ABSTRACT
The treatment of intractable vasogenic brain edema (VBE) caused by tumor and irradiation is challenging. Traditional intervention strategy includes dehydration and glucocorticoids accompanied by obvious side effects and minor effects after long-term use. Novel treatment needs to be found urgently. Recently, vascular endothelial growth factor (VEGF)/VEGFR pathway has been revealed to be essential in VBE. Therefore, tyrosine kinase inhibitor (TKI) targeting VEGFR which blocks (VEGF)/VEGFR signal might be effective. However, such reports have seldom been described. Herein, we documented a heavily-treated breast cancer patient experienced progressive aphasia, limb activity disorder and intermittent convulsion 10 months after radiotherapy of brain metastasis (BM). Cranial MRI demonstrated large peritumoral brain edema (PTBE). High dose of steriods and dehydration had no improvement. Apatinib with a dose of 250mg daily was initiated and all her discomforts disappeared after 10 days of use. The brain MRI of taking apatinib 5 weeks demonstrated remarkable shrinkage of edema. Our case indicates that apatinib, a potent small-molecular TKI targeted VEGFR2 is promising for intractable VBE for satisfactory efficacy and safety. This case is of great value in its rarity and because it provides new insight into PTBE management in clinical practice.
Keywords: Apatinib, peritumoral brain edema, intractable, radiotherapy, VEGF, VEGFR2, TKI
Introduction
Brain metastases (BM) is a common neurological complication of malignancy.1 With the improvement of anti-tumor treatment and extended survival of cancer patients, the incidence of BM keeps increasing overtime. PTBE is frequently encountered in BM, which belongs to VBE by most researchers. PTBE causes or aggravates neurological dysfunction and intracranial hypertension. Steroids has been applied to the treatment of PTBE since 1960. However, glucocorticoid therapy has numerous adverse effects, usually in a dose-dependent manner. Dehydration such as mannitol is useful for relieving intracranial hypertension but also accompanied with “rebound phenomenon”. Increased blood-brain barrier permeability is a key factor in the development of PTBE.2 Radiotherapy, as an irreplaceable part in the treatment of BM, induces blood vessel damage and ultimately aggravates VBE. The secretion of VEGF by tumor cells plays a critical role in VBE and VEGF antibody bevacizumab gained certain anti-edema effect.2 In view of high cost of bevacizumab, other novel VEGF inhibitors should be urgently explored to solve this difficult situation.
Case presentation
A 49-year-old Chinese female was admitted in January, 2015 for painless mass in left breast. The subsequent biopsy demonstrated invasive breast carcinoma with immunohistochemical results of ER(-), PR(-), HER-2(3+), Ki67(+ 20%) and P53(+ 90%)(Figure1). Systemic evaluation showed bilateral lung metastases (0.1–0.9cm) and hepatic metastases (0.7–1.0cm) in addition with primary left breast cancer (33.9 × 18.3mm) and left axillary lymph node involvement (56.8 × 33.0mm). The patient refused molecular targeted therapy for economic reasons and underwent chemotherapy (six cycles of TAC: Pirarubicin, Cyclophosphamide, Paclitaxel and one cycle of T: Paclitaxel). The response was evaluated as partial remission (PR).
Figure 1.
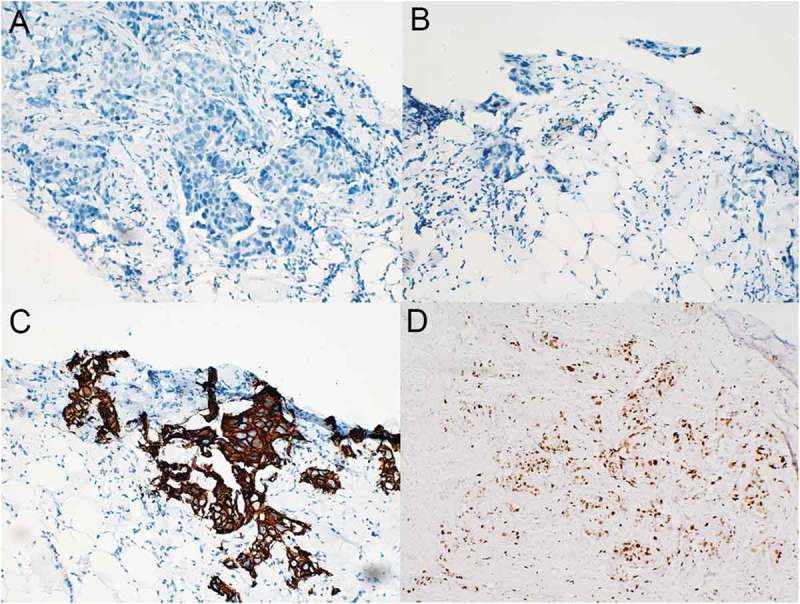
Immunohistochemical results of the primary invasive left breast carcinoma (original magnification, 200×). The tumor stained negative for both ER (A) and PR (B) but strongly positive for HER-2 (C) and exhibited a Ki-67 proliferation index of 20% (D) .
