Abstract
Esophageal Squamous Cell Carcinoma (ESCC) belongs to malignant tumor of human digestive system. It has greatly threatened human health both mentally and physically. Long non-coding RNAs have been discovered to be special molecular regulators in various cancers, including ESCC. LncRNA DUXAP10 is a newfound RNA, which is able to improve the progression of cancers 1–3. In this study, DUXAP10 was certified to be upregulated in ESCC tissues and cells. Besides, it was positively correlated with short survival time. Moreover, down-expression of DUXAP10 contributed to decreased cell proliferation and metastasis. Silenced DUXAP10 led to increased apoptosis rate and stagnation of cell cycle. Results of mechanism experiments suggested that DUXAP10 motivated ESCC progression through recruiting enhancer of zeste homolog 2 (EZH2) to the promoter of p21. Our findings suggested that the pseudogene-derived from lncRNA DUXAP10 drove the biological progression of ESCC. DUXAP10 was likely to be a potential therapeutic target for ESCC.
Key words: DUXAP10, p21, EZH2, proliferation, ESCC
Introduction
Esophageal cancer has become a common malignant tumor in this world. Moreover, it is a vital cause in cancer-related death 4. Unfortunately, the incidence of esophageal carcinoma is unceasingly increasing. Esophageal squamous cell carcinoma (ESCC) is considered to be the predominant histological subtype of esophageal cancer 5, 6. Asia, especially China is an area with quite high incidence of ESCC 7. The poor diagnosis of advanced stages resulted in the low survival rate within five years. The survival rate of advanced patients is less than 20% 8–10. Therefore, it is very urgent to improve treatment method of ESCC. More and more novel molecular markers for prognostic analysis and treatment emerged.
Long non-coding RNAs (lncRNAs) are commonly defined as a class of evolutionarily conserved RNA molecules with length more than 200 nucleotides. They are almost unable to code protein 11. Increasing evidences have been provided to prove the effect of lncRNAs on biological processes of cancers, such as tumorigenesis and development 12, 13. Moreover, dysregulation of lncRNAs is a key factor in initiation and progression of various tumors, including ESCC 14–16. Pseudogenes are always regarded as genomic loci that are similar with their parental genes. Moreover, they are nicknamed as ‘junk genes’ or ‘genomic fossil’ for their special regulatory model 17, 18. It is interested that a lot of pseudogenes can transcribe into lncRNAs 19. It has been certified that pseudogenes can exert functions at multiple levels (DNA, RNA or protein) in various biological processes 20, 21. Both DUXAP8 and RSU1P2 are pseudogenes, they have been reported to be regulators in cancers 22, 23. All the above examples validly proved the important role of pseudogenes in cancers. DUXAP10 is a pseudogene which derived from long non-coding RNA (lncRNA). LncRNA DUXAP10 have been proved to be greatly functional in bladder cancer 24 and non-small cell lung cancer 25. However, it is not been investigated in OSCC. In this study, we are dedicated in detecting the effect of DUXAP10 on OSCC progression. The result of our study obviously revealed a fact that DUXAP10 promoted tumorigenesis of OSCC though epigenetically silencing p21 expression.
Materials and methods
Tissue collection and ethics statement
ESCC specimens (totally 96 pairs) analyzed in this study are collected from those patients who underwent the primary resection surgery at Department of Thoracic Surgery, Jiangsu Cancer Hospital. All samples were snap frozen in liquid nitrogen and stored at −80 °C as soon as they were collected. This study had obtained the approval of the Research Ethics Committee of Jiangsu Cancer Hospital (Nanjing, Jiangsu, PR China). All patients had signed informed consent before this study. We performed all experiments with absolute obedience of relevant guidelines and rules.
Cell culture and transfection
All cells (KYSE30, KYSE510, KYSE180, KYSE150 and NE1) used in this study were bought from the American Type Culture Collection (Manassas, VA). All cell lines were preserved in RPMI 1640 Medium (Invitrogen, Shanghai, China) and supplemented with a complex (10% FBS+100 U/ml penicillin+100 mg/ml streptomycin) (Invitrogen) at a 37 °C with 5% CO2. Cells were typically seeded on six-well plates. The next day, they were transfected with specific shRNA or control shRNA using Lipofectamine 2000 (Invitrogen) in accordance with the instruction. After transfection, cells were harvested for next experiments.
