Abstract
The Chloride Channel (CLC) gene family is reported to be involved in vacuolar nitrate (NO3-) transport. Nitrate distribution to the cytoplasm is beneficial for enhancing NO3- assimilation and plays an important role in the regulation of nitrogen (N) use efficiency (NUE). In this study, genomic information, high-throughput transcriptional profiles, and gene co-expression analysis were integrated to identify the CLCs (BnaCLCs) in Brassica napus. The decreased NO3- concentration in the clca-2 mutant up-regulated the activities of nitrate reductase and glutamine synthetase, contributing to increase N assimilation and higher NUE in Arabidopsis thaliana. The genome-wide identification of 22BnaCLC genes experienced strong purifying selection. Segmental duplication was the major driving force in the expansion of the BnaCLC gene family. The most abundant cis-acting regulatory elements in the gene promoters, including DNA-binding One Zinc Finger, W-box, MYB, and GATA-box, might be involved in the transcriptional regulation of BnaCLCs expression. High-throughput transcriptional profiles and quantitative real-time PCR results showed that BnaCLCs responded differentially to distinct NO3- regimes. Transcriptomics-assisted gene co-expression network analysis identified BnaA7.CLCa-3 as the core member of the BnaCLC family, and this gene might play a central role in vacuolar NO3- transport in crops. The BnaCLC members also showed distinct expression patterns under phosphate depletion and cadmium toxicity. Taken together, our results provide comprehensive insights into the vacuolar BnaCLCs and establish baseline information for future studies on BnaCLCs-mediated vacuolar NO3- storage and its effect on NUE.
Introduction
Nitrogen (N) is a fundamental non-mineral macronutrient, which is essential for the growth and development of higher plants [1]. China is the largest N-consuming country worldwide; although massive amounts of N are applied annually as fertilizer, crop yields are declining in some areas [2–3]. Hence, enhancing plant N use efficiency (NUE) is critical for developing sustainable agriculture [4]. Oilseed rape (Brassica napus L.) is a staple oil crop and has a high N requirement. To maintain its optimum yield, relatively high amounts of N fertilizer (from 150 to 300 kg N hm-2) are applied to soils [5–6], but only 30–50% of the applied N fertilizer is taken up from soil by crops [7]. The average NUE is approximately 35% in China, which results in N surpluses that are detrimental to the environment [4–5]. Therefore, the development of N-efficient cultivars through genetic improvement of crop NUE is a cost-effective and environmental friendly way to reduce excessive N in soils [8].
Inorganic nitrate (NO3-) is a predominant N-containing anion absorbed by upland crops, such as B. napus, under aerobic conditions [1]. NO3- uptake, accumulation, and utilization have been reported to have close relationships with NUE [2, 9, 10, 11]. Four NO3- transporters, namely nitrate transporter 1 (NRT1), nitrate transporter 2 (NRT2), chloride channel (CLC) family proteins, and slow-type anion channel associated homologs (SLAC1/SLAH1-4), have been implicated in efficient N uptake and transport [12]. To cope with fluctuating NO3- concentrations in soils, NRT1 and NRT2, which are low-affinity (Km = 0.5 mM) and high-affinity (Km = 10–100 mM) transport systems, respectively, work together to ensure efficient NO3- uptake [13–14]. Once it has entered the roots, NO3- can be stored in situ or undergo long-distance transport to shoots where it can be assimilated or stored in vacuoles.
The 2NO3-/H+ antiporter AtCLCa responsible for NO3- storage in vacuoles first identified from Arabidopsis thaliana (A. thaliana) cells belongs to the CLC gene family [15]. Members of this family specifically transport chloride as Cl-/H+ antiporters or channels [16–17]. Geelen et al. (2000) found that the clca-1 mutant presented reduced NO3- content, and then De Angeli et al. (2006) first demonstrated that AtCLCa is a tonoplast-localized NO3- antiporter that is involved in the regulation of NO3- sequestration into vacuoles [15, 18]. Three of the six other members in the CLC gene family, namely AtCLCb, AtCLCc, and AtCLCg [19], are also localized to tonoplasts [20]. Although AtCLCb functions as a 2NO3-/H+ antiporter in Xenopus oocytes, it is still unknown if it is involved in vacuolar NO3- storage in plants [20]. While AtCLCc seems to be responsible for NO3- and chloride homeostasis, and essential for stomatal movement and salt tolerance by regulating chloride transport [21–22], AtCLCg participates in salt tolerance by altering chloride homeostasis in mesophyll cells [23]. Both the AtCLCd and AtCLCf proteins are localized to Golgi membranes. However, AtCLCd plays a crucial role in the regulation of luminal pH in the trans-Golgi network by transporting chloride or NO3- [24], whereas the function of AtCLCf remains elusive [25]. AtCLCe is situated in the thylakoid membrane of chloroplasts and participates in the regulation of photosynthetic electron transport [26].
B. napus is a staple oil crop worldwide. The allotetraploid B. napus (AnAnCnCn, ~1345 Mb, 2n = 4x = 38) was derived from the natural hybridization between Brassica rapa (ArAr, ~485 Mb, 2n = 2x = 20) [27] and Brassica oleracea (CoCo, ~630 Mb, 2n = 2x = 18) [28] ~7,500 years ago [29–31]. The allopolyploidy events occurring in B. napus resulted in more duplicated segments and homologous regions within the rapeseed genome [30] than those found in A. thaliana (~125 Mb, 2n = 2x = 10) (Arabidopsis Genome Initiative 2000), which also belongs to the Brassicaceae family.
B. napus has a higher requirement than other cereals for N-containing nutrients to maintain its optimal growth [8]. In rice, overexpression of OsNRT2.3b contributes to enhance NO3- uptake and grain yield in the field [32]. In A. thaliana, mutation of AtCLCa was shown to decrease NO3- concentrations in tissues and improve NUE [4]. However, the genome-wide organization of vacuolar NO3- transporter genes in B. napus, especially the CLC gene family, is poorly understood. Thus, in the present study, we aimed to provide: (i) a genome-wide identification of the CLC family members in Brassica sp. crops; (ii) a comprehensive analysis of the molecular characteristics of the tonoplast-localized NO3- transporter genes in B. napus; and (iii) the identification of the core members of the BnaCLC gene family and their transcriptional responses to different N regimes. The genome-wide identification and molecular characterization of the BnaCLC family members presented here will provide fundamental information for NUE improvement and for further research on the biological functions and evolutionary relationships within this gene family in B. napus and other crop species.
Materials and methods
Plant materials and growth conditions
The B. napus cultivar ‘Xiang-you 15’ (XY15) was provided by the Improvement Center of National Oil Crops (Hunan Branch, Changsha, Hunan Province, China).A. thaliana wild-type Wassilewskija (Ws) was used as control for the chloride channel accessory 2 (clca-2) mutant (FST 171A06), as described in De Angeli et al. (2006) [15]. Both plant lines were obtained from the Institute National de la Recherche Agronomique collection in France.
All plants were grown in an incubator set at 70% relative humidity, 16-h-light/8-h-dark cycle, and a constant temperature of 22°C. Plant lines were sowed in a matrix consisting of vermiculite and perlite at a ratio of 3:2. After germination, B. napus were transplanted and hydroponically grown in plastic boxes (2 L), as described by Han et al. (2016) [4]. These boxes were arranged in a completely randomized design with three biological replicates. The Hoagland nutrient solution (pH 5.8) provided to plants was replaced every 3 days. As described in Han et al. (2016) [4], Ws and clca-2 were transplanted and hydroponically grown in plastic boxes (550 mL), and the nutrient solution was replaced every 5 days. These boxes were arranged in a completely randomized design with four biological replicates.
Determination of NO3- and N concentrations
As described by Han et al. (2016) [4], plant tissues were sampled, bathed in boiling water for 30 min, and then assayed forNO3- content using a continuous-flow auto-analyzer (AA3, Seal Analytical Inc., Southampton, UK). The whole seedlings of hydroponically grown A. thaliana were sampled and oven dried at 105°C for 30 min, and then at 65°C until they reached a constant weight. N concentrations were determined using the Kjeldahl method [33], Total N = N concentration × biomass. The NUE value based on biomass was calculated based on the following formula: NUE = total biomass/ total N.
Determination of the activities of nitrate reductase (NR) and glutamine synthetase (GS)
The method for determining NR activity was slightly modified from that presented in previous studies [34–35]. Five milliliters of phosphate buffer (0.1M, pH7.5) was added to frozen root tissue samples (~0.3 g) and then ground to homogeneity using a chilled mortar and pestle in the presence of acid-washed sand. The homogenates were centrifuged at 2,000 ×g for 15 min at 4°C, and the resulting supernatant was assayed for NADH-dependent NR activity. The reaction mixture consisted of 0.4 mL supernatant, 0.1 M KNO3, and 3 mM NADH. The reaction was terminated after 30 min at 25°C by the addition of 1% sulfanilamide and α-naphthylamine. The amount of reaction product was measured at 540 nm using the UV-2600 spectrophotometer (Shimadzu Corp., Kyoto, Japan).
The activity of GS was determined by quantification of γ-glutamyl hydroxamate as described by Culimore and Sims. (1980) [36]. Five milliliters of Tris-HCl buffer (0.05M Tris-HCl, 0.5 mM MgCl2, 1.0mM EDTA, pH7.8) was added to frozen root tissue samples (~0.3 g) and then ground to homogeneity using a chilled mortar and pestle. The homogenates were centrifuged at 8,000 ×g for 15 min at 4 °C, and the resulting supernatant was used to analyze GS activity. The reaction mixture contained 1.2 mL supernatant, 0.25M imidazole buffer (pH 7.0), 0.3 M sodium glutamate, 0.3 mM ATP, 0.5M MgSO4, and 2 M hydroxylamine, and it was incubated for 15 min at 25°C. An acidic FeCl3 solution (0.088 M FeCl3, 0.67 M HCl, and 0.20 M trichloroacetic acid) was added to terminate the reaction, and the amount of Fe (III)-complex in the reaction product (γ-glutamyl hydroxamate) was measured at 540 nm using the UV-2600 spectrophotometer (Shimadzu Corp.).
Retrieval of the CLC family gene sequences
The CLC genes of Brassica species were identified based on their similarities to A. thaliana homologs. The genomic sequences, coding sequences (CDS), protein sequences, and gene IDs of the seven CLC genes (CLCa–g) were obtained from the Arabidopsis Information Resource (TAIR, https://www.arabidopsis.org/) database. We used the CLC gene sequences of A. thaliana as the seed sequences, and performed a basic local alignment search tool analysis using protein sequences as queries (BLASTp) to retrieve homologous gene sequences with an E value < 1e-10 in Brassica crops and other species. The databases used included The Brassica Database (BRAD) Version 1.1 (http://brassicadb.org/brad/) [37] for B. rapa, Bol Base Version 1.0 (http://119.97.203.210/bolbase/index.html) for B. oleracea [38], Genoscope (http://www.genoscope.cns.fr/brassicanapus/) for B. napus [30], and the National Center for Biotechnology Information (NCBI, www.ncbi.nlm.nih.gov) for other species [39]. In the present study, genes from Brassica species were named as follows: (abbreviation of the species name) + (chromosome number) + (period) + (name of gene homolog in A. thaliana). For example, BnaC6.CLCa-1 represents the CLCa gene on chromosome C6 of B. napus. All the data were retrieved on March 10, 2018.
Chromosomal localization of the CLC family genes in B. napus
The starting positions of all the CLC family genes in B. napus were obtained from BRAD using the complete nucleotide sequences of B. napus. The MapInspect software (http://www.softsea.com/review/MapInspect.html) was then used to draw chromosomal location diagrams for these genes.
