Abstract
Gut microbiota in a healthy population is shaped by various geographic, demographic and lifestyle factors. Although the majority of research remains focused on the bacterial community, recent efforts to include the remaining microbial members like viruses, archaea and especially fungi revealed various functions they perform in the gut. Using the amplicon sequencing approach we analysed bacterial and fungal gut communities in a Slovenian cohort of 186 healthy volunteers. In the bacterial microbiome we detected 253 different genera. A core microbiome analysis revealed high consistency with previous studies, most prominently showing that genera Faecalibacterium, Bacteroides and Roseburia regularly comprise the core community. We detected a total of 195 fungal genera, but the majority of these showed low prevalence and are likely transient foodborne contaminations. The fungal community showed a low diversity per sample and a large interindividual variability. The most abundant fungi were Saccharomyces cerevisiae and Candida albicans. These, along with representatives from genera Penicillium and Debaryomyces, cover 82% of obtained reads. We report three significant questionnaire-based host covariates associated with microbiota composition. Bacterial community was associated with age and gender. More specifically, bacterial diversity was increased with age and was higher in the female population compared to male. The analysis of fungal community showed that more time dedicated to physical activity resulted in a higher fungal diversity and lower abundance of S. cerevisiae. This is likely dependent on different diets, which were reported by participants according to the respective rates of physical activity.
Introduction
The human gut microbiota is a diverse community comprised of bacteria, fungi, archaea, viruses and protozoa. These microorganisms co-exist in a complex interdependence, shaped by countless microbe-microbe and microbe-host interactions. Bacteria dominate the gut microbiome, representing approximately 99.9% of the total cell population. Fungi, archaea and protozoa combine to fulfill the remaining 0.1% [1,2]. Although bacterial community remains the primary focus of gut microbiota research, recent studies on archaea [3,4], viruses [5,6] and fungi (mycobiome) [7–9] indicate potential involvement of these microbial groups in health and disease.
Studies on bacterial microbiota largely focus on the comparison between different patient populations and healthy controls, striving to define disease specific microbial patterns [10–12]. Major steps towards understanding the healthy microbiota were made in 2008 when both the American Human Microbiome Project (NIH) and the European MetaHIT project launched with the objective to optimize and standardize analytical methods, and increase the size of studied cohorts in order to better address the interindividual variability [1,13,14]. Human gut microbiota was, up until now, mostly studied in relation to age, diet and lifestyle-related changes [2,15–19] as well as human genetics by screening twin-pairs [20]. Noteworthy are two recent large cohort studies on healthy Belgian and Dutch populations, which succeeded to associate bacterial microbiome patterns with a comprehensive collection of host and environmental factors [21,22].
The fungal community received little attention up to date, especially in the healthy population. Reported concentrations of fungi in stool samples range from 0 to 109 CFU g-1 per stool, indicating high interindividual variability [23,24], but the discrepancies between culture-dependent and culture-independent methods question the reliability of these estimates [23,25,26]. Even at several magnitudes lower count compared to the bacteria, fungi show significant patterns in different gastrointestinal and other diseases, especially in the immune-compromised patient populations [27], as well as various interactions with the host immune system [28]. The beneficial effects of fungi in human gastrointestinal tract, on the other hand, are not well known. Certain commercially available probiotics already utilize the ability of Saccharomyces strains to limit the inflammatory response and increase immune health [29]. Certain filamentous fungi with the potential to metabolize complex plant-derived carbohydrates were primarily studied in insects [30] and rumen of cattle [31], but have also been detected in humans [8].
Here we present a study including a Slovenian cohort of 186 healthy volunteers. Both the bacterial and fungal gut community structures were analyzed in relation to a set of 13 host specific factors. We report age- and gender-associated patterns in the bacterial communities, and a weak association between the time dedicated to physical activity and the fungal community, which is likely influenced by different dietary habits coinciding with physical activity.
Methods
Stool samples were collected from 186 healthy volunteers from Maribor (Slovenia) and the surrounding area. Samples included in the final analysis were required to be from participants who were at least 18 years old and without any gastrointestinal infection or surgical procedure on the gastrointestinal tract 3 months prior to sample collection. Participants diagnosed with chronic inflammatory diseases were also excluded. Stool samples were collected together with a completed questionnaire and a written informed consent in accordance with the approval of the Republic of Slovenia National Medical Ethic Committee. Upon collection, each sample was anonymized, deidentified and was further processed only with a study code.
Questionnaire covered information on volunteers age, gender, body mass index, type of diet (regular, vegetarian, vegan, lactose free, gluten free or raw food), antibiotic therapy in last 3 months (yes or no), hospitalization in last 3 months (yes or no), digestion (regular, occasional constipation or regular constipation), physical activity (none or occasional exercise, exercise approximately once a week, exercise multiple times weekly or active athlete), surgical removal of cecum (yes or no), probiotics or prebiotics usage (yes or no), smoking (yes or no) and level of stress (1–5) (Table 1).
Table 1. Host/environmental factors with sample distribution and PERMANOVA analysis.
A list of host and environmental factors, which were collected with questionnaire, with corresponding distribution of samples inside their respective factor category. To the right we show the results of the PERMANOVA test (1000 permutations, Bray-Curtis distances), presented with the value for explained variance (R2) for each factor in relation to the bacterial or fungal community. Host/environmental factors with a significant P value, after adjustment with Benjamini-Hochberg correction (FDR < 0.05), are highlighted in grey.
| PERMANOVA | ||||
|---|---|---|---|---|
| Host factor | Categories | Distribution of samples | Explained variance (R2) in bacterial community | Explained variance (R2) in fungal community |
| Gender | Male | 69 (37.1%) |
0.01114 (P = 0.026) |
0.00446 |
| Female | 117 (62.9%) | |||
| Age | min 18, max 85, mean 45.2 |
0.01065 (P = 0.045) |
0.00387 | |
| Diet | Regular | 151 (81.2%) | 0.03633 | 0.05478 |
| Vegetarian | 23 (12.4%) | |||
| Vegan | 5 (2.7%) | |||
| Gluten free | 2 (1.1%) | |||
| Lactose free | 4 (2.2%) | |||
| Raw food diet | 1 (0.5%) | |||
| BMI | min 16.5, max 38.7, mean 24.8 | 0.0081 | 0.00622 | |
| Antibiotic therapy in the last 3 months | Yes | 12 (6.5%) | 0.00724 | 0.00519 |
| No | 174 (93.5%) | |||
| Hospitalization in the last 3 months | Yes | 5 (2.7%) | 0.0063 | 0.00333 |
| No | 181 (97.3%) | |||
| Digestion rate | Regular | 158 (84.9%) | 0.00598 | 0.00272 |
| Occasional constipation | 25 (13.4%) | |||
| Frequent constipation | 3 (1.6%) | |||
| Time dedicated to physical activity | Occasionally | 75 (40.3%) | 0.00578 |
0.04188 (P = 0.026) |
| Once per week | 49 (26.3%) | |||
| Multiple times per week | 58 (31.2%) | |||
| Active athlete | 4 (2.2%) | |||
| Cecum removal | Yes | 11 (5.9%) | 0.00579 | 0.00238 |
| No | 175 (94.1%) | |||
| Chronic disease | None | 166 (89.2%) | 0.01654 | 0.01203 |
| IBS | 3 (1.6%) | |||
| Unspecific | 17 (9.1%) | |||
| Probiotics or prebiotics usage in the last 3 months | Yes | 27 (14.5%) | 0.00441 | 0.00744 |
| No | 159 (85.5%) | |||
| Smoker | Yes | 20 (10.8%) | 0.00439 | 0.00433 |
| No | 166 (89.2%) | |||
| Stress | 1–5 | mean 3.151 | 0.00389 | 0.03556 |
Stool samples were collected in sterile containers. Faecal material was mixed thoroughly with an inoculation loop, a portion corresponding to approximately 50 μL was stored in 1 mL of Inhibitex buffer (QIAamp Fast Stool DNA Mini Kit, Qiagen) and frozen at -80°C until further use.