Total RNA isolation and qPCR assays
Total RNA was extracted from cultured cells with TRIzol reagent (Invitrogen, Carlsbad, CA). Both quantity and quality of RNA were measured by using NanoDrop2000c (Thermo Scientific, Waltham, MA, USA). For qPCR, 5 μg of RNA was reversely transcribed to cDNA with a Reverse Transcription Kit (Takara, Dalian, China) on ABI 7500. The mRNA expression was quantified by utilizing the SYBR® Premix Ex Taq™ (Takara, Dalian, China) on the Roche LightCycler 480 Real-Time PCR System (Applied Biosystems). The expression levels of mRNAs were relatively calculated using the 2−ΔΔCt method. GAPDH was chosen as the internal control.
Cell viability and colony formation assay
Reagent Kit I (MTT; Roche Applied Science) was used for detecting cell viability. KYSE30 and KYSE180 cells which had been transfected with shRNA or sh-NC were cultured in 96-well plates at a density of 3000 cells per well. Next, cell viability was assessed in accordance with the user guide. To perform colony formation assay, 500 cells were put into a six-well plate and maintained in a media containing 10% FBS. We replaced the medium every 4 days. Two weeks later, we used methanol to fix cells and 0.1% crystal violet (Sigma, Aldrich) to stain. At last, The visible colonies were manually counted. Each treatment group were measured in triplicate.
Flow cytometry
KYSE30 and KYSE180 cells which had been treated with shRNA or sh-NC were harvested after 48 h. Subsequently, based on the user guide, cells were stained with PI with the help of the CycleTESTTM PLUS DNA Reagent Kit (BD Biosciences). The result was analyzed with a flow cytometer (FACScan®; BD Biosciences). Finally, the percentages of the cells in different phases (G0-G1, S, and G2-M) were counted for comparison.
For apoptosis analysis, we treated ESCC cells with fluorescein isothiocyanate (FITC), Annexin V and propidium iodide (PI) avoid light at normal temperature in accordance with the recommendations of manufacturer. Subsequently, apoptotic condition of cells was analyzed by FACScan®
Western blot assay
ESCC cells were harvested after the required transfection. proteins were extracted from transfected cells and quantified using 12% polyacrylamide gradient SDS gel. Antibodies (Anti-GAPDH, anti-p21 and anti-EZH2) were all bought from Abcam (Hong Kong, China). ECL chromogenic substrate was used to were quantified by densitometry (Quantity One software; Bio-Rad). GAPDH was considered as the internal control.
Subcellular fractionation
To separate nuclear and cytosolic fractions of DUXAP10, PARIS Kit (Life Technologies) was put into use in accordance with the user guide. RNAs that were isolated from each fractions were then measured by qRT-PCR analysis.
RNA immunoprecipitation (RIP)
In order to investigate whether DUXAP10 can interact with EZH2, RNA immunoprecipitation was conducted in ESCC cells with the EZMagna RIP kit (Millipore, Billerica, MA, USA). Cells were completely lysed in RIP lysis buffer, and followed by incubation of magnetic beads conjugated with antibodies (EZH2 or control IgG) for 6 h at 4 °C. Then, washed again, and incubated beads with Proteinase K so that proteins could be removed. Finally, purified RNA was analyzed by qPCR.
Chromatin immunoprecipitation (ChIP)
ESCC cells were treated with formaldehyde followed by incubation for 10 minutes so that DNA-protein cross-links could be generated. Then, the cell lysates were sonicated so that the chromatin fragments could be generated. Next, they were immunoprecipitated with H3K4me2 and EZH2 (IgG was regarded as control). Finally, we recovered precipitated chromatin DNA and analyzed them with qPCR.
Statistically analysis
Statistical analyses in this study were performed using SPSS 22.0 (SPSS, Chicago, IL, USA). Differences between groups was assessed by Student's t-test and one-way ANOVA, The OS rate in different group (DUXAP10 high or low) was analyzed by using Kaplan–Meier method with the log-rank test. The interaction between clinical elements of ESCC patients and DUXAP10 expression was analyzed by generating Cox proportional hazards regression model. All data are represented as means ± SD. Data were statistically significant when P value less than 0.05.
Results
Overexpression of DUXAP10 is a significant prognostic factor in ESCC
To clearly understand the role of DUXAP10 in ESCC, we firstly measured the expression level of DUXAP10 in both ESCC tissues and the matched normal tissues. Unsurprisingly, it was strongly expressed in ESCC tissues (Figure 1A). Similarly, the expression of DUXAP10 was detected in four ESCC cells (KYSE30, KYSE510, KYSE180 and KYSE150) and one normal cell (NE1). It was discovered that DUXAP10 was expressed much stronger in ESCC cells than that in NE1 cell (Figure 1B). Next, we used the mean value of DUXAP10 expression as the cutoff value to divide samples into two groups. Based on above, Kaplan Meier analysis was utilized to analyze the correlation between survival time of ESCC patients and DUXAP10 expression. Obviously, the result indicated that the high expression group had the shorter survival time (Figure 1C). Next, we analyzed correlation between the DUXAP10 expression and clinical characteristics of ESCC patients. It was found that the level of DUXAP10 strongly related with TNM stage and Lymph Node Metastasis, but not with age or gender. (Table 1). According to the results of proportional hazards method analysis, we came to a conclusion that high level of DUXAP10 was an important prognostic factor in ESCC (P = 0.003, Table 2).