Multiple alignment and phylogenetic analysis of the BnaCLC family proteins
Multiple sequence alignments of the CLC protein sequences were performed using the ClustalW tool of MEGA 6.06 [40]. Based on these alignments, an unrooted phylogenetic tree, comprising 128 full-length CLC protein sequences, was constructed in MEGA 6.06 using the neighbor joining method, and Poisson correction, pairwise deletion, and bootstrapping (1000 replicates; random seeds) as the required parameters.
Characterization of conserved motifs and physiochemical characteristics of the BnaCLC family proteins
The protein sequences of A. thaliana and Brassica species were submitted to the Multiple Expectation maximization for Motif Elicitation (MEME) online software (http://meme-suite.org/tools/meme, Version 4.11.2) for characterizing the conserved motifs/domains [41]. Default parameters were generally used, except for the optimum motif width, which was set to 6–50 bp, and the maximum number of motifs, which was set to 10. The ExPASy ProtoParam (http://www.expasy.org/tools/protparam.html) [42] tool was used to obtain the number of amino acids, molecular weights (MWs, kDa), theoretical isoelectric points (pIs), grand average of hydropathy (GRAVY) values, and instability indexes (a protein with instability index > 40 was considered unstable) [43].
Evolution selection pressure analysis and functional divergence of the BnaCLC family genes
Pairwise alignments of gene CDS without stop codons were performed in ClustalW (http://www.clustal.org/clustal2/) [44], and then subject to the KaKs_Calculator toolkit (https://sourceforge.net/projects/kakscalculator2/) [27] to obtain the non-synonymous substitution rate (Ka), synonymous substitution rate (Ks), and Ka/Ks values by implementing the yn00 method [45]. The formula T = Ks/2λ (where λ = 1.5 × 10−8 for Brassicaceae) [46] was used to calculate the probable age of segmental duplication. The Detecting Variability in Evolutionary Rates among Genes software (DIVERGE) 3.0 (http://xungulab.com/software/diverge3/diverge3.html) [47] was used to detect gene functional divergence through multiple alignments of CDS and protein sequences.
Exon-intron structure of the BnaCLC family genes
The full-length CDS and genomic sequences of the CLC family genes were submitted to the online Gene Structure Display Server (GSDS) 2.0 (http://gsds.cbi.pku.edu.cn/) [48] to analyze their exon-intron structures.
Identification of putative cis-acting regulatory elements (CREs) in the BnaCLC gene promoters
A 2.0-kb fragment of each genomic sequence upstream of the start codon (ATG) was downloaded from the Brassica napus Genome Browser (http://www.genoscope.cns.fr/brassicanapus/) [30]. These sequences were submitted to PLACE 30.0 (http://www.dna.affrc.go.jp/PLACE/) [49] to examine putative CREs. The distributions of the over-presented CREs along the promoters were displayed in PROSITE (https://prosite.expasy.org/mydomains/). The enriched CREs were displayed using the word cloud generator WordArt (https://wordart.com/).
Transcriptional profiling and identification of core gene members within the BnaCLC family
The B. napus cultivar XY15 was used to identify the genome-wide mRNA transcriptome responses of this species to NO3- depletion and replenishment conditions. The hydroponic grown B. napus seedlings were cultivated and processed according to the method described by Han et al. (2016) [4]. Briefly, B. napus seedlings were cultivated under high NO3- solutions (9.0 mM) for 9 days and then transferred to NO3--free solutions for 3 days. For NO3--depletion treatments, the seedlings were cultivated in NO3--free solutions (control). For NO3--replenishment treatments, the seedlings were transferred to high NO3- (9.0 mM) for 6 h. The shoots and roots of the rapeseed seedlings subject to each treatment were then sampled. Each sample, including three independent biological replicates, was then used for high-throughput transcriptome profiling.
Gene co-expression analysis was used to identify gene interactions and the core genes within the BnaCLC family members. Default thresholds of Pearson correlation values were set (http://plantgrn.noble.org/DeGNServer/Analysis.jsp), and CYTOSCAPE 3.2.1 was used to construct the gene co-expression networks [50].
Transcriptional responses of the BnaCLC members under cadmium (Cd) toxicity and inorganic phosphate (Pi) depletion
Quantitative reverse-transcription PCR (qRT-PCR) was used to identify the relative gene expression of the BnaCLCa members. For the Cd toxicity treatment, the rapeseed plants were hydroponically cultivated in a Cd-free solution for 10 d, and then transferred to the 10 μM CdCl2 treatment for 6 h. For the inorganic phosphate (Pi) starvation treatment, the rapeseed seedlings were first grown under 250 μM Pi (KH2PO4) for10 d, and then transferred to the a Pi-free solution for 5 d. The shoots and roots were individually harvested and stored at -80 °C until RNA isolation.
After treatment with RNase-free DNase I, the total RNAs isolated from samples were used as templates for cDNA synthesis with the PrimeScript RT reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa, Shiga, Japan). The qRT-PCR assays for the detection of relative gene expression were performed using SYBR Premix Ex Taq II (TliRNaseH Plus) (TaKaRa) under an Applied Biosystems StepOne Plus Real-time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). The thermal cycles were as follows: 95°C for 3 min, followed by 40 cycles at 95°C for 10 s and 60°C for 30 s. Melting curve analysis to ensure primer specificity was conducted as follows: 95°C for 15 s, 60°C for 1 min, 60–95°C for 15 s (+0.3°C per cycle). Expression data were normalized using the public reference genes BnaEF1-α [51] and BnaGDI1 [52], and relative gene expression was calculated with the 2-ΔΔCT method [53].
Statistical analysis
Significant differences (P-value < 0.05) were determined by one-way analysis of variance(ANOVA), followed by Tukey’s honestly significant difference (HSD) multiple comparison tests, using the Statistical Package for the Social Sciences17.0 (SPSS, Chicago, IL, USA).
Availability of data and materials
We have upload the raw high-throughput sequencing data as supporting information files (S3 Table), in addition, rapeseed seeds that are required to reproduce these findings can be shared by contacting the corresponding author, Zhen-hua Zhang (zhzh1468@163.com) or Ying-peng Hua (yingpenghua89@126.com)
Results
Decreased vacuolar sequestration capacity (VSC) of NO3- results in enhanced NUE in the clca-2 mutant
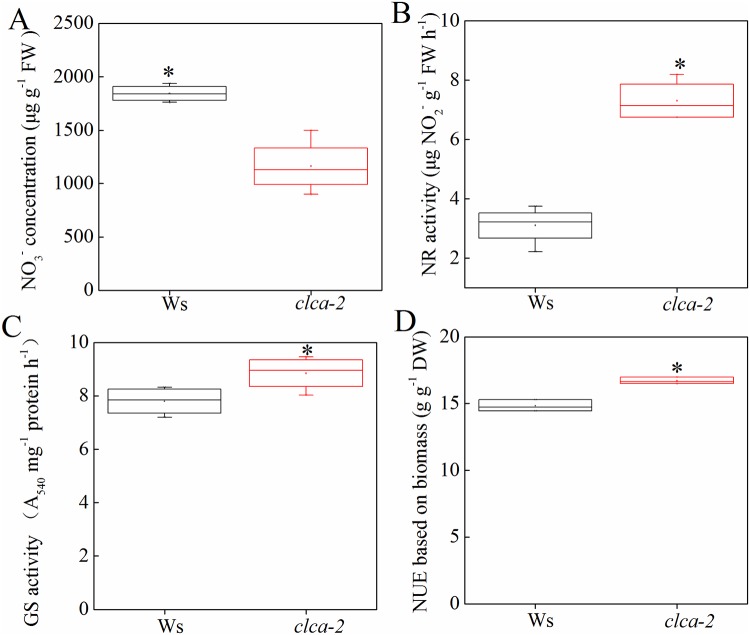
AtCLCa has been defined as a 2NO3-/H+ antiporter and participates in NO3- storage in vacuoles [15]. To investigate the effect of impaired NO3- VSC on NUE in the clca-2 mutant, we first determined the NO3- concentrations in both Ws and clca-2 plants. The results showed that NO3- concentration was significantly lower in the clca-2 mutant than in Ws (Fig 1A). Further, the activities of some key enzymes involved in N assimilation, namely NR and GS, were determined. They were significantly higher in the clca-2 mutant than in Ws (Fig 1B and 1C). These results suggested that there was more NO3- in the cytoplasm of the clca-2 mutant than in the cytoplasm of Ws, which induced the activities of NR and GS thereby contributing to enhance N assimilation and NUE (Fig 1D). Thus, the decreased NO3- VSC regulated by AtCLCa improved NUE in A. thaliana. However, a genome-wide analysis of the CLC gene family in B. napus was necessary to further explore the functions of these genes and to provide a theoretical basis for studying their effects on the NUE of this species.
Fig 1. Decreased NO3- concentration in the clca-2 mutant accelerated nitrogen (N) assimilation, resulting in higher N use efficiency (NUE).
Plants grown hydroponically were sampled for further analysis at the seedling stage. Conditions for hydroponics culture and characteristics of Arabidopsis thalina (A. thaliana) are defined in the “Methods”. The wild-type Wassilewskija (Ws) plants were used as the control for the CLC null mutant (clca-2). At the seedling stage, plants were sampled for NO3- assays (A), and the activities of nitrate reductase (NR) (B) and glutamine synthetase (GS) (C) were shown in Ws and clca-2. NUE values were calculated based on total biomass (shoot and root) per total N uptake, and the differences in NUE of Ws and clca-2 were shown in D. Bars indicate standard deviations (SD) of four biological replicates. The asterisks denote significant differences at P < 0.05.
Comparative analysis and phylogenetic relationships among the BnaCLC family genes
The sequences and gene IDs of the seven AtCLCs genes were used to perform BLAST searches against the genomes in the Brassica database. We found that B. rapa, B. oleracea, and B. napus had 11, 10, and 22 CLC homologs, respectively (S1 Table). This indicated that the BnaCLCs did not suffer gene loss or addition during the allopolyploidy process of the rapeseed genome (AnAnCnCn, 2n = 4x = 38). Similar to A. thaliana, the CLC genes identified in the three Brassica species can also be divided into seven subgroups (CLCa–g). Specifically, the 22 BnaCLC family genes comprised four BnaCLCas, four BnaCLCbs, four BnaCLCcs, two BnaCLCds, two BnaCLCes, four BnaCLCfs, and two BnaCLCgs.
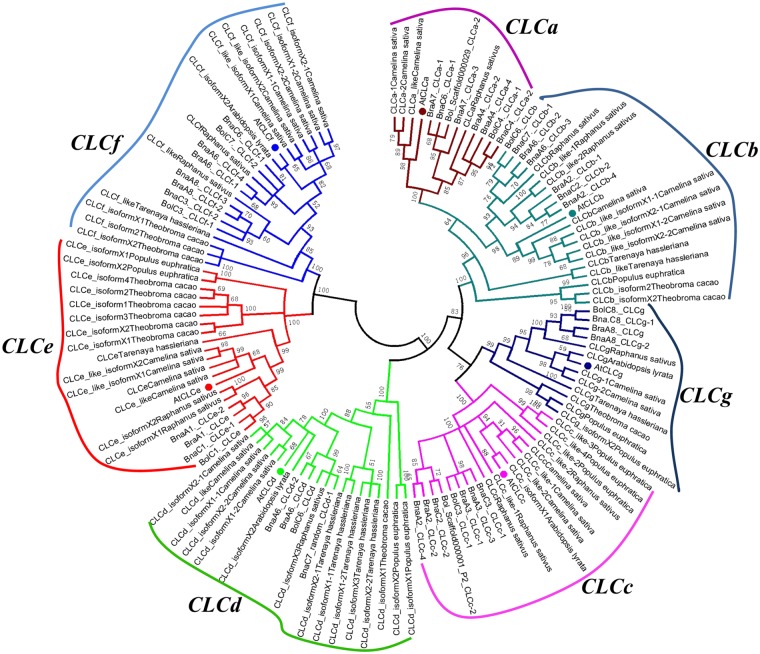
To determine the molecular evolution and phylogenetic relationships among the CLC proteins from several species, an unrooted phylogenetic tree was constructed based on the amino acid sequences of 128 CLC genes from 10 plant species (Fig 2): the four Brassica species plus Arabidopsis lyrata, Camelina sativa, Populus euphratica, Raphanus sativus, Tarenayahassleriana and Theobroma cacao. The CLC family genes were classified into seven clades regardless of plant species (Fig 2), which is consistent with the subfamily categorization. This indicated that CLC protein divergence occurred before organism speciation. Furthermore, in Brassica species, each subfamily member of the CLC family was closely clustered with the corresponding homologs in A. thaliana (Fig 2). Most of the CLC proteins within each subfamily had very short branch lengths (Fig 2), indicating a recent genetic divergence.