In September, 2015, her hepatic metastases progressed rapidly (1.0–1.9cm). Then she was enrolled in the clinical trial and randomly assigned to the control group (Lapatinib plus Capecitabine). In March, 2017, she complained of dizziness. The brain MRI with contrast revealed a lesion measuring about 3.1 × 2.7cm located in the left frontal lobe with circular enhancement and edema around, consistent with metastasis (Figure 2A,B). Local radiotherapy with a dose of 48Gy/16f was performed to the single BM and her discomfort disappeared. However, 3 months later, her disease progressed (lung metastases: 0.2–2.7cm, liver metastases: 1.2–4.3cm) and the BM shrank to 1.5 × 1.5cm. In August, the patient experienced recurrence in the left chest wall and further progression of liver metastases. Systemic therapy was switched to NP (Vinorelbine and Cisplatin) plus Herceptin. The lesions demontrated PR response to 4 cycles of NP plus Herceptin. Due to gastrointestinal side effects and grade 3 myelosuppression, the fifth and sixth chemotherapy regimen was adjusted to NC (Vinorelbine and Carboplatin) and N (Vinorelbine), respectively.
Figure 2.
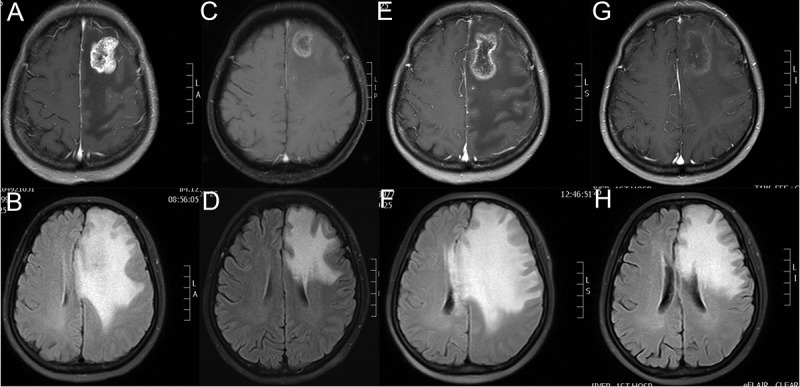
Brain MRIs of the patient revealed the size of BM lesion and PTBE. (A,C,E,G) The BM maximum diameter slice of T1-weighted MRI with contrast in 03/2017, 11/2017, 02/2018 and 03/2018 respectively. (B,D,F,H) The most obvious slice of PTBE in Flair-weighted MRI in 03/2017, 11/2017, 02/2018 and 03/2018 respectively. As Figure 1F shows, extensive PTBE caused progressive neurological deficits of the patient in 02/2018 which failed to be improved by high dose of steroids and dehydrants. As Figure 1H demonstrates, the PTBE shrank dramatically after use of apatinib 35 days.
In February, 2018, she complained of aphasia and intermittent convulsion. The brain MRI with contrast manifested a lesion in the left frontal lobe with obvious ring enhancement. Flair-weighted MRI showed large area of PTBE, resulting in compression and deformation of bilateral ventricle and midline structure shift to the right (Figure 2E,F). Comparing with the brain MRI in November, 2017 (Figure 2C,D), the BM demonstrated slight increase (2.5 × 2.0cm to 3.8 × 2.2cm) while PTBE increased significantly. Conventional intervention with dehydration (mannitol: 250ml, q8h, from 02/01/2018 to 02/07/2018; 250mg, q6h, from 02/08/2018 to 02/10/2018; glycerol fructose: 250ml, q12h, from 02/01/2018 to 02/10/2018) and glucocorticoid (dexamethasone: 20mg daily, from 02/08/2018 to 02/10/2018) was given. However, there was no improvement. The patient developed progressive aphasia and right-side limb activity disorder. Apatinib, with a dose of 250mg daily, was initiated. Surprisingly, after 3 days of use, the aphasia disappeared and she could communicate with others normally. After 10 days of use, her right-side limb activity recovered completely. The subsequent brain MRI (March, 16, 2018) revealed remarkable shrinkage of PTBE and no obvious change in the BM lesion (Figure 2G,H). Now, she takes apatinib 250mg daily and no common apatinib related side effects were observed.