Figure 1.
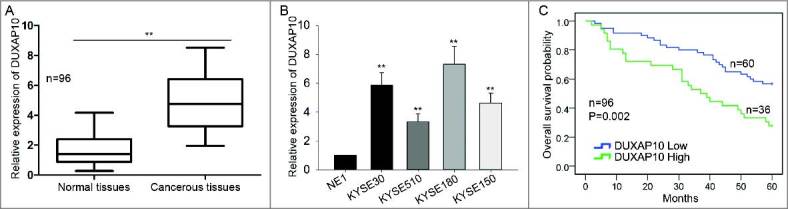
Overexpression of DUXAP10 is a significant prognostic factor in ESCC. A. The expression levels of DUXAP10 in ESCC tissues and in the normal tissues were tested with qRT-PCR. B. The expression of DUXAP10 was detected in ESCC cells (KYSE30, KYSE510, KYSE180 and KYSE150) and one normal cell (NE1). C. Kaplan Meier analysis was utilized to analyze the correlation between survival time of ESCC patients and DUXAP10 expression. Error bars represent the mean ± SD of at least three independent experiments. **P< 0.01 vs. control group.
Table 1.
Correlation between DUXAP10 expression and clinical features of ESCC patients. (n = 96)
| Variable | DUXAP10 Expression | P-value | |
| low | high | ||
| Histological Grade | |||
| Poor | 59 | 33 | 0.114 |
| Moderate-well | 1 | 3 | |
| TNM stage | |||
| I-II | 30 | 5 | <0.001** |
| III-IV | 30 | 31 | |
| Tumor Location | |||
| Upper | 39 | 29 | 0.105 |
| Middle+Lower | 21 | 7 | |
| Lymph Node Metastasis | |||
| Absent | 34 | 10 | |
| Present | 26 | 26 | 0.006** |
| Age | |||
| <60 | 36 | 24 | |
| ≥60 | 24 | 12 | 0.514 |
| Gender | |||
| Male | 15 | 10 | |
| Female | 45 | 26 | 0.764 |
Low/high by the sample mean. Pearson χ2 test. P<0.05 was considered statistically significant.
Table 2.
Multivariate analysis of prognostic parameters in ESCC patients by Cox regression analysis
| Variable | Category | P-value |
| DUXAP10 | low | 0.003** |
| high | ||
| Gender | Male | 0.392 |
| Female | ||
| Age | <60 | 0.449 |
| ≥60 | ||
| Lymph Node Metastasis | Absent | 0.007** |
| Present | ||
| Tumor Location | Upper | 0.395 |
| Middle+Lower | ||
| TNM stage | I-II | 0.002** |
| III-IV | ||
| Histological Grade | Poor | 0.717 |
| Moderate-well |
Proportional hazards method analysis showed a positive, independent prognostic importance of DUXAP10 expression (P = 0.003**), in addition to the independent prognostic impact of TNM stage (P = 0.002**) and Lymph Node Metastasis (P = 0.007**). P < 0.05 was considered statistically significant.
Knockdown of DUXAP10 negatively influences ESCC cell proliferation
According to Figure 1, we hypothesized that DUXAP10 was a tumor facilitator in ESCC. To validate this hypothesis, we interfered the expression of DUXAP10 in KYSE30 and KYSE180 cells with shRNAs (sh-DUXAP10#1, sh-DUXAP10#2, sh-DUXAP10#3 and sh-DUXAP10#4) for next functional assays. The optimal transfection efficiency was observed after two days (Figure 2A). sh-DUXAP10#1 and sh-DUXAP10#4 were selected for next experiments for their highest transfection efficiency. Then, we tried to investigate the effects of silenced DUXAP10 on ESCC cell proliferation. Results of MTT and colony formation assay manifested that the cell proliferation was significantly after DUXAP10 was down-expressed in KYSE30 and KYSE180 cells (Figure 2B-C).
Figure 2.