Fig 2. Phylogenetic tree of CLCs in diverse plant species.
A total of 118 protein sequences from 10 species including Brassica napus, Brassica rapa, Brassica oleracea, Arabidopsis thaliana, Arabidopsis lyrata, Camelina sativa, Populus euphratica, Raphanus sativus, Tarenaya hassleriana and Theobroma cacao were multi-aligned using the ClustalW program, and then an unrooted phylogenetic tree was constructed using the software MEGA6.06 with the neighbor-joining method. The percentage of replicate trees, in which the associated taxa clustered together in the bootstrap test (1000 replicates), are shown next to the branches.
Chromosomal localization and gene expansion patterns of the BnaCLC genes
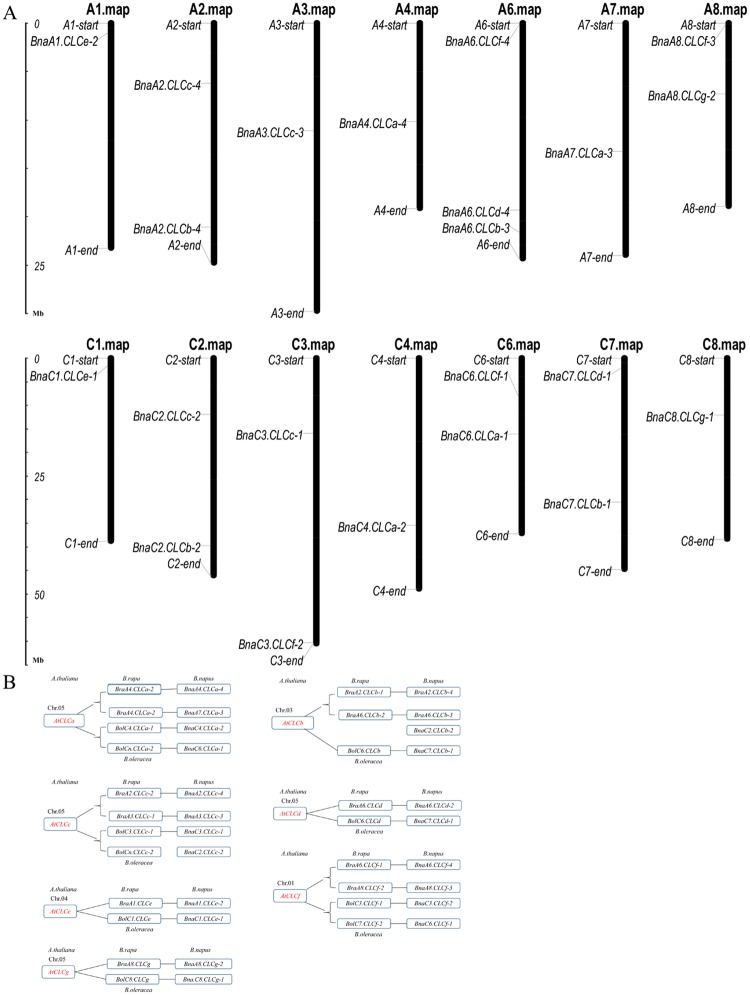
The CLC genes in B. napus were physically mapped onto 14 chromosomes (An subgenomes: An1, An2, An3, An4, An6, An7, and An8; Cn subgenomes: Cn1, Cn2, Cn3, Cn4, Cn6, Cn7, and Cn8). All BnaCLCs were evenly distributed on each of the An (11) and Co (11) genomes (Fig 3A). Comparative genomics revealed that the A. thaliana genome is divided into 24 ancestral crucifer blocks (labeled A-X) [54]. Here, the members of the seven CLC subfamilies were mapped to six blocks (Table 1). All the BnaCLCa and BnaCLCg members were mapped to the S block, and the members belonging to the other five CLC subfamilies were located within the L, Wb, Q, U, and C blocks, respectively (Table 1). The Brassica genomes resulting from whole genome triplication are separated into least fractionated (LF), moderate fractionated (MF1), and most fractionated (MF2) subgenomes [55]. There were 43 CLCs in B. rapa, B. oleracea, and B. napus. Approximately 37.2% (16) of the CLCs belonged to the LF subgenome, and the MF1 and MF2 subgenomes comprised 17 and 10 CLC family genes, accounting for 39.5% and 23.3%, respectively (Table 1). To further understand the CLC gene expansion patterns, we investigated gene duplication events in B. napus, which revealed that segmental duplication played an important role in the expansion of the BnaCLC family genes (Fig 3B).
Fig 3. Chromosomal location and expansion events of the CLC gene family in B. napus.
The starting positions of all BnaCLCs were collected from BRAD through BLASTn search, and the gene chromosomal location diagram was drawn using the MapInspect software (A). Evolutionary processes and expansion events of the CLC gene family in B. napus (B). The homologues between different Brassica species are connected by lines.
Table 1. Molecular characterization of the BnaCLC family proteins in Brassica crops (B. rapa, B. oleracea and B. napus).
| Gene name | Gene ID | Block | Subgenome | CDS (bp) | Amino acids | MW (kD) | pI | Instablility index | GRAVY |
|---|---|---|---|---|---|---|---|---|---|
| BnaC6.CLCa-1 | BnaC06g13300D | S | MF1 | 2313 | 770 | 84.74 | 8.14 | 36.55 | 0.250 |
| BnaC4.CLCa-2 | BnaC04g33870D | S | MF1 | 2331 | 776 | 85.36 | 8.11 | 36.40 | 0.264 |
| BnaA7.CLCa-3 | BnaA07g15180D | S | MF1 | 2313 | 770 | 84.67 | 7.91 | 36.76 | 0.257 |
| BnaA4.CLCa-4 | BnaA04g11860D | S | MF2 | 2331 | 776 | 85.43 | 7.89 | 36.60 | 0.256 |
| BraA7.CLCa-1 | Bra028478 | S | MF1 | 2313 | 770 | 84.74 | 8.14 | 36.90 | 0.253 |
| BraA4.CLCa-2 | Bra030328 | S | MF2 | 2331 | 776 | 85.44 | 8.11 | 36.60 | 0.256 |
| BolC4.CLCa-1 | Bol006752 | S | MF1 | 2331 | 776 | 85.36 | 8.11 | 36.40 | 0.264 |
| BolCn.CLCa-2 | Bol036409 | S | MF1 | 2313 | 770 | 84.74 | 8.14 | 36.55 | 0.250 |
| BnaC7.CLCb-1 | BnaC07g24030D | L | LF | 2352 | 783 | 86.28 | 7.55 | 37.77 | 0.254 |
| BnaC2.CLCb-2 | BnaC02g36720D | L | MF1 | 2352 | 783 | 86.10 | 8.64 | 34.02 | 0.305 |
| BnaA6.CLCb-3 | BnaA06g32380D | L | LF | 2352 | 783 | 86.47 | 7.27 | 38.22 | 0.240 |
| BnaA2.CLCb-4 | BnaA02g28670D | L | MF1 | 2343 | 780 | 85.86 | 8.72 | 33.57 | 0.284 |
| BraA2.CLCb-1 | Bra032985 | L | MF1 | 2352 | 783 | 86.12 | 8.65 | 33.28 | 0.291 |
| BraA6.CLCb-2 | Bra025253 | L | LF | 2352 | 783 | 86.44 | 7.27 | 38.11 | 0.240 |
| BolC6.CLCb | Bol042862 | L | LF | 2352 | 783 | 86.29 | 7.55 | 38.36 | 0.254 |
| BnaC3.CLCc-1 | BnaC03g27500D | Wb | MF1 | 2328 | 775 | 84.43 | 8.77 | 38.12 | 0.390 |
| BnaC2.CLCc-2 | BnaC02g16210D | Wb | MF2 | 2328 | 775 | 84.33 | 8.55 | 36.91 | 0.396 |
| BnaA3.CLCc-3 | BnaA03g23270D | Wb | MF1 | 2328 | 775 | 84.47 | 8.77 | 38.01 | 0.389 |
| BnaA2.CLCc-4 | BnaA02g11800D | Wb | MF2 | 2328 | 775 | 84.41 | 8.55 | 36.91 | 0.396 |
| BraA3.CLCc-1 | Bra000596 | Wb | MF1 | 2328 | 775 | 84.44 | 8.77 | 38.12 | 0.386 |
| BraA2.CLCc-2 | Bra022507 | Wb | MF2 | 2328 | 775 | 84.41 | 8.55 | 36.91 | 0.396 |
| BolC3.CLCc-1 | Bol015059 | Wb | MF1 | 2328 | 775 | 84.43 | 8.77 | 38.12 | 0.390 |
| BolCn.CLCc-2 | Bol045308 | Wb | MF2 | 2328 | 775 | 84.36 | 8.55 | 36.75 | 0.396 |
| BnaC7.CLCd-1 | BnaC07g49590D | Q | LF | 2409 | 802 | 88.15 | 8.55 | 41.83 | 0.217 |
| BnaA6.CLCd-2 | BnaA06g28170D | Q | LF | 2379 | 792 | 87.10 | 8.35 | 42.76 | 0.214 |
| BraA6.CLCd | Bra009891 | Q | LF | 2379 | 792 | 87.10 | 8.35 | 42.76 | 0.214 |
| BolC6.CLCd | Bol022298 | Q | LF | 2409 | 802 | 88.13 | 8.55 | 41.39 | 0.218 |
| BnaC1.CLCe-1 | BnaC01g03130D | U | LF | 2142 | 713 | 75.30 | 5.18 | 50.75 | 0.293 |
| BnaA1.CLCe-2 | BnaA01g01990D | U | LF | 2106 | 701 | 74.08 | 5.52 | 50.99 | 0.315 |
| BraA1.CLCe | Bra011615 | U | LF | 2103 | 700 | 74.03 | 5.35 | 53.74 | 0.306 |
| BolC1.CLCe | Bol029074 | U | LF | 3096 | 1031 | 110.71 | 5.56 | 46.06 | 0.164 |
| BnaC6.CLCf-1 | BnaC06g07440D | C | LF | 2346 | 781 | 83.71 | 6.46 | 40.09 | 0.019 |
| BnaC3.CLCf-2 | BnaC03g70680D | C | MF1 | 2298 | 765 | 81.95 | 7.57 | 43.97 | 0.091 |
| BnaA8.CLCf-3 | BnaA08g00400D | C | MF1 | 2292 | 763 | 81.90 | 6.80 | 43.86 | 0.071 |
| BnaA6.CLCf-4 | BnaA06g00250D | C | LF | 2358 | 785 | 83.99 | 6.28 | 40.90 | 0.021 |
| BraA6.CLCf-1 | Bra038007 | C | LF | 2358 | 785 | 83.98 | 6.28 | 41.14 | 0.023 |
| BraA8.CLCf-2 | Bra030845 | C | MF1 | 2331 | 776 | 83.28 | 6.31 | 43.48 | 0.057 |
| BolC3.CLCf-1 | Bol035224 | C | MF1 | 2355 | 784 | 83.94 | 6.22 | 41.72 | 0.059 |
| BolC7.CLCf-2 | Bol039017 | C | LF | 2346 | 781 | 83.69 | 6.46 | 40.09 | 0.022 |
| BnaC8.CLCg-1 | BnaC08g08120D | S | MF2 | 2295 | 764 | 83.90 | 8.82 | 41.49 | 0.461 |
| BnaA8.CLCg-2 | BnaA08g07280D | S | MF2 | 2295 | 764 | 83.92 | 8.86 | 41.75 | 0.468 |
| BraA8.CLCg | Bra038948 | S | MF2 | 2295 | 764 | 83.90 | 8.86 | 41.75 | 0.465 |
| BolC8.CLCg | Bol027031 | S | MF2 | 2295 | 764 | 83.84 | 8.75 | 40.96 | 0.463 |
Note: CDS, coding sequence; MW, molecular weight; pI, isoelectric point; GRAVY, grand average of hydropathy; LF, Least fractionated subgenome; MF1, medium fractionated subgenome; MF2, more fractionated genome.
