Abstract
Orbital cellulitis is the most common subset of orbital inflammatory disease. We describe a patient with necrotic choroidal melanoma who presented with orbital cellulitis. MRI revealed a mass lesion suggesting intraocular melanoma with no extrascleral extension. There was no metastasis on positron emission tomography-CT scan. Enucleation with orbital implant was performed. Histopathological analysis of the specimen revealed intraocular necrotic melanoma with very few recognisable melanoma cells. The necrotic subtype is more commonly associated with extrascleral extension, distant metastasis and poorer prognosis than other melanoma types. Sterile orbital cellulitis may rarely be a manifestation of ocular tumours in adults, and a high index of suspicion should be maintained to rule out the same.
Keywords: ultrasonography, cancer intervention
Background
Orbital cellulitis is the most common subset of orbital inflammatory disease. It is seen more frequently in children compared with adults and most commonly results from extension of sinus disease. The other sources of infection that are more common in adults are trauma, adjacent skin lesions, panophthalmitis and dental infections.1 Non-infectious or sterile orbital cellulitis may be seen in intraocular tumours like retinoblastoma in paediatric age groups and idiopathic orbital inflammatory disease in adults. Here we describe an interesting case of an adult presenting with orbital cellulitis secondary to an unexpected underlying aetiology.
Case presentation
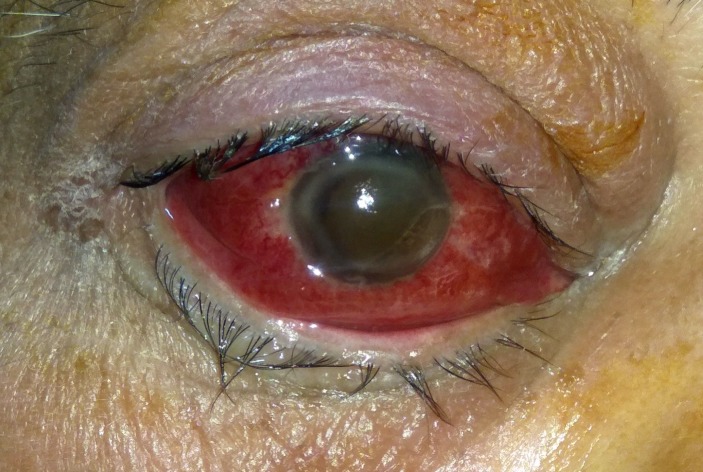
A 56-year-old healthy North Indian man with no history of any systemic illness presented to our centre with a 2-day history of sudden loss of vision in the right eye (OD) associated with severe pain, nausea, progressive proptosis, eyelid oedema and a 1-day history of low-grade fever with chills. There was no history of any periorbital trauma, pre-existing sinus disease or dental procedure. On examination, the best corrected visual acuity was no perception of light OD and 20/20, N6 in left eye (OS). Proptosis values (measured by Hertel’s exophthalmometry) were 24 mm on the right side and 21 mm on the left side. The patient had marked oedema of the upper and lower eyelids on the right side along with severe chemosis (figure 1) and total hyphema which precluded further intraocular examination. Extraocular movements were limited in all gazes. The pupil could not be examined in the OD, whereas the OS did not show any consensual reaction. Ocular examination findings were normal in the OS. Intraocular pressures were 56 mm Hg OD and 14 mm Hg OS. A clinical diagnosis of panophthalmitis with orbital cellulitis was reached, and the patient was started on intravenous antibiotics.
Figure 1.
Clinical photograph of the right eye showing lid oedema, chemosis and total hyphema.