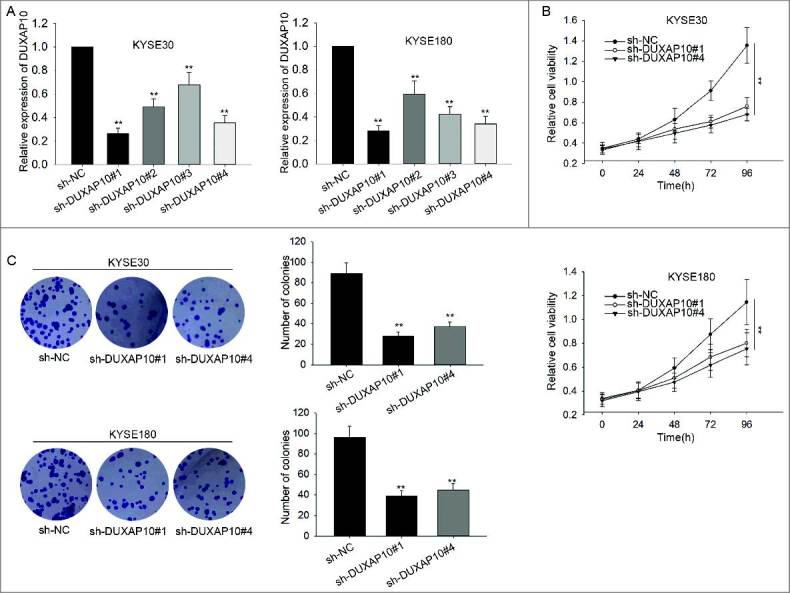
Knockdown of DUXAP10 negatively influences ESCC cell proliferation. A. The expression of DUXAP10 was interfered in KYSE30 and KYSE180 with shRNAs. The lowest expression was observed after transfection for about two days. B-C. Results of MTT and colony formation assay showed the decreased cell proliferation after DUXAP10 was down-expressed in KYSE30 and KYSE180 cells. Error bars represent the mean ± SD of at least three independent experiments. **P< 0.01 vs. control group.
The influence of silenced DUXAP10 on cell cycle distribution and cell apoptosis
To further explore the effects of DUXAO10 on cell cycle and apoptosis, flow cytometry analysis was conducted in two DUXAP10-downregulated ESCC cells. Not surprisingly, cell cycle was arrested at G0/G1 phase (Figure 3A). Additionally, cell apoptosis was accelerated (Figure 3B). All in all, silenced DUXAP10 led to cell cycle arrest and increased apoptosis of ESCC cells.
Figure 3.
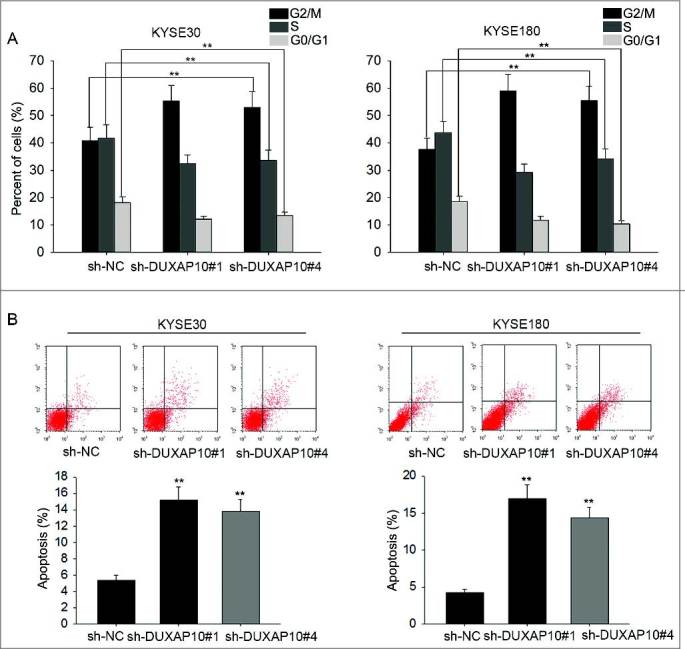
The influence of silenced DUXAP10 on cell cycle distribution and cell apoptosis. A. Cell cycle was stagnated at G0/G1 phase in DUXAP10 downregulated ESCC cells. B. cell apoptosis was accelerated in ESCC cells in which DUXAP10 was downregulated. Error bars represent the mean ± SD of at least three independent experiments. **P< 0.01 vs. control group.
