Abstract
The fertility of men with neurofibromatosis 1 (NF1) is reduced. Despite this observation, gonadal function has not been examined in patients with NF1. In order to assess the role of reduced neurofibromin in the testes, we examined testicular morphology and function in an Nf1+/- mouse model. We found that although Nf1+/- male mice are able to reproduce, they have significantly fewer pups per litter than Nf1+/+ control males. Reduced fertility in Nf1+/- male mice is associated with disorganization of the seminiferous epithelium, with exfoliation of germ cells and immature spermatids into the tubule lumen. Morphometric analysis shows that these alterations are associated with decreased Leydig cell numbers and increased spermatid cell numbers. We hypothesized that hyper-activation of Ras in Nf1+/- males affects ectoplasmic specialization, a Sertoli-spermatid adherens junction involved in spermiation. Consistent with this idea, we found increased expression of phosphorylated ERK, a downstream effector of Ras that has been shown to alter ectoplasmic specialization, in Nf1+/- males in comparison to control Nf1+/+ littermates. These data demonstrate that neurofibromin haploinsufficiency impairs spermatogenesis and fertility in a mouse model of NF1.
Introduction
Neurofibromatosis 1 (NF1) is a multisystem autosomal dominant condition that affects 1 in 3000 individuals. It is caused by germ-line mutations in the neurofibromin (NF1) gene, which is located on chromosome 17. There is significant variation in the clinical phenotype among affected individuals, but the most common manifestations are café-au-lait macules, iris Lisch nodules, multiple neurofibromas, axillary and inguinal freckling, skeletal abnormalities and learning disabilities [1]. Individuals with NF1 have fewer children, on average, than individuals in the general population, and this reduction in fertility is greater in males than females [2–6]. This reduction in reproductive fitness has been attributed to social rather than biological factors [2, 4], but gonadal function has rarely been examined in patients with NF1. One, German study [7] found decreased libido in males with NF1 and one French study [8] described testicular dystrophy.
Recent studies demonstrate that the reproductive period of individuals with NF1 is often reduced suggesting that gonadal function may be impaired. Children with NF1 have been found to enter puberty later than their unaffected siblings or children in the general population [9]. The onset of menstruation in girls with NF1 is delayed when compared to their unaffected mothers or unaffected girls in the general population [10]. Women with NF1 also often enter menopause earlier than expected [11]. In addition, a higher than expected occurrence of osteoporosis has been reported in men with NF1 [11], which may reflect alterations in the production of testicular hormones [12].
Spermatogenesis, the process by which spermatogonia become haploid spermatids, takes place in the seminiferous epithelium and is dependent on multiple cell-cell interactions [13, 14]. The seminiferous epithelium is composed of spermatogonia, spermatocytes, spermatids, mature spermatozoa and Sertoli cells. Spermatogonial stem cells lie on the basal lamina where they proliferate and differentiate into spermatocytes. Following two meiotic divisions, spermatocytes become round spermatids that eventually differentiate into elongated spermatids and then mature spermatozoa. Sertoli cells help regulate this process through the production of growth factors and cytokines. Interstitial Leydig cells and the hypothalamic-pituitary axis provide additional support for the process through the production of hormones [15–17]. Neurofibromin, the protein product of the NF1 gene, is highly expressed in developing and adult gonads [18], where its expression occurs during spermatogenesis and the estrus cycle [18]. Neurofibromin is a Ras GTPase-activating protein involved in regulation of the Ras/mitogen activated protein kinase (MAPK) signaling pathway that controls cell division [19]. Ras signaling is essential for proper spermatogenesis and is also important for the proliferation and migration of germ cells and maintaining the blood-testis-barrier [20–25].
Considering the importance of Ras signaling for the proliferation of spermatogonia and for the maintenance of junctional dynamics within Sertoli cells and spermatids [22, 26, 27], and its dysregulation in NF1 [19, 28], we hypothesized that haploinsufficiency of neurofibromin has negative effects on spermatogenesis in males with NF1. We tested this hypothesis by studying the effects of neurofibromin deficiency on cellular differentiation and migration within the seminiferous epithelium in a well-established mouse model of NF1, the Nf1Dsk9/+ mouse.
Materials & methods
Experimental animals
The NF1 mouse model used in this study has been described elsewhere [29]. Dsk9 is a missense mutation in the GTPase-activating protein related domain of neurofibromin. Nf1Dsk9/+ (subsequently referred to as “Nf1+/-”) mice were maintained on a mixed (C3HeB/FeJ x C57bl/6) background. All surgical and animal care procedures were approved by the University of British Columbia Committee on Ethics of Animal Experiments (IACUC Approval # A11-0117), and were conducted in accordance to the University’s Guidelines for Animal Experiments (https://animalcare.ubc.ca/animal-care-committee/policies-and-guidelines). For the breeding studies, male or female Nf1+/- mice were bred with Nf1+/+ control littermates. For breeding studies, we used three haremed cages in which female Nf1+/- mice were crossed to male Nf1+/+ control littermates and three haremed cages in which male Nf1+/- mice were crossed to female Nf1+/+ control littermate. We also examined breeding records from additional six cages using the same crosses that were not haremed.