Investigations
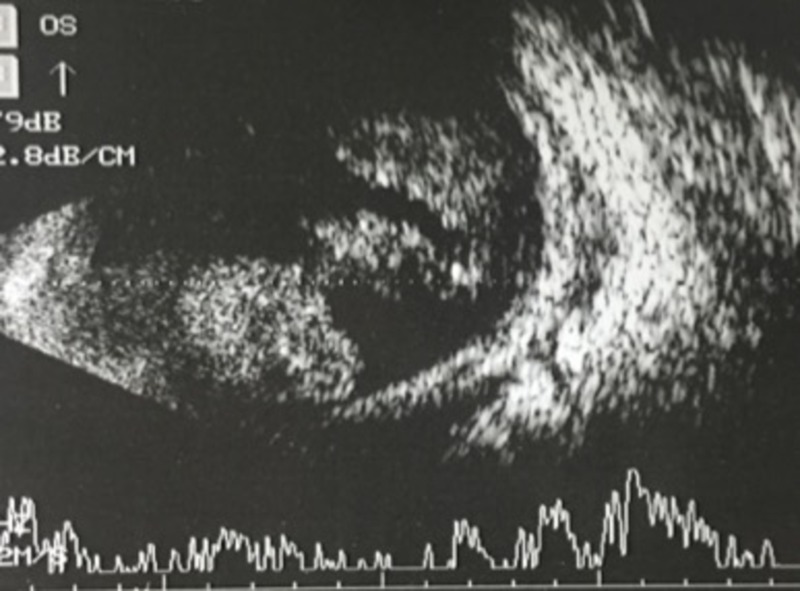
Ocular B-scan ultrasonography OD revealed a solid homogeneous mound-like mass in the vitreous cavity with moderate internal echoes, mixed with mobile moderate-to-high amplitude spikes suggestive of an intraocular mass with vitreous haemorrhage (figure 2). Contrast-enhanced MRI of the orbit revealed an intraocular polypoidal mass, anterior to the equator, which appeared hyperintense on T1-weighted and hypointense on T2-weighted scans (figure 3). T2-hyperintense signals surrounding the mass were suggestive of vitreous haemorrhage. No evidence of extrascleral invasion was observed. Whole body positron emission tomography (PET) CT showed 18-Fluorodeoxyglucose (18FDG)-avid, homogeneously enhancing asymmetric soft tissue thickening which encased the right eyeball circumferentially. Mild hyperdensity was seen in the posterior chamber of the right globe. However, there was no evidence of any metastatic foci.
Figure 2.
Ocular B-scan ultrasound of the right eye showing a solid homogeneous mass in the vitreous cavity with moderate internal echoes surrounded by vitreous haemorrhage.
Figure 3.
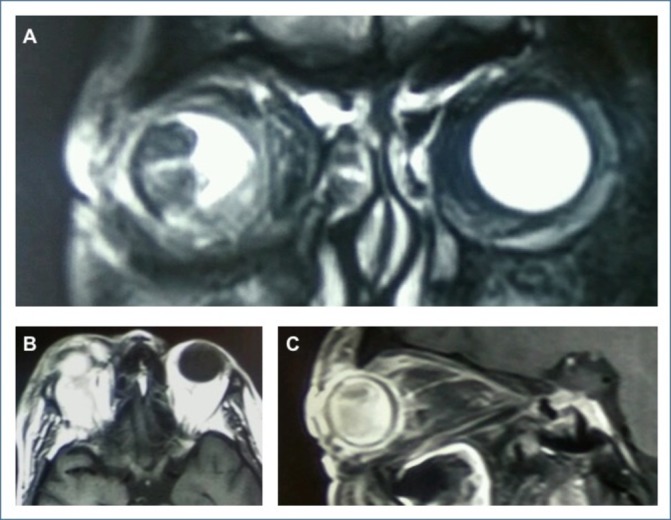
(A) T2-weighted coronal MRI scan of orbits showing hypointense polypoidal mass in the vitreous cavity. (B,C) T1-weighted axial and sagittal MRI scans of the right orbit showing contrast enhancement.