Isolation of the total bacterial DNA and high-throughput 16S rDNA amplicon sequencing
Total DNA was isolated from stool samples using QIAamp Fast Stool DNA Mini Kit (Qiagen, Hilden, Germany) with mechanical disruption (MagNA Lyser, speed 7000 for 70 s).
The bacterial community composition was determined by sequencing the V3V4 hypervariable region of the 16S rRNA gene using a broad-range set of primers Bakt_341F (5'-CCTACGGGNGGCWGCAG-3')–Bakt_805R (5'-GACTACHVGGGTATCTAATCC-3') [32]. The library preparation was performed according to the recommended Illumina 16S Metagenomic Sequencing Library Preparation manual protocol (Illumina, CA, USA). The fungal community composition was determined by sequencing the Internal Transcribed Spacer 2 (ITS2) using a broad-range set of primers ITS86F (5'-GTGAATCATCGAATCTTTGAA-3')–ITS4R (5'-TCCTCCGCTTATTGATATGC-3') [33]. The library was prepared according to the 16S Metagenomic Sequencing Library Preparation manual (Illumina, CA, USA) with the exception of using Q5 High-Fidelity DNA Polymerase (NEB, Massachusetts, USA) instead of the recommended KAPA HiFi HotStart ReadyMix (Kapa Biosystems, Massachusetts, USA). Sequencing was performed on the Illumina MiSeq platform with MiSeq Reagent Kit V3 (2x300 cycle, 10% PhiX).
Sequence data analysis
The analysis in mothur (v.1.36.1) [34,35] was done according to the MiSeq standard operating procedure (SOP) for Illumina paired end reads. The bacterial 16S rRNA reads were processed using the following criteria: i) reads were not allowed any ambiguous bases and the maximum homopolymer length was set to 8 base pairs (bp); ii) The reads were aligned against the Silva reference alignment (Release 123); iii) Chimeras were identified using the UCHIME algorithm; iv) The classification of reads was performed using the RDP training set (v.9) with 0.80 bootstrap threshold value; v) Sequences were clustered into operational taxonomic units (OTUs) at the 97% similarity cut-off. After quality filtering we obtained an average depth of 35484 reads per sample (min 297, max 89229). OTUs represented in the abundance less than 0.01% of total number of reads were removed followed by rarefying each sample to 3000 reads. Samples with less than 3000 reads were removed from further analysis (n = 1). Alternatively, normalization of contingency table as described by Lagkouvardos et al., 2017 [36] was tested, but did not significantly impact the final set of OTUs nor their relative abundance. Method reportedly introduces lower bias in the representation of low abundant OTUs, but this advantage was not observable in our dataset due to our conservative approach to remove OTUs with overall abundance less than 0.01%.
Fungal ITS2 reads were processed using following criteria: i) The reads were not allowed any ambiguous bases; ii) The removal of reads shorter than 205 bp or longer than 502 bp; iii) The removal of reads containing homopolymers longer than 12 bp; iv) ITSx software was used for binning in order to remove non-fungal reads; v) The reads were aligned pairwise using the Needleman-Wunsch method (rewards +1 for a match and penalizes with -1 and -2 for a mismatch and gap, respectively); vi) The sequences were clustered into operational taxonomic units (OTUs) at a 98% similarity cut-off; vii) The classification was inferred using UNITE ITS database (version 6) with 0.80 bootstrap threshold value. After quality filtering we obtained an average depth of 20901 reads per sample (min 504, max 66742). OTUs represented in the abundance less than 0.01% of total number of reads were removed followed by rarefying each sample to 950 reads. Samples with less than 950 reads were removed from further analysis (n = 6).
The sequence data was deposited in the form of combined paired end reads (contigs) on the Metagenomics RAST (MG-RAST) database server (http://metagenomics.anl.gov/) under the project access number mgp85661 (https://www.mg-rast.org/linkin.cgi?project=mgp85661). Seven samples (all fungal ITS2 metagenomes) did not meet the minimum criteria of 1000000 bp per sample as required by MG-RAST and are available along with metadata for all samples in Supporting Information (S1 Appendix).
The statistics and graphic representation were done in mothur (v 1.31.1) and R (version 3.1.3) using packages ‘ggplot2’ and ‘vegan’.
Core community analysis
The core community was defined in our study as selection of OTUs with a relative abundance of at least 0.1% and present in more than 95% of tested samples. To compare our results with already published data, we found 4 studies, in which comparable information was either reported in the article or could be extracted from supplementary information. These studies include 1) a study on combined Belgian Flemish Gut Flora Project (FGFP; discovery cohort; N = 1106) and the Dutch LifeLines-DEEP study (LLDeep; replication; N = 1135); The criteria for a core community was the presence of a genus in at least 95% of the tested population [21]; 2) A study on the collection of samples from the Human Microbiome Project (HMP, n = 238); The criteria for a core community was the presence of OTUs in at least 95% of the tested population [37]; 3) A study on a Mongolian cohort (n = 64); The criteria for a core community was the presence of OTUs in at least 90% of the tested population [38] and 4) a study on European individuals (n = 124). Authors used shotgun metagenomic sequencing, therefore the results were presented at the species taxonomic level. To ensure consistency, we used only information on genus taxonomy with the criteria of taxa being detected at the minimum 10% reference coverage and present in at least 95% of the tested samples [1].