Conserved domain analysis of the CLC family genes in Brassica species
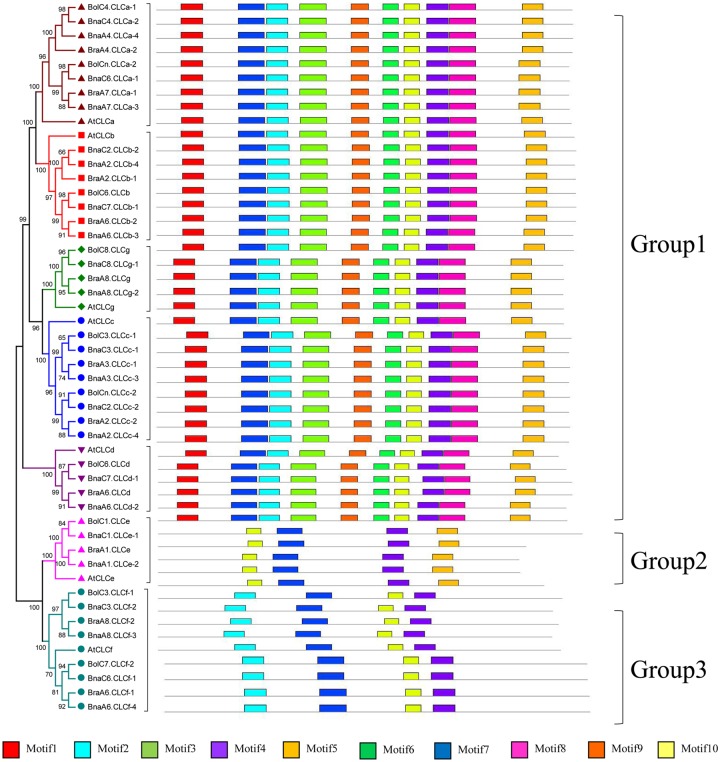
To characterize the conserved motifs in the CLC gene family, the MEME program was used to align the protein sequences of the CLC family genes. As shown in Fig 4, the CLC family genes were divided into three groups according to the classification of their conserved motifs. Group 1 comprised five subfamilies, namely CLCa, CLCb, CLCc, CLCd, and CLCg, which contained all of the 10 motifs predicted (S1A Fig). The CLCf and CLCe subfamilies were clustered in Group 2 and Group 3, respectively, and only four motifs were found in these two groups. All the CLC genes of Brassica species contained Motifs 4/7/10 (S1B Fig), demonstrating that these three motifs are highly conserved and might be used as markers for the identification and characterization of CLC genes in these crops. To further characterize these three highly conserved motifs, their short amino acid sequences were analyzed in WebLogo.
Fig 4. Characterization of the conserved motifs in the CLC proteins in A. thaliana and Brassica crops (B. rapa, B. oleracea and B. napus).
Conserved motifs were predicted by the MEME program. The boxes with different color represent different conserved motifs, and the gray lines indicate non-detected motifs in the CLC family genes.
Physicochemical characteristics of the CLC family genes and proteins in Brassica species
As shown in Table 1, the full-length CDS of the CLC genes in Brassica species range from 2,103 bp (BraA1.CLCe) to 3,096 bp (BolC1.CLCe), with deduced proteins of 700 to 1,031 amino acids. The predicted MWs ranged from 74.03kDa (BraA1.CLCe) to 110.71 kDa (BolC1.CLCe), which coincided with protein sizes. The pIs of the CLC proteins varied from 5.18 to 8.86, but most were above 7.0; exceptions were CLCe and CLCf. Half of the CLC proteins (23) showed instability indices (IIs) < 40.0, whereas the other half presented weak stabilities with IIs > 40.0. The GRAVY values of all CLCs, defined as the hydropathy values of all amino acids divided by the protein length, ranged from 0.019 (BnaC6.CLCf-1) to 0.468 (BnaA8.CLCg-2), indicating that all CLC genes in Brassica species are hydrophobic. Most of the CLC genes showed similar physicochemical parameters, except for the CLCf subfamily, whose GRAVY value was lower than 0.1.
Evolutionary selection pressures on the CLC proteins of Brassica species
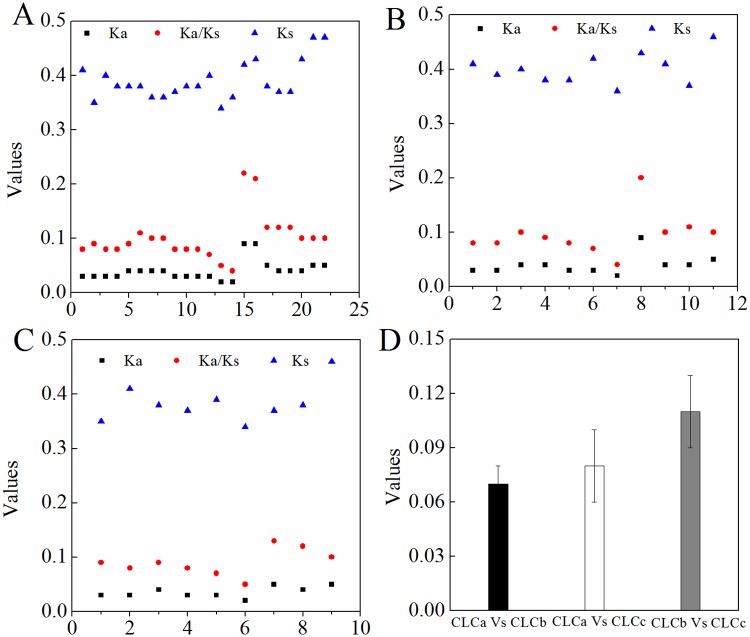
The Ks and Ka values are used to explore the mechanism of gene divergence after duplication [56]. To reveal the selective forces acting on the CLC proteins in Brassica species, we calculated the Ka/Ks for orthologous CLC gene pairs between the genomes of Brassicas pecies and A. thaliana. Generally, Ka/Ks > 1.0 indicates positive selection, Ka/Ks = 1.0 denotes neutral selection, and Ka/Ks < 1.0 shows negative or purifying selection [56]. As shown in Fig 5A–5C and S2 Table, the Ka/Ks ratios of the CLC genes in Brassica species were < 0.3. This suggested that a strong purifying selection pressure might have acted on the CLC genes of Brassica species to maintain gene function. To further investigate the functional divergence of BnaCLCs, the DIVERGE 3.0 program was used to estimate the type-II functional divergence (for eight or more homologs) of the BnaCLC homologs mainly located in the tonoplast. The coefficient of type-II functional divergence, θII, represents the level of gene functional divergence; it equals 0 when there is no type-II functional divergence and it equals 1 when divergence is very strong [57]. The results revealed that BnaCLCa, BnaCLCb, and BnaCLCc experienced a relatively strong functional divergence, as all of their θII coefficients were >0 (Fig 5D).
Fig 5. The synonymous substitution rates (Ks) and non-synonymous substitution rates (Ka) of the CLC family proteins in Brassica crops.
The values of Ks, Ka and Ka/Ks in B. rapa (A), B. oleracea (B) and B. napus (C). The DIVERGE v. 3.0 program was used to detect the type-II functional divergence of the homologs located in the tonoplast in B. napus (D).
Identification of gene structure and CREs in the promoters of the BnaCLC family genes
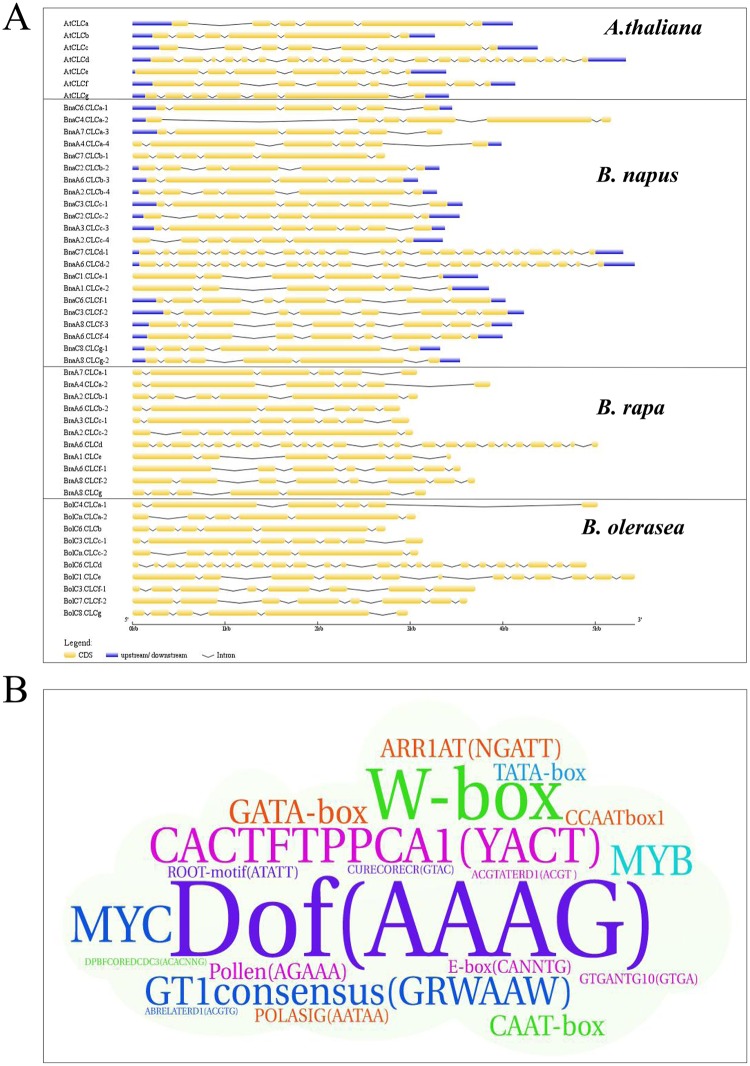
The number and organization of exon-intron structures are typical evolutionary imprints within certain gene families [57]. Therefore, the exon-intron structures of the CLC family genes from Brassica species were determined by aligning CDS with corresponding genomic sequences, which revealed that the exon-intron structures of the CLC genes in Brassica species were similar to those of their homologs in A. thaliana. Most of the CLC genes were disrupted by five to eight introns, the exception was the CLCd subfamily with 22 introns (Fig 6A). In B. napus, four BnaCLC subfamilies presented five introns, namely BnaCLCa/b/e/g. Four genes in the BnaCLCc subfamily contained six introns. The BnaCLCf subfamily also comprised four genes, but the number of introns varied. Specifically, BnaCLCf-1 and BnaCLCf-4 had seven introns, whereas BnaCLCf-2 and BnaCLCf-3 were disrupted by eight introns. The most unique gene in the BnaCLC family was BnaCLCds, which comprised two genes containing 22 introns.