DUXAP10 epigenetically silences p21 expression by binding with EZH2
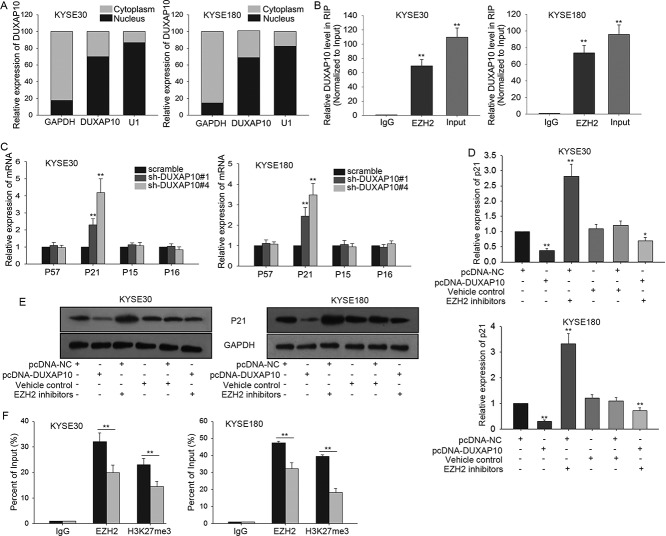
To clearly detect molecular mechanisms by which DUXAP10 promoted the formation of ESCC phenotype, fractionation assays were utilized to explore the specific localization of DUXAP10 in ESCC cells. Obviously, DUXAP10 was expressed much higher in nucleus rather than that in cytoplasm (Figure 4A), which suggested that DUXAP10 might regulate ESCC cell activities at transcription levels. EZH2 had been reported to bind with lncRNAs to modulate p21 in cancers 26, 27. We predicted that EZH2 was able to bind with DUXAP10 on internet (http://pridb.gdcb.iastate.edu/RPISeq/) (RF or SVM score > 0.5). Result of RIP assay suggested that DUXAP10 could interact with EZH2 in ESCC cells (Figure 4B). As we all know, some cell cycle-related proteins could largely inhibit tumor progression. In this study, we chose several of them (they are all the target of EZH2) to do loss of function detection. After DUXAP10 was downregulated, all those proteins were upregulated, especially p21 (Figure 4C). So p21 was chosen to do further study. In order to detect the relevance among DUXAP10, EZH2 and p21, rescue assay was adopted to explore the change on mRNA level and protein level of p21 in response to DUXAP10 upregulation and EZH2 downregulation. As a result, both mRNA level and protein level of p21 were largely reduced by transfection of pcDNA-DUXAP10 and were recovered again by adding EZH2 inhibitors (Figure 4D-E). To figure out whether DUXAP10 modulated p21 expression via interacting with EZH2, ChIP analysis was carried out in ESCC cells. Obviously, the results showed that EZH2 could directly bind to the promoter region of p21 and reconciled H3K4me2 with demethylation. Nevertheless, silenced DUXAP10 weakened the ability to bind and to modify H3K4me2 demethylation (Figure 4F).
Figure 4.
DUXAP10 epigenetically silences p21 expression by binding with EZH2. A. Fractionation assays were utilized to examine the localization of DUXAP10 in ESCC cells. B. RIP assay was conducted to demonstrate the combination between DUXAP10 and EZH2 in ESCC cells. C. P21 was mostly upregulated after DUXAP10 was silenced in ESCC cells. D-E. We could observe that the mRNA level and protein level of p21 was largely reduced by transfection of pcDNA-DUXAP10 while recovered again by adding EZH2 inhibitors. F. ChIP analysis showed us interference of DUXAP10 expression weakened the ability to bind and to modify H3K4me2 demethylation. Error bars represent the mean ± SD of at least three independent experiments. **P< 0.01 vs. control group.
Modulation of DUXAP10-EZH2-p21 axis affects cell proliferation, cell cycle and cell apoptosis
To further investigate the effect of DUXAP10-EZH2-p21 axis on ESCC cell activities. The rescue assays were conducted in ESCC cells in which sh-p21 had been transfected. Firstly, MTT assay was performed in p21-downregulated KYSE30 cell, the result manifested that the increased cell proliferation largely weakened by adding EZH2 inhibitors, while recovered again with EZH2 inhibitors+pcDNA-DUXAP10 (Figure 5A). Next, flow cytometry analysis was applied to assess the change on cell cycle and cell apoptosis in KYSE30/sh-p21 cell. The motivated cell cycle was stagnated by EZH2 inhibitors, while was motivated again by transfection of EZH2 inhibitors+pcDNA-DUXAP10 (Figure 5B). Moreover, decreased apoptosis was induced by adding EZH2 inhibitors while was receded again by transfection of EZH2 inhibitors+pcDNA-DUXAP10 (Figure 5C).