Discussion
BM is frequently encountered in clinical practice. 8–10% of cancer patients suffered from symptomatic BM according to population-based data.3,4 Peritumoral edema, the most common concomitant of BM, adversely affects the life quality and survival of BM patients. PTBE can further aggravate the original space occupying effect of the tumor, induce or aggravate the corresponding symptoms of nerve loss when involving the functional areas of the brain, result in intracranial hypertension, and in severe cases, brain hernia. PTBE can affect tumor exposure and increase the difficulty of tumor resection. Mortality and disability rate of brain tumor patients with PTBE increased significantly.
Several molecular mechanisms have been reported to underlie the PTBE formation. BM, as like brain injury, primary brain tumor and inflammation, destroys the blood-brain barrier (BBB), which makes the plasma macromolecular substance permeate through the vascular lumen to the brain cell space. Therefore, it is considered that the damage of function and structure of BBB is the pathological basis of PTBE.2 Treatment for BM, radiotherapy particularly, inevitably cause vascular endothelial damage and lead to the BBB breakdown.5,6 Radiation causes swelling, exudation, degeneration, and exfoliation of vascular endothelial cells.
VEGF/VEGFR pathway has been revealed to be closely associated with the development of PTBE.7,8 Central nervous system neoplastic cells typically secretes multiple cytokines to act on the endothelial cells within or around the tumor.2 VEGF is one of the most important mediators of vascular permeability.9,10 VEGFR family has three members: VEGFR1, VEGFR2 and VEGFR3.11 VEGFR2 monitors the function of vascular endothelium.11 When VEGF ligand binds to the extracellular segment of VEGFR2, specific intracellular tyrosine residues phosphorylated. After binding of PLC-γ to pY1175, PIP2 hydrolyzes and generates the second messengers IP3 and diacylglycerol (DAG). DAG activates PKC whilst IP3 acts on receptors in the endoplasmic reticulum (E.R.), increasing the concentration of intracellular calcium. Activated PKC and increased intracellular calcium activate endothelial nitric oxide synthase (eNOS) then promote the generation of nitric oxide (NO). Increased intracellular calcium also boost the cytosolic phospholipase A (cPLA) and prostaglandin production. NO and prostaglandin cause vascular cell permeablility.11,12 Despite of tumor-derived VEGF, the local microenvironmental disorders due to tumor or radiotherapy, cause hypoxia which stimulates the astrocyte and vascular endothelial cell to produce VEGF.5 Furthermore, newly formed microvessels in the tumor are lack of tight connection. VEGF inhibits the function of tight junction associated protein claudin-1, which destroys the tight junction and leads to increased vascular permeability13 (Figure 3).
Figure 3.
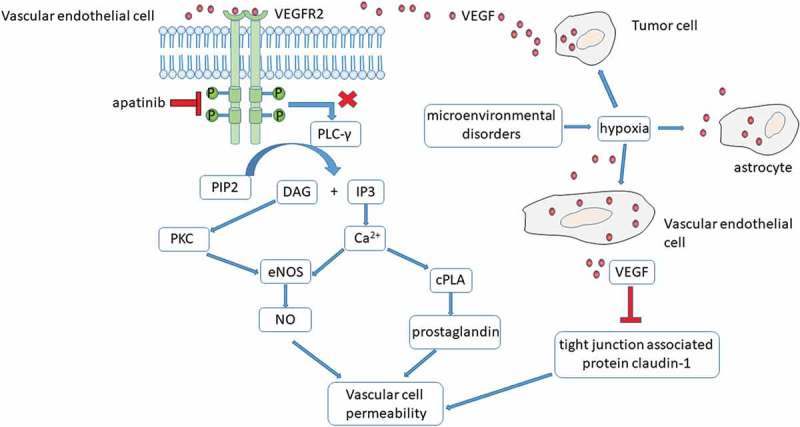
The mechanism of VEGF mediated increase in vascular permeability and aptinib treatment in the intractable PTBE.
