroids without improvement. The patient described a 6-week history of progressive arm swelling, redness and pain. He had low-grade fever but denied chills, sweats, fatigue or other systemic symptoms. He denied sick contacts or recent travel. Prior to the onset of symptoms, he had spent a significant amount of time performing yard work and sustained several minor scratches on his arms from thorns and brush. Pertinent medical history included a 4-year history of rheumatoid arthritis treated with adalimumab. He was on methotrexate initially after diagnosis, but due to deranged liver function tests, he had been on adalimumab monotherapy for almost 4 years. The patient was a former smoker but denied alcohol or illicit drug use. Family and surgical history were not pertinent to his presenting complaint. On physical examination, the patient was afebrile and non-toxic appearing. The ventral aspect of the left forearm and the hand showed significant tense oedema with scattered erythematous patches, some areas of desquamation but without any open wounds or drainage (figure 1). The movement of the hand on the concerned side was not affected. The affected area did not extend proximal to the forearm. The remainder of the complete physical examination was unremarkable.
Figure 1.
Areas of cellulitis on the left hand and forearm.
Investigations
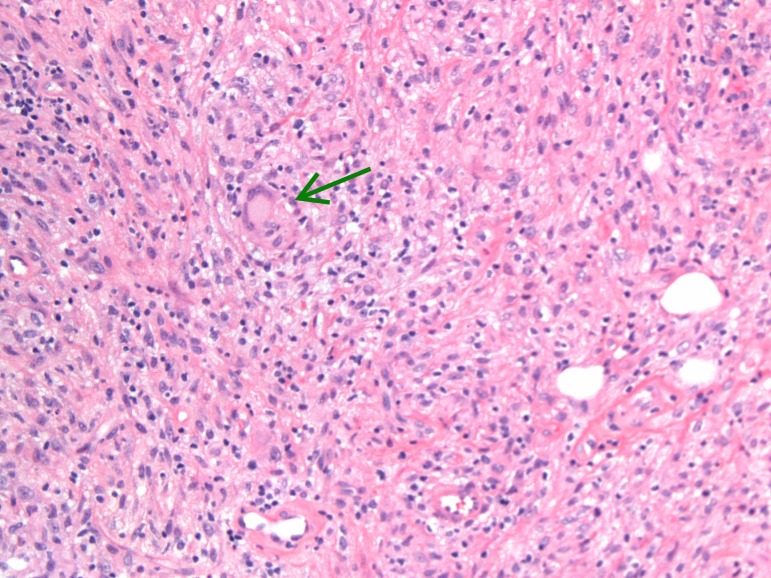
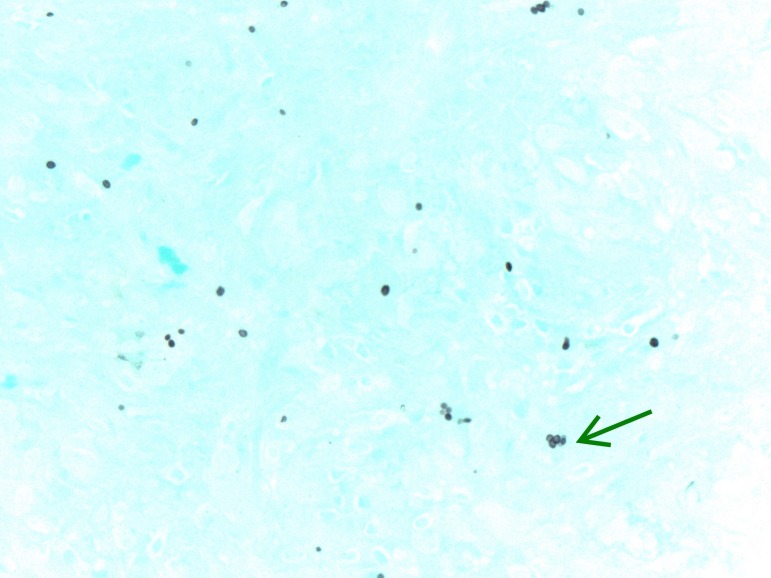
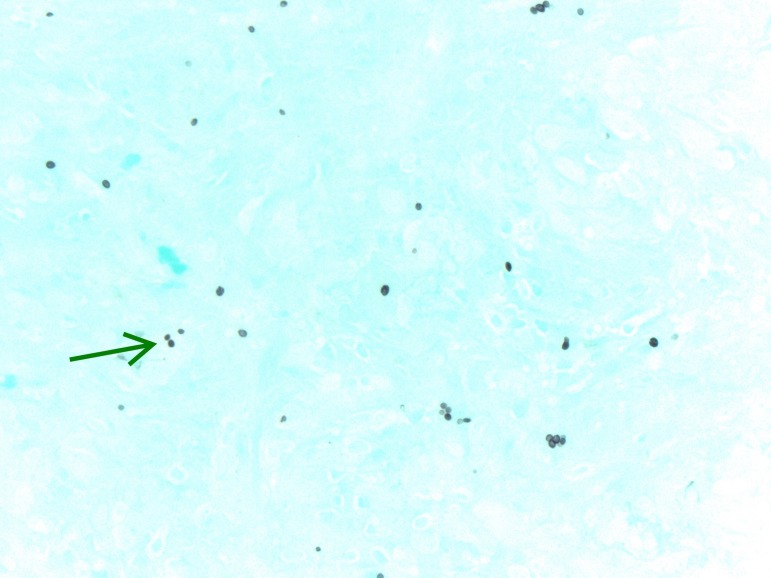
Initial laboratory work was significant for unremarkable complete blood count and complete metabolic panel, elevated high sensitivity C reactive protein level and normal procalcitonin level. Plain film X-ray of left hand revealed significant subcutaneous oedema without bony abnormalities. MRI of the left forearm revealed postgadolinium enhancement of the flexor compartment on axial T1-weighted imaging with consideration of myositis, infectious or inflammatory aetiologies. Blood cultures were negative on admission. Histoplasma and Blastomyces whole blood PCR were sent after no improvement on antibiotics and returned negative. The patient then underwent localised surgical biopsy and the surgeon found copious amounts of tan, amorphous, friable material without purulence. Fungal smear, acid fast smear, fungal cultures and histopathology were sent from the surgical specimen along with serum and urine Histoplasma studies. The fungal smear, acid-fast smear, Histoplasma urine antigen, Histoplasma serum antigen and Histoplasma serum antibody (by immunodiffusion) all returned negative. Histopathology testing revealed chronic non-necrotising granulomatous inflammation, with multi nucleated giant cells (figure 2). Staining of the tissue revealed organisms consistent with Histoplasma (figures 3 and 4). Subsequently, Histoplasma serum antibody (by complement fixation) and fungal culture from the biopsy specimen returned positive, establishing the diagnosis of PCH.
