Abstract
Ozurdex (Allergan, Irvine, California, USA) is a biodegradable sustained release intravitreal implant containing 0.7 mg dexamethasone in a solid polymer drug delivery system. In the UK, it is approved for use in patients with macular oedema secondary to retinal vein occlusion, diabetic maculopathy and non-infectious uveitis. Although the implant is meant to be injected into the vitreous cavity, it can be inadvertently injected into the crystalline lens. This can also migrate into the anterior chamber, under altered anatomical conditions of the anterior segment. We report a case of incompletely penetrated dexamethasone implant, in a patient undergoing treatment for macular oedema secondary to retinal vein occlusion. The partially penetrated implant was managed conservatively with a good outcome.
Keywords: macula, retina
Background
Complications arising from intravitreal steroid (Ozurdex) implant are known. Inadvertent injection of the implant into the crystalline lens has been reported in the literature. There has been no reported case of incomplete scleral penetration of Ozurdex implant. The management of this is relevant and, in this context, it may be safe to observe without active intervention by surgical manipulation.
Case presentation
A 71-year-old white woman attended the urgent eye care department 6 days following an intravitreal injection of dexamethasone implant (Ozurdex) into her right eye for a branch retinal vein occlusion. This was her third implant to date. Although the procedure was recorded as being uneventful, the patient was concerned about a raised ‘white spot’ in the superotemporal aspect of her globe. She did not have any pain or visual disturbance. The white spot was noted by the patient on the same day following the injection. The patient was bilaterally pseudophakic and had received 12 previous intravitreal injections of ranibizumab (Lucentis) to the same eye, as well as macular grid laser for the same condition. She also received sectoral scatter laser for new vessels elsewhere (NVE), which had responded adequately with complete regression of the vessels. She had a medical history of systemic hypertension and hypercholesterolaemia, for which she took bendroflumethiazide and simvastatin.
On examination, the patient’s best-corrected Snellen visual acuity was 6/6 in the right eye. Slit lamp examination of her eye revealed a firm, raised well-defined whitish nodule measuring about 1mm×1 mm in size with dilated overlying conjunctival vessels. This raised area was subconjunctival with no evidence of disruption of the overlying conjunctiva (figures 1 and 2). There was no anterior chamber or vitreous activity. Intraocular pressure by applanation tonometry measured 14 mm Hg. Dilated fundal examination showed flame shaped and deep retinal haemorrhages in the distribution of the vein occlusion as well as old healed laser scars. The implant was not visible. Ultra-widefield photography was performed which showed the implant firmly stationed in the superotemporal quadrant in relation to the white nodule in the conjunctiva (figure 3). The implant was not free floating in the vitreous cavity as one would expect with an intra ocular Ozurdex implant. This confirmed that the raised area on the outer sclera was indeed due to an incompletely penetrated Ozurdex implant. The case was discussed with the vitreoretinal surgeon, (VR) and given that the implant was subconjunctival observation was recommended.
Figure 1.
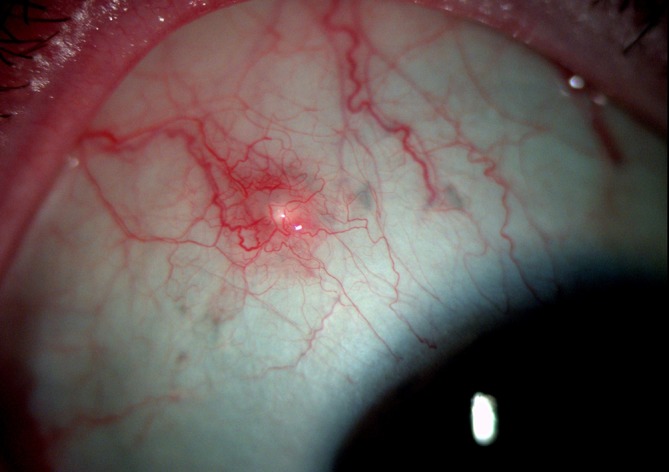
Localised conjunctival hyperaemia over protruding Ozurdex implant.
Figure 2.