Results and discussion
Analysis of bacterial community
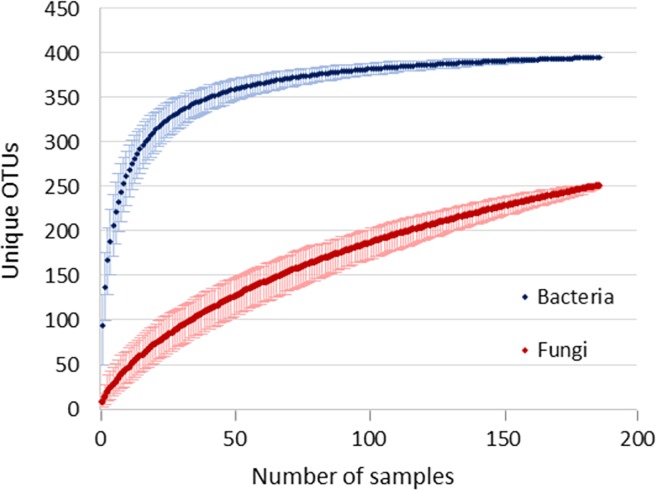
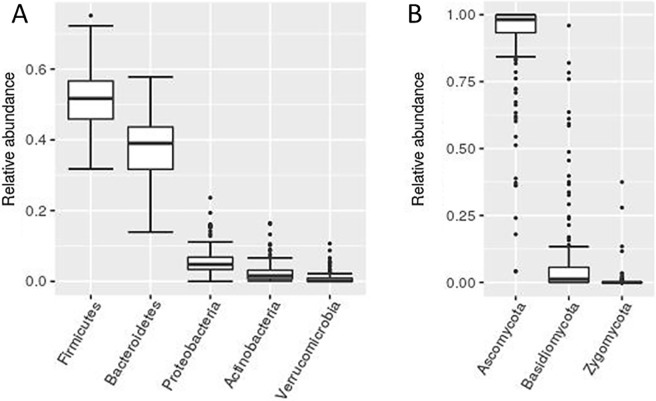
Amplicon sequencing approach targeting V3V4 variable region of 16S rRNA gene was used to investigate the bacterial communities in a group of 186 healthy volunteers from the Slovenian population. After quality filtering we obtained an overall richness of 27852 OTUs corresponding to 253 bacterial genera. This falls short off the projected richness of 294 genera (Chao1 richness index), which would require an estimated 702 additional samples to reach. After the removal of low abundance OTUs (overall relative abundance < 0.01%) and subsampling the remainder to 3000 reads per sample, we obtained 395 bacterial OTUs (Fig 1), on average 121.2 OTUs per sample (S1 Table). Out of 7 detected bacterial phyla, Firmicutes and Bacteroidetes show the highest abundance as well as interindividual variability (Fig 2A), but in contrast to some related studies, we found no significant correlation between the Firmicutes/Bacteroidetes ratio and host/environmental factors [39,40].
Fig 1. Rarefaction curves for bacterial and fungal OTUs.
Rarefaction curves for bacterial (blue) and fungal (red) OTUs were calculated by rarefying both bacterial and fungal community to 950 reads/sample (only for this specific analysis we rarefied bacterial community to 950 reads/sample in order for the rarefaction curves to be comparable). Plotted data points represent the mean value of OTUs for the respective number of samples (1000 iterations) with a 95% confidence interval.
Fig 2. Bacterial and fungal phyla relative abundance.
A box plot presentation of relative abundances of bacterial (A) and fungal (B) phyla. Only phyla with an overall relative abundance greater than 1% are shown.
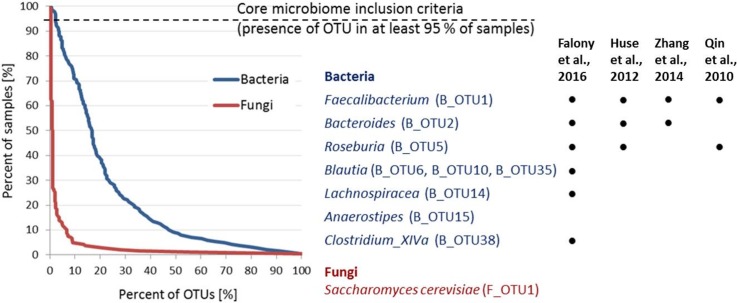
The bacterial core community, defined as OTUs with relative abundance of at least 0.1% and present in 95% of samples or more, consists of 9 OTUs, classified into 7 different bacterial genera (Fig 3). The two most abundant core OTUs correspond to the genus Faecalibacterium (B_OTU1; B_ and F_ prefixes before OTU indicate bacterial and fungal OTU, respectively) and Bacteroides (B_OTU2). The remaining 7 OTUs all classify to the family Lachnospiraceae, with the most prevalent being genus Blautia (B_OTU6, B_OTU10, B_OTU35) along with single representatives from genera Roseburia (B_OTU5), Lachnospiracea (B_OTU14), Anaerostipes (B_OTU15) and Clostridium XIVa (B_OTU38) (Fig 3, S1 Table).
Fig 3. Core microbiome analysis.
The core microbiome is shown as a percent of samples (%) that include the corresponding percent (%) of fungal (red) and bacterial (blue) OTUs. To the right is the list of bacterial (n = 9) and fungal (n = 1) OTUs, which meet the criteria for inclusion into the core community. We compared our observed core taxa with four other studies also reporting core communities (information on studied cohorts and core community inclusion criteria are included in Materials and Methods). The dot indicates that the core taxa identified in our cohort was also reported by respective study at genus or family taxonomic level.
The bacterial core microbiome in healthy population was previously analyzed in different studies, including European [1,21], American [37] and Mongolian cohorts [38]. Despite the different methodologies used, we observed a high degree of consensus with their results, especially in the case of the combined dataset from FGFP (Belgian Flemish healthy cohort), LLDeep (Dutch healthy cohort) and some other U.K. and U.S. studies [21] (Fig 3). Among the 14 core genera reported in this combined dataset, 6 coincide with our set of core OTUs, although only at the family taxonomic level in case of Lachnospiraceae. Genus Anaerostipes was the only additionally detected genus in our analysis while 7 genera were detected by Falony et al., but not by us [21]. Comparing our results to all four other studies we found that at this core inclusion criteria, Faecalibacterium always comprises core community, while Bacteroides and Roseburia each failed to be included into core community by only one of the other four studies. It should be noted that in the study by Qin et al., Bacteroides vulgatus missed the core community inclusion criteria by a single study participant. Other core genera reported by four comparator studies vary substantially among investigated cohorts [21,37] (Fig 3). Recently, researchers became more inclined to investigate the bacterial “functional core” using shotgun metagenomic approach [41]. Functional profile showed more redundancy among individuals [42] as a result of same metabolic traits being performed by a variety of different bacterial groups. Still, our findings in congregation with others support, to some extent, the “outdated” idea of a taxonomical core. Observed core genera either indicate a common microbial evolution [43] or a possession of a unique set of traits which facilitate their persistence in the gut despite the alternating environment. Defining the core community at the species level and identifying unique metabolic traits of these taxa might further elucidate this observations.
Age and gender associated differences in bacterial community
We identified gender and age to be significantly associated with the bacterial microbiome, jointly explaining 2.2% of the interindividual bacterial community variation (Permutational multivariate analysis of variance (PERMANOVA) using Bray-Curtis distances, false discovery rate (FDR) < 0.05). All host/environmental factors, collected with the questionnaire, and PERMANOVA test results are shown in Table 1.
