Abstract
The hepatitis C virus (HCV) has affected an estimate of 80 million individuals worldwide and is a strain of public health. Around 25–30% of patients in Europe and the US infected with HIV are coinfected with HCV. Despite treatment modalities containing a NS3/4A protease inhibitor in combination with pegylated interferon and ribavirin prior to 2013 improved SVR rates, the amount of severe side effects was high. Nowadays, oral direct-acting antivirals (DAAs) combination therapy offers excellent treatment efficacy, safety and tolerability. This review focuses on current literature and clinical evidence and their impact regarding NS3/4A protease inhibitors. In addition, pitfalls in treatment from HIV- and HBV-coinfected patients will also be discussed. In the era of DAA treatment, the third-generation pan-genotypic NS3/4A protease inhibitors (mainly grazoprevir, glecaprevir and voxilaprevir) show a high antiviral activity and genetic resistance barrier with cure rates of over 95% when combined with an NS5A inhibitor, irrespectively of baseline resistance associated variants (RASs) being present. These new key components of DAA combination therapy are impressive options to eradicate HCV in the so called difficult-to-treat population (e.g. compensated cirrhosis, end-stage renal disease and patients who failed previous DAA treatment).
Keywords: direct-acting antiviral, hepatitis C, HIV, NS3/4A protease inhibitor
Introduction
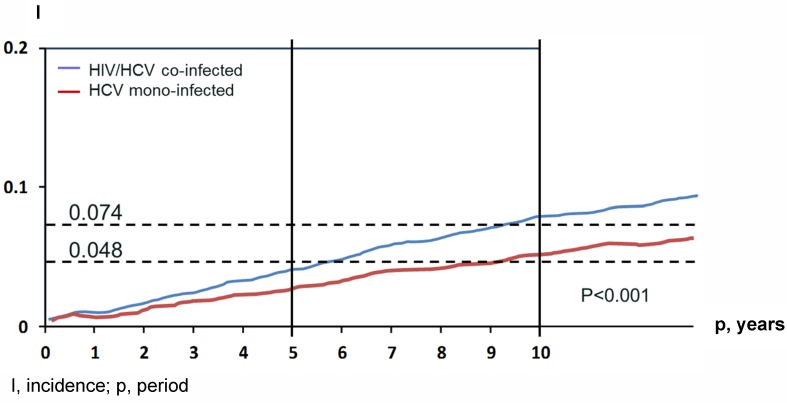
Approximately 80 million individuals worldwide are estimated to have a chronic hepatitis C virus (HCV) infection [1]. With around 150.000 new cases every year in Western Europe and in the United States, chronic HCV infection is a public health burden [2]. Chronic hepatitis C is strongly associated with the development of cirrhosis, end-stage liver disease and hepatocellular carcinoma (HCC) (Figure 1 (Fig. 1)). Antiviral therapy can prevent these complications [3], [4]. In HIV infected patients, HCV coinfection is a major cause of non-AIDS related morbidity and mortality [5]. Around 25–30% of HIV-positive patients are coinfected with chronic HCV infection in Europe and the USA [6]. In the past decade, standard of care was the administration of interferon (IFN) in association with ribavirin (RBV). Most HCV genotype 1-infected patients achieved in only 40–50% a sustained virological response (SVR) [7], [8], [9] while in genotype 2- or 3-infected patients viral eradication was achieved in ~80% [10]. Since, the development of new antiviral drugs with direct action against the virus, in particular NS3/4A protease inhibitors, started a new era of HCV treatment [11]. Up to now, amazingly high SVR rates of oral direct-acting antivirals (DAAs) combination therapy were reported with an average above 95% at least for genotypes 1 and 4 [12], offering an excellent treatment efficacy, safety and tolerability. These modern all oral therapy consists of a combination of inhibitors of NS5B polymerase, NS3/4A protease and the NS5A replication complex [13], [14]. Protease inhibitors can be generally divided into four sections [13]. This review focuses on protease inhibitors approved in the past 4 years, particularly the second (mainly simeprevir and asunaprevir) and third generation inhibitors (mostly paritaprevir, grazoprevir, glecaprevir and voxilaprevir) will be highlighted.
Figure 1. Comparison of the incidence of hepatic decompensation and HCC in HCV/HIV co-infected and HCV mono-infected veterans.
adapted from Lo et al. 2012 [71]
The NS3/4A protease
HCV belongs to the Flaviviridae family and is a small 55 nm virus with a lipid envelope and a single-stranded RNA viral genome with around 9,600 nucleotides [15], [16]. The positive strand RNA genome includes a 5’-non-coding region with an internal ribosome entry site, an open reading frame that encodes structural (core, envelope 1, 2, p7) and non-structural (NS2, NS3, NS4A, NS4B, NS5A, NS5B) proteins and a 3’-non-coding region. The internal ribosome entry site causes the translation of a polyprotein precursor which is processed into mature structural and non-structural proteins [17]. The non-structural NS3-region consists of an N-terminal serine protease and a C-terminal RNA helicase. The NS4A peptide is an important co-factor for the polyprotein maturation. NS4A holds the NS3 protease domain very close to the membrane and serves as a molecular tether that anchors the HCV replication machinery complex together at the cellular membrane [16] (Figure 2 (Fig. 2)). NS4A and NS3 both together form a complex imparting proteolysis of the HCV polyprotein, which splits junctions between the non-structural proteins. The polyprotein itself is required for the replication as well [18]. All NS3 protease inhibitors are linked with the active site of the enzyme [19]. Up-to-date, it is still challenging to design a pan-genotypic NS3 protease inhibitor.
Figure 2. HCV lifecycle and potential targets for DAAs.
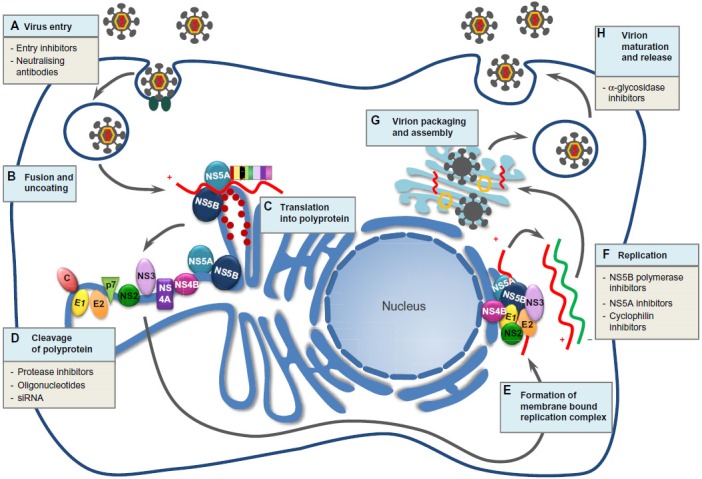
adapted from Holmes et al. 2015 [96] (CC BY-NC 3.0 licence https://creativecommons.org/licenses/by-nc/3.0/)
Resistance to NS3 protease
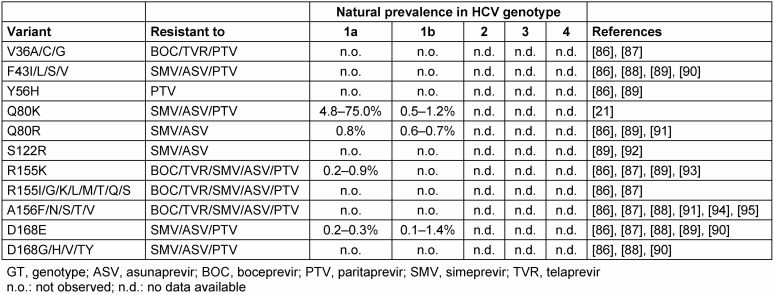
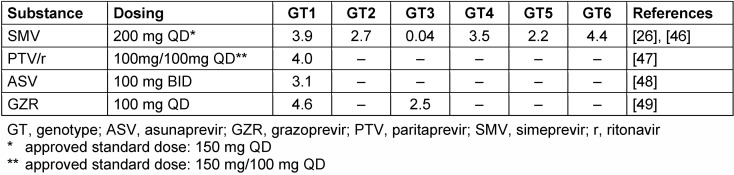
The high replication rate of HCV generates a heterogeneous virus population in infected patients [20]. The heterogeneity within NS3, NS5A and NS5B areas is a relevant access in the interaction with DAAs. Amino acid polymorphisms associated with suboptimal efficacy of DAAs are denominated as resistance associated substitutions (RASs). RASs can be linked with virological treatment failure. Many RASs are related to a replicative impairment. This is an explanation for the low likeliness of detectable pre-existence RASs as well as a relatively rapid replacement by wild-type virus after quitting protease inhibitor therapy. Despite the low prevalence of NS3 associated RASs in genotype 1 patients (Table 1 (Tab. 1)), there is one important exception. Interestingly, the Q80K has no loss of replicative fitness in numerous patients and is likely to be found with a high prevalence in DAA naïve patients [19]. This variant is linked with different levels of resistance to some protease inhibitors like simprevir, asunaprevir and paritaprevir. Q80K is mainly present in genotype 1a that confers low level resistance to in vitro SMV activity [21]. Finally, it is the most common polymorphism among the NS3 RASs linked to low activity of NS3/4A protease inhibitors [22]. Q80K prevalence vary by country with a low overall in genotype 1 infected patients in Europe (up to 19%) compared to North America (48%), respectively [21]. Little is known about RASs for other genotypes. The absence of known RASs for a certain NS3 protease inhibitor does not lead to a high direct-acting antiviral activity. Telaprevir, one of the breakthrough structures, has no impact in genotype 3 patients in a clinical trial, although there were no pre-existing RASs noticed [23]. In conclusion, based on in vitro studies, all currently approved NS3 protease inhibitors were designed for HCV genotype 1. The potency against HCV genotype 3 is clearly lower [19]. Table 2 (Tab. 2) shows an overview of the clinical antiviral activity in monotherapy of the recently approved most important HCV protease inhibitors.