Treatment, outcome and follow-up
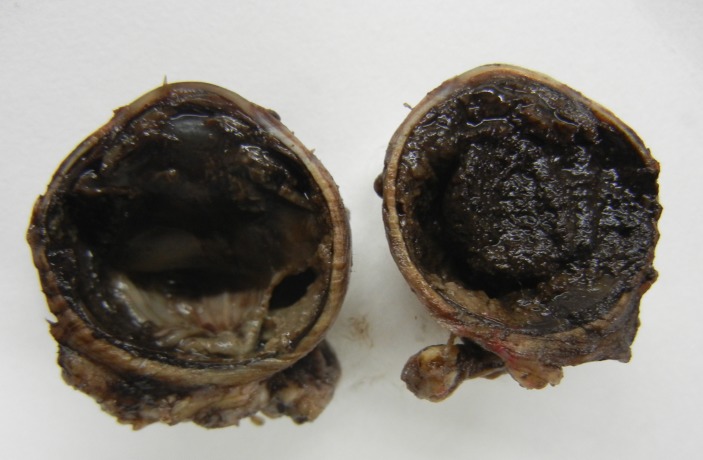
A diagnosis of sterile orbital cellulitis secondary to underlying choroidal melanoma was made, and systemic steroids were started. By the second day of treatment, eyelid oedema and conjunctival chemosis had started resolving. After resolution of orbital cellulitis signs, enucleation (myoconjunctival technique) with a primary non-porous orbital implant was performed. Gross examination of the enucleated globe revealed a 18×18×16 mm pigmented mass in the vitreous cavity involving the iris and ciliary body (figure 4). Histopathological analysis showed presence of 90% necrosis of tumour tissue with very few viable cells (melanoma cells with dark nuclei, pigmented macrophages, erythrocytes). No microscopic scleral invasion or vascular invasion was identified. There was a rich inflammatory cell infiltrate with vascular proliferation extraocularly. At the 6-month follow-up, the patient had no signs of orbital recurrence or systemic metastases.
Figure 4.
Gross specimen of the enucleated eye showing pigmented tumour mass involving most of the vitreous cavity.
Discussion
Uveal melanoma is the most common intraocular malignancy in adults.2 Its most common presenting features are blurred vision, visual field defect, photopsia, floaters, metamorphopsia and pain. However, a large number of patients are asymptomatic too, and may be incidentally diagnosed on routine fundus examination.3 Unlike retinoblastoma which is known to present with orbital cellulitis, sterile orbital inflammation is rarely seen in association with choroidal melanoma. It is important to note that the necrotic subtype is mostly associated with extrascleral extension of the tumour.4 5
In the current case, there was no extrascleral extension on imaging and histopathology. To the best of our knowledge, only two such cases have been described in literature.6 7 The aetiology of this orbital inflammation is most likely the release of inflammatory mediators and cytokines, such as tumour necrosis factor and interleukin-6. Necrotic tissue acts as a potent inciting factor for severe inflammation leading to the development of scleritis, episcleritis and even panophthalmitis in patients with necrotic uveal melanoma.8 Necrotic melanoma is categorised with the mixed type tumours in the modified Callender classification which are associated with worse prognosis.9 Necrotic tumours have a higher chance of associated extrascleral extension than non-necrotic tumours.10 Furthermore, extrascleral extension may have higher chances of distant metastases.11 Reported 15-year survival for patients with necrotic tumours is 40% compared with 81% in those with spindle A type tumours.12 Orbital cellulitis in a case of melanoma should raise suspicion of extrascleral extension, metastases and likely worse prognosis.
PET scan may help in excluding systemic sites of metastasis in these suspicious cases of choroidal melanoma; however, the most important investigation in these patients is orbital MRI. Extrascleral extension of the tumour can only be confirmed on histopathology. In the current case, despite the presence of orbital inflammation, there was no distant metastasis on PET or extraocular extension in histopathology. Therefore, adjuvant radiotherapy was not indicated in our patient.