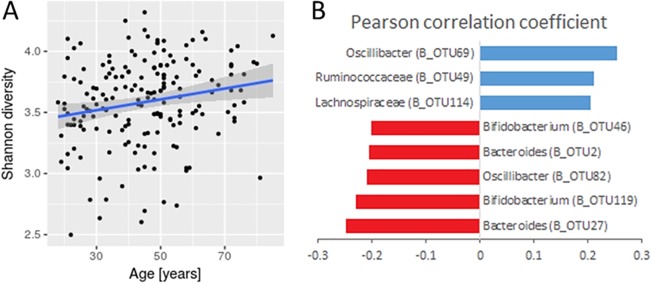
We observed an age-associated increase in bacterial richness and diversity (Pearson's r = 0.217, P = 0.003 and r = 0.213, P = 0.003 for Chao1 and Shannon indices, respectively) (Fig 4A). The Pearson correlation test most prominently showed an age-related decrease in the genera Bifidobacterium (B_OTU46, B_OTU119) and Bacteroides (B_OTU2, B_OTU27), and an increase in representatives from the genus Clostridiales (B_OTU49, B_OTU69, B_OTU114) and unclassified Proteobacteria (B_OTU93) (Fig 4B).
Fig 4. Age-associated changes in bacterial communities.
Graph shows bacterial community Shannon diversity index in relation to age. The linear regression indicates the increase of the Shannon diversity with age and is presented with a 95% confidence interval (A) (Pearson's r = 0.213, P = 0.003). The bar plot shows Pearson correlations of bacterial OTUs that significantly increase (blue) or decrease (red) in relative abundance with age (FDR < 0.05) (B).
Multiple studies have looked into the dynamics of microbiota throughout the human lifespan with mostly contradicting results. As previously reported, we also observed an age-related increase in Proteobacteria and a decrease in the genera Bifidoacterium and Bacteroides [44–46]
However, we did not confirm the often-reported age-associated decrease of Faecalibacterium and Clostridium cluster XI [47,48]. In addition, a significant increase in known short chain fatty acids producers from families Ruminococacceae and Lachnospiraceae observed in this study to some extent contradict the previously reported lower availability of total SCFAs in the elderly population [19]. The decline in SCFAs levels and other changes in metabolome were suggested to be linked with a transition from a saccharolytic metabolism, typical in adults, towards a predominantly putrefactive metabolism [49]. But these changes might very well be environment dependent and therefore vary between cohorts based on subjects long-term and short-term dietary habits [50].
Contrary to common narrative [47,49], we observed a slight increase in bacterial community diversity and richness with age, but similar trends have already been reported by others [22,51]. The discrepancies between studies likely arise from generation-specific dietary habits and lifestyle. Therefore, longitudinal studies, accounting for interindividual variability and age-related physiological changes, are needed to improve our knowledge on aging microbiota.
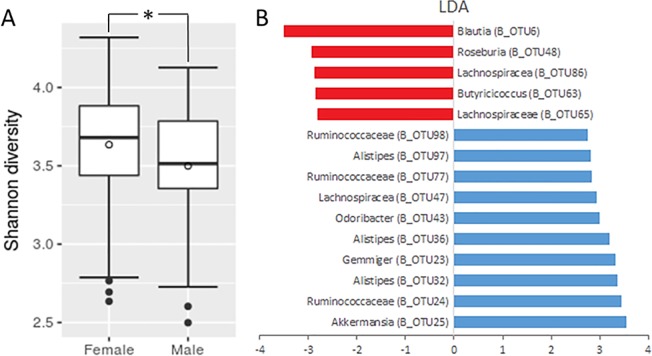
Bacterial communities in our cohort significantly differ between males and females (AMOVA, P < 0.001), with females showing a slightly higher Shannon diversity (Kruskal-Wallis test, P = 0.014) (Fig 3A) (Fig 5A). In the male population, we observed a significant increase in the abundance of several OTUs from the order Clostridiales (B_OTU6, B_OTU48, B_OTU63, B_OTU65, B_OTU86), while females most prominently display higher abundance of Akkermansia (B_OTU25) in addition to multiple OTUs corresponding to the family Ruminococcaceae (B_OTU24, B_OTU77, B_OTU98) and genus Alistipes (B_OTU32, B_OTU34, B_OTU97) (Fig 5B).
Fig 5. Gender-associated differences in bacterial communities.
A box plot presentation of Shannon diversity indices in females compared to males (Kruskal-Wallis test, P = 0.014) (A). The bar plot presents LEfSe results showing LDA values for OTUs, which were significantly increased in males (red) and females (blue).
Gender often ranks high among host covariates associated with bacterial microbiota [21,22,52,53]. In agreement with our findings, Borgo et al. and Zhernakova et al. already reported higher bacterial diversity in female populations. It should be noted that Borgo et al. reported gender specific changes in bacterial diversity exclusively for mucosa-associated microbiota, while lumen-associated microbiota showed no alpha or beta diversity distinction between genders [22,53]. Specific gender-associated patterns in bacterial community vary among studies. Our observed changes are predominantly characterized by a rearrangement of several taxa corresponding to Firmicutes, while Dominianni et al. most notably showed a female-associated decrease in Bacteroidetes and Borg et al. reports a female mucosa-associated enrichment with Bifidobacterium and a depletion in Veillonellaceae [52,53]. These patterns do not seem to be diet-related, since dietary groups in our cohort showed a balanced distribution between genders. Additionally, both host covariates (gender and diet) showed non-related changes in bacterial communities (nested PERMANOVA, P = 0.370). Nevertheless, it should be noted, that energy metabolism varies between genders even under the same dietary regime [54,55]. In agreement with previous studies, we conclude that gut microbiota shows gender-specific patterns, but these seem to be cohort specific. Better understanding will follow from an incorporation of gender-associated differences in physiology [56] and immune response [57] into gut microbiota studies.
Analysis of fungal communities
Amplicon sequencing of ITS2 spacer region was used to investigate fungal communities. After quality filtering we obtained an overall richness of 2158 OTUs corresponding to 195 fungal genera, which is just one short of predicted 196 (Chao1 richness index), indicating that the majority of the present fungal genera were most likely detected. After the removal of low abundance OTUs (overall relative abundance < 0.01%) and rarefying the remainder to 950 reads per sample we obtained 251 fungal OTUs (Fig 1), on average 7.5 OTUs per sample (S2 Table). Analysed fungal community is largely dominated by representatives from Ascomycota (Fig 2B), further emphasized by the fact that the 4 most abundant fungal OTUs, Saccharomyces cerevisiae (F_OTU1), Candida albicans (F_OTU2) and unclassified species from genera Penicillium (F_OTU3) and Debaryomyces (F_OTU4) (detected in 98.9%, 61.8%, 21% and 50% of samples, respectively), together cover over 80% of total obtained reads. In comparison, it requires top 82 bacterial OTUs to reach the same total read coverage. Fungal communities exhibit low diversity and high interindividual variability. Individual fungal OTUs appear on average in 5.6 out of 186 tested samples (3%). Consequently, only Saccharomyces cerevisiae met the criteria for inclusion in the core community (Fig 3, S2 Table). However, it is highly likely that substantial proportion of S. cerevisiae sequences were food derived.