Table 1. Natural prevalence of NS3 protease-inhibitors associated RAVs detected by population sequencing .
adapted from Sarrazin 2016 [19]
Table 2. Clinical antiviral activity of the most important approved protease inhibitors (mean or median maximum HCV viral load decline after 3–14 days’ monotherapy [log10 IU/ml].
adapted from Sarrazin 2016 [19]
Protease inhibitors
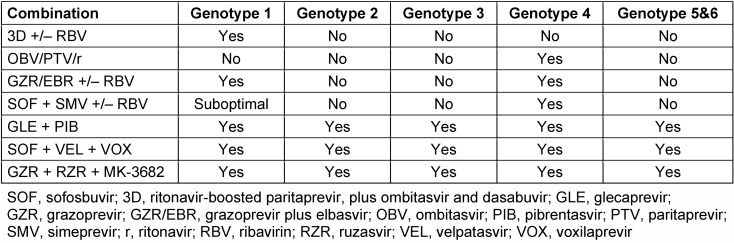
In the course of the past years, four new protease inhibitors have been approved for the treatment of chronic hepatitis C infection (Table 3 (Tab. 3)).
Table 3. NS3/4A protease inhibitors containing IFN-free combination treatment regimens and their treatment recommendations as per genotype.
adapted from EASL Recommendations on Treatment of Hepatitis C 2016 [14]
Simeprevir (SMV)
Simeprevir has been approved as a once-daily oral NS3/4A protease inhibitor in 2013. The efficacy has been originally established in combination with pegIFN alfa and ribavirin, in HCV genotype 1 infected subjects with compensated liver disease, including cirrhosis [24]. A high antiviral activity was shown in clinical monotherapy trials for SMV in HCV genotype 1, 4 and 6 infected patients. However, activity was low in HCV genotype 2, 3 and 5 patients because of the presence of pre-existing baseline RASs [25], [26]. SMV was the first second-generation protease inhibitor for use in combination with the NS5B polymerase inhibitor sofosbuvir (SOF). Published data from this once-daily regime showed first-time SVR12>90% in genotype 1 patients, irrespectively of ribavirin add-on or the treatment duration (12 weeks vs. 24 weeks) [27]. Finally, these results were a major step forward for the inception of the era of DAA treatment for patients with chronic hepatitis C infection. In the meantime, additional results from two phase 3 trials were published [28], [29]. In the OPTIMIST-1 trial, treatment-naïve and -experienced patients without cirrhosis were randomly allocated to 12 versus 8 weeks of the SMV plus SOF regimen. The SVR 12 rate was 97% versus 83%, respectively. Furthermore, the relapse rate was significantly higher in the 8 weeks’ course. But there was no difference in SVR12 based on genotype 1 subtype or presence of the baseline Q80K RAS [28]. The Optimist-2 trial was designed as a single-arm, open-label investigation for 12 weeks of SMV plus SOF in treatment-naïve or -experienced patients with cirrhosis. Patients without baseline Q80K resistance had similar SVR12 rates, irrespectively from genotype 1 subtype. Nevertheless, patients with subtype 1a infection and the presence of the Q80K RAS had decreased SVR12 rates (74%) [29]. To date, treatment regimens containing SMV are no longer recommended by internatiol and national guidelines.
Asunaprevir (ASV)
Asunaprevir is a second generation NS3 protease inhibitor and was initially approved in Japan for use in combination with pegylated IFN and ribavirin. ASV is active against genotype 1, 4, 5 and 6 in vitro [30]. The HALLMARK-DUAL trial investigated the safety and efficacy of daclatasvir (DCV), a NS5A inhibitor, once daily plus ASV 100 mg twice daily or placebo for 12 weeks in chronically infected patients with HCV genotype 1b with or without cirrhosis. Even though SVR rates were all-over high in the study population, there were statistically significant lower SVR rates for patients with the presence of pre-existing baseline RASs compared to patients without any baseline RASs (39% versus 92% SVR), respectively. Baseline RASs were detected in 13% of the patients [31]. Overall SVR rates were about 80%. Due to the extensive use of this suboptimal regimen in Japan, DAA failures occurred in approximately 20%. ASV was not approved in the US and Europe.
Paritaprevir (PTV)
Paritaprevir is another NS3/4A protease inhibitor (co-dosed with ritonavir (r) as a booster) that was approved for use in combination of the NS5A inhibitor ombitasvir (OBV) and non-nucleoside NS5B polymerase inhibitor dasabuvir (DSV) with or without ribavirin in 2014 [32]. Because of the multitarget antiviral activity, there are important drug-drug interactions, which should be considered before initiating therapy. Furthermore, this combination is not recommendend in patients with Child-Pugh B decompensated cirrhosis or with compensated cirrhosis but with previous episodes of decompensation and with Child-Pugh C decompensated cirrhosis because of the significant higher protease inhibitor concentrations in this difficult-to-treat population [14]. Based on results of major phase III trials, treatment duration of this regimen for patients infected with HCV subtype 1b with or without compensated cirrhosis (without RBV) and subtype 1a without (with RBV) cirrhosis will be 12 weeks. Patients infected with subtype 1a with compensated cirrhosis should receive this combination with ribavirin for 24 weeks [33], [34], [35], [36]. Treatment duration can be shortened from 12 weeks to 8 weeks for patients infected with subtype 1b without cirrhosis, with caution for patients with advanced fibrosis (METAVIR score F3) [37]. In summary, the combination of PTV/r, OBV with DSV with or without RBV is highly potent in patients with chronic HCV infection with genotype 1 with or without cirrhosis. As well, the combination of PTV/r, OBV and RBV without DSV is highly efficacious in patients with HCV genotype 4 infection. In the PEARL-1 and AGATE-1 trials, patients achieved not less than 97% SVR12 rates [38], [39]. Despite the fact that resistance associated polymorphisms will occur fast in monotherapy of the single substance, the combination supplies a high genetic barrier to resistance. Based on a large clinical trial program with more than 2,500 patients, baseline resistance data will be available for nearly 700 patients. None of the patients had RASs within all three agents whereas the prevalence of RASs within one substance was up to 33% in HCV subtype 1a infected patients [40]. The existence of Q80K mutation as a baseline RAS in genotype 1 infected patients showed no difference in the entire group, which is elucidated by the low-level resistance to PTV [21]. However, in the 8-week course SVR12 rates were lower in patients infected with HCV subtype 1a with the pre-existing Q80K mutations (74% vs. 87%), respectively [40], [41]. RASs can be detected in approximately 85% of patients with treatment failure, however NS3 RASs will be observed in only 9% of patients after around 1 year. In especial NS5A and NS5B RASs show a tendency to subsist [42].
Grazoprevir (GZR)
Grazoprevir in combination with the NS5A inhibitor Elbasvir (EBR) was approved for the treatment of HCV infected patients with genotype 1 and 4 by the FDA in 2016 [43]. GZR should be active against all HCV genotypes in vitro, but up to now there is only data from experiments performed recently with HCV genotype 1, 2 and 3 available [44]. However, the genetic resistance barrier for the most approved NS3 protease inhibitors is low in monotherapy, a higher barrier was described for GZR caused by the higher antiviral activity against typical RASs emerging in patients with treatment (PTV/r+OBV,DSV +/- RBV) failure, namely R155-, A156- and D168-mutation [26], [45], [46], [47], [48], [49]. Based on pharmacokinetic data from non-HCV infected patients with cirrhosis, the combination of GZR and EBR is contraindicated in patients with Child-Pugh B and Child-Pugh C hepatic impairment. No dose advantage is required in patients with all stages of renal impairment [14].
Even though high SVR12 rates (~97%) were across all treatment arms for treatment-naïve and -experienced patients, commonly with genotype 1 and 4 infection, in phase 2 and phase 3 trials [50], [51], [52], [53], [54], [55], there are major restrictions for treatment-naïve and -experienced patients with subtype 1a with or without cirrhosis as well for treatment-experienced genotype 4 patients with or without cirrhosis. Patients in this special population with a HCV RNA level at baseline >800,000 IU/ml should receive the combination for 16 weeks plus RBV if no NS5A resistance test can be performed [14], [43]. Interestingly, RASs in the NS3-region had no impact on SVR. But relevant pre-existing RASs were reported in up to 10% in subtype 1a infected patients. Notably treatment-naïve patients with subtype 1a infection and the presence of baseline RASs had significantly lower SVR rates (22%) compared to patients without baseline RASs (98%), respectively [55]. However, only the prevalence of baseline RASs with a >5fold resistance (M/L28T/A, Q/R30E/H/RG/K/LD, L31M/V/F, H58D, Y93C/H) is relevant in subtype 1a patients [56], [57]. Despite the high antiviral activity of this third-generation protease inhibitor, some major restrictions should be considered. Resistance tests at baseline for pre-existing RASs should be mandatory in patients with HCV subtype 1a whereas there is no necessity in subtype 1b. Recently, promising data were presented for the new 3-drug combination, MK3. MK3 is a three-drug regimen which is formulated into a fixed-dose combination tablet, taken orally twice daily, without regard to food. This triplet consists of the NS5B polymerase inhibitor uprifosbuvir (MK-3682), GZR and the next-generation NS5A inhibitor Ruzasvir (MK-8408). This regimen for 8 or 12 weeks demonstrated high SVR rates (97%) in treatment-naïve patients infected with HCV genotype 1. MK3 was also highly effective in treatment-naïve genotype 2 patients. In conclusion, this triplet regimen is also effective in GT3 treatment-naïve or experienced patients. Additionally, this new combination with RBV add-on and a prolonged treatment duration over 16 weeks offers an option for the very difficult-to-treat population who previously experienced DAA failure with the emergent of NS3 and NS5A RASs [58].