Tissue isolation procedure
Nf1+/- and Nf1+/+control littermate mice were weighed prior to surgical procedures. Animals were sacrificed using isoflurane followed by cervical dislocation at 6 to 7 months of age. The testes and epididymis were isolated from the lower abdominal cavity and placed in ice-cold HEPES-PSS buffer (PH 7.4) containing 10mM HEPES, 6mM glucose, 1.8mM CaCl, 130mM NaCl, 4mM KCl, 4mM NaHCO3, 1.2mM MgSO4, 1.2mM KH2PO4, and 0.03mM EDTA. The testes were cleaned of fat, weighed, and either fixed in 10% formalin or flash-frozen in liquid nitrogen and stored at -80°C for histological staining and Western blotting, respectively. Within 30 minutes of isolation, the cauda epididymis, where mature sperm are stored, was cleaned of fat and dissected from the corpus epididymis and the vas deferens. The cauda epididymis was minced and placed into 500 μL of HEPES-PSS buffer for 30 minutes in order to release spermatozoa into the buffer. Spermatozoa were centrifuged at 15000 rpm for 1 minute and re-suspended in 500 μL of HEPES-PSS buffer. Sperm were counted in a hemocytometer using 10 μL of the sperm suspension. A minimum of 100 sperms per chamber were counted to reduce statistical dispersion.
Histology
Following fixation in 10% formalin, testis samples were dehydrated and embedded in paraffin. Five-micrometer transverse sections were stained using standard hematoxylin and eosin or Masson trichrome staining protocols (Sigma Aldrich, ON, Canada). Sections were viewed and imaged using an Olympus BX61 upright light microscope and Q imaging Retiga Exi camera with InVivo 3.2.0 software (Media Cybernetics). Computerized quantitative morphometric analysis was performed using Image-Pro Plus software (Media Cybernetics).
Testicular morphometric analysis
Testicular volume was measured using the formula for a prolate (elongated) ellipsoid () [30], where “a” is the major axis (polar diameter) and “b” is the minor axis (equatorial radius). The width and equatorial length of the testes were measured on testicular histological images using Image J1.45s, version 1.44p (NIH, Bethesda, MD). We found that testicular volume was significantly correlated with mouse size (R2 = 0.5, p = 0.02). Therefore, we divided testicular volume by the body weight of each mouse to yield volume per unit weight to normalize for mouse size.
Seminiferous tubule size was measured at a final magnification of 200X using Image-Pro Plus software (Media Cybernetics, Inc., Rockville, MD). To ensure comprehensive coverage of the testes, five rectangles of equal dimensions were randomly placed on the image. Within each of five grids placed on the histology image, 400 equidistant points were counted by manually tagging the cell type on which the point fell. A total of 2000 points were counted in each testis. If the point fell on white space within the seminiferous tubule, it was characterized as ‘intratubular space’, and if the point fell on white space outside the tubules, it was characterized as ‘interstitial space’. The lumen of the tubules was included as part of the spermatid count. Distinction was not made between a round or elongating spermatid. Germ cell count included both spermatogonia and spermatocytes. Sertoli cells, germ cells, and round and elongated spermatids in the seminiferous tubules were expressed as a percentage of the total cell population of the tubule. The cross section of the tubule in which cells were enumerated also included intracellular space. The density and distribution of Sertoli cells, germ cells, spermatids and Leydig cells within the testis were measured on a complete section at a final magnification of 200X using Image-Pro Plus software. Within each of the five rectangles, five tubules were randomly selected and the diameter of each axis (i.e. the lengths and widths) were measured. The five rectangles covered over 70% of the section and each rectangle had between 15–25 tubules within it so five tubules covered at least 20% of the tubules present. Tubules with a length-to-width ratio less than or equal to 1.5 were considered to be round or nearly round and were included in the analysis of seminiferous tubule size. At least 25 tubules were measured per testis (50 tubules per mouse) for both Nf1+/+ control and Nf1+/- mice.
Western blotting
Flash-frozen isolated testes from Nf1+/+and Nf1+/- mice were homogenized in a pre-chilled stainless-steel mortar and pestle. The resulting tissue powder was mixed in 50 μL of ice-cold lysis buffer containing 50mM pyrophosphate, 50mM NaF, 50mM NaCl, 5mM EDTA, 5mM EGTA, 100μM Na3VO4, 10mM HEPES (pH 7.4), 0.1% Triton X-100, 10 μg/mL leupeptin, and 1mM phenylmethylsulfonyl fluoride. Extracted protein (40 μg) was fractionated by gel electrophoresis in 9% sodium dodecyl sulfate-polyacrylamide gels, transferred to nitrocellulose membranes, and then blocked for 1 hour with PBS containing 5% skim milk and 0.2% Tween-20. Following overnight incubation (at 4°C) with specific primary antibody, the membrane was incubated with secondary antibody for 1 hour at room temperature. Immunoblots were visualized with an enhanced chemiluminescence detection system following the protocol provided by the manufacturer (Pierce Biotechnology, Rockford, IL, USA).
Western blotting was performed with rabbit primary antibodies for p44/42 MAPK (ERK1/2), p44/42 MAPK phosphorylated at threonine 202 and tyrosine 204 (p-ERK1/2), or phosphatidylinositol 3-kinase (PI3K), along with anti-rabbit conjugated secondary antibody, all purchased from New England Biolabs (Whitby, Ontario, Canada).
Statistical analysis
Data are reported as means ± SEM from at least five mice. Differences between Nf1+/+ and Nf1+/- groups were analyzed by 2-tailed Student’s t tests and were graphed using GraphPad 5 Prism software (San Diego, Calif., USA). Statistical significance was defined as p ≤ 0.05.