Fig 6. Gene structures and identification of the putative cis-acting regulatory elements (CREs) of the CLC family genes in B. napus.
The exon-intron structures of the CLCs were determined by the alignments of coding sequences with corresponding genomic sequence (A). The yellow boxes represent exons, blue boxes indicate upstream or down- stream, and the lines represent introns. The diagram is obtained using GSDS Web server. Over-presentation of the CREs in the promoters of the BnaCLC family genes is delineated by the Word Art program (B). The bigger the font size is, the larger the CRE number is.
Transcription factors (TFs) can bind to CREs in the promoters of the genes they regulate and play vital roles in the transcriptional regulation of plant growth [58]. To further understand the transcriptional regulation of CREs in BnaCLC genes, we analyzed the CREs present in their promoter sequences (Fig 6B). Among the identified CREs, the DNA-binding One Zinc Finger (Dof)-type CRE was the most enriched (Fig 6B). It has been reported that Dof-type CREs are important in the transcriptional regulation of plant growth under N supply stress [59]. Moreover, excluding the common CREs, such as TATA-box, CAAT-box, and light-responsive elements (e.g., GATA-box and GT1CONSENSUS) [60], some phytohormone responsive elements were identified. These included ARR1AT (NGATT), cytokinin-responsive elements, and abscisic acid-responsive elements, such as ABRELATERD1, ACGTATERD1, MYB, and MYC [61]. The copper-responsive element (CURE) was the only mineral nutrient-responsive element detected in the BnaCLC genes.
Transcriptional profiling and identification of core BnaCLCs in response to different NO3- levels
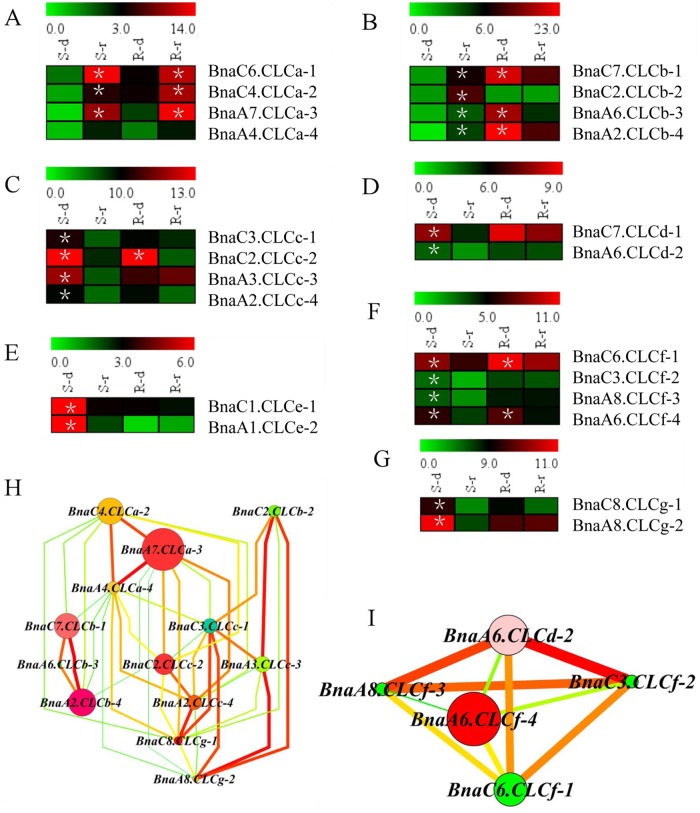
A high-throughput RNA-sequencing analysis was performed to investigate the molecular responses of the BnaCLCs to different NO3- levels. The shoots and roots of ‘XY15’ were sampled under NO3--depletion and NO3--replenishment conditions. The expression levels of BnaCLCas were strongly induced by NO3- replenishment in both shoots and roots (Fig 7A). It has been reported that the response of AtCLCb is identical to that of AtCLCa [20], whereas the response of BnaCLCbs to NO3- replenishment is different from that of BnaCLCas. In the present study, the transcript abundances of BnaCLCbs in the shoots were induced by NO3-, which was different from that in roots (Fig 7B). The other BnaCLCs, including BnaCLCc–g, were significantly down-regulated by NO3- replenishment in the shoots. In the roots, the transcript levels of BnaC2.CLCc-2, BnaC6.CLCf-1, and BnaA6.CLCf-4 were significantly down-regulated by resupplying NO3-, but the others showed no obvious changes after NO3- replenishment (Fig 7C–7G).
Fig 7. Expression profiling of the BnaCLCs in response to NO3- depletion and resupply in B. napus (A-G).
The shoots (S) and roots (R) were sampled separately for RNA seq. S-d shows shoot sample under NO3--depletion treatment, and S-r indicates shoot sample under NO3--resupply treatment. R-d represents root sample under NO3--depletion treatment, and R-r indicates root sample under NO3--resupply treatment. The asterisks denote significant differences at P < 0.05. Co-expression network analysis of the BnaCLCs (H, I). Cycle nodes represent genes, and the size of the nodes represents the power of the interaction among the nodes by degree value. Edges between two nodes represent interactions between genes.
To assess the core gene(s) among BnaCLCs, a gene co-expression network was constructed according to their protein subcellular localization. It has been reported that AtCLCa, AtCLCb, AtCLCc, and AtCLCg are localized to the tonoplasts [15, 20, 22, 23]. BnaA7.CLCa-3 was identified as the core member among the four subfamilies, and it was significantly induced by 20-fold and 6-fold in the shoots and roots, respectively, after NO3- replenishment (Fig 7H). In A. thaliana, AtCLCd and AtCLCf are localized to the Golgi membranes [24–25]. The gene co-expression network identified BnaA6.CLCf-4 as the core member in these two subfamilies, and its expression was suppressed by approximately 1.5-fold after resupplying NO3- (Fig 7I). AtCLCe is localized to the thylakoid membrane of chloroplasts [26], and two members were identified in the BnaCLCe subfamily. BnaC1.CLCe-1 showed higher transcript abundance than BnaA1.CLCe-2; thus, we deduced it was the core member in this subfamily (Fig 7E).
Transcriptional responses of the BnaCLC family members to Pi depletion
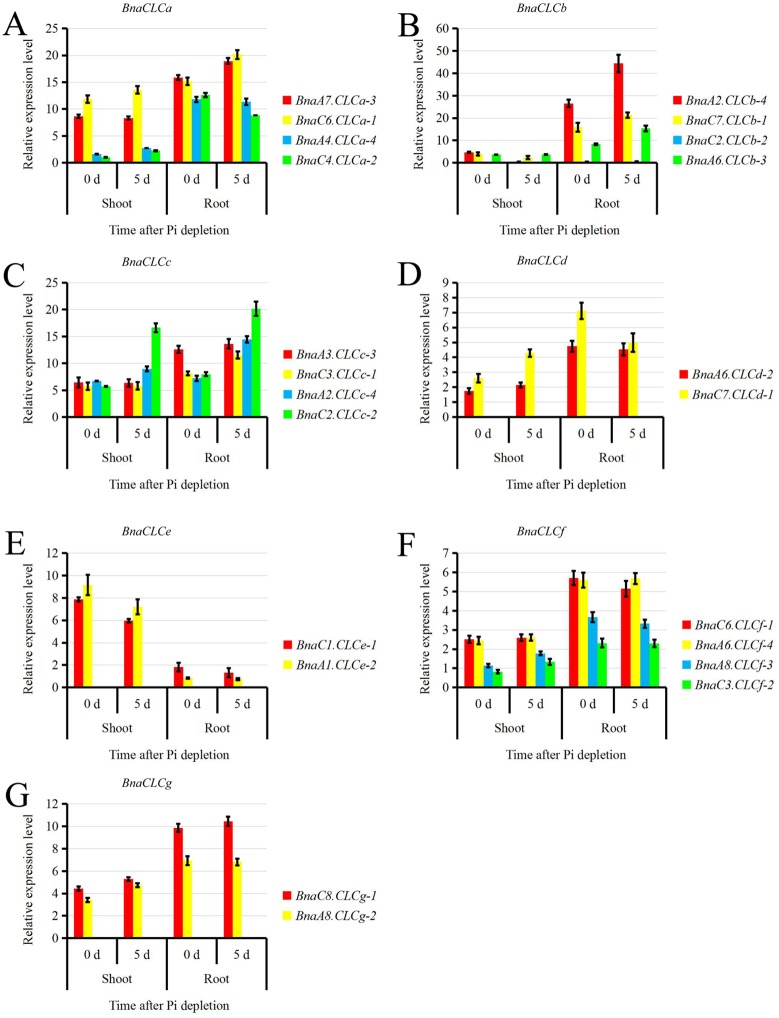
Elser et al. (2007) showed that plants respond more strongly when N and Pi are added simultaneously than when either of them is added alone [62]. Because the interaction between N and Pi has been widely studied, we investigated the relative expression of the BnaCLCs in response to Pi depletion and found that they responded differently (Fig 8). The expression of the core member BnaA7.CLCa-3 in the BnaCLC family was up-regulated in the root by Pi depletion (Fig 8A), and the relative expressions of BnaCLCbs in the root also significantly increased after Pi depletion, except for BnaC2.CLCb-2 (Fig 8B). Among the BnaCLCc subfamily, BnaC2.CLCc-2 and BnaA2.CLCc-4 were induced by Pi depletion both in shoots and roots (Fig 8C). However, BnaCLCes were down-regulated whereas BnaCLCgs were up-regulated by Pi depletion in both shoots and roots (Fig 8E and 8G).
Fig 8. Relative expression of the BnaCLC family members under phosphate (Pi) depletion.
Relative expression levels of BnaCLCa (A), BnaCLCb (B), BnaCLCc (C), BnaCLCd (D), BnaCLCe (E), BnaCLCf (F), and BnaCLCg (G), as revealed by the qRT-PCR assays. For the Pi starvation treatment, the rapeseed seedlings were first grown under 250 μM Pi (KH2PO4) for 10 d, and then transferred to a Pi-free solution for 5 d. Bars indicate the standard deviation (SD) of three biological replicates.
Transcriptional responses of the BnaCLC family members to Cd toxicity
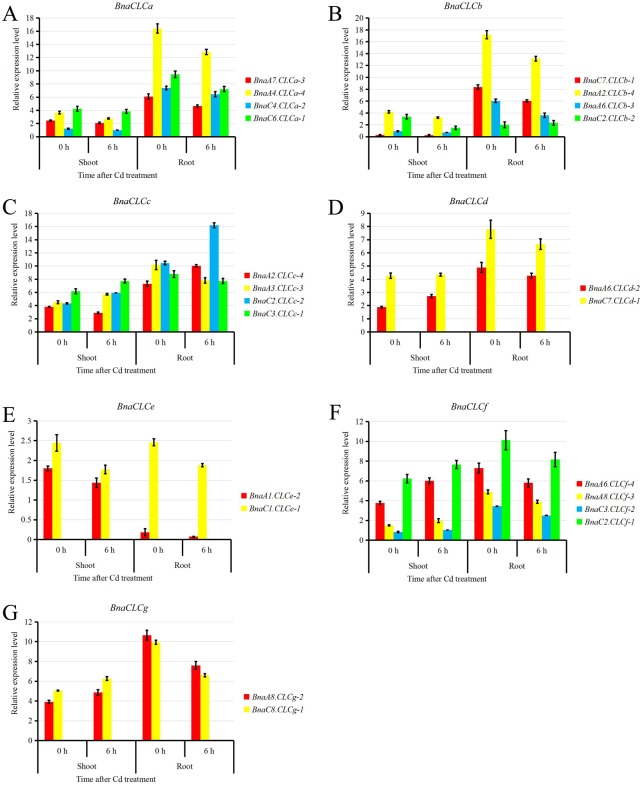
Previous studies demonstrated that AtCLCs play important roles in the regulation of abiotic and biotic stresses [22, 23, 63]. Thus, we investigated the relative expression of BnaCLCs under Cd stress and found that the relative expression of the BnaCLCa and BnaCLCb members was down-regulated by Cd stress in the root but no significant change was observed in the shoot (Fig 9A and 9B). Whereas BnaC3.CLCc-1 and BnaA3.CLCc-3 were slightly down-regulated, BnaC2.CLCc-2 and BnaA2.CLCc-4 were up-regulated by Cd in the root (Fig 9C); BnaC3.CLCc-1, BnaC2.CLCc-2, and BnaA3.CLCc-3 were up-regulated whereas BnaA2.CLCc-4 was down-regulated under Cd stress in the shoot (Fig 9C). The BnaCLCd, BnaCLCf and BnaCLCg subfamilies showed similar responses to Cd stress, as the relative expressions of these genes were up-regulated in the shoot and down-regulated in the root (Fig 9D, 9F and 9G). The BnaCLCe genes were suppressed by Cd stress in both shoot and root (Fig 9E).