Glecaprevir (GLE, formerly called ABT493)
Glecaprevir is a new pan-genotypic NS3/4A third generation protease inhibitor which was evaluated as a fixed-dose combination with pibrentasvir (PIB, formerly called ABT-530), an NS5A inhibitor. This regimen was approved by the FDA in August 2017. This next generation regimen demonstrated a high barrier to resistance in vitro and is potent against the common NS3 variants (e.g. 80, 150, 168) and NS5A variants (e.g. 28, 30, 31 and 93) [59]. In a 3-day monotherapy trial of patients treated with GLE the presence of baseline RASs did not appear to affect the viral load decline during therapy. Patients with HCV subtype 1a and the presence of Q80K at baseline were showing an remarkable decline of viral load [59]. Interestingly, GLE is highly active against wild-type HCV subtype 1a and subtype 1b, as well as subtype 3a. The antiviral activity grants a high level of resistance compared to most second- and third generation protease inhibitors [60]. In part 1 and 2 of the SURVEYOR-I and SURVEYOR-II phase 2 trial, the combination of GLE and PIB without add-on RBV demonstrated high efficacy in patients without cirrhosis across all 6 major HCV genotypes. In patients infected with HCV genotype 1 and 3 with presence of cirrhosis, the fix-dosed regimen with or without RBV achieved SVR rates of 96%–100%. Baseline substitutions had minimal impact on SVR12 rates. The presence of NS5A Y93H mutation at baseline did not affect SVR rates in patients with HCV genotype 3 infection. However, the presence of A30K RAS at baseline increases the risk of treatment failure in genotype 3 infected patients [61], [62]. Patients with HCV genotype 1 or 4 and prior DAA failure were evaluated in the MAGELLAN-1 part 2 trial. They were stratified by HCV genotype and prior DAA experience for receiving 12 or 16 weeks GLE/PIB. The majority had broad representation of baseline NS5A RASs, between 9–11% had dual class resistance. Patients with prior failure to protease inhibitor and NS5A inhibitor containing therapy had significantly lower SVR rates compared to patients with prior failure to PI containing regimen (81% vs. 100%). The presence of NS5A and NS3 RASs at baseline had a strong impact on SVR rates [63]. These findings provide a basis for resistance testing after DAA failure if GLE/PIB is considered as a retreatment option. Nevertheless, this fixed-dose combination represents a major improvement in next generation DAA therapy for treatment-naïve patients and in certain cases as a retreatment option for patients with prior DAA failure. In particular, patients with kidney insufficiency can be easily treated with this once daily regime [64].
Voxilaprevir (VOX, formerly called GS-9857)
Voxilaprevir is a NS3/4A protease inhibitor which recently was approved by the FDA as a once-daily single in combination with an NS5B inhibitor, velpatasvir, and an NS5A inhibitor, voxilaprevir (SOF/VEL/VOX) in July 2017. VOX showed potent in vitro activity against HCV genotypes 1–6 and an improved resistance profile against frequently genotype 1 NS3 RASs compared to other NS3/4A protease inhibitors, respectively [65]. In a randomized, dose ranging phase 1 study, VOX demonstrated a potent antiviral activity with or without the presence of commonly observed NS3 mutations with resistance to protease inhibitors [66]. The New Drug Application (NDA) is based on data from the POLARIS-1 phase 3 trial and the POLARIS-4 phase 3 trial, which reported high SVR rates (97%) in patients with HCV genotypes 1–6, including those who failed to prior treatment with an NS5A-containing regimen. The POLARIS-1 study evaluated the treatment combination of SOF, VEL and VOX as a fixed dose single-tablet regime for 12 weeks in patients who previously received an NS5A inhibitor. Overall high SVR rates (96%) were shown in this difficult-to-treat population. Among the group of relapsers, no treatment emergent RASs were reported. Six relapses were observed in patients with cirrhosis at baseline, no relapse in patients without any clinical signs of cirrhosis. Latterly presented data from the POLARIS-4 trial showed formidable high SVR12 rates (97%) in DAA experienced patients infected with HCV genotype 1–3 with or without cirrhosis who had not previously been treated with an NS5A inhibitor. Baseline RASs did not affect treatment outcome. Impressively, no treatment RASs emerged in patients treated with SOF/VEL/VOX [67]. The NDA is further supported by two additional phase 3 studies (POLARIS-2 and POLARIS-3) in which DAA-naïve HCV-infected patients received 8 weeks of SOF/VEL/VOX. The so-called POLARIS 2-trial compared the treatment with the single-tablet combination of SOF, VEL and VOX for 8 weeks to treatment with SOF and VEL for 12 weeks in patients infected with HCV genotype 1–6 with and without cirrhosis who have not previously been treated with a DAA. Treatment with the single-tablet regimen resulted in high SVR rates in genotype 1–6 DAA-naïve patients. But higher relapse rates were reported for this combination in the 8 weeks’ arm compared to the arm being treated with SOF and VEL for 12 weeks, particularly in HCV subtype 1a patients with the presence of Q80K RAS at baseline. Despite the fact of high susceptibility from Q80K in vitro to VOX, patients who received SOF/VEL/VOX for 8 weeks had significant lower SVR rates with the presence of Q80K (88% vs. 94%). Data from the POLARIS-3 trial reported high SVR rates (96%) in patients infected with HCV genotype 3 and cirrhosis, irrespectively from the existence of Y93H at baseline. Interestingly, no treatment associated RASs were discovered in the SOF/VEL/VOX arm whereas in the SOF/VEL arm failures evolved an Y93H. The most common adverse events among patients who received SOF/VEL/VOX were headache, fatigue, diarrhea and nausea [68]. In conclusion, lower relapse rates can be expected for this single-tablet 3-drug regimen compared to the combination of SOF and VEL. Up to date, there is no distinct evidence if this regimen is superior to the dual combination of GLE and PIB in treatment naïve patients. However, this fixed-dosed regimen will become important in salvage therapy and in difficult-to-treat treatment-naïve patients.
HIV/HCV coinfection
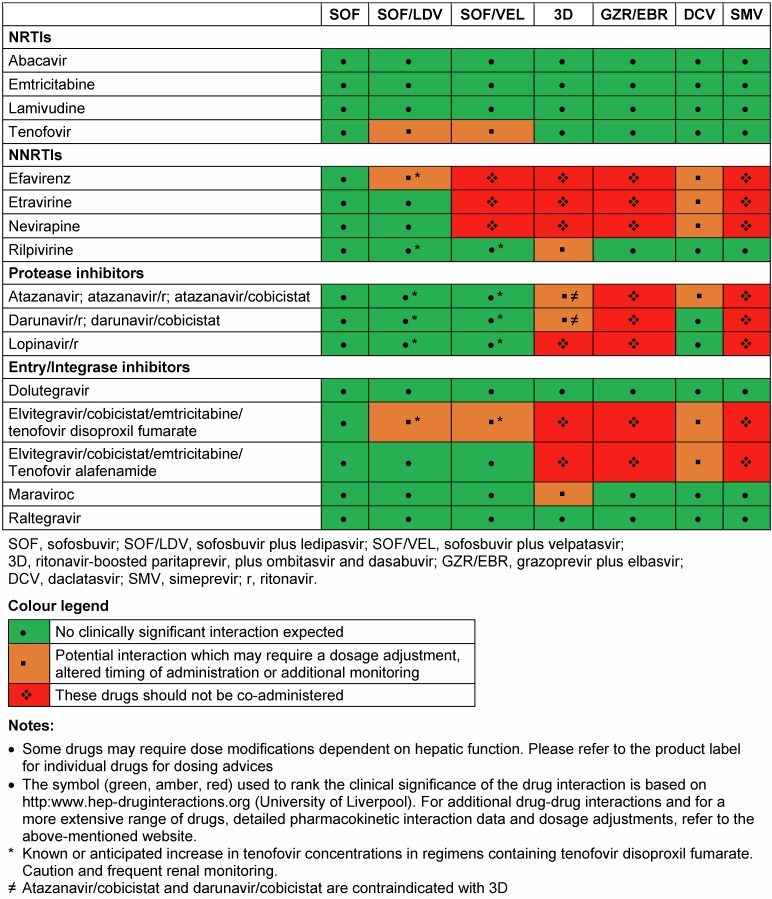
While AIDS-related mortality has decreased, liver related mortality due to HCV coinfection has emerged as a major cause for non-AIDS associated morbidity and mortality [69], [70]. Data from the Veterans Aging Cohort Study Virtual Cohort showed a higher risk of hepatic decompensation and death in HIV coinfected patients compared to HCV-monoinfected individuals. Notably the risk of decompensation was higher for coinfected patients with advanced liver fibrosis, severe anemia and non-black race [71]. Consequently, the recently published and updated EASL and AASLD guidelines highly recommend treating all HCV coinfection patients with prioritization. HIV-coinfected patients should be treated and retreated the same as patients without HIV infection [14], [72], [73]. However, there are a couple of restrictions in consequence of drug-drug interaction between the combined antiretroviral therapy (cART) and DAAs. Especially NS3/4A protease inhibitors interact with boosters like ritonavir and cobistat, HIV protease inhibitors (PI), non-nucleoside reverse-transcriptase inhibitors (NNRTIs) and entry inhibitors (most notably maraviroc). A switch of the cART before starting HCV treatment with DAAs is mainly forced by PTV/r, SMV and GZR in most patients. Nevertheless, since integrase inhibitors containing cART regimens are recommended as a first-line therapy, a favorable interaction profile is increasing. Dose adjustments are necessary if the NS5A inhibitor DCV is coadministrated with ritonavir-boosted atazanavir or efavirenz. Table 4 (Tab. 4) summarizes and highlights the most important drug-drug interactions. For additional drug-drug interactions, data the interaction checker from the university of Liverpool represents a very well established online tool (http://www.hep-druginteractions.org), also available in app format (Liverpool HEP iChart). Real-life cohorts evaluated the safety and efficacy of DAAs in HIV coinfected patients. A lately presented prospective multicohort study from Spain demonstrated a worse respond to DAA-based therapy in HIV-coinfected patients compared to HCV-monoinfected patients (95 vs. 97%), respectively [74]. The underlying reason of this major finding was unclear. However, encouraging SVR12 rates were shown for HIV-coinfected individuals in the German Hepatitis C-registry. At baseline, cirrhosis was less frequent in HIV-coinfected patients and the majority was male. Overall, there were no differences in SVR rates between HIV-coinfected and HCV-monoinfected patients [75]. In summary, these recently presented data give strong evidence that there is no need to recommend different treatment options and regimens for HIV-coinfected individuals. Nonetheless, there is one important exception. In the setting of acute HCV infection, SVR rates were significantly lower in patients with HIV-coinfection (77%, 20/26), who received a fixed-dose combination of SOF and LDV, compared to HCV-monoinfected patients (100%, 20/20), respectively [5], [6]. To date, all DAA regimens are not approved for treatment in patients with acute HCV-infection. Furthermore, there are still unacknowledged questions such as the relevance of transmitted RASs in the era of DAA treatment.