Figure 5.
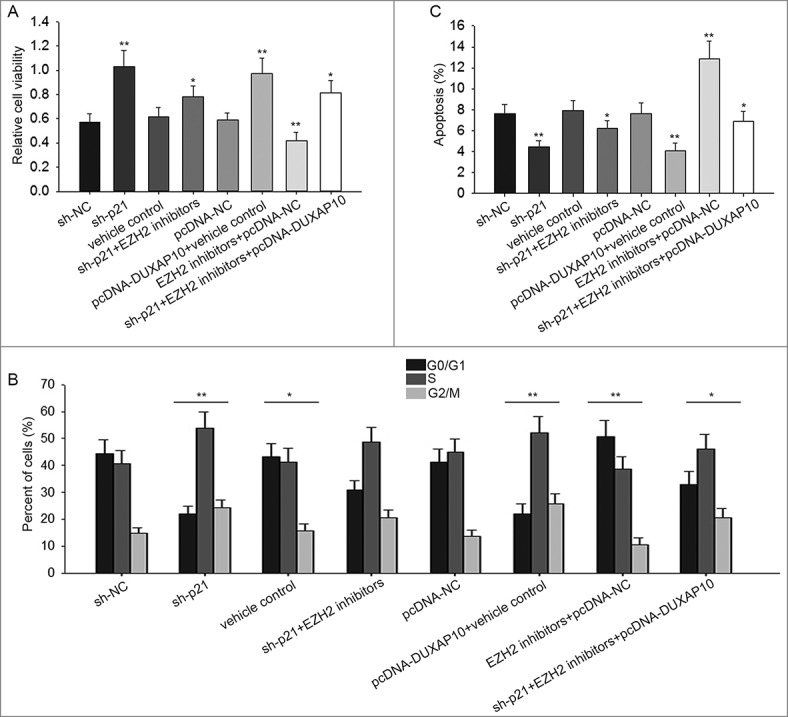
Modulation of DUXAP10-EZH2-p21 axis affects cell proliferation, cell cycle and cell apoptosis. A. Result of MTT assay in p21 downregulated KYSE30 cell manifested the increased cell proliferation largely reduced by adding EZH2 inhibitors, while recovered again with EZH2 inhibitors+pcDNA-DUXAP10. B. Results of flow cytometry analysis made us realize that the accelerated cell cycle was stagnated by EZH2 inhibitors, while was motivated again by transfection of EZH2 inhibitors+pcDNA-DUXAP10. C. Decreased apoptosis was promoted by adding EZH2 inhibitors while was suppressed again by transfection of EZH2 inhibitors+pcDNA-DUXAP10. Error bars represent the mean ± SD of at least three independent experiments. **P< 0.01 vs. control group.
Discussion
According to the previous report, we knew that pseudogene was originally interpreted by Jacq and colleagues in 1977 28. Pseudogenes were originally called as junk DNA for they were unable to code protein 29, 30. More and more evidences were provided to reveal a fact that a lot of pseudogene transcripts were part of lncRNAs family and involved in various biological processes of cancer 31, 32. Nevertheless, pseudogenes were still not fully elucidated. Previous studies disclosed that DUXAP10 was up-regulated in NSCLC and could accelerate cell activities in cancer. There were two direct target of DUXAP10 (RRAD and LATS2) found in NSCLC 25. Based on all above, we knew the importance of DUXAP10 in cancer. This study focused on the specific role of DUXAP10 in ESCC.
Many evidences suggested that lncRNAs (including pseudogene RNAs) are able to modulate expression of their target genes through binding with RNA-binding proteins or competitively binding with common miRNAs 33–36. For example, lncRNA HOXA11-AS contributed to proliferation and invasion of gastric cancer via scaffolding PRC2, LSD1 and DNMT1 37. Additionally, EZH2 has been proved to be a histone methyltransferase that could be responsible for H3K27me3 trimethylation of target genes 38. According to the previous reports, nearly 20% of human lncRNAs are identify to physically interact with EZH2 39, 40. Therefore, EZH2 is thought to be a significant gene which participates in tumorigenesis of human cancers. In this study, DUXAP10 was found to bind with EZH2, thus to silence p21 expression. DUXAP10 was overexpressed in ESCC tissues and cells. And knockdown of DUXAP10 negatively modulated cell proliferation, induced cell cycle arrest and accelerate cell apoptosis of ESCC. Therefore, the oncogenic role of DUXAP10 was initially found. Next, we discovered that DUXAP10 was located in nucleus of ESCC cells. RIP assay were conducted to uncover the binding relation between DUXAP10 and ZEH2. To obtain further evidence, the expression levels of targets of EZH2 were also detected by qRT-PCR. p21 was found to be the most remarkable one, so it was chosen to do the next assay. p21 has been reported in many kinds of researches for its anti-oncogenic function. In order to prove the actual role of p21 in ESCC, rescue assays were performed. The results manifested that p21 could reverse the oncogenic function of DUXAP10. Oppositely, the anti-oncogenic function of p21 also could be reversed by DUXAP10. Therefore, interactions among p21, DUXAP10 and EZH2 in ESCC were clearly manifested. Our findings would be valuable for early diagnosis and treatment of ESCC. We thought our study was significant and moderately novel. We would do more experiments and researches in the future.