Results
Effects of Nf1 mutation on mouse litter size and sperm count
To assess the fertility of Nf1+/- male mice, we determined the number of pups produced when Nf1+/- males were crossed to Nf1+/+ control females, and Nf1+/+ control males were crossed to Nf1+/- females. As shown in Table 1, the average number of pups per litter from Nf1+/- males was significantly reduced compared to that of Nf1+/+ control male littermates (p = 0.007); however, the amount of time between litters was not significantly different (p = 0.75). We saw no evidence of post-natal death or other indications that Nf1+/- males were more aggressive with pups than Nf1+/+ control males. Epididymal sperm counts in Nf1+/- mice were significantly higher than those of Nf1+/+ control mice (p = 0.02) (Table 1).
Table 1. Testicular Functional Parameters in Nf1+/+ and Nf1+/- mice.
Values listed in each column are mean ± SEM. Bold numbers indicate p≤ 0.05 and are considered to be statistically significant.
| Testicular Functional Parameter | Nf1+/+ male x Nf1+/- female | Nf1+/- male x Nf1+/+ female | p-value |
|---|---|---|---|
| Average Litter Size | 8.67 ± 0.76 | 4.00 ± 1.2 | 0.007 |
| Days between Litters | 33.4 ± 4.7 | 31.2 ± 4.9 | 0.75 |
| Sperm Count (x106 spermatozoa/ml) | 8.8 ± 5.5 | 15 ± 2.3 | 0.02 |
Histological changes in seminiferous tubules
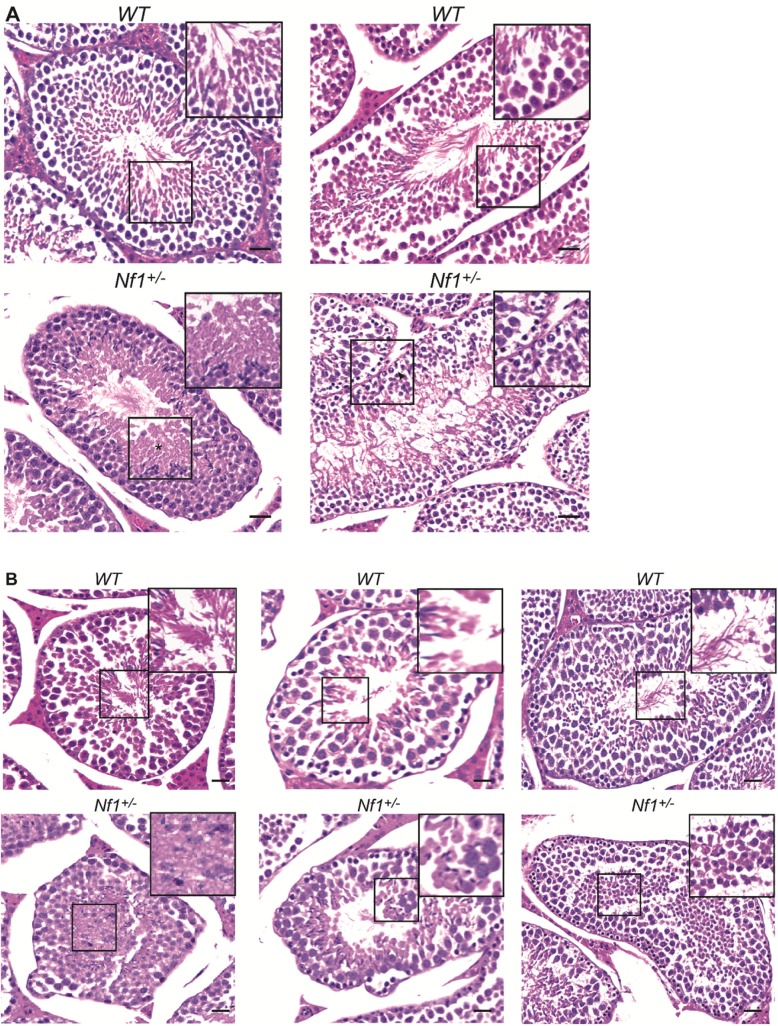
To determine whether these changes in fertility were associated with morphological changes in the testes, we performed histological examination of the testis in Nf1+/- males compared to Nf1+/+ control males. We found varying amounts of mild to moderate degeneration of seminiferous tubules in all Nf1+/- males. Mild alterations include detachment of the germinal epithelium from the basal lamina, vacuolation, and residual bodies (Fig 1A). Moderate alterations include disorganization of the seminiferous epithelium, with exfoliation of germ cells and immature spermatids into the lumen (Fig 1B), suggesting that spermiation may be affected.
Fig 1. Seminiferous tubule degeneration in Nf1+/- mice.
(A) Photomicrographs from Nf1+/+ (upper panels) and Nf1+/- (lower panels) seminiferous tubules taken at 200X showing mild to moderate degeneration of seminiferous tubules in Nf1+/- mice (detachment of germ cells from the basal epithelium, vacuolation of the seminiferous tubule and presence of atypical residual bodies. (B) Photomicrographs from Nf1+/+ (upper panels) and Nf1+/- (lower panels) testes taken at 200X showing moderate alterations in spermatogenesis in Nf1+/- mice (exfoliation of immature round spermatids and germ cells into the lumen of seminiferous tubules). (Scale bar; 50μm).