Table 4. Drug interactions between HCV DAAs and HIV antiretrovirals.
adapted from EASL Recommendations on Treatment of Hepatitis C 2016 [14]
HBV/HCV coinfection
Even though the prevalence of hepatitis B virus (HBV) coinfection varies, there are populations at high risk for acquiring both infections due to common routes of transmission. The prevalence of HBV/HCV coinfection in two US Veterans Administration cohorts was 42–67% [76]. Lately, the FDA warns about the risk of HBV becoming an active infection again in any patient who has a current or previous infection with HBV and is treated with certain DAA medicines for hepatitis C virus [77]. HBV reactivation is defined as the increase in HBV-DNA in a patient with inactive or resolved HBV infection. Reactivation can occur spontaneously, but is mostly triggered by immunodeficiency due to HIV or immunosuppressive therapy. In total, 29 cases were collected globally. Interestingly, HBV reactivation can occur early after starting DAA therapy, the median time to occurrence of reactivation was 46 days. Three patients developed decompensated liver failure. There was no HCV genotype or drug specific risk but rather a general risk for HBV reactivation under DAA therapy. Most patients with HBV reactivation were patients with known chronic HBV infection and a positive HBs-Antigen. However, HBV reactivation has also been reported in HBV/HCV coinfected patients treated with IFN-based HCV therapy. But compared to patients under IFN based therapy, development of hepatitis was more likely in DAA treated patients [78]. The mechanism through which HBV reactivation occurs with DAAs is currently unknown. In a prospective, multicenter cohort at 14 sites in Taiwan patients with chronic HBV/HCV coinfection treated with LDV/SOF for 12 weeks were observed for HBV reactivation. Treatment with LDV/SOF was associated with silent HBV viral reactivation in 63% of patients (70/111). Nevertheless, no patient experienced clinical signs or symptoms of HBV reactivation [79]. Results from a retrospective analysis of HBV reactivation in 62,920 American veterans showed varying severety of reactivation. However, the clinical presence of liver failure was rare [80]. In conclusion, HBV screening is mandatory for all HCV patients before initiating treatment with DAAs. Especially patients with anti-HBc should be monitored more closely during DAA treatment and post-treatment follow-up. Lately, the updated EASL guideline recommends nucleos(t)ide analogue prophylaxis for HBsAg-positive patients undergoing DAA therapy until week 12 post DAA [81].
Conclusion
Due to high antiviral activity and high genetic resistance barrier NS3/4A protease inhibitors are an important element of HCV treatment. In combination with DAAs of one or two other classes they can stop different stages of viral life cycle and therefore they can achieve SVR rates above 95% [12]. Protease inhibitors are well tolerated and have a low incidence of side effects, also in patients with renal impairment and compensated cirrhosis. HCV infected patients with genotype 3 represent up to 30% of all HCV infections worldwide. In past years of DAA treatment they belong to the difficult-to-treat subgroup [82]. Numerous NS3/4A protease inhibitors exhibit significantly lower potency against HCV genotype 3, caused by polymorphisms between genotypes in the drug target altering the intermolecular dynamics of the protein-inhibitor complex [83]. But reported results from new treatment combinations of pan-genotypic protease inhibitors, namely GZR, GLE and VOX, look promising and showed high SVR rates in this difficult-to-treat population irrespectively in the most cases from baseline RASs [60], [61], [63], [66], [67], [68], [64], [62]. A baseline resistance test was recommended from experts to patients receiving a treatment combination of a second-generation protease inhibitor, mainly SMV, and a NS5A-inhibitor with a relative low barrier to resistance. To date, in the era of new pan-genotypic third generation NS3/4A protease inhibitors, the presence of baseline RASs or even the onset of RASs during treatment does not affect SVR rates in most cases at all. But however, there is still a demand of carefulness, because SVR rates were significantly lower in HCV genotype 1a patients receiving SOF/VEL/VOX for 8 weeks with the presence of Q80K RAS at baseline and in HCV genotype 3 patients with NS5A baseline RASs who received GLE/PIB. Anyway, these regimens are excellent tools to treat patients who failed previous treatment with DAAs, including therapy with an NS5A-inhibitor or NS3/4A protease inhibitor. These combination therapies should be evaluated in a real-life setting and trials need to validate whether resistance testing prior therapy is clinically relevant or not.
However, guidelines recommend genotype testing prior to HCV treatment initiation [14], [72], testing is very expensive and is not widely available in low and middle-income countries because it requires advanced equipment. The new DAA combinations with a pan-genotypic third generation NS3/4A protease inhibitor represent an opportunity to treat HCV infected patients without prior genotype testing in low and middle-income countries. Finally, diagnostic simplification and cost-reduction will be the key to enable implementation of HCV screening and treatment in those countries [84]. Current prices of these regimens are variable and unaffordable globally. Hopefully, this situation will improve in future and these novel third generation NS3/4A protease inhibitors will be accessible for all HCV infected patients.
Summing up, the new third generation NS3/4A protease inhibitors in combination with other DAAs can be a substantial step in the eradication of HCV in the so called difficult-to-treat population (e.g. compensated cirrhosis, end-stage renal disease and patients who failed previous DAA treatment). Anyway, a real decrease of HCV infections in global population demands available HCV testing in low-income countries, access to health care and treatment. All together we should ensure that these lifesaving treatments become accessible to all those who need them.
Abbreviations
3D: ritonavir-boosted paritaprevir, plus ombitasvir and dasabuvir
ASV: asunaprevir
cART: combined antiretroviral therapy
BOC: boceprevir
DAA: direct-acting antiviral
DCV: daclatasvir
DSV: dasabuvir
EBR: elbasvir
GLE: glecaprevir
HBV: hepatitis B virus
HCV: hepatitis C virus
HCC: hepatocellular carcinoma
IFN: interferon
LDV: ledipasvir
NDA: new drug application
NNRTI: non-nucleoside reverse-transcriptase inhibitor
OBV: ombitasvir
PIB: pibrentrasvir
PTV: paritaprevir
r: ritonavir
RAS: resistance associated substitutions
RBV: ribavirin
SMV: simeprevir
SOF: sofosbuvir
SVR: sustained virological response
TVR: telaprevir
VEL: velpatasvir
VOX: voxilaprevir
Notes
Competing interests
-
de Leuw P, M.D., Specialist for Internal Medicine & Infect. Diseases
Consultancies/speaker’s bureau/travel support for Bristol-Myers Squibb GmbH & Co. KGaA, Gilead Sciences GmbH, GlaxoSmithKline GmbH & Co.KG, Hexal AG, MSD Sharp & Dohme GmbH, Janssen-Cilag GmbH
-
Stephan C, M.D., Professor for Internal Medicine & Infect. Diseases Consultant
Receipt of grants/research supports: MSD
Receipt of honoraria or consultation fees (any): AbbVie, MSD, ViiV, BMS, Gilead, Janssen, Astellas, Stada
Receipt of travel grants within last 2 years: Janssen, Gilead Sciences, BMS
References
- 1.World Health Organization. Global report on access to hepatitis C treatment: Focus on overcoming barriers. Geneva: WHO; 2016. [cited 2017 Mar 5]. Available from: http://www.who.int/iris/handle/10665/250625. [Google Scholar]
- 2.World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. Geneva: WHO; 2014. [cited 2016 Oct 3]. Available from: http://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/ [PubMed] [Google Scholar]
- 3.Veldt BJ, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, Zeuzem S, Manns MP, Hansen BE, Schalm SW, Janssen HL. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007 Nov;147(10):677–684. doi: 10.7326/0003-4819-147-10-200711200-00003. [DOI] [PubMed] [Google Scholar]