Conflict of interest
None.
Funding Statement
These study was supported by Jiangsu Provincial Special Program of Medical Science Funding (BE2017759).
Reference
- 1.Zhang Q, Wang WW, Xu TH, Xu ZF. Highly expressed long non-coding RNA DUXAP10 promotes proliferation of ovarian cancer. European review for medical and pharmacological sciences 2018; 22:314–21. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Yu X, Wei C, Nie F, Huang M, Sun M. Over-expression of oncigenic pesudogene DUXAP10 promotes cell proliferation and invasion by regulating LATS1 and beta-catenin in gastric cancer. Journal of experimental & clinical cancer research: CR 2018; 37:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lian Y, Xiao C, Yan C, Chen D, Huang Q, Fan Y, Li Z, Xu H. Knockdown of pseudogene derived from lncRNA DUXAP10 inhibits cell proliferation, migration, invasion, and promotes apoptosis in pancreatic cancer. Journal of cellular biochemistry 2018; 119:3671–82. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 5.Lam AK. Molecular biology of esophageal squamous cell carcinoma. Critical reviews in oncology/hematology 2000; 33:71–90. [DOI] [PubMed] [Google Scholar]
- 6.Wu CC, Chen CJ. Esophageal carcinoma. The New England journal of medicine 2015; 372:1472. [DOI] [PubMed] [Google Scholar]
- 7.Crew KD, Neugut AI. Epidemiology of upper gastrointestinal malignancies. Seminars in oncology 2004; 31:450–64. [DOI] [PubMed] [Google Scholar]
- 8.Isono K, Sato H, Nakayama K. Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology 1991; 48:411–20. [DOI] [PubMed] [Google Scholar]
- 9.Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. International journal of cancer 1999; 83:18–29. [DOI] [PubMed] [Google Scholar]
- 10.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis 2010; 31:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA biology 2013; 10:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huarte M, Rinn JL. Large non-coding RNAs: missing links in cancer? Human molecular genetics 2010; 19:R152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Molecular cancer 2011; 10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Wu Z, Mei Q, Li X, Guo M, Fu X, Han W. Long non-coding RNA HOTAIR, a driver of malignancy, predicts negative prognosis and exhibits oncogenic activity in oesophageal squamous cell carcinoma. Br J Cancer 2013; 109:2266–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li JY, Ma X, Zhang CB. Overexpression of long non-coding RNA UCA1 predicts a poor prognosis in patients with esophageal squamous cell carcinoma. International journal of clinical and experimental pathology 2014; 7:7938–44. [PMC free article] [PubMed] [Google Scholar]
- 16.Hao Y, Wu W, Shi F, Dalmolin RJ, Yan M, Tian F, Chen X, Chen G, Cao W. Prediction of long noncoding RNA functions with co-expression network in esophageal squamous cell carcinoma. BMC Cancer 2015; 15:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 2010; 465:1033–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Proudfoot N. Pseudogenes. Nature 1980; 286:840–1. [DOI] [PubMed] [Google Scholar]
- 19.Grander D, Johnsson P. Pseudogene-Expressed RNAs: Emerging Roles in Gene Regulation and Disease. Current topics in microbiology and immunology 2016; 394:111–26. [DOI] [PubMed] [Google Scholar]
- 20.Xiao-Jie L, Ai-Mei G, Li-Juan J, Jiang X. Pseudogene in cancer: real functions and promising signature. Journal of medical genetics 2015; 52:17–24. [DOI] [PubMed] [Google Scholar]
- 21.Poliseno L, Marranci A, Pandolfi PP. Pseudogenes in Human Cancer. Frontiers in medicine 2015; 2:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma HW, Xie M, Sun M, Chen TY, Jin RR, Ma TS, Chen QN, Zhang EB, He XZ, De W, et al. The pseudogene derived long noncoding RNA DUXAP8 promotes gastric cancer cell proliferation and migration via epigenetically silencing PLEKHO1 expression. Oncotarget 2017; 8:52211–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Guo X, Que S, Yang X, Fan H, Liu M, Li X, Tang H. LncRNA RSU1P2 contributes to tumorigenesis by acting as a ceRNA against let-7a in cervical cancer cells. Oncotarget 2017; 8:43768–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lv XY, Ma L, Chen JF, Yu R, Li Y, Yan ZJ, Cheng Y, Ma Q. Knockdown of DUXAP10 inhibits proliferation and promotes apoptosis in bladder cancer cells via PI3K/Akt/mTOR signaling pathway. International journal of oncology 2018; 52:288–94. [DOI] [PubMed] [Google Scholar]