Effects of Nf1 mutation on spermatid maturation
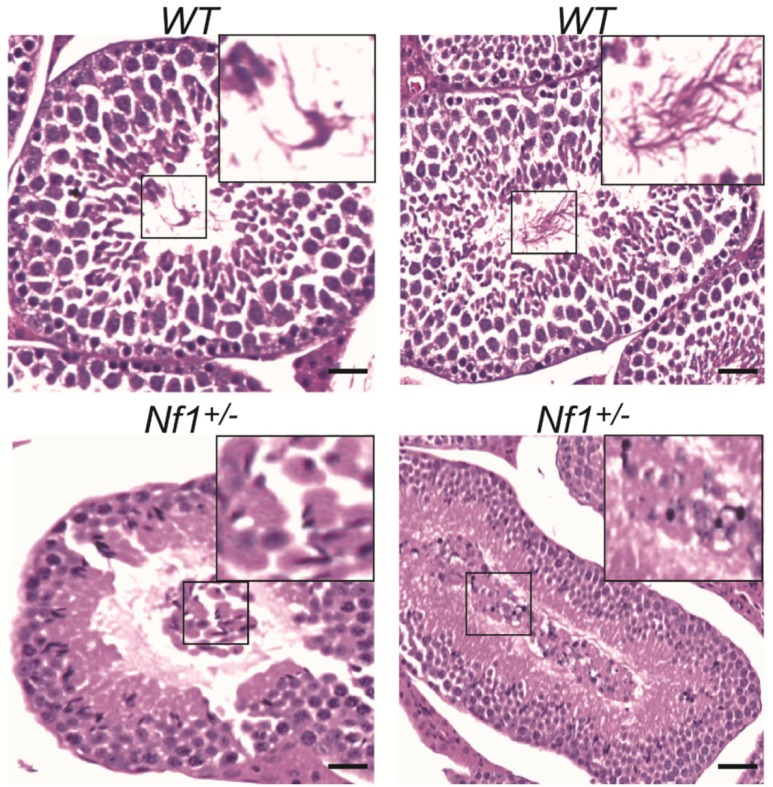
Further suggesting an important role for neurofibromin during spermiation, we also observed abnormal spermatids with enlarged heads and atypical residual bodies in Nf1+/- mice, which appear to be associated with the release of immature spermatids into the lumen (Fig 2).
Fig 2. Alterations in spermiation in Nf1+/- mice.
Photomicrographs from Nf1+/- testes taken at 200X showing abnormal spermatid morphology (*) (enlarged heads and presence of residual bodies) associated with alterations in spermiation in Nf1+/- mice. (Scale bar; 50μm).
Progressive differentiation and migration of spermatocytes is required for the production of mature spermatozoa. To further examine this process, we quantified the number of Leydig cells, Sertoli cells, spermatocytes, and spermatids in the seminiferous epithelium of Nf1+/- and Nf1+/+ control mice (Table 2).
Table 2. Testicular structural parameters in Nf1+/+ control and Nf1+/- mice.
Values listed in each column are mean ± SEM. Values represent the average per field counted, except for testicular volume and seminiferous tubule area, which are per testis or per tubule, respectively. Bold numbers indicate p≤ 0.05.
| Testicular Structural Parameters | Nf1+/+ n = 5 |
Nf1+ n = 6 |
p-value |
|---|---|---|---|
| Number of Leydig cells | 28.2 ± 0.71 | 24.2 ± 1.2 | 0.02 |
| Amount of Interstitial Space | 68.7 ± 6.7 | 74.6 ± 3.2 | 0.43 |
| Number of Sertoli cells | 0.027 ± 0.004 | 0.025 ± 0.005 | 0.75 |
| Number of Germ cells | 0.32 ± 0.01 | 0.34 ± 0.008 | 0.13 |
| Number of Round and Elongated Spermatids | 0.51 ± 0.02 | 0.56 ± 0.008 | 0.03 |
| Testicular Volume | 2.73 ± 0.09 | 3.11 ± 0.14 | 0.05 |
| Seminiferous Tubule Area | 52720 ± 3208 | 47990 ± 2138 | 0.25 |
Leydig cells secrete testosterone and cytokines required for spermatogenesis. The number of Leydig cells present in Nf1+/- mice was significantly lower than in Nf1+/+ control littermates (p = 0.02) (Table 2). This reduction did not result in a significant increase in the interstitial space in Nf1+/- mice and was not associated with any difference in the number of Sertoli cells. The total number of germ cells also did not differ between Nf1+/- and Nf1+/+ control mice, although the number of immature (round or elongated) spermatids was significantly increased in Nf1+/- mice compared to Nf1+/+ control (p = 0.03) (Table 2). Testicular volume was also significantly increased among Nf1+/- mice (p = 0.05) (Table 2). This difference does not appear to be a result of alterations in the size of the seminiferous tubules, as we did not observe a significant difference in the seminiferous tubule area between Nf1+/- and Nf1+/+ control mice (Table 2).
Effects of the Nf1 mutation on signaling pathways within seminiferous epithelium
As loss of neurofibromin may cause hyperactivation of the RAS/MAPK/ERK pathway [16, 19, 28], which is involved in regulation of seminiferous epithelium [20–23, 31]. We examined the effects of neurofibromin deficiency on expression and activation of the signaling components of the phosphoinositide 3-kinase (PI3K) and ERK pathway in the testes, and showed that Nf1 mutation resulted in increased phosphorylation and activation of ERK, while having no effect on total ERK protein expression (Fig 3). In addition, we observed a significant increase in total PI3K expression in Nf1+/- mice when compared to Nf1+/+ control mice (Fig 3).