- 4.Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, De Santo JL, Lee WM, Di Bisceglie AM, Bonkovsky HL, Dienstag JL, Morishima C, Lindsay KL, Lok AS HALT-C Trial Group. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010 Sep;52(3):833–844. doi: 10.1002/hep.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rockstroh JK, Bhagani S, Hyland RH, Yun C, Dvory-Sobol H, Zheng W, Brainard DM, Ingiliz P, Lutz T, Boesecke C, Nelson M. Ledipasvir-sofosbuvir for 6 weeks to treat acute hepatitis C virus genotype 1 or 4 infection in patients with HIV coinfection: an open-label, single-arm trial. Lancet Gastroenterol Hepatol. 2017 May;2(5):347–353. doi: 10.1016/S2468-1253(17)30003-1. [DOI] [PubMed] [Google Scholar]
- 6.Deterding K, Spinner C, Schott E, Welzel T, Gerken G, Klinker H, Spengler U, Wiegand J, Schulze zur Wiesch J, Pathil A, Cornberg M, Umgelter A, Zöllner C, Zeuzem S, von der Leyen H, von Witzendorff D, Manns MP, Wedemeyer H HepNet Acute HCV/V Study Group. Six weeks of sofosbuvir/ledipasvir (SOF/LDV) are sufficient to treat acute hepatitis C virus genotype 1 monoinfection: the HEPNET acute HCV IV study. J Hepatol. 2016 Feb;64:S211. doi: 10.1016/S0168-8278(16)00177-X. [DOI] [PubMed] [Google Scholar]
- 7.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001 Sep 22;358(9286):958–965. doi: 10.1016/S0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 8.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002 Sep;347(13):975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 9.McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, Nyberg LM, Lee WM, Ghalib RH, Schiff ER, Galati JS, Bacon BR, Davis MN, Mukhopadhyay P, Koury K, Noviello S, Pedicone LD, Brass CA, Albrecht JK, Sulkowski MS IDEAL Study Team. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009 Aug;361(6):580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 10.Zeuzem S, Berg T, Moeller B, Hinrichsen H, Mauss S, Wedemeyer H, Sarrazin C, Hueppe D, Zehnter E, Manns MP. Expert opinion on the treatment of patients with chronic hepatitis C. J Viral Hepat. 2009 Feb;16(2):75–90. doi: 10.1111/j.1365-2893.2008.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coilly A, Dumortier J, Botta-Fridlund D, Latournerie M, Leroy V, Pageaux GP, Agostini H, Giostra E, Moreno C, Roche B, Antonini TM, Guillaud O, Lebray P, Radenne S, Saouli AC, Calmus Y, Alric L, Debette-Gratien M, De Ledinghen V, Durand F, Duvoux C, Samuel D, Duclos-Vallée JC. Multicenter Experience with Boceprevir or Telaprevir to Treat Hepatitis C Recurrence after Liver Transplantation: When Present Becomes Past, What Lessons for Future? PLoS ONE. 2015;10(9):e0138091. doi: 10.1371/journal.pone.0138091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rockstroh JK. Summary from AASLD 2015 for Hepatitis C Beyond 95% SVR cure rates: still room for improvement? 66th Annual Meeting of the American Association for the Study of Liver Diseases. 66th Annual Meeting of the American Association for the Study of Liver Diseases; 2015 Nov 13-17; Boston, MA. [cited 2015 Dec 29]. Available from: http://www.natap.org/2015/AASLD/AASLD_165.htm. [Google Scholar]
- 13.McCauley JA, Rudd MT. Hepatitis C virus NS3/4a protease inhibitors. Curr Opin Pharmacol. 2016 Oct;30:84–92. doi: 10.1016/j.coph.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 14.European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol. 2017 Jan;66(1):153–194. doi: 10.1016/j.jhep.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Houghton M, Weiner A, Han J, Kuo G, Choo QL. Molecular biology of the hepatitis C viruses: implications for diagnosis, development and control of viral disease. Hepatology. 1991 Aug;14(2):381–388. doi: 10.1002/hep.1840140227. [DOI] [PubMed] [Google Scholar]
- 16.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Kräusslich HG, Mizokami M, Bartenschlager R, Liang TJ. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005 Jul;11(7):791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007 Jun;5(6):453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- 18.Grakoui A, McCourt DW, Wychowski C, Feinstone SM, Rice CM. Characterization of the hepatitis C virus-encoded serine proteinase: determination of proteinase-dependent polyprotein cleavage sites. J Virol. 1993 May;67(5):2832–2843. doi: 10.1128/jvi.67.5.2832-2843.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarrazin C. The importance of resistance to direct antiviral drugs in HCV infection in clinical practice. J Hepatol. 2016 Feb;64(2):486–504. doi: 10.1016/j.jhep.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Mo H, Hedskog C, Lawitz E, Brainard DM, Yang J, Delaney W, Worth A, Miller MD. Antiviral response and resistance analysis of treatment-naïve HCV infected patients receiving multiple doses of the NS3 protease inhibitor GS-9256. Antiviral Res. 2017 Apr;140:151–157. doi: 10.1016/j.antiviral.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Sarrazin C, Lathouwers E, Peeters M, Daems B, Buelens A, Witek J, Wyckmans Y, Fevery B, Verbinnen T, Ghys A, Schlag M, Baldini A, De Meyer S, Lenz O. Prevalence of the hepatitis C virus NS3 polymorphism Q80K in genotype 1 patients in the European region. Antiviral Res. 2015 Apr;116:10–16. doi: 10.1016/j.antiviral.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Bae A, Sun SC, Qi X, Chen X, Ku K, Worth A, Wong KA, Harris J, Miller MD, Mo H. Susceptibility of treatment-naive hepatitis C virus (HCV) clinical isolates to HCV protease inhibitors. Antimicrob Agents Chemother. 2010 Dec;54(12):5288–5297. doi: 10.1128/AAC.00777-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Meyer S, Ghys A, Foster GR, Beumont M, Van Baelen B, Lin TI, Dierynck I, Ceulemans H, Picchio G. Analysis of genotype 2 and 3 hepatitis C virus variants in patients treated with telaprevir demonstrates a consistent resistance profile across genotypes. J Viral Hepat. 2013 Jun;20(6):395–403. doi: 10.1111/jvh.12046. [DOI] [PubMed] [Google Scholar]
- 24.Full Prescribing Information for Olyiso™. FDA; 2017. [cited 2017 Feb 25]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/205123s001lbl.pdf. [Google Scholar]
- 25.Lenz O, Vijgen L, Berke JM, Cummings MD, Fevery B, Peeters M, De Smedt G, Moreno C, Picchio G. Virologic response and characterisation of HCV genotype 2-6 in patients receiving TMC435 monotherapy (study TMC435-C202) J Hepatol. 2013 Mar;58(3):445–451. doi: 10.1016/j.jhep.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 26.Moreno C, Berg T, Tanwandee T, Thongsawat S, Van Vlierberghe H, Zeuzem S, Lenz O, Peeters M, Sekar V, De Smedt G. Antiviral activity of TMC435 monotherapy in patients infected with HCV genotypes 2-6: TMC435-C202, a phase IIa, open-label study. J Hepatol. 2012 Jun;56(6):1247–1253. doi: 10.1016/j.jhep.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 27.Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, DeJesus E, Pearlman B, Rabinovitz M, Gitlin N, Lim JK, Pockros PJ, Scott JD, Fevery B, Lambrecht T, Ouwerkerk-Mahadevan S, Callewaert K, Symonds WT, Picchio G, Lindsay KL, Beumont M, Jacobson IM. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014 Nov 15;384(9956):1756–1765. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 28.Kwo P, Gitlin N, Nahass R, Bernstein D, Etzkorn K, Rojter S, Schiff E, Davis M, Ruane P, Younes Z, Kalmeijer R, Sinha R, Peeters M, Lenz O, Fevery B, De La Rosa G, Scott J, Witek J. Simeprevir plus sofosbuvir (12 and 8 weeks) in hepatitis C virus genotype 1-infected patients without cirrhosis: OPTIMIST-1, a phase 3, randomized study. Hepatology. 2016 Aug;64(2):370–380. doi: 10.1002/hep.28467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawitz E, Matusow G, DeJesus E, Yoshida EM, Felizarta F, Ghalib R, Godofsky E, Herring RW, Poleynard G, Sheikh A, Tobias H, Kugelmas M, Kalmeijer R, Peeters M, Lenz O, Fevery B, De La Rosa G, Scott J, Sinha R, Witek J. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: A phase 3 study (OPTIMIST-2) Hepatology. 2016 Aug;64(2):360–369. doi: 10.1002/hep.28422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McPhee F, Sheaffer AK, Friborg J, Hernandez D, Falk P, Zhai G, Levine S, Chaniewski S, Yu F, Barry D, Chen C, Lee MS, Mosure K, Sun LQ, Sinz M, Meanwell NA, Colonno RJ, Knipe J, Scola P. Preclinical Profile and Characterization of the Hepatitis C Virus NS3 Protease Inhibitor Asunaprevir (BMS-650032) Antimicrob Agents Chemother. 2012 Oct;56(10):5387–5396. doi: 10.1128/AAC.01186-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manns M, Pol S, Jacobson IM, Marcellin P, Gordon SC, Peng CY, Chang TT, Everson GT, Heo J, Gerken G, Yoffe B, Towner WJ, Bourliere M, Metivier S, Chu CJ, Sievert W, Bronowicki JP, Thabut D, Lee YJ, Kao JH, McPhee F, Kopit J, Mendez P, Linaberry M, Hughes E, Noviello S HALLMARK-DUAL Study Team. All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet. 2014 Nov 1;384(9954):1597–1605. doi: 10.1016/S0140-6736(14)61059-X. [DOI] [PubMed] [Google Scholar]