Fig 9. Relative expression of the BnaCLC family members under cadmium (Cd) toxicity.
Relative expression levels of BnaCLCa (A), BnaCLCb (B), BnaCLCc (C), BnaCLCd (D), BnaCLCe (E), BnaCLCf (F), and BnaCLCg (G), as revealed by the qRT-PCR assays. For the Cd toxicity treatment, the rapeseed seedlings were first grown under a Cd-free solution for 10 d, and then transferred to 10 μM CdCl2 for 6 h. Bars indicate the standard deviation (SD) of three biological replicates.
Discussion
Genome-wide characterization of the CLC genes in B. napus
The CLC proteins are first characterized as 2Cl−/1H+ antiporters specifically involved in chloride transport [16–17]. De Angeli et al. (2006) demonstrated that AtCLCa is a tonoplast-located 2NO3-/1H+ antiporter that participates in the regulation of NO3- storage in vacuoles [15]. The CLC proteins can be found in all organisms, and seven AtCLCs have been identified in A. thaliana [12]. Our results revealed higher activity of NR and GS in the clca-2 mutant, whose N assimilation was thus accelerated, and the NUE was also significantly higher in the clca-2 mutant (Fig 1). Nitrate is a major N form and it can be reduced to NH4+ by NR and nitrite reductase (NiR), and then synthesized into amino acids through the GS/glutamine-2-oxoglutarate aminotransferase (GOGAT) pathway [64–65]. More importantly, the activity of both NR and GS can be induced by NO3- [66]. Less NO3- was transported to the vacuoles of the clca-2 mutant than to the vacuoles of Ws, and the NO3- in the cytoplasm induced the activities of NR and GS. Thus, a higher NUE was found in theclca-2 mutant [4]. Moreover, high-NUE genotypes showed higher activities of NR and GS, which accelerated NO3- assimilation in B. napus [4, 8]. Therefore, high NR and GS activities contribute to enhance NUE in both A. thaliana and B. napus. NO3- status affects the activity of NR and GS in plants [66], and it has been reported that the CLC genes play vital roles in the regulation of NO3- homeostasis. However, the functions of the CLC family genes and their effects on NUE were rarely reported in B. napus. To achieve a better understanding of the biological function of CLC genes, we performed a comprehensive analyses of this family in B. napus. In addition, B. napus is a main oil crop that originated from the natural hybridization between the intact genomes of B. oleracea and B. rapa. Due to the genome complexity and high N demand of B. napus, it is important to investigate the molecular functions of the BnaCLC genes.
In the present study, 22 CLC family genes were identified in B. napus based on the corresponding homologs in A. thaliana (S1 Table). All these genes were divided into the same seven subfamilies as the CLCs in A. thaliana (i.e., AtCLCa-g) (Fig 2), and they were evenly distributed in seven chromosomes of the An subgenome and seven chromosomes of the Cn subgenome (Fig 3A). This further supported the hypothesis that the B. napus genome results from the hybridization of B. rapa (Ar) and B. oleracea (Co) genomes [67]. Genome duplication, segmental duplication, and tandem duplication are the main factors contributing to the expansion of gene families [54, 68]. Our results revealed that whole genome duplication and segmental duplication were the driving forces of the CLC gene expansion in B. napus (Fig 3B). All the BnaCLCs genes underwent strong purifying selection, as evidenced by Ka/Ks ratios lower than 1.0 (Fig 4A–4C; S2 Table), which indicated that the CLC gene function was highly preserved. Moreover, obvious gene functional divergence occurred among the CLCa, CLCb and CLCc subfamilies in B. napus (Fig 4D).
Genome-wide identification and transcriptomics-assisted gene co-expression network analysis characterized the core members of the BnaCLC gene family
The compartmentalization of NO3- in organelles is critical for plant physiology [4]. It has been reported that several AtCLCs are involved in NO3- transport into vacuoles, such as AtCLCa, AtCLCb and AtCLCc [15, 20, 21]. Our results revealed that N assimilation was enhanced in the clca-2 mutants, which contributed to its higher NUE (Fig 1). High-throughput transcriptomics was performed to explore the molecular responses of the BnaCLCs to different NO3- supply levels (Fig 7). It showed that the transcript abundance of BnaCLCas in both shoot and root was significantly enhanced by NO3- after N starvation (Fig 7A), which was similar to the response of AtCLCa to NO3- in A. thaliana [18]. Gene CLCb, a close relative of CLCa, was weakly upregulated (1.5–2.0-fold) by NO3- [20, 69, 70]. The BnaCLCb transcripts were significantly induced and repressed by NO3- in the shoot and root of B. napus, respectively (Fig 7B). However, the other five BnaCLCs, namely BnaCLCc, BnaCLCd, BnaCLCe, BnaCLCf and BnaCLCg, were repressed by NO3- replenishment (Fig 7C–7G). In A. thaliana, decreased expression of AtCLCc mRNA was also observed within 0.6 h when Ca(NO3)2 was supplied to N-starved plants [18]. The different responses of BnaCLCs to NO3- might be related to anion specificity. For example, AtCLCa specifically mediates NO3- transport because the proline in its selectivity filter motif GXGIP plays an important role in NO3- selectivity [71]. However, AtCLCc has a broader anion specificity, including chloride, malate, and citrate [15, 18].
Sets of BnaCLCs found in duplicated segments could have redundant/duplicate gene functions; therefore, the core members should be characterized. Based on their different subcellular localizations, gene co-expression analysis identified BnaA7.CLCa-3,BnaA6.CLCf-4, and BnaC1.CLCe-1 as the core members of the BnaCLC gene family (Fig 7H and 7I). AtCLCa functions as a 2NO3-/1H+ antiporter and plays a vital role in NO3- transportation into vacuoles [15], and AtCLCe is reported to function in the regulation of photosynthetic electron transport [26]; however, the gene function of AtCLCf is still unknown [25]. Therefore, we propose that BnaA7.CLCa-3 is the most important member of the BnaCLC gene family. In addition, BnaA7.CLCa-3 response to NO3- was similar to that of AtCLCa (Fig 7A), suggesting that BnaA7.CLCa-3 might play a crucial role in the transportation of NO3- and regulation of NUE in B. napus.
Potential regulation of BnaCLCs and their contribution to NUE improvement and tolerance to biotic or abiotic stresses
We identified a set of CREs in the promoters of the CLC family genes in B. napus (Fig 6B). The most abundant were Dof, W-box, MYB and GATA-box. The Dof, GATA-box, and MYB TFs have been implicated in the molecular responses of plants to N status [72–74]. For instance, overexpression of ZmDOF1 resulted in increased N content and improved growth in transgenic A. thaliana plants under low N conditions [75–76]. Rice OsDOF18, the most homologous gene to ZmDOF1 [77–78], modulates ammonium uptake by inducing ammonium transporter genes [74]. W-box (AGCT) is an emerging player in plant signaling and has been reported to play important roles in plant responses to various biotic and abiotic stresses [79]. Our results revealed that W-box was one of the most abundant CREs in the promoter regions of the CLC family genes in B. napus (Fig 6B). Previous studies have shown that AtCLCs play key roles in responses to biotic and abiotic stresses. For example, AtCLCc is involved in stomatal movement and contributes to salt tolerance [22]. Although AtCLCg shares a high degree of identity (62%) with AtCLCc, and both are important for tolerance to excess chloride, their functions are not redundant [23]. Furthermore, AtCLCd negatively regulates pathogen-associated molecular pattern triggered immunity [63]. Our results showed that the BnaCLCs showed distinct responses to nutrient depletion and heavy metal stress. N and P are essential nutrients for plant growth and the interaction between them was thoroughly discussed by Agren et al. (2012) [80]. In addition, Li et al. (2009) revealed that low-Pi stress down-regulated the genes coding for NR and GS, which weakened N assimilation [81]. Our results revealed that most of the BnaCLCs were up-regulated by Pi depletion (Fig 8). The CLC genes play vital roles in NO3- storage and its function loss promoted N assimilation [4]. Up-regulation of the BnaCLCs under Pi depletion might increase NO3- storage in vacuoles, which results in less NO3- allocated to the cytoplasm, and NO3- storage in the vacuole weakens its assimilation. Notably, low-Pi stress influenced N assimilation through different pathways. In addition, the BnaCLCs showed distinct expression patterns under Cd toxicity, and thus might play pivotal roles in the regulation of Cd detoxification (Fig 9). The vacuole occupies 60–95% of the mature plant cell, and itis not only the site for storing nutrients, but also an important organelle for sequestering toxic heavy metals, such as Cd [2, 82, 83]. The proton pumps in the tonoplast, vacuolar H+-pyrophosphatase (V-PPase) and the vacuolar H+-ATPase (V-ATPase), are responsible for establishing ΔH+ between cytoplasm and vacuoles [84–85], providing the driving force for ion transmembrane transport. AtCLCa is a tonoplast-localized 2NO3-/H+ antiporter that is involved in the regulation of NO3- sequestration into vacuoles [15]. Vacuolar compartmentalization is central for heavy metal homeostasis. It depends on two vacuolar pumps (V-ATPase and V-PPase) and on a set of tonoplast transporters, which are directly driven by proton motive force and primary ATP-dependent pumps [83]. Hence, both NO3- and Cd sequestration into vacuoles rely on the proton motive force established by V-ATPase and V-PPase. When NO3- storage is decreased, lower proton driving force is required. Our results showed that the BnaCLCs were down-regulated by Cd (Fig 9), which decreased NO3- transportation into vacuole. Consequently, the proton driving force might diverted to transport Cd into the vacuole and improve Cd tolerance of B. napus. However, further research is required to reveal the functions of BnaCLCs in Cd detoxification.
Conclusion
Through genome-wide analysis of the CLC family genes in B. napus, a total of 22 BnaCLCs were identified in the rapeseed genome. We found that genome-wide duplication and segmental duplication of the CLC genes contributed to a relatively large CLC gene family in B. napus. These genes showed high orthologous relationships with corresponding AtCLC homologs, and they were classified into seven subgroups. AtCLCa has been reported to transport NO3- into vacuoles. We found that the clca-2 mutant showed enhanced N assimilation ability and high NUE. Therefore, to further explore the potential roles of BnaCLCs in vacuolar NO3- transport, a high-throughput transcriptomics analysis was performed. The results revealed that different BnaCLCs showed distinct responses to NO3- levels. Nevertheless, the general responses of BnaCLCa and BnaCLCc to NO3- replenishment were the same as AtCLCa and AtCLCc. In addition, gene co-expression analysis revealed that BnaA7.CLCa-3 was the core member of the BnaCLC family. Its expression was up-regulated when exposed to both NO3- resupply and Pi depletion, and it was down-regulated by Cd toxicity. Moreover, two enriched CREs, including Dof and W-box, were abundant in the promoter regions of BnaCLCs, which might contribute to NUE improvement and stress tolerance. These findings provide a fundamental basis for improving crops’ NUE and tolerance to diverse stresses through genetic engineering of the CLC genes.
