Description
Aggressive angiomyxoma (AA) is a rare mesenchymal tumour that sporadically arises in soft-tissues of perineum and pelvis. It was first described by Steeper and Rosay1 in 1983 and approximately 300 cases of this neoplasm have been reported in literature since then. It usually presents in premenopausal women, with a peak incidence during the fourth decade of life, and is frequently clinically mistaken with more common benign perineal lesions such as Bartholin’s gland and Gartner’s duct cysts, fibromas, lipomas and leiomyomas. Also, AA may resemble other rare mesenchymal neoplasms as vaginal angiomyofibroblastomas and desmoid tumours which should always be included in the differential diagnosis.
Recurrence and local invasion are the principal causes of morbidity associated to this entity and have been described in 35%–72% of cases.2
Regarding physiopathology, thorough analysis of tumour biology has revealed AA has complex hormonal regulation. Histological samples evidence the expression of oestrogen and progesterone receptors in neoplastic tissue, which seem to play a role in several cellular proliferative pathways as suggested by clinical response to suppressive hormonal therapy.3
As for treatment, there is no global consensus on the preferred management for these lesions, but surgical resection represents the mainstay of care. Wide local excision is sometimes difficult to achieve due to the neoplasm’s infiltrative features and its recurrent nature, which also complicates the clinical scenario occasionally requiring of extensive approaches and perineal reconstructions to attain satisfactory results. Taking this into consideration, several neoadjuvant strategies have been used to decrease recurrence rates and to facilitate removal. Gonadotropin-releasing hormone (GnRH) analogues, aromatase inhibitors and oestrogen/progesterone receptor blockers have been found useful in this context. In some previously reported cases, GnRH analogue therapy resulted in considerable decrease of neoplastic tissue, and even in complete regression of small growths.4 However, it is not yet elucidated whether long-term treatment with this medications is curative or whether recurrence will inevitably appear after discontinuing therapy. Also, radiation and angiographic embolisation have shown successful results in specific scenarios.5 The outcomes of this strategies have not been properly evaluated because of the limited amount of cases for study, and evidence relies on small series and case reports.
A 39-year-old woman with no other medical history besides this current symptom, attended our centre for assessment in November 2016. She had a soft perineal tumour which was initially detected in 2003 measuring 4×3 cm, and had already been subjected to three uncomplicated local resection procedures in public hospitals of Mexico city following subsequent recurrences in 2006 (measuring 4×3 cm), 2008 (measuring 6×7 cm) and 2010 (measuring 5×6 cm).
She referred the lesions had reappeared and progressively grow since 2011, and that she had not received any further treatment from the time of her last surgical intervention (2010).
Physical examination revealed a bulky, soft and mobile vulvar bilobulated tumour extending from the right labia majora to the perianal region with no associated lymphadenopathy.
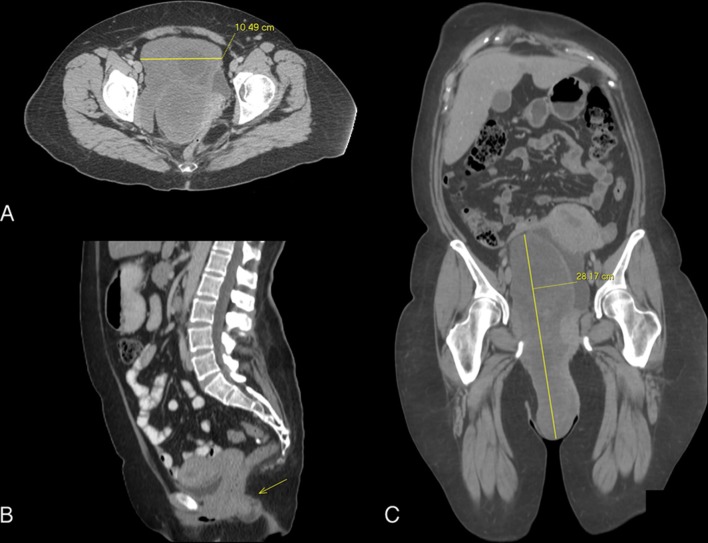
An initial incisional biopsy was obtained, confirming the diagnosis of AA on microscopic analysis. Regarding our diagnostic approach, CT scan revealed a heterogeneous pelvic tumour with copious internal vascularity and multiple hypodense cystic zones suggestive of necrosis. The growth measured 28.1×10.4 cm and extended towards the vaginal canal displacing the uterus, urinary bladder and perineal soft-tissues medially (figure 1). Surgical resection was planned aiming for complete removal.