- 32.Full Prescribing Information for VIEKIRA PAK™. FDA; 2014. [cited 2017 Feb 26]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206619lbl.pdf. [Google Scholar]
- 33.Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, Tam E, Marinho RT, Tsai N, Nyberg A, Box TD, Younes Z, Enayati P, Green S, Baruch Y, Bhandari BR, Caruntu FA, Sepe T, Chulanov V, Janczewska E, Rizzardini G, Gervain J, Planas R, Moreno C, Hassanein T, Xie W, King M, Podsadecki T, Reddy KR PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014 May;370(21):1983–1992. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 34.Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, Aguilar H, Xiong J, Pilot-Matias T, DaSilva-Tillmann B, Larsen L, Podsadecki T, Bernstein B. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014 Apr;370(17):1594–1603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 35.Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, Shiffman ML, Wedemeyer H, Berg T, Yoshida EM, Forns X, Lovell SS, Da Silva-Tillmann B, Collins CA, Campbell AL, Podsadecki T, Bernstein B. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014 May;370(21):1973–1982. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 36.Feld JJ, Moreno C, Trinh R, Tam E, Bourgeois S, Horsmans Y, Elkhashab M, Bernstein DE, Younes Z, Reindollar RW, Larsen L, Fu B, Howieson K, Polepally AR, Pangerl A, Shulman NS, Poordad F. Sustained virologic response of 100% in HCV genotype 1b patients with cirrhosis receiving ombitasvir/paritaprevir/r and dasabuvir for 12 weeks. J Hepatol. 2016 Feb;64(2):301–307. doi: 10.1016/j.jhep.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Welzel TM, Zeuzem S, Dumas EO, Asselah T, Shaw D, Hazzan R, Forns X, Pilot-Mathias T, Lu W, Cohen DE, Feld J. GARNET: High SVR Rates Following 8-Week Treatment With Ombitasvir/ Paritaprevir/Ritonavir + Dasabuvir in Patients With HCV Genotype 1b Infection. EASL – AASLD Special Conference: New perspectives in hepatitis c virus infection – the roadmap for cure; 2016 Sep 23-24; Paris, France. [Google Scholar]
- 38.Pol S, Reddy KR, Baykal T, Hezode C. Interferon-free regimens of ombitasvir and ABT-450/r with or without ribavirin in patients with HCV genotype 4 infection: PEARL-I study results. Hepatology. 2014;60:1129. [Google Scholar]
- 39.Asselah T, Hezode C, Qaqish RB, Elkhashab T, Hassanein T, Papatheodoridis G, Alqahtanis S, Pilot-Matias T, Yu Y, Redman R, Mobashery N. High SVR rates in patients with genotype 4 chronic hepatitis C infection and compensated cirrhosis with ombitasvir/paritaprevir/ritonavir co-administered with ribavirin (AGATE-I) J Hepatol. 2016;64(2):S827. doi: 10.1016/S0168-8278(16)01618-4. [DOI] [Google Scholar]
- 40.Krishnan P, Tripathi R, Schnell G, Reisch T, Beyer J, Irvin M, Xie W, Larsen L, Pod-sadecki T, Pilot-Matias T, Collins C. Pooled analysis of resistance in patients treated with ombitasvir/ABT-450/r and dasabuvir with or without ribavirin in Phase 2 and Phase 3 clinical trials. Hepatology. 2014 Oct;60(suppl 1):1134A–1135A. [Google Scholar]
- 41.Krishnan P, Tripathi R, Schnell G, Reisch T, Beyer J, Irvin M, Xie W, Larsen L, Cohen D, Podsadecki T, Pilot-Matias T, Collins C. Resistance analysis of baseline and treatment-emergent variants in hepatitis C virus genotype 1 in the AVIATOR study with paritaprevir-ritonavir, ombitasvir, and dasabuvir. Antimicrob Agents Chemother. 2015 Sep;59(9):5445–5454. doi: 10.1128/AAC.00998-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnan P, Tripathi R, Schnell G, Reisch T, Beyer J, Dekhtyar T, Irvin M, Xie W, Larsen L, Podsadecki T, Pilot-Matias T, Collins C. Long-term follow-up of treatment-emergent resistance-associated variants in NS3, NS5A and NS5B with paritaprevir/r, ombitasvir- and dasabuvir-based regimens. J Hepatol. 2015 Apr;62(suppl 2):S220. doi: 10.1016/S0168-8278(15)30071-4. [DOI] [Google Scholar]
- 43.Full prescribing information for ZEPATIER™. FDA; 2016. [cited 2017 Feb 26]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208261Orig1s000lbl.pdf. [Google Scholar]
- 44.Summa V, Ludmerer SW, McCauley JA, Fandozzi C, Burlein C, Claudio G, Coleman PJ, Dimuzio JM, Ferrara M, Di Filippo M, Gates AT, Graham DJ, Harper S, Hazuda DJ, Huang Q, McHale C, Monteagudo E, Pucci V, Rowley M, Rudd MT, Soriano A, Stahlhut MW, Vacca JP, Olsen DB, Liverton NJ, Carroll SS. MK-5172, a selective inhibitor of hepatitis C virus NS3/4a protease with broad activity across genotypes and resistant variants. Antimicrob Agents Chemother. 2012 Aug;56(8):4161–4167. doi: 10.1128/AAC.00324-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howe AY, Black S, Curry S, Ludmerer SW, Liu R, Barnard RJ, Newhard W, Hwang PM, Nickle D, Gilbert C, Caro L, DiNubile MJ, Mobashery N. Virologic resistance analysis from a phase 2 study of MK-5172 combined with pegylated interferon/ribavirin in treatment-naive patients with hepatitis C virus genotype 1 infection. Clin Infect Dis. 2014 Dec;59(12):1657–1665. doi: 10.1093/cid/ciu696. [DOI] [PubMed] [Google Scholar]
- 46.Reesink HW, Fanning GC, Farha KA, Weegink C, Van Vliet A, Van 't Klooster G, Lenz O, Aharchi F, Mariën K, Van Remoortere P, de Kock H, Broeckaert F, Meyvisch P, Van Beirendonck E, Simmen K, Verloes R. Rapid HCV-RNA decline with once daily TMC435: a phase I study in healthy volunteers and hepatitis C patients. Gastroenterology. 2010 Mar;138(3):913–921. doi: 10.1053/j.gastro.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 47.Pilot-Matias T, Tripathi R, Cohen D, Gaultier I, Dekhtyar T, Lu L, Reisch T, Irvin M, Hopkins T, Pithawalla R, Middleton T, Ng T, McDaniel K, Or YS, Menon R, Kempf D, Molla A, Collins C. In vitro and in vivo antiviral activity and resistance profile of the hepatitis C virus NS3/4A protease inhibitor ABT-450. Antimicrob Agents Chemother. 2015 Feb;59(2):988–997. doi: 10.1128/AAC.04227-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pasquinelli C, McPhee F, Eley T, Villegas C, Sandy K, Sheridan P, Persson A, Huang SP, Hernandez D, Sheaffer AK, Scola P, Marbury T, Lawitz E, Goldwater R, Rodriguez-Torres M, Demicco M, Wright D, Charlton M, Kraft WK, Lopez-Talavera JC, Grasela DM. Single- and multiple-ascending-dose studies of the NS3 protease inhibitor asunaprevir in subjects with or without chronic hepatitis C. Antimicrob Agents Chemother. 2012 Apr;56(4):1838–1844. doi: 10.1128/AAC.05854-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petry AS, Fraser IP, O’Mara E, Van Dyck K, Nachbar RB, De Lepeleire IM, Robberechts M, Han L, Palcza J, Moiseev VS, Kobalava ZD, Wagner FD, Uhle M, Wagner JA. Safety and antiviral activity of MK-5172, a next generation HCV NS3/4A protease inhibitor with a broad HCV genotypic activity spectrum and potent activity against known resistance mutants, in genotype 1 and 3 HCV-infected patients. Hepatology. 2011;54(4):531a. [Google Scholar]
- 50.Buti M, Gordon SC, Zuckerman E, Lawitz E, Calleja JL, Hofer H, Gilbert C, Palcza J, Howe AY, DiNubile MJ, Robertson MN, Wahl J, Barr E, Forns X. Grazoprevir, Elbasvir, and Ribavirin for Chronic Hepatitis C Virus Genotype 1 Infection After Failure of Pegylated Interferon and Ribavirin With an Earlier-Generation Protease Inhibitor: Final 24-Week Results From C-SALVAGE. Clin Infect Dis. 2016 Jan 1;62(1):32–36. doi: 10.1093/cid/civ722. [DOI] [PubMed] [Google Scholar]
- 51.Forns X, Gordon SC, Zuckerman E, Lawitz E, Calleja JL, Hofer H, Gilbert C, Palcza J, Howe AY, DiNubile MJ, Robertson MN, Wahl J, Barr E, Buti M. Grazoprevir and elbasvir plus ribavirin for chronic HCV genotype-1 infection after failure of combination therapy containing a direct-acting antiviral agent. J Hepatol. 2015 Sep;63(3):564–572. doi: 10.1016/j.jhep.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Gamal N, Andreone P. Grazoprevir/elbasvir fixed-dose combination for hepatitis C. Drugs Today. 2016 Jul;52(7):377–385. doi: 10.1358/dot.2016.52.7.2510258. [DOI] [PubMed] [Google Scholar]