Fig 3. Up-regulation of PI3K expression and ERK phosphorylation in Nf1+/- mice.
Western blot analysis of protein expression showing up-regulation in PI3K and phosphorylated ERK (pERK) with similar ERK expression in Nf1+/- testes compared to Nf1+/+ testes.
Discussion
Despite reproductive fitness being reduced in individuals with NF1 [2–4, 6], gonadal function has not been well characterized. Delayed puberty [9] and early menopause [11] suggest that reproductive function may be altered in people with NF1.
The present work is the first time that testicular morphology and function have been examined in Nf1+/- mice. Interestingly, we observed a 50% reduction in the average litter size from Nf1+/- males crossed to Nf1+/+ control females when compared to Nf1+/+ control males crossed to Nf1+/- females. This suggests fertility may be reduced in Nf1+/- males, but we cannot rule out the possibility that the effect observed is due to an increase in the fertility of Nf1+/- females when paired to Nf1+/+ control males. However, as described below, the reduced litter size in these crosses was associated with changes in spermatogenesis and sperm quality in Nf1+/- males, supporting a role for Nf1 in male fertility.
Changes in spermatogenesis and/or sperm quality may be associated with alterations in the seminiferous epithelium, which are known to affect fertility rates [32, 33]. In models of testicular toxicity, the disorganized seminiferous epithelium appears to result from alterations in Sertoli-spermatocyte and Sertoli-spermatid junctions [14, 17, 34, 35]. Spermatogenesis depends on the maintenance and restructuring of cellular junctions between Sertoli cells, spermatocytes, and spermatids [36]. In the testis, cellular junctions regulate the permeability of amino acids, hormones, and growth factors, and create an immune barrier essential for prevention of antigen responses [33].
Coexisting tight junctions and a special adherens junction, known as the basal ectoplasmic specialization, form the blood-testis barrier [36]. Spermatocyte differentiation depends on the migration of immature spermatocytes across the blood-testis barrier and progressive migration toward the seminiferous tubule lumen. During this process, Sertoli-Sertoli, Sertoli-spermatocyte, and Sertoli-spermatid cell junctions are rapidly remodeled to enable differentiation and migration of spermatocytes and to maintain the integrity of the blood-testis barrier [21]. Alterations in Sertoli-spermatocyte junctions cause exfoliation of germ cells [14], while exfoliation of immature spermatids into the seminiferous tubule lumen suggests that Sertoli-spermatid junctions are affected as round immature spermatids would normally associate with Sertoli cells via the ectoplasmic specialization [37]. We observed exfoliation of spermatocytes and round and elongating spermatids into the lumen, suggesting that neurofibromin haploinsufficiency may affect junctional dynamics in the testis.
Further contributing to the exfoliation of immature round and elongated spermatids in Nf1+/- mice, we also found that the number of Leydig cells was also reduced in Nf1+/- mice. Leydig cells produce testosterone which reinforces the tight junctions in the blood-testis barrier [15, 21, 38] and regulates ES assembly and disassembly [14, 16, 37]. Testosterone withdrawal promotes stage specific-detachment of round spermatids from the rat seminiferous epithelium through an ERK-dependent mechanism [39]. Nf1+/- mice also demonstrated enlarged sperm heads and atypical residual bodies, which further supports a role for neurofibromin in junctional dynamics. Alterations in spermatid morphology usually result from disassembly of tubulobulbar complexes, a specialized junction between Sertoli cells and elongated spermatids [40].
ERK phosphorylation is associated with the basal ectoplasmic specialization, a Sertoli-spermatocyte adherens junction that regulates spermatogenesis, and the apical ectoplasmic specialization, a Sertoli-spermatid adherens junction involved in spermiation [15, 32]. Phosphorylation of ERK results in decreased levels of the adhesion protein complexes, cadherin/catenin and nectin/afadin, resulting in loss of ectoplasmic specialization adhesion [14, 25]. Increased phosphorylation of ERK because of neurofibromin haploinsufficiency is, therefore, likely to affect proliferation of immature spermatogonia and affect ES adhesion. In support of this hypothesis, we found that in Nf1+/- mice, there was an abnormal release of immature round spermatids from the seminiferous epithelium and an increase in spermatids were associated with a significant increase in ERK phosphorylation without affecting total ERK protein expression.
Though alterations in spermiation may result in lower sperm counts, we found that caudal epididymal sperm counts in Nf1+/- mice were significantly increased. It is conceivable that the hyperactivation of Ras affects survival of spermatids and contributes to a higher sperm count yet lower quality sperm. Activation of ERK is associated with reduction in sperm quality [21] and alterations in sperm quality are known to reduce the number of offspring produced [41, 42]. The presence of abnormal spermatid morphology such as atypical residual bodies in the spermatids of Nf1+/- mice further supports the hypothesis that Nf1+/- mice produce lower quality sperm. Reduced reproductive fitness with an increased sperm count is not frequently observed, but has been demonstrated in progesterone receptor knockout mice (PRKO) with a similar phenotype to Nf1+/- male mice [43, 44]. Similar to Nf1+/- mice, PRKO mice demonstrate delayed spermatogenesis [43] with alterations in Leydig cells [44]. While sperm quality was not directly assessed in this model, in humans reduced progesterone receptor expression is known to affect sperm quality [45].