Figure 2.
Photomicrograph showing fibrovascular tissue with chronic and non-necrotising granulomatous inflammation (green arrow shows multinucleated giant cell).
Figure 3.
Silver stain (GMS) showing organisms morphologically consistent with Histoplasma species-green arrow. GMS, Grocott’s methenamine silver.
Figure 4.
GMS showing narrow-based budding yeast consistent with Histoplasma (arrow). GMS, Grocott’s methenamine silver.
Differential diagnosis
The differential diagnosis in this case included bacterial cellulitis, disseminated fungal infection, localised or disseminated mycobacterial infection, inflammatory myopathy and skin manifestations of rheumatoid arthritis. The failure to respond to broad-spectrum antibiotics and the known association with invasive atypical infections in the setting of immunosuppression with tumour necrosis factor (TNF) alpha inhibitor therapy led to early consideration of unusual organisms.
Treatment
The patient was initially treated in the outpatient setting with oral clindamycin without improvement. Given his history of rheumatoid arthritis and his lack of response to antibiotics, he was prescribed a course of steroids as an outpatient, again with no improvement. In the inpatient setting, he was started on the fifth-generation cephalosporin, ceftaroline to cover staphylococcal species (including Methicillin resistant Staphylococcus aureus) and streptococcal species. Adalimumab was discontinued on admission to the hospital. After confirmation of H. capsulatum, the patient was prescribed a 6-month course of itraconazole, with close monitoring of liver function tests and serum itraconazole levels.
Outcome and follow-up
The patient completed nearly 6 months of itraconazole therapy with complete clinical resolution. Itraconazole therapy was discontinued shortly before completion due to concern for possible cardiotoxicity. He continues to follow up with Infectious disease team and not had any recurrence of symptoms. No signs of pulmonary histoplasmosis or disseminated histoplasmosis were documented during the initial evaluation or subsequently in follow-up. He has been again started on methotrexate and this time he seems to be tolerating it fine.