- 53.Lawitz E, Gane E, Pearlman B, Tam E, Ghesquiere W, Guyader D, Alric L, Bronowicki JP, Lester L, Sievert W, Ghalib R, Balart L, Sund F, Lagging M, Dutko F, Shaughnessy M, Hwang P, Howe AY, Wahl J, Robertson M, Barr E, Haber B. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015 Mar 21;385(9973):1075–1086. doi: 10.1016/S0140-6736(14)61795-5. [DOI] [PubMed] [Google Scholar]
- 54.Sulkowski M, Hezode C, Gerstoft J, Vierling JM, Mallolas J, Pol S, Kugelmas M, Murillo A, Weis N, Nahass R, Shibolet O, Serfaty L, Bourliere M, DeJesus E, Zuckerman E, Dutko F, Shaughnessy M, Hwang P, Howe AY, Wahl J, Robertson M, Barr E, Haber B. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015 Mar 21;385(9973):1087–1097. doi: 10.1016/S0140-6736(14)61793-1. [DOI] [PubMed] [Google Scholar]
- 55.Zeuzem S, Ghalib R, Reddy KR, Pockros PJ, Ben Ari Z, Zhao Y, Brown DD, Wan S, DiNubile MJ, Nguyen BY, Robertson MN, Wahl J, Barr E, Butterton JR. Grazoprevir-Elbasvir Combination Therapy for Treatment-Naive Cirrhotic and Noncirrhotic Patients With Chronic Hepatitis C Virus Genotype 1, 4, or 6 Infection: A Randomized Trial. Ann Intern Med. 2015 Jul;163(1):1–13. doi: 10.7326/M15-0785. [DOI] [PubMed] [Google Scholar]
- 56.Kwo P, Gane E, Peng C-Y, Pearlman B, Vireling J, Serfaty L, Buti M, Shafran S, Stryszak P, Lin L, Gress J, Robertson M, Wahl J, Barr E, Haber B. Efficacy and safety of grazoprevir/elbasvir +/- RBV for 12 weeks in patients with HCV G1 or G4 infection who previously failed peginterferon/RBV: C-EDGE treatment-experienced trial. J Hepatol. 2015;62:S674. doi: 10.1016/S0168-8278(15)31088-6. [DOI] [Google Scholar]
- 57.Zeuzem S, Ghalib R, Reddy KR, Pockros PJ, Ari ZB, Zhao Y, Brown D, DiNubile M, Robertson M, Wahl J, Barr E, Butterton J. The phase 3 C-EDGE treatment-naive (TN) study of a 12-weeek oral regimen of grazoprevir (GZR, MK-5172)/elbasvir (EBR, MK-8742) in patients with chronic HCV genotype (GT) 1, 4 or 6 infection. J Hepatol. 2015;62:S213. doi: 10.1016/S0168-8278(15)30056-8. [DOI] [Google Scholar]
- 58.Wyles D, Wedemeyer H, Ben-Ari Z, Gane EJ, Hansen JB, Jacobson IM, Laursen AL, Luetkemeyer A, Nahass R, Pianko S, Zeuzem S, Jumes P, Huang HC, Butterton J, Robertson M, Wahl J, Barr E, Joeng HK, Martin E, Serfaty L C-CREST Part C and C-SURGE Investigators. Grazoprevir, ruzasvir, and uprifosbuvir for hepatitis C virus after NS5A treatment failure. Hepatology. 2017 Jul 7; doi: 10.1002/hep.29358. [DOI] [PubMed] [Google Scholar]
- 59.Lawitz EJ, O’Riordan WD, Asatryan A, Freilich BL, Box TD, Overcash JS, Lovell S, Ng TI, Liu W, Campbell A, Lin CW, Yao B, Kort J. Potent Antiviral Activities of the Direct-Acting Antivirals ABT-493 and ABT-530 with Three-Day Monotherapy for Hepatitis C Virus Genotype 1 Infection. Antimicrob Agents Chemother. 2015 Dec;60(3):1546–1555. doi: 10.1128/AAC.02264-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ng T, Pilot-Matias T, Tripathi R, Schnell G, Reisch T, Beyer J, Dekhtyar T, Wang S, Mensa F, Kort J, Collins C. Analysis of HCV genotype 2 and 3 variants in patients treated with combination therapy of next generation HCV direct-acting antiviral agents ABT-493 and ABT-530. J Hepatol. 2016;64:S409–S410. doi: 10.1016/S0168-8278(16)00653-X. [DOI] [Google Scholar]
- 61.Gane E, Poordad F, Wang S, Asatryan A, Kwo PY, Lalezari J, Wyles DL, Hassanein T, Aguilar H, Maliakkal B, Liu R, Lin CW, Ng TI, Kort J, Mensa FJ. High Efficacy of ABT-493 and ABT-530 Treatment in Patients With HCV Genotype 1 or 3 Infection and Compensated Cirrhosis. Gastroenterology. 2016 Oct;151(4):651–659.e1. doi: 10.1053/j.gastro.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 62.Foster GR, Gane E, Asatryan A, Asselah T, Ruane PJ, Pol S, Poordad F, Stedman Ca, Dore G, Roberts SK, Kaita K, Vierling J, Vargas HE, Kort J, Lin CW, Liu R, NG T, Mensa F. Endurance-3: Safety and Efficacy of Glecaprevir/Pibrentasvir Compared To Sofosbuvir Plus Daclatasvir In Treatment-Naïve Hcv Genotype 3-Infected Patients Without Cirrhosis. J Hepatol. 2017;66(1):S33. doi: 10.1016/S0168-8278(17)30326-4. [DOI] [Google Scholar]
- 63.Poordad F, Pol S, Asatryan A, Buti M, Shaw D, Hézode C, Felizarta F, Gordon SC, Pianko S, Fried MW, Bernstein DE, Gallant J, Lin CW, Ley Y, NG TI, Pilot-Matias T, Kort J, Mensa F. Magellan-1, Part 2: Glecaprevir/Pibrentasvir for 12 Or 16 Weeks In Patients With Chronic Hcv Genotype 1 Or 4 And Prior Direct-Acting Antiviral Treatment Failure. The International Liver Congress (EASL); 2017 Apr 22; Amsterdam, The Netherlands. [Google Scholar]
- 64.Gane E, Lawitz E, Pugatch D, Papatheodoridis G, Bräu N, Brown A, Pol S, Leroy V, Persico M, Moreno C, Colombo M, Yoshida EM, Nelson DR, Collins C, Lei Y, Kosloski M, Mensa FJ. Glecaprevir and Pibrentasvir in Patients with HCV and Severe Renal Impairment. N Engl J Med. 2017 Oct;377(15):1448–1455. doi: 10.1056/NEJMoa1704053. [DOI] [PubMed] [Google Scholar]
- 65.Taylor J, Appleby T, Barauskas O, Chen X, Dvory-Sobol H, Gong R, Lee J, Nejati E, Schultz B, Wang Y, Yang C, Yu M, Zipfel S, Chan K. P0899: preclinical profile of the pangenotypic HCV NS3/4A protease inhibitor GS-9857. J Hepatol. 2015 Apr;62(suppl 2):S681. doi: 10.1016/S0168-8278(15)31102-8. [DOI] [Google Scholar]
- 66.Rodriguez-Torres M, Glass S, Hill J, Freilich B, Hassman D, Di Bisceglie AM, Taylor JG, Kirby BJ, Dvory-Sobol H, Yang JC, An D, Stamm LM, Brainard DM, Kim S, Krefetz D, Smith W, Marbury T, Lawitz E. GS-9857 in patients with chronic hepatitis C virus genotype 1-4 infection: a randomized, double-blind, dose-ranging phase 1 study. J Viral Hepat. 2016 Aug;23(8):614–622. doi: 10.1111/jvh.12527. [DOI] [PubMed] [Google Scholar]
- 67.Bourlière M, Gordon SC, Flamm SL, Cooper CL, Ramji A, Tong M, Ravendhran N, Vierling JM, Tran TT, Pianko S, Bansal MB, de Lédinghen V, Hyland RH, Stamm LM, Dvory-Sobol H, Svarovskaia E, Zhang J, Huang KC, Subramanian GM, Brainard DM, McHutchison JG, Verna EC, Buggisch P, Landis CS, Younes ZH, Curry MP, Strasser SI, Schiff ER, Reddy KR, Manns MP, Kowdley KV, Zeuzem S POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, Velpatasvir, and Voxilaprevir for Previously Treated HCV Infection. N Engl J Med. 2017 Jun;376(22):2134–2146. doi: 10.1056/NEJMoa1613512. [DOI] [PubMed] [Google Scholar]
- 68.Jacobson IM, Lawitz E, Gane EJ, Willems BE, Ruane PJ, Nahass RG, Borgia SM, Shafran SD, Workowski KA, Pearlman B, Hyland RH, Stamm LM, Svarovskaia E, Dvory-Sobol H, Zhu Y, Subramanian GM, Brainard DM, McHutchison JG, Bräu N, Berg T, Agarwal K, Bhandari BR, Davis M, Feld JJ, Dore GJ, Stedman CAM, Thompson AJ, Asselah T, Roberts SK, Foster GR. Efficacy of 8 Weeks of Sofosbuvir, Velpatasvir, and Voxilaprevir in Patients With Chronic HCV Infection: 2 Phase 3 Randomized Trials. Gastroenterology. 2017 Jul;153(1):113–122. doi: 10.1053/j.gastro.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 69.Puoti M, Moioli MC, Travi G, Rossotti R. The burden of liver disease in human immunodeficiency virus-infected patients. Semin Liver Dis. 2012 May;32(2):103–113. doi: 10.1055/s-0032-1316473. [DOI] [PubMed] [Google Scholar]
- 70.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44(Suppl 1):S6–S9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Lo Re V, Tate J, Kallan M, Lim J, Goetzet M, et al. Increased risk of hepatic decompensation and hepatocellular carcinoma in HIV/HCV-co-infected patients compared to HCV-mono-infected patients despite combination antiretroviral therapy. XIX International AIDS Conference; 2012 Jul 22-27; Washington, DC. [Google Scholar]
- 72.American Association for the study of liver diseases; Infectious Diseases Society of America. HCV GUIDIANCE: Recommendations for testing, managing, and treating hepatitis C. http://www. [cited 2017 Mar 3]. Available from: http://www.hcvguidelines.org/full-report-view. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arends JE, Lieveld FI, Boeijen LL, de Kanter CT, van Erpecum KJ, Salmon D, Hoepelman AI, Asselah T, Ustianowski A. Natural history and treatment of HCV/HIV coinfection: Is it time to change paradigms? J Hepatol. 2015 Nov;63(5):1254–1262. doi: 10.1016/j.jhep.2015.06.034. [DOI] [PubMed] [Google Scholar]