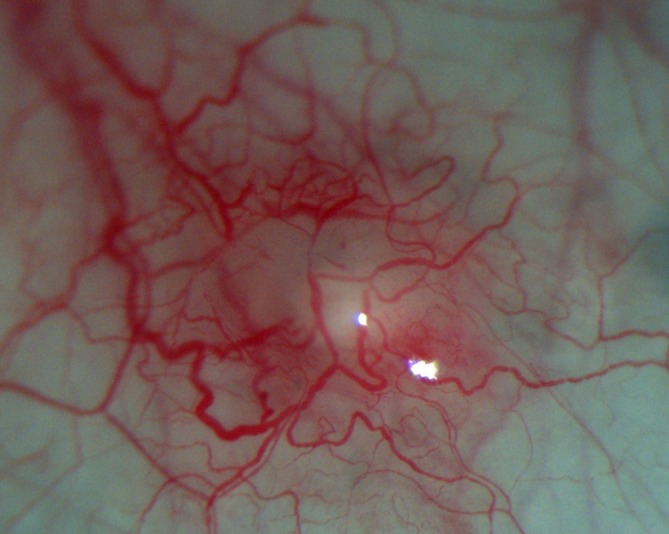
At higher magnification, the intact conjunctiva is demonstrable over the protrusion.
Figure 3.
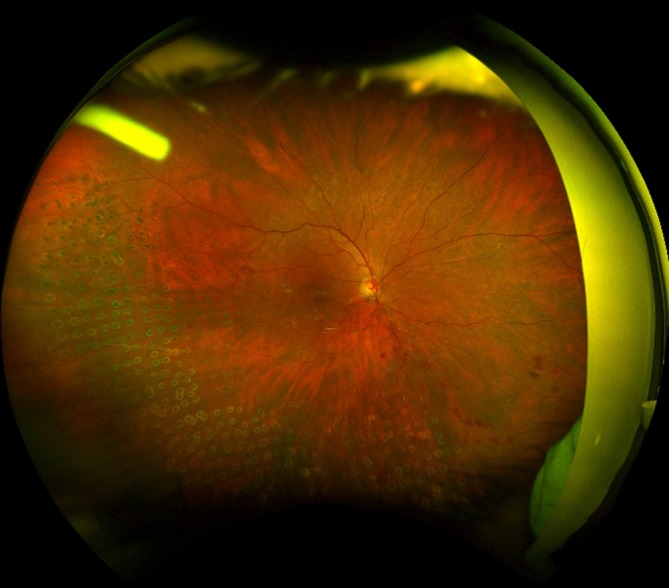
Ultra-widefield photography (Optos) confirms the presence of Ozurdex implant, corresponding to injection site superotemporally.
The patient was reviewed 3 weeks later. The protruding implant was still visible, with no signs of infection or an inflammatory response in the vitreous or anterior chamber. However, the patient’s optical coherence tomography (OCT) scan did demonstrate an increase in retinal thickness and new intraretinal cyst formation. Further observation was advised. At 2 months’ follow-up, the raised white area in the conjunctiva was no longer visible, instead a flat greyish scar was visible at the site of the implant (figure 4). The implant had presumably disintegrated by that time. An OCT scan of the macula showed a suboptimal response. OCT-angiography established the diagnosis of macular ischaemia and hence the inadequate response.
Figure 4.
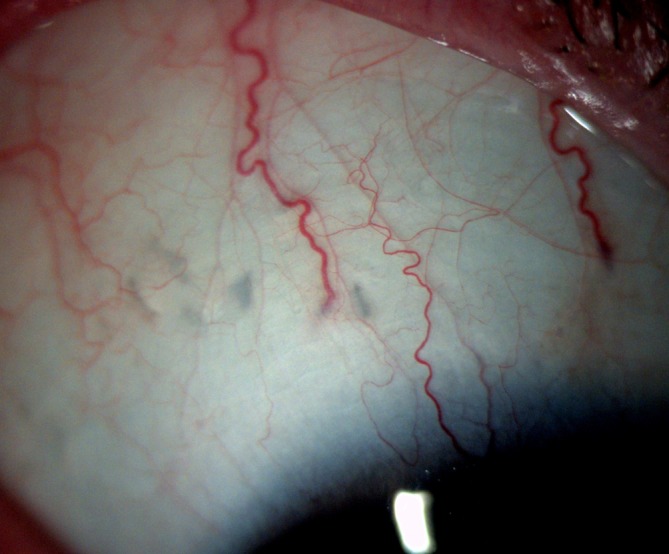
Two months postinjection showing resolution of conjunctival inflammation and disappearance of the incompletely penetrated implant. Note multiple grey scars in the sclera from previous injection.