Figure 1.
CT scan with intravenous contrast showing axial, (A) sagittal (B) and coronal (C) views of the pelvic region displaying a heterogeneous hypodense cystic growth (Yellow arrow) with solid components extending towards the vaginal canal, medially displacing the uterus, urinary bladder, right adnexal structures and perineal soft-tissues.
The procedure was carried out via a transperineal approach. A skin incision was performed delimitating the extrinsic portion of the tumour. Then, it was carefully dissected from the lateral vaginal wall, urinary bladder, uterus and right adnexal structures without compromising their integrity, intending to give wide margins to the surgical piece. After complete excision, meticulous homoeostasis was performed followed by closure of the wound by approximating the skin flaps. The tumour was densely vascularised and adhered to adjacent tissues, which especially made it a lengthy and difficult resection. Nevertheless, the patient was maintained stable and the procedure went on without complications lasting for 235 min with an operative blood loss of 700 mL (figure 2).
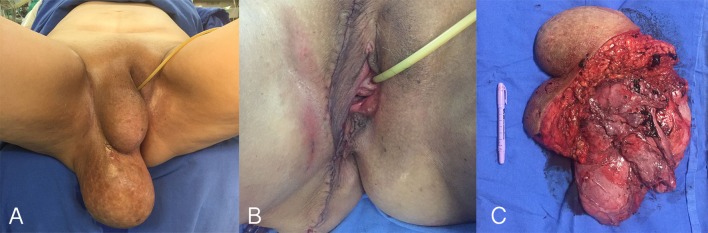
Figure 2.
Perineal region of the patient before surgical resection. (A) Image taken during the immediate postoperative period displaying a closed vertical perineal incision and no observable signs of remaining tumour. (B) Macroscopic image of the excised lesion. Notice the 26×22×7 cm bulky, tan-pink, rubbery, non-encapsulated, poorly circumscribed lobulated mass with a glistening surface blended with surrounding tissues (C).
A 26×22×7 cm tumour was obtained displaying positive margins on microscopic examination. Macroscopically the tumour was a bulky, poorly circumscribed mass that blended with surrounding tissues and had a tan-pink colour and rubbery consistency with a glistening surface (figure 2). Microscopically H&E stain disclosed a mesenchymal neoplasm formed by fibromyxoid stroma and fusiform spindle-shaped tumour cells with ill-defined cytoplasmic borders and no atypia or mitosis, along with abundant collagen fibres and dilated hyalinised thick walled vessels.
Immunohistochemistry revealed the presence of oestrogen and progesterone receptors, and showed reactivity for desmin, smooth muscle actin, vimentin and CD 34. More than 80% of neoplastic cells were found in the sample with a Ki-67 of less than 1% (figure 3).
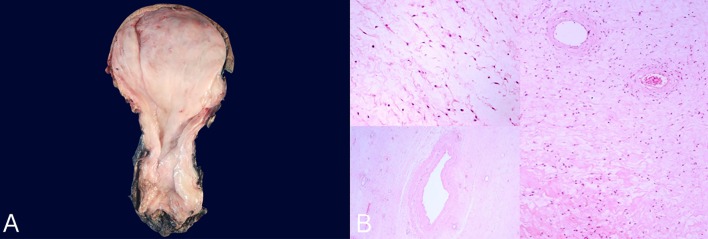
Figure 3.
(A) Clean specimen (26×22×7 cm) consisting of a bulky, tan-pink, rubbery, lobulated mass with a glistening surface. (B)H&E stain (×20) showing mesenchymal neoplasm formed by fibromyxoid stroma and fusiform spindle-shaped tumour cells with ill-defined cytoplasmic borders, no atypia or mitosis and abundant collagen fibres with dilated hyalinised thick walled vessels.