To conclude, choroidal melanoma may rarely present as orbital cellulitis, especially the necrotic type. Presentation of orbital cellulitis in adults must be carefully investigated with orbital imaging, to exclude possible intraocular tumours. Furthermore, a high index of suspicion is warranted for detecting extraocular extension and metastasis in these cases.
Learning points.
Choroidal melanoma may present clinically as orbital cellulitis.
In these cases, extraocular extension and metastasis should be looked for.
Necrotic melanomas have worse prognosis than other types.
Footnotes
Patient consent for publication: Obtained.
Contributors: All authors have been associated with collection of patient findings and writing the manuscript. PS and SS were involved in writing the manuscript. MB looked after arranging all the data and information of the patient’s management. RM supervised the entire process and finally reviewed the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Verity DH, Rose GE. Infectious processes of the orbit : Albert DM, Miller JW, Jakobiec’s Principles and practices of Ophthalmology. 3rd edition Philadelphia, USA: Elsevier, 2008:2961–71. [Google Scholar]
- 2. Singh AD, Topham A. Incidence of uveal melanoma in the United States: 1973-1997. Ophthalmology 2003;110:956–61. 10.1016/S0161-6420(03)00078-2 [DOI] [PubMed] [Google Scholar]
- 3. Krantz BA, Dave N, Komatsubara KM, et al. Uveal melanoma: epidemiology, etiology, and treatment of primary disease. Clin Ophthalmol 2017;11:279–89. 10.2147/OPTH.S89591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fraser DJ, Font RL. Ocular inflammation and hemorrhage as initial manifestations of uveal malignant melanoma. Incidence and prognosis. Arch Ophthalmol 1979;97:1311–4. 10.1001/archopht.1979.01020020053012 [DOI] [PubMed] [Google Scholar]
- 5. Biswas J, Ahuja VK, Shanmugam MP, et al. Malignant melanoma of the choroid presenting as orbital cellulitis: report of two cases with a review of the literature. Orbit 1999;18:123–30. 10.1076/orbi.18.2.123.2718 [DOI] [PubMed] [Google Scholar]
- 6. Nair AG, Kaliki S, Ali MJ, et al. Intraocular malignant melanoma of the choroid presenting as orbital cellulitis. Int Ophthalmol 2014;34:647–50. 10.1007/s10792-013-9836-1 [DOI] [PubMed] [Google Scholar]
- 7. Nalcaci S, Palamar M, Yaman B, et al. Choroidal malignant melanoma with no extraocular extension presenting as orbital cellulitis. Orbit 2016;35:285–7. 10.1080/01676830.2016.1176216 [DOI] [PubMed] [Google Scholar]
- 8. Moshari A, Cheeseman EW, McLean IW. Totally necrotic choroidal and ciliary body melanomas: associations with prognosis, episcleritis, and scleritis. Am J Ophthalmol 2001;131:232–6. [DOI] [PubMed] [Google Scholar]
- 9. McLean IW, Foster WD, Zimmerman LE, et al. Modifications of callender’s classification of uveal melanoma at the armed forces institute of pathology. Am J Ophthalmol 1983;96:502–9. 10.1016/S0002-9394(14)77914-0 [DOI] [PubMed] [Google Scholar]
- 10. Hykin PG, McCartney AC, Plowman PN, et al. Postenucleation orbital radiotherapy for the treatment of malignant melanoma of the choroid with extrascleral extension. Br J Ophthalmol 1990;74:36–9. 10.1136/bjo.74.1.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paul EV, Parnell BL, Fraker M. Prognosis of malignant melanomas of the choroid and ciliary body. Int Ophthalmol Clin 1962;2:387–402. 10.1097/00004397-196206000-00007 [DOI] [Google Scholar]
- 12. Bujara K. Necrotic malignant melanomas of the choroid and ciliary body. Graefes Arch Clin Exp Ophthalmol 1982;219:40–3. 10.1007/BF02159979 [DOI] [PubMed] [Google Scholar]