- 74.Neukam K, Suàrez-Santamaria M, Rivero-Juárez A, Romero-Palacios A, Ortega E, de los Santos-Gil I, Cucurull J, Vera-Méndez F, Pineda JA. HIV coinfection impairs the response to DAA-based HCV therapy. J Hepatol. 2016;64(2):S219. doi: 10.1016/S0168-8278(16)00190-2. [DOI] [Google Scholar]
- 75.Rockstroh J, Lutz T, Mauss S, Cordes C, Hillenbrand H, Moll A, Pfeiffer-Vornkahl H, Cornberg M, Manns MP, Baumgarten A German Hepatitis C-Registry. SVR12 rates under DAA-based HCV therapy from the National German Cohort Study: Does HIV co-infection impair the response to DAA combination therapy?. AASLD The Liver Meeting® 2016; 2016 Nov 11-15; Boston, MA. [Google Scholar]
- 76.Tyson GL. Prevalence and determinants of hepatitis B co-infection among a United States Veterans Administration cohort with hepatitis C. Ann Arbor: The University of Texas School of Public Health, ProQuest Dissertations Publishing; 2012. [Google Scholar]
- 77.Bersoff-Matcha SJ, Cao K, Jason M, Ajao A, Jones SC, Meyer T, Brinker A. Hepatitis B Virus Reactivation Associated With Direct-Acting Antiviral Therapy for Chronic Hepatitis C Virus: A Review of Cases Reported to the U.S. Food and Drug Administration Adverse Event Reporting System. Ann Intern Med. 2017 Jun;166(11):792–797. doi: 10.7326/M17-0377. [DOI] [PubMed] [Google Scholar]
- 78.Chen G, Wang C, Chen J, Ji D, Wang Y, Wu V, Karlberg J, Lau G. Hepatitis B reactivation in hepatitis B and C coinfected patients treated with antiviral agents: A systematic review and meta-analysis. Hepatology. 2017 Jul;66(1):13–26. doi: 10.1002/hep.29109. [DOI] [PubMed] [Google Scholar]
- 79.Liu CJ, Chuang WL, Sheen IS, Wang HY, Chen CY, Tseng KC, Chang TT, Massetto B, Yang J, Camus G, Zhang F, Brainard DM, McHutchison JG, Hu TH, Hsu YC, Lo GH, Chu CJ, Chen JJ, Peng Cy, Chein RN, Chen PJ. Ledipasvir/Sofosbuvir for 12 Weeks Is Safe and Effective in Patients with Chronic Hepatitis C and Hepatitis B Coinfection: A Phase 3 Study in Taiwan. J Hepatol. 2017;66(1):S56–S57. doi: 10.1016/S0168-8278(17)30374-4. [DOI] [Google Scholar]
- 80.Belperio PS, Shahoumian TA, Mole LA, Backus LI. Evaluation of hepatitis B reactivation among 62,920 veterans treated with oral hepatitis C antivirals. Hepatology. 2017 Jul;66(1):27–36. doi: 10.1002/hep.29135. [DOI] [PubMed] [Google Scholar]
- 81.European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017 Aug;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 82.Kattakuzhy S, Levy R, Rosenthal E, Tang L, Wilson E, Kottilil S. Hepatitis C genotype 3 disease. Hepatol Int. 2016 Nov;10(6):861–870. doi: 10.1007/s12072-016-9748-z. [DOI] [PubMed] [Google Scholar]
- 83.Soumana DI, Kurt Yilmaz N, Ali A, Prachanronarong KL, Schiffer CA. Molecular and Dynamic Mechanism Underlying Drug Resistance in Genotype 3 Hepatitis C NS3/4A Protease. J Am Chem Soc. 2016 Sep;138(36):11850–11859. doi: 10.1021/jacs.6b06454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cohn J, Roberts T, Amorosa V, Lemoine M, Hill A. Simplified diagnostic monitoring for hepatitis C, in the new era of direct-acting antiviral treatment. Curr Opin HIV AIDS. 2015 Sep;10(5):369–373. doi: 10.1097/COH.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 85.Kim JL, Morgenstern KA, Lin C, Fox T, Dwyer MD, Landro JA, Chambers SP, Markland W, Lepre CA, O'Malley ET, Harbeson SL, Rice CM, Murcko MA, Caron PR, Thomson JA. Crystal structure of the hepatitis C virus NS3 protease domain complexed with a synthetic NS4A cofactor peptide. Cell. 1996 Oct 18;87(2):343–355. doi: 10.1016/S0092-8674(00)81351-3. [DOI] [PubMed] [Google Scholar]
- 86.Bartels DJ, Sullivan JC, Zhang EZ, Tigges AM, Dorrian JL, De Meyer S, Takemoto D, Dondero E, Kwong AD, Picchio G, Kieffer TL. Hepatitis C virus variants with decreased sensitivity to direct-acting antivirals (DAAs) were rarely observed in DAA-naive patients prior to treatment. J Virol. 2013 Feb;87(3):1544–1553. doi: 10.1128/JVI.02294-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuntzen T, Timm J, Berical A, Lennon N, Berlin AM, Young SK, Lee B, Heckerman D, Carlson J, Reyor LL, Kleyman M, McMahon CM, Birch C, Schulze Zur Wiesch J, Ledlie T, Koehrsen M, Kodira C, Roberts AD, Lauer GM, Rosen HR, Bihl F, Cerny A, Spengler U, Liu Z, Kim AY, Xing Y, Schneidewind A, Madey MA, Fleckenstein JF, Park VM, Galagan JE, Nusbaum C, Walker BD, Lake-Bakaar GV, Daar ES, Jacobson IM, Gomperts ED, Edlin BR, Donfield SM, Chung RT, Talal AH, Marion T, Birren BW, Henn MR, Allen TM. Naturally occurring dominant resistance mutations to hepatitis C virus protease and polymerase inhibitors in treatment-naïve patients. Hepatology. 2008 Dec;48(6):1769–1778. doi: 10.1002/hep.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vallet S, Viron F, Henquell C, Le Guillou-Guillemette H, Lagathu G, Abravanel F, Trimoulet P, Soussan P, Schvoerer E, Rosenberg A, Gouriou S, Colson P, Izopet J, Payan C ANRS AC11 HCV Group. NS3 protease polymorphism and natural resistance to protease inhibitors in French patients infected with HCV genotypes 1-5. Antivir Ther (Lond) 2011;16(7):1093–1102. doi: 10.3851/IMP1900. [DOI] [PubMed] [Google Scholar]
- 89.Gaudieri S, Rauch A, Pfafferott K, Barnes E, Cheng W, McCaughan G, Shackel N, Jeffrey GP, Mollison L, Baker R, Furrer H, Günthard HF, Freitas E, Humphreys I, Klenerman P, Mallal S, James I, Roberts S, Nolan D, Lucas M. Hepatitis C virus drug resistance and immune-driven adaptations: relevance to new antiviral therapy. Hepatology. 2009 Apr;49(4):1069–1082. doi: 10.1002/hep.22773. [DOI] [PubMed] [Google Scholar]
- 90.Dietz J, Susser S, Berkowski C, Perner D, Zeuzem S, Sarrazin C. Consideration of Viral Resistance for Optimization of Direct Antiviral Therapy of Hepatitis C Virus Genotype 1-Infected Patients. PLoS ONE. 2015;10(8):e0134395. doi: 10.1371/journal.pone.0134395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lenz O, Verbinnen T, Fevery B, Tambuyzer L, Vijgen L, Peeters M, Buelens A, Ceulemans H, Beumont M, Picchio G, De Meyer S. Virology analyses of HCV isolates from genotype 1-infected patients treated with simeprevir plus peginterferon/ribavirin in Phase IIb/III studies. J Hepatol. 2015 May;62(5):1008–1014. doi: 10.1016/j.jhep.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 92.Palanisamy N, Danielsson A, Kokkula C, Yin H, Bondeson K, Wesslén L, Duberg AS, Lennerstrand J. Implications of baseline polymorphisms for potential resistance to NS3 protease inhibitors in Hepatitis C virus genotypes 1a, 2b and 3a. Antiviral Res. 2013 Jul;99(1):12–17. doi: 10.1016/j.antiviral.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 93.Howe JA, Long J, Black S, Chase R, McMonagle P, Curry S, Thompson S, DiNubile MJ, Howe AY. Clinical Implications of Detectable Baseline Hepatitis C Virus-Genotype 1 NS3/4A-Protease Variants on the Efficacy of Boceprevir Combined With Peginterferon/Ribavirin. Open Forum Infect Dis. 2014 Sep;1(2):ofu078. doi: 10.1093/ofid/ofu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hézode C, Hirschfield GM, Ghesquiere W, Sievert W, Rodriguez-Torres M, Shafran SD, Thuluvath PJ, Tatum HA, Waked I, Esmat G, Lawitz EJ, Rustgi VK, Pol S, Weis N, Pockros PJ, Bourlière M, Serfaty L, Vierling JM, Fried MW, Weiland O, Brunetto MR, Everson GT, Zeuzem S, Kwo PY, Sulkowski M, Bräu N, Hernandez D, McPhee F, Wind-Rotolo M, Liu Z, Noviello S, Hughes EA, Yin PD, Schnittman S. Daclatasvir plus peginterferon alfa and ribavirin for treatment-naive chronic hepatitis C genotype 1 or 4 infection: a randomised study. Gut. 2015 Jun;64(6):948–956. doi: 10.1136/gutjnl-2014-307498. [DOI] [PubMed] [Google Scholar]
- 95.Dore GJ, Lawitz E, Hézode C, Shafran SD, Ramji A, Tatum HA, Taliani G, Tran A, Brunetto MR, Zaltron S, Strasser SI, Weis N, Ghesquiere W, Lee SS, Larrey D, Pol S, Harley H, George J, Fung SK, de Lédinghen V, Hagens P, McPhee F, Hernandez D, Cohen D, Cooney E, Noviello S, Hughes EA. Daclatasvir plus peginterferon and ribavirin is noninferior to peginterferon and ribavirin alone, and reduces the duration of treatment for HCV genotype 2 or 3 infection. Gastroenterology. 2015 Feb;148(2):355–366. doi: 10.1053/j.gastro.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 96.Holmes JA, Thompson AJ. Interferon-free combination therapies for the treatment of hepatitis C: current insights. Hepat Med. 2015;7:51–70. doi: 10.2147/HMER.S55864. [DOI] [PMC free article] [PubMed] [Google Scholar]