Compared to bacteria, fungi introduce the additional problem of differentiating between gut commensals and transient colonizers, which derive primarily from food. Generally, the minimum criteria to consider a fungus as a potential commensal, is its successful growth at 37°C [58]. Although multiple species meet this criteria, some studies rightfully question the ability of fungi to persist in the gut microbiota [9]. Still, it is important to note that mucosa-associated fungi reportedly show more stability compared to luminal communities [59]. Yeast S. cerevisiae is usually among the most abundant fungi detected in the gut [9,60] and is also the most prevalent as well as abundant fungal OTU in our study. It was shown though, that despite the ability to grow at 37°C, S. cerevisiae does not persist in the gut for more than about 3 days [61], and is therefore not considered a true commensal. Fungi most often reported to colonize the gut include representatives from the genera Candida, Malassezia, Cladosporium and yeast from the Dipodascaceae family [8,62]. Other commonly detected fungi in the gut, which are not able to grow at 37°C, include foodborne species from the genera Debaryomyces and Penicillium. These fungi are often used at different food processing stages or are present as unwanted food contaminants. They can also comprise normal skin or oral microbiota [15,63–66]. A recent publication investigated the composition changes of fungal communities during periods of controlled diet [9]. The authors demonstrated the importance of food and the oral cavity as the major sources of commonly detected fungi, showing that switching to a S. cerevisiae free diet or improving oral hygiene resulted in a significant decrease of S. cerevisiae or C. albicans abundance in stool, respectively [9].
Differences in fungal communities associated with physical activity
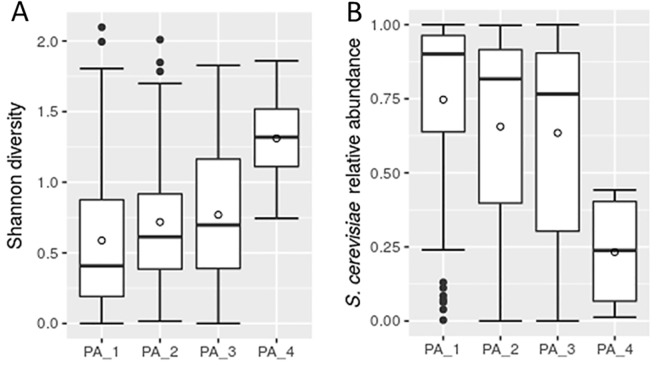
Physical activity was the only significant covariate associated with fungal microbiome, explaining 4.2% of interindividual fungal community variation (PERMANOVA using Bray-Curtis distances, false discovery rate (FDR) < 0.05). The physical activity factor was defined in the questionnaire with 4 categories depending on the time participants dedicated to sport and recreational activities, ranging from none or occasional exercise to an active athlete. We found, that more frequent physical activity correlated with an increase in the total fungal diversity (Spearman's r = 0.217, P = 0.003) and a decrease in the abundance of S. cerevisiae (F_OTU1) (Spearman's r = -0.217, P = 0.003) (Fig 6). Additionally we report, that S. cerevisiae was significantly associated with lower overall fungal community diversity (Pearson's r = 0.712, P<0.001) (S1 Fig), further supported by negative correlations it exhibits with highly abundant C. albicans (F_OTU2) and unclassified Debaryomyces (F_OTU4) (S3 Fig).
Fig 6. Changes in fungal microbiota associated with the reported rate of physical activity.
Box plots presenting the Shannon diversity indices (A) and S. cerevisiae abundance (B) according to the rate of reported physical activity. Physical activity was defined with four categories, i.e. PA_1—occasional exercise; PA_2—exercise approximately once a week; PA_3—exercise multiple times weekly; PA_4—active athlete.
Distinct patterns, associated with physical activity, were recently reported in relation to bacterial communities. Authors mainly emphasized the physical activity-associated increase in bacterial diversity and the decrease in relative abundance of Bacteroides [67,68], but we did not observe any related changes in the bacterial community analysed in our cohort. We would like to note, that the physical activity variable showed correlation with different dietary habits these groups reported. Most notably, there were significant discrepancies in the proportion of participants with diet exempting or reducing meat consumption (veganism and vegetarianism). The proportion of participants reporting veganism/vegetarianism was 8.7%, 8.9%, 29.3% and 50% for the 4 groups ascending by the time dedicated to physical activity. Diet was also the second most prominent covariate in the PERMANOVA test (explained variance (R2) of 5.5%, P = 0.007) (Table 1), but did not manage to meet the false discovery rate (FDR) significance threshold.
The specific nature of fungal community compositions, especially the low richness and the high interindividual variability, makes it challenging to determine significant correlations with host factors. Conclusions from related studies confirm this observation, as Nash et al. were unable to correlate any host factors with fungal community, and Auchtung et al. reported high temporal variability, mainly attributed to short term diet [8,9]. To our knowledge, the only other group that reported fungal community associated host covariates in a healthy population was Strati et al., where authors showed age- and gender-associated changes in fungal community, more specifically higher fungal richness in females compared to males, and in adults compared to earlier stages of life [69].
Associations within and between bacterial and fungal communities
The Pearson correlation test was used to identify associations inside bacterial and fungal sets of OTUs as well as between bacteria and fungi. A total of 41, 77 and 59 associations were found for bacteria vs. bacteria, fungi vs. fungi and bacteria vs. fungi comparisons, respectively (FDR < 0.05) (S2 and S4 Figs).
The bacterial communities were dominated by positive correlations with the only exception of a weak negative correlation between Blautia (B_OTU2) and Prevotella (B_OTU4). We found no indications of closely related bacterial groups exhibiting stronger correlations and the number of correlations per individual phylum was proportional to its respective relative abundance (S2 Fig).
Associations in the fungal community must be inspected with care because of the bias introduced by low prevalence fungi. Here, strong associations occur as a result of co-occurrence in a small fraction of samples, potentially originating from the same food source. Consequently, we observed significantly more associations in fungal community compared to bacterial. When focusing solely on high prevalence fungi, we identified a negative correlation between S. cerevisiae (F_OTU1) and both the C. albicans (F_OTU2) and the unclassified Debaryomyces (F_OTU4) to be the most prominent (S3 Fig).
Detected associations between bacteria and fungi span across the top 6 most abundant bacterial phyla (Firmicutes, Bacteroides, Actinobactria, Proteobacteria, Verrucomicrobia and Tenericutes) and top 2 most abundant fungal phyla (Ascomycota and Basidiomycota). Dominated by positive correlations, they show random distribution and no preference towards any particular taxonomic group (S4 Fig).