Supporting information
(DOCX)
(DOCX)
(RAR)
The 10 conserved motifs predicted by the MEME program (A). Characterization of the three common motifs (motifs 4/7/10) in the CLC proteins of A. thaliana and Brassica species (B), as obtained in Weblogo. The larger the font, the more conserved is the motif.
(DOCX)
Abbreviations
- ANOVA
analysis of variance
- BLAST
basic local alignment search tool
- Cd
cadmium
- CDS
coding sequences
- CLC
Chloride Channel
- CREs
cis-acting regulatory elements
- CURE
copper-responsive element
- Dof
DNA-binding One Zinc Finger
- GS
glutamine synthetase
- HSD
honestly significant difference
- IIs
instability indices
- MWs
molecular weights
- N
Nitrogen
- NCBI
National Center for Biotechnology Information
- NO3-
inorganic nitrate
- NR
nitrate reductase
- NRT
nitrate transporter
- NUE
N use efficiency
- Pi
inorganic phosphate
- PIs
isoelectric points
- TFs
transcription factors
- V-ATPase
vacuolar H+-ATPase
- V-PPase
vacuolar H+-pyrophosphatase
- VSC
vacuolar sequestration capacity
Data Availability
The data underlying the results presented in the study are available within the manuscript and Supporting Information files.
Funding Statement
This study was financially supported by the National Key Research and Development Program of China (2017YFD0200100 and 2017YFD0200103), National Natural Science Foundation of China (31801923, 31101596 and 31372130), Hunan Provincial Recruitment Program of Foreign Experts, National Oilseed Rape Production Technology System of China, “2011 Plan” supported by the Ministry of Education of China and Double First-class Construction Project of Hunan Agricultural University (kxk201801005).
References
- 1.Wang YY, Cheng YH, Chen KE, Tsay YF. Nitrate transport, signaling, and use efficiency. Annu Rev Plant Biol. 2018; 69: 85–122. 10.1146/annurev-arplant-042817-040056 [DOI] [PubMed] [Google Scholar]
- 2.Shen QR, Tang L, Xu YC. A review on the behavior of nitrate in vacuoles of plants. Acta Pedologica Sinica. 2003; 40: 465–470. [Google Scholar]
- 3.Xu GH, Fan XR, Miller AJ. Plant nitrogen assimilation and use efficiency. Annu Rev Plant Biol. 2012; 63: 153–182. 10.1146/annurev-arplant-042811-105532 [DOI] [PubMed] [Google Scholar]
- 4.Han YL, Song HX, Liao Q, Yu Y, Jian SF, Lepo JE, et al. Nitrogen use efficiency is mediated by vacuolar nitrate sequestration capacity in roots of Brassica napus. Plant Physiol. 2016; 170: 1684–1698. 10.1104/pp.15.01377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rathke GW, Behrens T, Diepenbrock W. Integrated nitrogen management strategies to improve seed yield, oil content and nitrogen efficiency of winter oilseed rape (Brassica napus L.): a review. Agr, Ecosyst and Environ. 2006; 117: 80–108. [Google Scholar]
- 6.Hua YP, Zhou T, Liao Q, Song HX, Guan CY, Zhang ZH. Genomics-assisted identification and characterization of the genetic variants underlying differential nitrogen use efficiencies in allotetraploid rapeseed genotypes. G3 (Bethesda). 2018; 8: 2757–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Dun X, Shi J, Wang X, Liu G, Wang H. Genetic dissection of root morphological traits related to nitrogen use efficiency in Brassica napus L. under two contrasting nitrogen conditions. Front Plant Sci. 2017; 8: 1709 10.3389/fpls.2017.01709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang GL, Ding GD, Li L, Cai H, Ye X, Zou J, et al. Identification and characterization of improved nitrogen efficiency in interspecific hybridized new-type Brassica napus. Ann Bot. 2014; 114: 549–559. 10.1093/aob/mcu135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han YL, Liao Q, Yu Y, Song HX, Liu Q, Rong XM, et al. Nitrate reutilization mechanisms in the tonoplast of two Brassica napus genotypes with different nitrogen use efficiency. Acta Physiol Plant. 2015; 37: 42. [Google Scholar]
- 10.Tang Y, Sun X, Hu C, Tan Q, Zhao X. Genotypic differences in nitrate uptake, translocation and assimilation of two Chinese cabbage cultivars [Brassica campestris L. ssp. Chinensis (L.)]. Plant Physiol Biochem. 2013; 70: 14–20. 10.1016/j.plaphy.2013.04.027 [DOI] [PubMed] [Google Scholar]
- 11.Zhang ZH, Huang HT, Song HX, Liu Q, Rong XM, Peng JW, et al. Research advances on nitrate nitrogen reutilization by proton pump of tonoplast and its relation to nitrogen use efficiency. Aust J Crop Sci. 2012; 6: 1377–1382. [Google Scholar]
- 12.Krapp A, David LC, Chardin C, Girin T, Marmagne A, Leprince AS, et al. Nitrate transport and signalling in Arabidopsis. J Exp Bot. 2014; 65: 789–798. 10.1093/jxb/eru001 [DOI] [PubMed] [Google Scholar]
- 13.Miller AJ, Smith SJ. Cytosolic nitrate ion homeostasis: could it have a role in sensing nitrogen status? Ann Bot. 2008; 101: 485–489. 10.1093/aob/mcm313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsay YF, Chiu CC, Tsai CB, Ho CH, Hsu PK. Nitrate transporters and peptide transporters. FEBS Lett. 2007; 581: 2290–2300. 10.1016/j.febslet.2007.04.047 [DOI] [PubMed] [Google Scholar]
- 15.De Angeli A, Monachello D, Ephritikhine G, Frachisse JM, Thomine S, Gambale F, et al. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature. 2006; 442: 939–942. 10.1038/nature05013 [DOI] [PubMed] [Google Scholar]
- 16.Jentsch TJ. CLC chloride channels and transporters, from genes to protein structure, pathology and physiology. Crit Rev Biochem Mol. 2008; 43: 3–36. [DOI] [PubMed] [Google Scholar]
- 17.Lisal J, Maduke M. Proton-coupled gating in chloride channels. Philos T R Soc B. 2009; 364: 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geelen D, Lurin C, Bouchez D, Frachisse JM, Lelievre F, Courtial B, et al. Disruption of putative anion channel gene AtCLCa in Arabidopsis suggests a role in the regulation of nitrate content. Plant J. 2000; 21: 259–267. [DOI] [PubMed] [Google Scholar]
- 19.Zifarelli G, Pusch M. CLC transport proteins in plants. FEBS Lett. 2010; 584: 2122–2127. 10.1016/j.febslet.2009.12.042 [DOI] [PubMed] [Google Scholar]
- 20.Von der Fecht-Bartenbach J, Bogner M, Dynowski M, Ludewig U. CLC-b-mediated NO3-/H+ exchange across the tonoplast of Arabidopsis vacuoles. Plant Cell Physiol. 2010; 51: 960–968. 10.1093/pcp/pcq062 [DOI] [PubMed] [Google Scholar]
- 21.Harada H, Kuromori T, Hirayama T, Shinozaki K, Leigh RA. Quantitative trait loci analysis of nitrate storage in Arabidopsis leading to an investigation of the contribution of the anion channel gene, AtCLC-c, to variation in nitrate levels. J Exp Bot. 2004; 55: 2005–2014. 10.1093/jxb/erh224 [DOI] [PubMed] [Google Scholar]
- 22.Jossier M, Kroniewicz L, Dalmas F, Thiec D, Ephritikhine G, Thomine S, et al. The Arabidopsis vacuolar anion transporter, AtCLCc, is involved in the regulation of stomatal movements and contributes to salt tolerance. Plant J. 2010; 64: 563–576. 10.1111/j.1365-313X.2010.04352.x [DOI] [PubMed] [Google Scholar]
- 23.Nguyen CT, Agorio A, Jossier M, Depré S, Thomine S, Filleur S. Characterization of the Chloride Channel-Like, AtCLCg, involved in chloride tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2016; 57: 764–75. 10.1093/pcp/pcv169 [DOI] [PubMed] [Google Scholar]
- 24.Von der Fecht-Bartenbach J, Bogner M, Krebs M, Stierhof YD, Schumacher K, Ludewig U. Function of the anion transporter AtCLC-d in the trans-Golgi network. Plant J. 2007; 50: 466–474. 10.1111/j.1365-313X.2007.03061.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marmagne A, Vinauger-Douard M, Monachello D, de Longevialle AF, Charon C, Allot M, et al. Two members of the Arabidopsis CLC (chloride channel) family, AtCLCe and AtCLCf, are associated with thylakoid and Golgi membranes, respectively. J Exp Bot. 2007; 58: 3385–93. 10.1093/jxb/erm187 [DOI] [PubMed] [Google Scholar]
- 26.Herdean A, Nziengui H, Zsiros O, Solymosi K, Garab G, Lundin B, et al. The Arabidopsis Thylakoid thylakoid Chloride chloride Channel AtCLCe functions in chloride homeostasis and regulation of photosynthetic electron transport. Front Plant Sci. 2016; 7: 115 10.3389/fpls.2016.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang DP, Zhang YB, Zhang Z, Zhu J, Yu J. KaKs_Calculator 2.0: a toolkit incorporating gamma-series methods and sliding window strategies. Genomics Proteomics Bioinformatics. 2010; 8: 77–80. 10.1016/S1672-0229(10)60008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu S, Liu Y, Yang X, Tong C, Edwards D, Parkin IA, et al. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat Commun. 2014; 5: 3930 10.1038/ncomms4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayer PE, Hurgobin B, Golicz AA, Chan CK, Yuan Y, Lee H, et al. Assembly and comparison of two closely related Brassica napus genomes. Plant Biotechnol J. 2017; 15: 1602–1610. 10.1111/pbi.12742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chalhoub B, Denoeud F, Liu S, Parkin IA, Tang H, Wang X, et al. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science. 2014; 345: 950–953. [DOI] [PubMed] [Google Scholar]
- 31.Sun F, Fan G, Hu Q, Zhou Y, Guan M, Tong C, et al. The high-quality genome of Brassica napus cultivar ‘ZS11’ reveals the introgression history in semi-winter morphotype. Plant J. 2017; 92: 452–468. 10.1111/tpj.13669 [DOI] [PubMed] [Google Scholar]
- 32.Fan XR, Tang Z, Tan Y, Zhang Y, Luo B, Yang M, et al. Overexpression of a pH-sensitive nitrate transporter in rice increases crop yields. Proc Natl Acad Sci USA. 2016; 113: 7118–23. 10.1073/pnas.1525184113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi WM, Xu WF, Li SM, Zhao XQ, Dong GQ. Responses of two rice cultivars differing in seedling-stage nitrogen use efficiency to growth under low-nitrogen conditions. Plant Soil. 2010; 326: 291–302. [Google Scholar]
- 34.Fan XR, Jia L, Li Y, Smith SJ, Miller AJ, Shen QR. Comparing nitrate storage and remobilization in two rice cultivars that differ in their nitrogen use efficiency. J Exp Bot. 2007; 58: 1729–1740. 10.1093/jxb/erm033 [DOI] [PubMed] [Google Scholar]
- 35.Huang CB, Wang ZH, Wang XY, Li SX. Nitrate accumulation and reduction in Spinach and their relations to plant growth. J Agro-Environ Sci. 2011; 30: 613–618. [Google Scholar]
- 36.Cullimore JV, Sims AP. An association between photorespiration and protein catabolism: Studies with Chlamydomonas. Planta. 1980; 150: 392–396. 10.1007/BF00390175 [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Wu J. Liang J, Cheng F, Wang X. Brassica database (BRAD) version 2.0: integrating and mining Brassicaceae species genomic resources. Database 2015: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu J, Zhao M, Wang X, Tong C, Huang S, Tehrim S, et al. Bolbase: a comprehensive genomics database for Brassica oleracea. BMC Genomics. 2013; 14: 664 10.1186/1471-2164-14-664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012; 40: D1178–D1186. 10.1093/nar/gkr944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013; 30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009; 37: W202–W208. 10.1093/nar/gkp335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkins MR, Gasteiger E, Bairoch A, Sanchez JC, Williams KL, Appel RD, et al. Protein identification and analysis tools on the ExPASy server. Methods Mol Biol. 1999; 112:531–52. [DOI] [PubMed] [Google Scholar]