Discussion
Histoplasmosis is the most common endemic fungal infection in the USA. The majority of infection with the causative agent of histoplasmosis, H. capsulatum, is asymptomatic and clinically benign. However, clinically significant disease does occur and its development is influenced by host factors including immune status and degree of inoculum. Histoplasmosis occurs in several distinct clinical entities, most commonly isolated pulmonary infection and disseminated disease that can be life threatening.1 Involvement in almost every organ has been described in the context of dissemination, however, localised extrapulmonary disease without evidence of dissemination is rare. A small number of case reports exist in the current literature that present cases of PCH. In all the cases, evidence for pulmonary disease or dissemination is absent and most cases describe a history of direct inoculation as was present in this case.2–8
The clinical presentation of PCH is significantly variable on review of the available case reports but typically involves ulcerative nodules. Case reports of PCH mimicking classic cellulitis (as in the patient presented here) do not appear to exist. Case reports of PCH in both immunocompromised and immunocompetent patients exist, however, there are not case reports of PCH occurring in patients on TNF alpha inhibitors (such as adalimumab as in this patient). Bhattacharya et al described a case of PCH following penetrating injury presenting as multiple painless nodules in an immunocompetent patient.2 Krunic et al published a report of PCH presenting as fever and multiple noduloulcerative lesions following local trauma in a woman with diabetes and vascular dementia.3 Interestingly, two case reports exist detailing cases of PCH in patients with rheumatoid arthritis (as in the case presented here), one on long-term steroids and the other on the disease-modifying agent, methotrexate. Both cases presented with nodular ulcerative lesions.4 5
Data on diagnostic testing are well described for the more common clinical entities of histoplasmosis (isolated pulmonary and disseminated forms).6–8 However no studies have evaluated the available laboratory tests for H. capsulatum in the setting of PCH. In the case presented here, definitive identification of H. capsulatum as the causative organism required surgical biopsy with direct visualisation using Grocott’s methenamine silver and periodic acid–Schiff staining along with fungal culture. Of the serological and urine tests that were ordered, only Histoplasma serum antibody (by complement fixation) was positive. Without evidence to focus diagnostic testing for suspected PCH, a broad approach is necessary to maximise sensitivity. Possibly most important is the early use of histopathology and culture as a diagnostic tool in atypical cases of cellulitis. PCH provides an accessible target for biopsy than the more common forms of histoplasmosis and the typical serological markers used for diagnosis of pulmonary and disseminated disease are likely to be inadequately sensitive in PCH.
Treatment of PCH appears to be effective based on the published case reports. Oral fluconazole, itraconazole and intravenous amphotericin B were all successfully used in at least one case report. Oral itraconazole or fluconazole was more commonly chosen presumably because of ease of use and fewer adverse effects.2–5
Whether our patient was more predisposed to this infection due to the anti-TNF alpha therapy or the disease process itself (rheumatoid arthritis itself causes immunological alteration), cannot be completely ascertained and hence is a limitation with our report. And although Histoplasma infection can be a reactivation syndrome in immunocompromised patients, especially in patients on anti-TNF alpha therapy, we believe our patient had primary site infection as symptoms occurred 4 years of adalimumab therapy, and there was a clear history of direct skin injury few weeks prior.
Learning points.
Primary cutaneous histoplasmosis (PCH) is a rare clinical entity caused by Histoplasma capsulatum in the absence of pulmonary or disseminated disease that appears to be caused by direct inoculation.
PCH typically presents as ulcerative nodules but can present as diffuse swelling and erythema mimicking classic cellulitis.
The diagnosis should be suspected in patients with a compatible clinical history, immunosuppression (including tumour necrosis factor alpha inhibitors), and those with a lack of response to antibacterial agents.
Diagnostic testing should include urine and serological testing but most importantly relies on surgical biopsy for histopathology and culture.
Itraconazole, fluconazole and amphotericin B have been successfully used to treat PCH.
Footnotes
Patient consent for publication: Obtained.
Contributors: MSK: resident physician on case and wrote the initial manuscript. VP: consultant involved in care, edited the case and provided the images.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Benedict K, Mody RK. Epidemiology of histoplasmosis outbreaks, United States, 1938-2013. Emerg Infect Dis 2016;22:370–8. 10.3201/eid2203.151117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhattacharya JB, Rani P, Aggarwal R, et al. . Primary cutaneous histoplasmosis masquerading as lepromatous leprosy. J Clin Diagn Res 2017;11:ED01–ED02. 10.7860/JCDR/2017/19676.9145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krunic AL, Carag H, Medenica MM, et al. . A case of primary cutaneous histoplasmosis in a patient with diabetes and multi-infarct dementia. J Dermatol 2002;29:797–802. 10.1111/j.1346-8138.2002.tb00226.x [DOI] [PubMed] [Google Scholar]
- 4. Romano C, Castelli A, Laurini L, et al. . Case report. Primary cutaneous histoplasmosis in an immunosuppressed patient. Mycoses 2000;43(3-4):151–4. 10.1046/j.1439-0507.2000.00563.x [DOI] [PubMed] [Google Scholar]
- 5. Lise MLZ, Staub HL. Primary cutaneous histoplasmosis in an immunocompromised patient with long-standing rheumatoid arthritis. Revista da Associação Médica Brasileira 2016;62:816–7. 10.1590/1806-9282.62.09.816 [DOI] [PubMed] [Google Scholar]
- 6. Babady NE, Buckwalter SP, Hall L, et al. . Detection of blastomyces dermatitidis and histoplasma capsulatum from culture isolates and clinical specimens by use of real-time PCR. J Clin Microbiol 2011;49:3204–8. 10.1128/JCM.00673-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hage CA, Ribes JA, Wengenack NL, et al. . A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis 2011;53:448–54. 10.1093/cid/cir435 [DOI] [PubMed] [Google Scholar]
- 8. Wheat J, French ML, Kohler RB, et al. . The diagnostic laboratory tests for histoplasmosis: analysis of experience in a large urban outbreak. Ann Intern Med 1982;97:680–5. [DOI] [PubMed] [Google Scholar]