Conclusions
The variability in the healthy human gut microbiota remains largely unexplained despite increasing effort to decipher the microbial patterns with host-specific and environmental factors. In this study we analysed bacterial and fungal communities in a cohort of 186 healthy individuals. Consistent with previous studies [8,9,62], we also report a low per sample fungal diversity, accompanied by a high interindividual variability, which is most likely a consequence of foodborne transient fungi. Out of 13 questionnaire-based host and environmental factors we report 3 significant host covariates. These are age- and gender-associated differences in bacterial communities and the rate of physical activity associated differences in fungal community. Fungal community is known to be largely affected by short-term diet, therefore we assume that the observed patterns highly depend on different diets individuals reported according to the time they dedicated to recreational activities and sport. We identified seven bacterial core genera, out of which three (Faecalibacterium, Bacteroides and Roseburia) commonly appear as core candidates in related studies originating from different geographic regions, including European, American and Mongolian cohorts. The consensus on these core genera, especially in such a variety of studied populations, suggests their pivotal role in the gut, that up to date remains undisclosed.
Supporting information
Bar plot shows Pearson correlation coefficient for fungal OTUs that significantly increase (blue) and decrease (red) with fungal community Shannon diversity index.
(TIF)
Coloured squares on the heat map indicate significant Pearson correlations (false discovery rate (FDR) < 0.05) between bacterial OTUs. Blue shades indicate positive and red shades indicate negative correlation.
(TIF)
Coloured squares on the heat map indicate significant Pearson correlations (false discovery rate (FDR) < 0.05) between fungal OTUs. Blue shades indicate positive and red shades indicate negative correlation.
(TIF)
Coloured squares on the heat map indicate significant Pearson correlations (false discovery rate (FDR) < 0.05) between bacterial and fungal OTUs. Blue shades indicate positive and red shades indicate negative correlation.
(TIF)
Table with all bacterial OTUs, percent of samples they appear in, percent of total number of obtained reads they include and their respective taxonomical classification (phylum and the highest taxonomical level to which they reliably classify). Numbers in the parenthesis in the taxonomy column indicate the percent of identity between OTU representative read and the best match in the RDP training set (v.9) reference base. Members of core community are highlighted with grey.
(XLSX)
Table with all fungal OTUs, percent of samples they appear in, percent of total number of obtained reads they include and their respective taxonomical classification (phylum and the highest taxonomical level to which they reliably classify). Numbers in the parenthesis in the taxonomy column indicate the percent of identity between OTU representative read and best match in the UNITE reference base. Members of core community are highlighted with grey.
(XLSX)
(ZIP)
Acknowledgments
The authors acknowledge the financial support from the Slovenian Research Agency (research core funding P3-0387 and PhD program 'Mladi raziskovalci'). Authors also acknowledge the technical support of Aleksander Kocuvan.
Data Availability
The sequence data was deposited in the form of combined paired end reads (contigs) on the Metagenomics RAST (MG-RAST) database server (http://metagenomics.anl.gov/) under the project access number mgp85661 (https://www.mg-rast.org/linkin.cgi?project=mgp85661). Seven samples (all fungal ITS2 metagenomes) did not meet the minimum criteria of 1000000 bp per sample as required by MG-RAST and are available along with metadata in Supporting Information (S1 Appendix).
Funding Statement
The authors acknowledge the financial support from the Slovenian Research Agency (research core funding P3-0387 and PhD program 'Mladi raziskovalci').
References
- 1.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010. March 4;464(7285):59–65. 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016. January;14(1):20–32. 10.1038/nrmicro3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis JD, Chen EZ, Baldassano RN, Otley AR, Griffiths AM, Lee D, et al. Inflammation, Antibiotics, and Diet as Environmental Stressors of the Gut Microbiome in Pediatric Crohn’s Disease. Cell Host Microbe. 2015. October 14;18(4):489–500. 10.1016/j.chom.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chehoud C, Albenberg LG, Judge C, Hoffmann C, Grunberg S, Bittinger K, et al. A Fungal Signature in the Gut Microbiota of Pediatric Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015. August;21(8):1948–56. 10.1097/MIB.0000000000000454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kernbauer E, Ding Y, Cadwell K. An enteric virus can replace the beneficial function of commensal bacteria. Nature. 2014. December 4;516(7529):94–8. 10.1038/nature13960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norman JM, Handley SA, Baldridge MT, Droit L, Liu CY, Keller BC, et al. Disease-specific Alterations in the Enteric Virome in Inflammatory Bowel Disease. Cell. 2015. January 29;160(3):447–60. 10.1016/j.cell.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokol H, Leducq V, Aschard H, Pham H-P, Jegou S, Landman C, et al. Fungal microbiota dysbiosis in IBD. Gut. 2016. February 3;gutjnl-2015-310746. 10.1136/gutjnl-2015-310746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nash AK, Auchtung TA, Wong MC, Smith DP, Gesell JR, Ross MC, et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome. 2017. November 25;5(1):153 10.1186/s40168-017-0373-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auchtung TA, Fofanova TY, Stewart CJ, Nash AK, Wong MC, Gesell JR, et al. Investigating Colonization of the Healthy Adult Gastrointestinal Tract by Fungi. mSphere. 2018. April 25;3(2):e00092–18. 10.1128/mSphere.00092-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. Phimister EG, editor. New England Journal of Medicine. 2016. December 15;375(24):2369–79. 10.1056/NEJMra1600266 [DOI] [PubMed] [Google Scholar]