- 43.Guruprasad K, Reddy BV, Pandit MW. Correlation between stability of a protein and its dipeptide composition: a novel approach for predicting in vivo stability of a protein from its primary sequence. Protein Eng. 1990; 4: 155–161. [DOI] [PubMed] [Google Scholar]
- 44.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and clustal X version 2.0. Bioinformatics. 2007; 23: 2947–1948. 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- 45.Yang Z, Nielsen R. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol Biol Evol. 2000; 17: 32–43. 10.1093/oxfordjournals.molbev.a026236 [DOI] [PubMed] [Google Scholar]
- 46.Blanc G, Wolfe KH. Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell. 2004; 16: 1667–1678. 10.1105/tpc.021345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu X, Zou Y, Su Z, Huang W, Zhou Z, Arendsee A, et al. An update of DIVERGE software for functional divergence analysis of protein family. Mol Biol Evol. 2013; 30: 1713–1719. 10.1093/molbev/mst069 [DOI] [PubMed] [Google Scholar]
- 48.Hu B, Jin J, Guo AY, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015; 31: 1296–1297. 10.1093/bioinformatics/btu817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999; 27: 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kohl M, Wiese S, Warscheid B. Cytoscape: software for visualization and analysis of biological networks. Methods Mol Biol. 2011; 696: 291–303. 10.1007/978-1-60761-987-1_18 [DOI] [PubMed] [Google Scholar]
- 51.Maillard A, Etienne P, Diquélou J, Trouverie J, Billard V, Yvin JC, et al. Nutrient deficiencies in Brassica napus modify the ionomic composition of plant tissues: a focus on cross-talk between molybdenum and other nutrients. J Exp Bot. 2016; 67: 5631–5641. 10.1093/jxb/erw322 [DOI] [PubMed] [Google Scholar]
- 52.Yang HL, Liu J, Huang SM. Selection and evaluation of novel reference genes for quantitative reverse transcription PCR (qRT-PCR) based on genome and transcriptome data in Brassica napus L. Gene. 2014; 538: 113–122. 10.1016/j.gene.2013.12.057 [DOI] [PubMed] [Google Scholar]
- 53.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔ CT Method. Methods. 2001; 25: 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 54.Schauser L, Wieloch W, Stougaard J. Evolution of NIN-Like proteins in Arabidopsis, rice, and Lotus japonicus. J Mol Evol. 2005; 60: 229–237. 10.1007/s00239-004-0144-2 [DOI] [PubMed] [Google Scholar]
- 55.Cheng F, Wu J, Fang L, Wang X. Syntenic gene analysis between Brassica rapa and other Brassicaceae species. Front Plant Sci. 2012; 3: 198 10.3389/fpls.2012.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nekrutenko A, Makova KD, Li WH. The KA/KS ratio test for assessing the protein-coding potential of genomic regions: an empirical and simulation study. Genome Res. 2002; 12: 198–202. 10.1101/gr.200901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hua YP, Zhou T, Song HX, Guan CY, Zhang ZH. Integrated genomic and transcriptomic insights into the two-component high-affinity nitrate transporters in allotetraploid rapeseed. Plant Soil. 2018; 427: 245–268. [Google Scholar]
- 58.Wittkopp PJ, Kalay G. Cis-regulatory elements: molecular mechanisms and evolutionary processes underlying divergence. Nat Rev Genet. 2011; 6: 59–69. [DOI] [PubMed] [Google Scholar]
- 59.Noguero M, Atif RM, Ochatt S, Thompson RD. The role of the DNA-binding One Zinc Finger (DOF) transcription factor family in plants. Plant Sci. 2013; 209: 32–45. 10.1016/j.plantsci.2013.03.016 [DOI] [PubMed] [Google Scholar]
- 60.Guo L, Yu Y, Xia X, Yin W. Identification and functional characterization of the promoter of the calcium sensor gene CBL1 from the xerophyte Ammopiptanthus mongolicus. BMC Plant Biol. 2010; 10: 18 10.1186/1471-2229-10-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sahito ZA, Wang L, Sun Z, Yan Q, Zhang X, Jiang Q, et al. The miR172c-NNC1 module modulates root plastic development in response to salt in soybean. BMC Plant Biol. 2017; 17: 229 10.1186/s12870-017-1161-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, et al. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett. 2007; 10: 1135–1142. 10.1111/j.1461-0248.2007.01113.x [DOI] [PubMed] [Google Scholar]
- 63.Guo W, Zuo Z, Cheng X, Sun J, Li H, Li L, et al. The chloride channel family gene CLCd negatively regulates pathogen-associated molecular pattern (PAMP)-triggered immunity in Arabidopsis. J Exp Bot. 2014; 65: 1205–1215. 10.1093/jxb/ert484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bernard SM, Habash DZ. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol. 2009; 182: 608–620. 10.1111/j.1469-8137.2009.02823.x [DOI] [PubMed] [Google Scholar]
- 65.Xu G, Fan X, Miller AJ. Plant nitrogen assimilation and use efficiency. Annu Rev Plant Biol. 2012; 63: 153–182. 10.1146/annurev-arplant-042811-105532 [DOI] [PubMed] [Google Scholar]
- 66.Balotf S, Kavoosi G, Kholdebarin B. Nitrate reductase, nitrite reductase, glutamine synthetase, and glutamate synthase expression and activity in response to different nitrogen sources in nitrogen-starved wheat seedlings. Biotechnol Appl Biochem. 2016; 63:220–9. 10.1002/bab.1362 [DOI] [PubMed] [Google Scholar]
- 67.Nagaharu U. Genomic analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. J Jap Bot. 1935; 7: 389–452. [Google Scholar]
- 68.He Y, Mao S, Gao Y, Zhu L, Wu D, Cui Y, et al. Genome-Wide identification and expression analysis of WRKY transcription factors under multiple stresses in Brassica napus. PLoS One. 2016; 11: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gifford ML, Dean A, Gutierrez RA, Coruzzi GM, Birnbaum KD. Cell-specific nitrogen responses mediate developmental plasticity. Proc Natl Acad Sci USA. 2008; 105: 803–808. 10.1073/pnas.0709559105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang R, Okamoto M, Xing X, Crawford NM. Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1,000 rapidly responding genes and new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism. Plant Physiol. 2003; 132: 556–567. 10.1104/pp.103.021253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wege S, Jossier M, Filleur S, Thomine S, Barbier-Brygoo H, Gambale F, et al. The proline 160 in the selectivity filter of the Arabidopsis NO3-/H+ exchanger AtCLCa is essential for nitrate accumulation in planta. Plant J. 2010; 63: 861–869. 10.1111/j.1365-313X.2010.04288.x [DOI] [PubMed] [Google Scholar]
- 72.Hudson D, Guevara D, Yaish MW, Hannam C, Long N, Clarke JD, et al. GNC and CGA1 modulate chlorophyll biosynthesis and glutamate synthase (GLU1/Fd-GOGAT) expression in Arabidopsis. PLoS One. 2011; 6: e26765 10.1371/journal.pone.0026765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Imamura S, Kanesaki Y, Ohnuma M, Inouye T, Sekine Y, Fujiwara T, et al. R2R3-type MYB transcription factor, CmMYB1, is a central nitrogen assimilation regulator in Cyanidioschyzon merolae. Proc Natl Acad Sci USA. 2009; 106: 12548–12533. 10.1073/pnas.0902790106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu Y, Yang W, Wei J, Yoon H, An G. Transcription factor OsDOF18 controls ammonium uptake by inducing ammonium transporters in rice roots. Mol Cells. 2017; 40: 178–185. 10.14348/molcells.2017.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kurai T, Wakayama M, Abiko T, Yanagisawa S, Aoki N, Ohsugi R. Introduction of the ZmDof1 gene into rice enhances carbon and nitrogen assimilation under low-nitrogen conditions. Plant Biotechnol J. 2011; 9: 826–837. 10.1111/j.1467-7652.2011.00592.x [DOI] [PubMed] [Google Scholar]
- 76.Yanagisawa S, Akiyama A, Kisaka H, Uchimiya H, Miwa T. Metabolic engineering with Dof1 transcription factor in plants: Improved nitrogen assimilation and growth under low nitrogen conditions. Proc Natl AcadSci USA. 2004; 101: 7833–7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kushwaha H, Gupta S, Singh VK, Rastogi S, Yadav D. Genome wide identification of Dof transcription factor gene family in sorghum and its comparative phylogenetic analysis with rice and Arabidopsis. Mol Biol Rep. 2010; 38: 5037–5053. 10.1007/s11033-010-0650-9 [DOI] [PubMed] [Google Scholar]
- 78.Lijavetzky D, Carbonero P, Vicente-Carbajosa J. Genomewide comparative phylogenetic analysis of the rice and Arabidopsis Dof gene families. BMC Evol Biol. 2003; 23: 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Banerjee A, Roychoudhury A. WRKY proteins: signaling and regulation of expression during abiotic stress responses. Scientific World J. 2015; 2015: 807560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agren GI, Wetterstedt JA, Billberger MF. Nutrient limitation on terrestrial plant growth—modeling the interaction between nitrogen and phosphorus. New Phytol. 2012; 194: 953–60. 10.1111/j.1469-8137.2012.04116.x [DOI] [PubMed] [Google Scholar]
- 81.Li L, Qiu X, Li X, Wang S, Lian X. The expression profile of genes in rice roots under low phosphorus stress. Sci China C Life Sci. 2009; 52: 1055–1064. 10.1007/s11427-009-0137-x [DOI] [PubMed] [Google Scholar]
- 82.Baetz U, Eisenach C, Tohge T, Martinonia E, Angeli AD. Vacuolar chloride fluxes impact ion content and distribution during early salinity stress. Plant Physiology. 2016; 172: 1167–1181. 10.1104/pp.16.00183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharma SS, Dietz KJ, Mimura T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell and Environment. 2016; 39: 1112–1126. [DOI] [PubMed] [Google Scholar]
- 84.Martinoia E, Maeshima M, Neuhaus HE. Vacuolar transporters and their essential role in plant metabolism. Journal of Experimental Botany. 2007; 58: 83–102. 10.1093/jxb/erl183 [DOI] [PubMed] [Google Scholar]
- 85.Migocka M, Kosieradzka A, Papierniak A, Maciaszczyk-Dziubinska E, Posyniak E, Garbiec A, Filleur S. Two metal-tolerance proteins, MTP1and MTP4, are involved in Zn homeostasis and Cd sequestration in cucumber cells. Journal of Experimental Botany. 2015; 66: 1001–1015. 10.1093/jxb/eru459 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(RAR)
The 10 conserved motifs predicted by the MEME program (A). Characterization of the three common motifs (motifs 4/7/10) in the CLC proteins of A. thaliana and Brassica species (B), as obtained in Weblogo. The larger the font, the more conserved is the motif.
(DOCX)
Data Availability Statement
The data underlying the results presented in the study are available within the manuscript and Supporting Information files.
We have upload the raw high-throughput sequencing data as supporting information files (S3 Table), in addition, rapeseed seeds that are required to reproduce these findings can be shared by contacting the corresponding author, Zhen-hua Zhang (zhzh1468@163.com) or Ying-peng Hua (yingpenghua89@126.com)