After surgical resection, considering the particular nature of the tumour, we broadly discussed the potential benefits of receiving adjuvant chemotherapy in order to decrease the risk of another recurrence. Different treatment options were thoroughly discussed, and considering the fact that our patient had no further desires of pregnancy, therapy with a GnRH analogue was chosen. Also, before starting treatment, we openly disclosed the risks of developing side effects from this medication, (such as oedema, mild central nervous system symptoms, metabolic and endocrine disturbances, and alterations in liver function tests) and explained to our patient, she would have to be referred to an endocrinologist for regular follow-ups with laboratory tests and imaging studies to screen for abnormalities. Treatment with monthly leuprolide (3.75 mg) was started in February 2016 and has continued until submission of this paper without eventualities. Tumourous recurrence has been excluded with serial clinical evaluations and MRIs (figure 4).
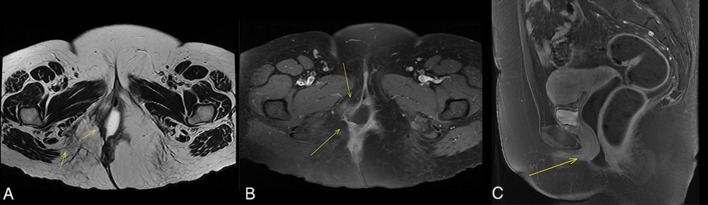
Figure 4.
MRI scan with intravenous contrast (gadolinium) showing (A) T1-weighted and (B) T2-weighted (B Coronal and C Saggital) images displaying no signs of tumour recurrence. Yellow arrows point to the previously affected field and show only a discrete zone of diffuse hyperintensity compatible with postsurgical changes.
AAs are often confused with more common lesions of the perineal area, possibly because their actual extension is never evident until appropriate imaging workup is obtained, which seldom occurs in the first instance in most public hospitals of low-income and middle-income countries as ours. This emphasises the importance of obtaining an initial biopsy for definitive diagnosis before committing to a specific treatment plan in order to be able to consider adjuvant treatment if appropriate. As mentioned before, several useful strategies have been described in the literature, each one with its particular indications; yet, because of the extremely low incidence of the lesion, evidence supporting each approach is weak. In our case, considering the antecedents and particular nature of the tumour and after discussing alternatives with the patient, we decided to offer hormonal blockade thoroughly explaining the secondary effects this therapy could have. After 2 years of follow-up, our patient is satisfied with her outcome and has shown no side effects or complications related to treatment.
Learning points.
Aggressive angiomyxoma (AA) is a rare locally aggressive mesenchymal tumour that arises in the perineal region of women in reproductive ages.
AA has high recurrence rates and has shown variable responses to several adjuvant therapies such as hormonal blockade, radiotherapy and angioembolisation.
Evidence supporting this approaches is scarce because of the limited availability of cases for study.
Footnotes
Contributors: JA-F, CR-C, JHR-Q and HM-F were responsible for planning of the case and conceived the original idea. JA-F, CR-C and JHR-Q wrote the manuscript. HM-F helped supervise and conduct the project. The design of the manuscript was done by JA-F, CR-C, JHR-Q and HM-F. Aquiring of data was achieved by JA-F and CR-C and aquiring and preparing of the images was done by JHR-Q and HM-F. The interpretation of data was performed by JA-F, CR-C, JHR-Q and HM-F.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Steeper TA, Rosai J. Aggressive angiomyxoma of the female pelvis and perineum. Report of nine cases of a distinctive type of gynecologic soft-tissue neoplasm. Am J Surg Pathol 1983;7:463–75. [DOI] [PubMed] [Google Scholar]
- 2. Ma J, Wan J, Jiang B, et al. Huge abdominal and perineal aggressive angiomyxoma: a misdiagnosed case report and literature review. Cell Mol Biol 2018;64:110–3. 10.14715/cmb/2018.64.6.18 [DOI] [PubMed] [Google Scholar]
- 3. Malukani K, Varma AV, Choudhary D, et al. Aggressive angiomyxoma in pregnancy: a rare and commonly misdiagnosed entity. J Lab Physicians 2018;10:245 10.4103/JLP.JLP_179_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chong C. Aggressive angiomyxoma. Nurse Pract 2016;41:1–4. 10.1097/01.NPR.0000490398.27464.0b [DOI] [PubMed] [Google Scholar]
- 5. Brzezinska BN, Clements AE, Rath KS, et al. A persistent mass: a case of aggressive Angiomyxoma of the vulva. Gynecol Oncol Rep 2018;24:15–17. 10.1016/j.gore.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]