- 11.Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol. 2017. October;14(10):573–84. 10.1038/nrgastro.2017.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu X, Ma J, Jiao C, Mao X, Zhao X, Lu M, et al. Alterations in the mucosa-associated fungal microbiota in patients with ulcerative colitis. Oncotarget. 2017. November 20;8(64):107577–88. 10.18632/oncotarget.22534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wylie KM, Truty RM, Sharpton TJ, Mihindukulasuriya KA, Zhou Y, Gao H, et al. Novel Bacterial Taxa in the Human Microbiome. PLOS ONE. 2012. June 13;7(6):e35294 10.1371/journal.pone.0035294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aagaard K, Petrosino J, Keitel W, Watson M, Katancik J, Garcia N, et al. The Human Microbiome Project strategy for comprehensive sampling of the human microbiome and why it matters. The FASEB Journal. 2012. November 19;27(3):1012–22. 10.1096/fj.12-220806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014. January;505(7484):559–63. 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012. May 9;486(7402):222–7. 10.1038/nature11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salazar N, Valdés-Varela L, González S, Gueimonde M, de los Reyes-Gavilán CG. Nutrition and the gut microbiome in the elderly. Gut Microbes. 2016. November 3;8(2):82–97. 10.1080/19490976.2016.1256525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conlon MA, Bird AR. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients. 2014. December 24;7(1):17–44. 10.3390/nu7010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salazar N, Arboleya S, Valdés L, Stanton C, Ross P, Ruiz L, et al. The human intestinal microbiome at extreme ages of life. Dietary intervention as a way to counteract alterations. Front Genet. 2014;5:406 10.3389/fgene.2014.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodrich JK, Davenport ER, Beaumont M, Jackson MA, Knight R, Ober C, et al. Genetic determinants of the gut microbiome in UK Twins. Cell Host Microbe. 2016. May 11;19(5):731–43. 10.1016/j.chom.2016.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, et al. Population-level analysis of gut microbiome variation. Science. 2016;352(6285):560–564. 10.1126/science.aad3503 [DOI] [PubMed] [Google Scholar]
- 22.Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352(6285):565–569. 10.1126/science.aad3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huseyin CE, Rubio RC, O’Sullivan O, Cotter PD, Scanlan PD. The Fungal Frontier: A Comparative Analysis of Methods Used in the Study of the Human Gut Mycobiome. Frontiers in Microbiology. 2017; 8: 1432 10.3389/fmicb.2017.01432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulze J, Sonnenborn U. Yeasts in the Gut: From Commensals to Infectious Agents. Dtsch Arztebl Int. 2009. December;106(51–52):837–42. 10.3238/arztebl.2009.0837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Chen Z, Guo R, Chen N, Lu H, Huang S, et al. Correlation between gastrointestinal fungi and varying degrees of chronic hepatitis B virus infection. Diagnostic Microbiology and Infectious Disease. 2011. August;70(4):492–8. 10.1016/j.diagmicrobio.2010.04.005 [DOI] [PubMed] [Google Scholar]
- 26.Scanlan PD, Marchesi JR. Micro-eukaryotic diversity of the human distal gut microbiota: qualitative assessment using culture-dependent and -independent analysis of faeces. ISME J. 2008. July 31;2(12):1183–93. 10.1038/ismej.2008.76 [DOI] [PubMed] [Google Scholar]
- 27.Limon JJ, Skalski JH, Underhill DM. Commensal Fungi in Health and Disease. Cell Host & Microbe. 2017. August;22(2):156–65. 10.1016/j.chom.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wheeler ML, Limon JJ, Underhill DM. Immunity to commensal fungi: detente and disease. Annual Review of Pathology: Mechanisms of Disease. 2017;12:359–385. 10.1146/annurev-pathol-052016-100342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kourelis A, Kotzamanidis C, Litopoulou-Tzanetaki E, Papaconstantinou J, Tzanetakis N, Yiangou M. Immunostimulatory activity of potential probiotic yeast strains in the dorsal air pouch system and the gut mucosa. J Appl Microbiol. 2010. July;109(1):260–71. 10.1111/j.1365-2672.2009.04651.x [DOI] [PubMed] [Google Scholar]
- 30.Geib SM, Filley TR, Hatcher PG, Hoover K, Carlson JE, Jimenez-Gasco M del M, et al. Lignin degradation in wood-feeding insects. Proc Natl Acad Sci USA. 2008. September 2;105(35):12932–7. 10.1073/pnas.0805257105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sirohi SK, Choudhury PK, Dagar SS, Puniya AK, Singh D. Isolation, characterization and fibre degradation potential of anaerobic rumen fungi from cattle. Ann Microbiol. 2013. September 1;63(3):1187–94. 10.1007/s13213-012-0577-6 [DOI] [Google Scholar]
- 32.Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013. January;41(1):e1 10.1093/nar/gks808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Op De Beeck M, Lievens B, Busschaert P, Declerck S, Vangronsveld J, Colpaert JV. Comparison and Validation of Some ITS Primer Pairs Useful for Fungal Metabarcoding Studies. PLoS ONE. 2014. June 16;9(6):e97629 10.1371/journal.pone.0097629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl Environ Microbiol. 2009. January 12;75(23):7537–41. 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lagkouvardos I, Fischer S, Kumar N, Clavel T. Rhea: a transparent and modular R pipeline for microbial profiling based on 16S rRNA gene amplicons. PeerJ. 2017; 5:e2836 10.7717/peerj.2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huse SM, Ye Y, Zhou Y, Fodor AA. A Core Human Microbiome as Viewed through 16S rRNA Sequence Clusters. PLoS One. 2012;7(6):e34242 10.1371/journal.pone.0034242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Guo Z, Lim AAQ, Zheng Y, Koh EY, Ho D, et al. Mongolians core gut microbiota and its correlation with seasonal dietary changes. Sci Rep. 2014. May 16;4:5001 10.1038/srep05001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koliada A, Syzenko G, Moseiko V, Budovska L, Puchkov K, Perederiy V, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017. May 22;17(1):120 10.1186/s12866-017-1027-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré J, et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009. June 9;9:123 10.1186/1471-2180-9-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamady M, Knight R. Microbial community profiling for human microbiome projects: Tools, techniques, and challenges. Genome Res. 2009. July;19(7):1141–52. 10.1101/gr.085464.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012. September 13;489(7415):220–30. 10.1038/nature11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davenport ER, Sanders JG, Song SJ, Amato KR, Clark AG, Knight R. The human microbiome in evolution. BMC Biology. 2017. December 27;15:127 10.1186/s12915-017-0454-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Claesson MJ, O’Sullivan O, Wang Q, Nikkilä J, Marchesi JR, Smidt H, et al. Comparative Analysis of Pyrosequencing and a Phylogenetic Microarray for Exploring Microbial Community Structures in the Human Distal Intestine. PLoS ONE. 2009. August 20;4(8):e6669 10.1371/journal.pone.0006669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011. March 15;108(Suppl 1):4586–91. 10.1073/pnas.1000097107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tiihonen K, Ouwehand AC, Rautonen N. Human intestinal microbiota and healthy ageing. Ageing Res Rev. 2010. April;9(2):107–16. 10.1016/j.arr.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 47.Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, et al. Through Ageing, and Beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLOS ONE. 2010. May 17;5(5):e10667 10.1371/journal.pone.0010667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, et al. Differences in Fecal Microbiota in Different European Study Populations in Relation to Age, Gender, and Country: a Cross-Sectional Study. Appl Environ Microbiol. 2006. February;72(2):1027–33. 10.1128/AEM.72.2.1027-1033.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodmansey EJ, McMurdo MET, Macfarlane GT, Macfarlane S. Comparison of Compositions and Metabolic Activities of Fecal Microbiotas in Young Adults and in Antibiotic-Treated and Non-Antibiotic-Treated Elderly Subjects. Appl Environ Microbiol. 2004. October;70(10):6113–22. 10.1128/AEM.70.10.6113-6122.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Senghor B, Sokhna C, Ruimy R, Lagier J-C. Gut microbiota diversity according to dietary habits and geographical provenance. Microbiome. 2018. April; 7:1–9. 10.1016/j.humic.2018.01.001 [DOI] [Google Scholar]