Investigation
Widefield photography (Optos).
Differential diagnosis
Conjunctival cyst, foreign body.
Treatment
Routine follow-up. No specific treatment related to the abnormal position of the implant.
Outcome and follow-up
Complete recovery from the partially penetrated dexamethasone implant. There was no untoward effect on the vision related to this. She is receiving further intravitreal injections for her retinal pathology with satisfactory visual outcome.
Discussion
We describe a case of a partially penetrated Ozurdex implant. The patient presented to the urgent care department, 6 days following the intravitreal injection although she had noticed the white spot in her eye on the same day. Steroids tend to mask signs of inflammation and even infection can go unnoticed unless the patient notices floaters and or visual disturbance. As the patient had no evidence of endophthalmitis, and the conjunctiva had healed over the injection site, observation was recommended rather than surgically manipulate the implant. The intraocular pressure was also normal, suggesting anatomical integrity of the globe. The implant naturally dissolved over time, without any adverse sequelae.
Management of complication from cases, arising out of intralenticular placement of Ozurdex with or without cataract formation has been described.1–3 Spontaneous migration, of an Ozurdex implant into the anterior chamber in the presence of anterior chamber implant has been described. There are, however, no reports of management of a partially inserted Ozurdex implant. As the conjunctiva is displaced during injection, the entry site is covered by tenons capsule and with the conjunctiva healed over the entry site, the risk of a lid and conjunctival flora being transmitted to the injection site is minimal. At the initial review, the option of opening the conjunctiva and removing the residual extruding Ozurdex was considered. However, this would potentially expose the injection site and increase the risk of endophthalmitis. The other strategy would be, to remove the Ozurdex implant by pars plana vitrectomy. This has been described in a case of endophthalmitis following Ozurdex implant.4This may be appropriate in the setting where intraocular infection has developed. Since there were no signs of infection in our patient, a conservative approach was adopted. This is the first reported case demonstrating that in circumstances where there is no endophthalmitis and the conjunctiva is fully healed over a partially penetrated implant, it is acceptable to adopt a conservative approach and observe patients without surgical intervention.
Abnormal position of the implant does not affect the efficacy of the implant, as has been demonstrated in cases of intralenticular placement of the implant even with a cataract formation.1 3 Anterior chamber migration is more sinister as it may potentially lead to corneal decompensation.5 To the best of our knowledge, this is the first reported case of management of an incompletely penetrated intravitreal Ozurdex implant. From our case, we believe that incompletely penetrated Ozurdex implant may be left alone without any ill effect, if there are no signs of intra ocular infection.
Learning points.
Incomplete scleral penetration of Ozurdex implant is extremely rare.
In the absence of intraocular infection or discomfort, this may be left alone without any untoward effect.
These patients may need close supervision as intraocular infection may be masked by the steroid.
Footnotes
Contributors: TS was involved in following up the patient and also involved in collecting data and initial draft of the case report. VR was involved in concept of the case report, management of the patient and finalising the draft and proof reading of the case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Regan KA, Blake CR, Lukowski ZL, et al. . Intralenticular Ozurdex® - One Year Later. Case Rep Ophthalmol 2017;8:590–4. 10.1159/000485318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berarducci A, Sian IS, Ling R. Inadvertent dexamethasone implant injection into the lens body management. Eur J Ophthalmol 2014;24:620–2. 10.5301/ejo.5000436 [DOI] [PubMed] [Google Scholar]
- 3. Clemente-Tomás R, Hernández-Pérez D, Neira-Ibáñez P, et al. . Intracrystalline Ozurdex®: therapeutic effect maintained for 18 months. Int Ophthalmol 2017. 10.1007/s10792-017-0780-3 [DOI] [PubMed] [Google Scholar]
- 4. Arıkan Yorgun M, Mutlu M, Toklu Y, et al. . Suspected bacterial endophthalmitis following sustained-release dexamethasone intravitreal implant: a case report. Korean J Ophthalmol 2014;28 275–7. 10.3341/kjo.2014.28.3.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Madi HA, Morgan SJ, Ghosh S. Corneal graft failure due to migration of Ozurdex™ implant into the anterior chamber. Am J Ophthalmol Case Rep 2017;8:25–7. 10.1016/j.ajoc.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]