Neurofibromin is a negative regulator of Ras, and neurofibromin haploinsufficiency leads to activation of the Ras pathway [1, 19, 28]. Activation of Ras is known to trigger phosphorylation of ERK, which promotes spermatogonial stem cell proliferation in mice [26, 27] and is involved in differentiation of spermatogonia through regulation of adherens junctions. We speculate that the hyperactivation of ERK may also contribute to abnormal loss of ectoplasmic specialization adhesion during spermiation. Further highlighting the importance of ERK in spermatogenesis, MAP3K, the human ortholog to ERK1, is highly expressed in Leydig cells and moderately expressed throughout the seminiferous tubules in adult males (see https://www.proteinatlas.org/ENSG00000102882-MAPK3/tissue/testis#img).
Together, the results from this study demonstrate that Nf1+/- mice have alterations in the seminiferous epithelium, and suggests that neurofibromin is involved in proliferation of the seminiferous epithelium, as well as regulation of the junctions vital to spermatogenesis. The precise mechanism of the alterations observed here is unclear, but Nf1+/- mice do appear to demonstrate defects in spermatogenesis. Thus, the observed alterations in the seminiferous epithelium of Nf1+/- mice may be related to reduction in fertility, but further investigation is needed to establish the causal relationship.
At this point, we do not know if similar alterations are present in patients with NF1, testosterone levels and spermatogenesis are rarely examined unless a symptomatic testicular abnormality is also present [46–48]. However, fertility is reduced [3], puberty is delayed, and osteoporosis appears to occur more frequently than expected in men with NF1 [11] suggesting defects in gonadal function or endocrine regulation.
Although there are discrepancies in spermatogenesis between human and mice, in light of the facts and findings detailed in this study, investigation of testicular function in patients with NF1 may be warranted. These studies should explore sperm counts, testosterone levels, and morphometric measurements of the testes as well as testicular pathology. With this knowledge, patients can be counselled to make decisions regarding their reproductive potential. Examination of ovarian structure and function in female Nf1+/- mice is also worth pursuing, as we are unaware of any studies that have examined it.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Jett K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1. Genetics in medicine: official journal of the American College of Medical Genetics. 2010;12(1):1–11. Epub 2009/12/23. 10.1097/GIM.0b013e3181bf15e3 . [DOI] [PubMed] [Google Scholar]
- 2.Crowe FW SW, Neel JV. A Clinical, Pathologicala and Genetic Study of Multiple Neurofibromatosis. Springfield, IL: Charles C Thomas; 1956. [Google Scholar]
- 3.Huson SM, Compston DA, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. II. Guidelines for genetic counselling. Journal of medical genetics. 1989;26(11):712–21. Epub 1989/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samuelsson B, Akesson HO. Relative fertility and mutation rate in neurofibromatosis. Hereditas. 1988;108(2):169–71. Epub 1988/01/01. . [DOI] [PubMed] [Google Scholar]
- 5.Samuelsson B, Riccardi VM. Neurofibromatosis in Gothenburg, Sweden. III. Psychiatric and social aspects. Neurofibromatosis. 1989;2(2):84–106. Epub 1989/01/01. . [PubMed] [Google Scholar]
- 6.Takano T, Kawashima T, Yamanouchi Y, Kitayama K, Baba T, Ueno K, et al. Genetics of neurofibromatosis 1 in Japan: mutation rate and paternal age effect. Human genetics. 1992;89(3):281–6. Epub 1992/05/01. . [DOI] [PubMed] [Google Scholar]
- 7.Meyhofer W. ["Pseudoeunuchoidism" in Recklinghausen's generalized neurofibromatosis]. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete. 1966;17(3):108–13. Epub 1966/03/01. . [PubMed] [Google Scholar]
- 8.Bricaire H, Buge A, Leprat J, Valcke JC. [Testicular Dystrophy in Recklinghausen's Neurofibromatosis]. La semaine des hopitaux: organe fonde par l'Association d'enseignement medical des hopitaux de Paris. 1965;41:757–65. Epub 1965/03/14. . [PubMed] [Google Scholar]
- 9.Armstrong L, Jett K, Birch P, Kendler DL, McKay H, Tsang E, et al. The generalized bone phenotype in children with neurofibromatosis 1: a sibling matched case-control study. American journal of medical genetics Part A. 2013;161A(7):1654–61. Epub 2013/05/29. 10.1002/ajmg.a.36001 . [DOI] [PubMed] [Google Scholar]
- 10.Virdis R, Street ME, Bandello MA, Tripodi C, Donadio A, Villani AR, et al. Growth and pubertal disorders in neurofibromatosis type 1. Journal of pediatric endocrinology & metabolism: JPEM. 2003;16 Suppl 2:289–92. Epub 2003/05/06. . [PubMed] [Google Scholar]
- 11.Tucker T, Schnabel C, Hartmann M, Friedrich RE, Frieling I, Kruse HP, et al. Bone health and fracture rate in individuals with neurofibromatosis 1 (NF1). Journal of medical genetics. 2009;46(4):259–65. Epub 2008/12/11. 10.1136/jmg.2008.061895 . [DOI] [PubMed] [Google Scholar]