- 51.Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao J, et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016. May 25; 16:90 10.1186/s12866-016-0708-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dominianni C, Sinha R, Goedert JJ, Pei Z, Yang L, Hayes RB, et al. Sex, Body Mass Index, and Dietary Fiber Intake Influence the Human Gut Microbiome. PLoS One. 2015. April 15;10(4):e0124599 10.1371/journal.pone.0124599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borgo F, Garbossa S, Riva A, Severgnini M, Luigiano C, Benetti A, et al. Body Mass Index and Sex Affect Diverse Microbial Niches within the Gut. Front Microbiol. 2018. February 14; 9: 213 10.3389/fmicb.2018.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leblanc V, Hudon A-M, Royer M-M, Corneau L, Dodin S, Bégin C, et al. Differences between men and women in dietary intakes and metabolic profile in response to a 12-week nutritional intervention promoting the Mediterranean diet. J Nutr Sci. 2015. April 13;4:e13 10.1017/jns.2015.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu BN, O’Sullivan AJ. Sex Differences in Energy Metabolism Need to Be Considered with Lifestyle Modifications in Humans. J Nutr Metab. 2011;2011:391809 10.1155/2011/391809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freire AC, Basit AW, Choudhary R, Piong CW, Merchant HA. Does sex matter? The influence of gender on gastrointestinal physiology and drug delivery. International Journal of Pharmaceutics. 2011. August 30;415(1):15–28. 10.1016/j.ijpharm.2011.04.069 [DOI] [PubMed] [Google Scholar]
- 57.Ahonen T, Vanhala M, Kautiainen H, Kumpusalo E, Saltevo J. Sex Differences in the Association of Adiponectin and Low-Grade Inflammation With Changes in the Body Mass Index From Youth to Middle Age. Gender Medicine. 2012. February 1;9(1):1–8. 10.1016/j.genm.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 58.Hallen-Adams HE, Suhr MJ. Fungi in the healthy human gastrointestinal tract. Virulence. 2016. October 13;8(3):352–8. 10.1080/21505594.2016.1247140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luan C, Xie L, Yang X, Miao H, Lv N, Zhang R, et al. Dysbiosis of Fungal Microbiota in the Intestinal Mucosa of Patients with Colorectal Adenomas. Sci Rep. 2015. January 23;5:7980 10.1038/srep07980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoffmann C, Dollive S, Grunberg S, Chen J, Li H, Wu GD, et al. Archaea and Fungi of the Human Gut Microbiome: Correlations with Diet and Bacterial Residents. PLoS ONE. 2013. June 17;8(6):e66019 10.1371/journal.pone.0066019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elmer GW, McFarland LV, Surawicz CM, Danko L, Greenberg RN. Behaviour of Saccharomyces boulardii in recurrent Clostridium difficile disease patients. Aliment Pharmacol Ther. 1999. December;13(12):1663–8. [DOI] [PubMed] [Google Scholar]
- 62.Hallen-Adams HE, Kachman SD, Kim J, Legge RM, Martínez I. Fungi inhabiting the healthy human gastrointestinal tract: a diverse and dynamic community. Fungal Ecology. 2015. June;15:9–17. 10.1016/j.funeco.2015.01.006 [DOI] [Google Scholar]
- 63.Bullerman LB. SPOILAGE | Fungi in Food–An Overview. In: Caballero B, editor. Encyclopedia of Food Sciences and Nutrition (Second Edition). 2018. May 16 10.4315/0362-028X.JFP-18-031 [DOI] [Google Scholar]
- 64.Gori K, Sørensen LM, Petersen MA, Jespersen L, Arneborg N. Debaryomyces hansenii strains differ in their production of flavor compounds in a cheese-surface model. Microbiologyopen. 2012. June;1(2):161–8. 10.1002/mbo3.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang E, Tanaka T, Tajima M, Tsuboi R, Nishikawa A, Sugita T. Characterization of the skin fungal microbiota in patients with atopic dermatitis and in healthy subjects. Microbiol Immunol. 2011. September;55(9):625–32. 10.1111/j.1348-0421.2011.00364.x [DOI] [PubMed] [Google Scholar]
- 66.Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, et al. Characterization of the Oral Fungal Microbiome (Mycobiome) in Healthy Individuals. PLoS Pathog. 2010. January 8;6(1):e1000713 10.1371/journal.ppat.1000713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Šket R, Treichel N, Kublik S, Debevec T, Eiken O, Mekjavić I, et al. Hypoxia and inactivity related physiological changes precede or take place in absence of significant rearrangements in bacterial community structure: The PlanHab randomized trial pilot study. PLOS ONE. 2017. December 6;12(12):e0188556 10.1371/journal.pone.0188556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clarke SF, Murphy EF, O’Sullivan O, Lucey AJ, Humphreys M, Hogan A, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014. December;63(12):1913–20. 10.1136/gutjnl-2013-306541 [DOI] [PubMed] [Google Scholar]
- 69.Strati F, Di Paola M, Stefanini I, Albanese D, Rizzetto L, Lionetti P, et al. Age and Gender Affect the Composition of Fungal Population of the Human Gastrointestinal Tract. Frontiers in Microbiology. 2016. August 3;7:1227 10.3389/fmicb.2016.01227 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bar plot shows Pearson correlation coefficient for fungal OTUs that significantly increase (blue) and decrease (red) with fungal community Shannon diversity index.
(TIF)
Coloured squares on the heat map indicate significant Pearson correlations (false discovery rate (FDR) < 0.05) between bacterial OTUs. Blue shades indicate positive and red shades indicate negative correlation.
(TIF)
Coloured squares on the heat map indicate significant Pearson correlations (false discovery rate (FDR) < 0.05) between fungal OTUs. Blue shades indicate positive and red shades indicate negative correlation.
(TIF)
Coloured squares on the heat map indicate significant Pearson correlations (false discovery rate (FDR) < 0.05) between bacterial and fungal OTUs. Blue shades indicate positive and red shades indicate negative correlation.
(TIF)
Table with all bacterial OTUs, percent of samples they appear in, percent of total number of obtained reads they include and their respective taxonomical classification (phylum and the highest taxonomical level to which they reliably classify). Numbers in the parenthesis in the taxonomy column indicate the percent of identity between OTU representative read and the best match in the RDP training set (v.9) reference base. Members of core community are highlighted with grey.
(XLSX)
Table with all fungal OTUs, percent of samples they appear in, percent of total number of obtained reads they include and their respective taxonomical classification (phylum and the highest taxonomical level to which they reliably classify). Numbers in the parenthesis in the taxonomy column indicate the percent of identity between OTU representative read and best match in the UNITE reference base. Members of core community are highlighted with grey.
(XLSX)
(ZIP)
Data Availability Statement
The sequence data was deposited in the form of combined paired end reads (contigs) on the Metagenomics RAST (MG-RAST) database server (http://metagenomics.anl.gov/) under the project access number mgp85661 (https://www.mg-rast.org/linkin.cgi?project=mgp85661). Seven samples (all fungal ITS2 metagenomes) did not meet the minimum criteria of 1000000 bp per sample as required by MG-RAST and are available along with metadata in Supporting Information (S1 Appendix).