- 25.Wei CC, Nie FQ, Jiang LL, Chen QN, Chen ZY, Chen X, Pan X, Liu ZL, Lu BB, Wang ZX. The pseudogene DUXAP10 promotes an aggressive phenotype through binding with LSD1 and repressing LATS2 and RRAD in non small cell lung cancer. Oncotarget 2017; 8:5233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang F, Peng H. LncRNA-ANCR regulates the cell growth of osteosarcoma by interacting with EZH2 and affecting the expression of p21 and p27. Journal of orthopaedic surgery and research 2017; 12:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Gao C, Yang Y, Li G, Dong J, Ai Y, Chen N, Li W. Long Noncoding RNA CRNDE/PRC2 Participated in the Radiotherapy Resistance of Human Lung Adenocarcinoma Through Targeting p21 Expression. Oncol Res 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacq C, Miller JR, Brownlee GG. A pseudogene structure in 5S DNA of Xenopus laevis. Cell 1977; 12:109–20. [DOI] [PubMed] [Google Scholar]
- 29.Graur D, Zheng Y, Azevedo RB. An evolutionary classification of genomic function. Genome biology and evolution 2015; 7:642–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balakirev ES, Ayala FJ. Pseudogenes: are they “junk” or functional DNA? Annual review of genetics 2003; 37:123–51. [DOI] [PubMed] [Google Scholar]
- 31.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nature reviews Genetics 2009; 10:155–9. [DOI] [PubMed] [Google Scholar]
- 32.Groen JN, Capraro D, Morris KV. The emerging role of pseudogene expressed non-coding RNAs in cellular functions. The international journal of biochemistry & cell biology 2014; 54:350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q, Hu X, Zhang X, Dai L, Duan X, Zhou C, Ao Y. The TMSB4 Pseudogene LncRNA Functions as a Competing Endogenous RNA to Promote Cartilage Degradation in Human Osteoarthritis. Mol Ther: the journal of the American Society of Gene Therapy 2016; 24:1726–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Zhu H, Wu X, Xie X, Huang G, Xu X, Li S, Xing C. Downregulated pseudogene CTNNAP1 promote tumor growth in human cancer by downregulating its cognate gene CTNNA1 expression. Oncotarget 2016; 7:55518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scarola M, Comisso E, Pascolo R, Chiaradia R, Marion RM, Schneider C, Blasco MA, Schoeftner S, Benetti R. Epigenetic silencing of Oct4 by a complex containing SUV39H1 and Oct4 pseudogene lncRNA. Nat Com 2015; 6:7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang MD, Chen WM, Qi FZ, Xia R, Sun M, Xu TP, Yin L, Zhang EB, De W Shu YQ. Long non-coding RNA ANRIL is upregulated in hepatocellular carcinoma and regulates cell proliferation by epigenetic silencing of KLF2. J Hematol Oncol 2015; 8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun M, Nie F, Wang Y, Zhang Z, Hou J, He D, Xie M, Xu L, De W Wang Z, et al. LncRNA HOXA11-AS Promotes Proliferation and Invasion of Gastric Cancer by Scaffolding the Chromatin Modification Factors PRC2, LSD1, and DNMT1. Cancer Res 2016; 76:6299–310. [DOI] [PubMed] [Google Scholar]
- 38.Chang CJ, Hung MC. The role of EZH2 in tumour progression. British journal of cancer 2012; 106:243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proceedings of the National Academy of Sciences of the United States of America 2009; 106:11667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun NX, Ye C, Zhao Q, Zhang Q, Xu C, Wang SB, Jin ZJ, Sun SH, Wang F, Li W. Long noncoding RNA-EBIC promotes tumor cell invasion by binding to EZH2 and repressing E-cadherin in cervical cancer. PLoS One 2014; 9:e100340. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]