- 12.Banu J. Causes, consequences, and treatment of osteoporosis in men. Drug design, development and therapy. 2013;7:849–60. Epub 2013/09/07. 10.2147/DDDT.S46101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia W, Mruk DD, Lee WM, Cheng CY. Cytokines and junction restructuring during spermatogenesis—a lesson to learn from the testis. Cytokine & growth factor reviews. 2005;16(4–5):469–93. Epub 2005/07/19. 10.1016/j.cytogfr.2005.05.007 . [DOI] [PubMed] [Google Scholar]
- 14.Xia W, Wong CH, Lee NP, Lee WM, Cheng CY. Disruption of Sertoli-germ cell adhesion function in the seminiferous epithelium of the rat testis can be limited to adherens junctions without affecting the blood-testis barrier integrity: an in vivo study using an androgen suppression model. Journal of cellular physiology. 2005;205(1):141–57. Epub 2005/05/10. 10.1002/jcp.20377 . [DOI] [PubMed] [Google Scholar]
- 15.Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacological reviews. 2012;64(1):16–64. Epub 2011/11/01. 10.1124/pr.110.002790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong CH, Cheng CY. The blood-testis barrier: its biology, regulation, and physiological role in spermatogenesis. Current topics in developmental biology. 2005;71:263–96. Epub 2005/12/14. 10.1016/S0070-2153(05)71008-5 . [DOI] [PubMed] [Google Scholar]
- 17.Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. Journal of cell science. 2004;117(Pt 5):783–98. Epub 2004/01/22. 10.1242/jcs.00900 . [DOI] [PubMed] [Google Scholar]
- 18.Gutmann DH, Geist RT, Wright DE, Snider WD. Expression of the neurofibromatosis 1 (NF1) isoforms in developing and adult rat tissues. Cell growth & differentiation: the molecular biology journal of the American Association for Cancer Research. 1995;6(3):315–23. Epub 1995/03/01. . [PubMed] [Google Scholar]
- 19.McCormick F, Martin GA, Clark R, Bollag G, Polakis P. Regulation of ras p21 by GTPase activating proteins. Cold Spring Harbor symposia on quantitative biology. 1991;56:237–41. Epub 1991/01/01. . [DOI] [PubMed] [Google Scholar]
- 20.Almog T, Lazar S, Reiss N, Etkovitz N, Milch E, Rahamim N, et al. Identification of Extracellular Signal-regulated Kinase 1/2 and p38 MAPK as Regulators of Human Sperm Motility and Acrosome Reaction and as Predictors of Poor Spermatozoan Quality. Journal of Biological Chemistry. 2008;283(21):14479–89. 10.1074/jbc.M710492200 [DOI] [PubMed] [Google Scholar]
- 21.Almog T, Naor Z. Mitogen activated protein kinases (MAPKs) as regulators of spermatogenesis and spermatozoa functions. Molecular and cellular endocrinology. 2008;282(1–2):39–44. Epub 2008/01/08. 10.1016/j.mce.2007.11.011 . [DOI] [PubMed] [Google Scholar]
- 22.Bruscoli S, Velardi E, Di Sante M, Bereshchenko O, Venanzi A, Coppo M, et al. Long glucocorticoid-induced leucine zipper (L-GILZ) protein interacts with ras protein pathway and contributes to spermatogenesis control. J Biol Chem. 2012;287(2):1242–51. Epub 2011/11/24. 10.1074/jbc.M111.316372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasegawa K, Namekawa SH, Saga Y. MEK/ERK signaling directly and indirectly contributes to the cyclical self-renewal of spermatogonial stem cells. Stem cells (Dayton, Ohio). 2013;31(11):2517–27. Epub 2013/07/31. 10.1002/stem.1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia W, Cheng CY. TGF-beta3 regulates anchoring junction dynamics in the seminiferous epithelium of the rat testis via the Ras/ERK signaling pathway: An in vivo study. Developmental biology. 2005;280(2):321–43. Epub 2005/05/11. 10.1016/j.ydbio.2004.12.036 . [DOI] [PubMed] [Google Scholar]
- 25.Wong CH, Cheng CY. Mitogen-activated protein kinases, adherens junction dynamics, and spermatogenesis: a review of recent data. Developmental biology. 2005;286(1):1–15. Epub 2005/09/13. 10.1016/j.ydbio.2005.08.001 . [DOI] [PubMed] [Google Scholar]
- 26.He Z, Jiang J, Kokkinaki M, Golestaneh N, Hofmann MC, Dym M. Gdnf upregulates c-Fos transcription via the Ras/Erk1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem cells (Dayton, Ohio). 2008;26(1):266–78. Epub 2007/10/27. 10.1634/stemcells.2007-0436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Kanatsu-Shinohara M, Morimoto H, Kazuki Y, Takashima S, Oshimura M, et al. Genetic reconstruction of mouse spermatogonial stem cell self-renewal in vitro by Ras-cyclin D2 activation. Cell stem cell. 2009;5(1):76–86. Epub 2009/07/03. 10.1016/j.stem.2009.04.020 . [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, He Y, Sharma R, Xing W, Estwick SA, Wu X, et al. Hyperactive RAS/PI3-K/MAPK Signaling Cascade in Migration and Adhesion of Nf1 Haploinsufficient Mesenchymal Stem/Progenitor Cells. International journal of molecular sciences. 2015;16(6):12345–59. Epub 2015/06/04. 10.3390/ijms160612345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deo M, Huang JL, Fuchs H, de Angelis MH, Van Raamsdonk CD. Differential effects of neurofibromin gene dosage on melanocyte development. The Journal of investigative dermatology. 2013;133(1):49–58. Epub 2012/07/20. 10.1038/jid.2012.240 . [DOI] [PubMed] [Google Scholar]
- 30.Lin CC, Huang WJ, Chen KK. Measurement of testicular volume in smaller testes: how accurate is the conventional orchidometer? Journal of andrology. 2009;30(6):685–9. Epub 2009/07/07. 10.2164/jandrol.108.006460 . [DOI] [PubMed] [Google Scholar]
- 31.Almog T, Naor Z. The role of Mitogen activated protein kinase (MAPK) in sperm functions. Molecular and cellular endocrinology. 2010;314(2):239–43. Epub 2009/05/27. 10.1016/j.mce.2009.05.009 . [DOI] [PubMed] [Google Scholar]
- 32.Chapin RE, Wine RN, Harris MW, Borchers CH, Haseman JK. Structure and control of a cell-cell adhesion complex associated with spermiation in rat seminiferous epithelium. Journal of andrology. 2001;22(6):1030–52. Epub 2001/11/10. . [DOI] [PubMed] [Google Scholar]
- 33.Yan HH, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22(6):1945–59. Epub 2008/01/15. 10.1096/fj.06-070342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee NP, Mruk DD, Wong CH, Cheng CY. Regulation of Sertoli-germ cell adherens junction dynamics in the testis via the nitric oxide synthase (NOS)/cGMP/protein kinase G (PRKG)/beta-catenin (CATNB) signaling pathway: an in vitro and in vivo study. Biology of reproduction. 2005;73(3):458–71. Epub 2005/04/29. 10.1095/biolreprod.105.040766 . [DOI] [PubMed] [Google Scholar]
- 35.Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-beta3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003;144(4):1139–42. Epub 2003/03/18. 10.1210/en.2002-0211 . [DOI] [PubMed] [Google Scholar]
- 36.Yan HH, Cheng CY. Blood-testis barrier dynamics are regulated by an engagement/disengagement mechanism between tight and adherens junctions via peripheral adaptors. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(33):11722–7. Epub 2005/08/09. 10.1073/pnas.0503855102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Donnell L, Stanton PG, Bartles JR, Robertson DM. Sertoli cell ectoplasmic specializations in the seminiferous epithelium of the testosterone-suppressed adult rat. Biology of reproduction. 2000;63(1):99–108. Epub 2000/06/22. . [DOI] [PubMed] [Google Scholar]
- 38.Hedger M, Klug J, Frohlich S, Muller R, Meinhardt A. Regulatory cytokine expression and interstitial fluid formation in the normal and inflamed rat testis are under leydig cell control. Journal of andrology. 2005;26(3):379–86. Epub 2005/05/04. 10.2164/jandrol.04149 . [DOI] [PubMed] [Google Scholar]
- 39.O'Donnell L, McLachlan RI, Wreford NG, de Kretser DM, Robertson DM. Testosterone withdrawal promotes stage-specific detachment of round spermatids from the rat seminiferous epithelium. Biology of reproduction. 1996;55(4):895–901. Epub 1996/10/01. . [DOI] [PubMed] [Google Scholar]
- 40.O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. Spermiation: The process of sperm release. Spermatogenesis. 2011;1(1):14–35. Epub 2011/08/26. 10.4161/spmg.1.1.14525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shao M, Ghosh A, Cooke VG, Naik UP, Martin-DeLeon PA. JAM-A is present in mammalian spermatozoa where it is essential for normal motility. Developmental biology. 2008;313(1):246–55. Epub 2007/11/21. 10.1016/j.ydbio.2007.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma W, Baumann C, Viveiros MM. Lack of protein kinase C-delta (PKCdelta) disrupts fertilization and embryonic development. Molecular reproduction and development. 2015;82(10):797–808. Epub 2015/07/24. 10.1002/mrd.22528 . [DOI] [PubMed] [Google Scholar]
- 43.Schneider JS, Burgess C, Sleiter NC, DonCarlos LL, Lydon JP, O'Malley B, et al. Enhanced sexual behaviors and androgen receptor immunoreactivity in the male progesterone receptor knockout mouse. Endocrinology. 2005;146(10):4340–8. Epub 2005/07/09. 10.1210/en.2005-0490 . [DOI] [PubMed] [Google Scholar]
- 44.Lue Y, Wang C, Lydon JP, Leung A, Li J, Swerdloff RS. Functional role of progestin and the progesterone receptor in the suppression of spermatogenesis in rodents. Andrology. 2013;1(2):308–17. Epub 2013/02/15. 10.1111/j.2047-2927.2012.00047.x . [DOI] [PubMed] [Google Scholar]
- 45.Gadkar S, Shah CA, Sachdeva G, Samant U, Puri CP. Progesterone receptor as an indicator of sperm function. Biology of reproduction. 2002;67(4):1327–36. Epub 2002/09/26. . [DOI] [PubMed] [Google Scholar]
- 46.Ganem JP, Workman KR, Shaban SF. Testicular microlithiasis is associated with testicular pathology. Urology. 1999;53(1):209–13. Epub 1999/01/14. . [DOI] [PubMed] [Google Scholar]
- 47.Kume H, Tachikawa T, Teramoto S, Isurugi K, Kitamura T. Bilateral testicular tumour in neurofibromatosis type 1. Lancet (London, England). 2001;357(9253):395–6. Epub 2001/02/24. 10.1016/s0140-6736(05)71531-2 . [DOI] [PubMed] [Google Scholar]
- 48.Raygada M, Arthur DC, Wayne AS, Rennert OM, Toretsky JA, Stratakis CA. Juvenile xanthogranuloma in a child with previously unsuspected neurofibromatosis type 1 and juvenile myelomonocytic leukemia. Pediatric blood & cancer. 2010;54(1):173–5. Epub 2009/09/29. 10.1002/pbc.22297 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.