Steroids are the mainstay of treatment in PTBE for the symptoms relief and cost-effectiveness. However, the obvious toxicity of steriods limit their unrestricted use. Hypertension, hyperglycemia and hypokalemia are common side effects of glucocorticoids use. Long-term intake might give rise to osteoporosis, necrosis of femoral head, peptic ulcer and active bleeding, suppressed immunity, all kinds of opportunistic infections and even fatal infection.2 Therefore, new agents with steroid-sparing effect is in great need to be explored, aiming to minimize the steroids’ administration when possible. The deep understanding of molecular determinants in the PTBE is critical for us to find out the alternatives which can replace or at least reduce the dose of steroid.2 Bevacizumab, a recombinant humanized monoclonal antibody targeted VEGF, can achieve a radiological relief and reduction of PTBE in glioblastoma.14 Bevacizumab, has been proved effective in radiation-induced brain necrosis.15,16 However, the high cost of Bevacizumab limit its wide application. Furthermore, the adverse effects of Bevacizumab need more attention in view of high rate of occurence.17
Apatinib, a small-molecular TKI against VEGFR2, potently blocks the VEGF/VEGFR2 signal transduction, and then reduce the permeability of blood vessels. Comparing with other TKI such as Sorafenib, Sunitinib or Pazopanib, apatinib showed a strong inhibitory effect of VEGFR2 with IC50 of 2nM.18 The IC50 of Sorafenib, Sunitinib or Pazopanib was 90, 10, 30, respectively.19–21 Apatinib has been approved for the third-line or later-phase treatment for recurrent or advanced gastric adenocarcinoma. However, its application in PTBE has never been documented. After extensive literature review in Pubmed, only one article documented the dramatic effect of apatinib in refractory radiation-induced brain edema.5 Our case focuses on the key mechanism that VEGF/VEGFR2 regulated PTBE and illustrates the potent role of apatinib in PTBE. Additionally, apatinib has a high cost- effectiveness and generally mild toxicity such as hypertension, proteinuria and hand foot syndrome which can be easily controlled by supportive management. Thus, apatinib is promising in the handling of PTBE and need to be further testified by large-scale clinical trial.
Conclusion
PTBE is common and hardly solved by conventional steroid and dehydration. Novel approach is in urgent need. VEGF/VEGFR2 pathway is fundamental in the regulation of vascular cell permeablility, which resulting in the development of PTBE. Apatinib, a potent and highly selective TKI targeting VEGFR2, is dramatically effective in PTBE demonstrated by our case. Apatinib is promising in the treatment of PTBE for satisfactory effect, toxicity and cost-effectivity. In the future, clinical trial will be launched to clarify apatinib in PTBE.
Funding Statement
This study was supported by research program from the National Natural Science Foundation of China (Grant No. 81602659) and Science and Technology Department of Jilin Province (Grant No.20160101028JC) Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal; Science and Technology Department of Jilin Province [20160101028JC]; National Natural Science Foundation of China (NSFC) [81602659].
References
- 1.Soffietti R, Abacioglu U, Baumert B, Combs SE, Kinhult S, Kros JM, Marosi C, Metellus P, Radbruch A, Villa Freixa SS, et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol. 2017. April 10;19(2):162–174. Epub PubMed PMID: 28391295; PubMed Central PMCID: PMCPMC5620494. doi: 10.1093/neuonc/now241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth P, Regli L, Tonder M, Weller M.. Tumor-associated edema in brain cancer patients: pathogenesis and management. Expert Rev Anticancer Ther. 2013. October 25;13(11):1319–1325. Epub PubMed PMID: 24152171. doi: 10.1586/14737140.2013.852473. [DOI] [PubMed] [Google Scholar]
- 3.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan detroit cancer surveillance system. J Clin Oncol. 2004. July 16;22(14):2865–2872. Epub PubMed PMID: 15254054. doi: 10.1200/jco.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 4.Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002. August 14;94(10):2698–2705. Epub PubMed PMID: 12173339. [DOI] [PubMed] [Google Scholar]
- 5.Hu WG, Weng YM, Dong Y, Li XP, Song QB. Apatinib in refractory radiation-induced brain edema: A case report. Medicine (Baltimore). 2017. November 18;96(46). e7358 Epub PubMed PMID: 29145238; PubMed Central PMCID: PMCPMC5704783. doi: 10.1097/md.0000000000007358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hladik D, Tapio S. Effects of ionizing radiation on the mammalian brain. Mutat Res. 2016. December 07;770(Pt B):219–230. Epub PubMed PMID: 27919332. doi: 10.1016/j.mrrev.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 7.Piazza M, Munasinghe J, Murayi R, Edwards N, Montgomery B, Walbridge S, Merrill M, Chittiboina P. Simulating vasogenic brain edema using chronic VEGF infusion. J Neurosurg. 2017. January 07;127(4):905–916. Epub PubMed PMID: 28059647; PubMed Central PMCID: PMCPMC5542877. doi: 10.3171/2016.9.jns1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou J, Kshettry VR, Selman WR, Bambakidis NC. Peritumoral brain edema in intracranial meningiomas: the emergence of vascular endothelial growth factor-directed therapy. Neurosurg Focus. 2013. December03;35(6):E2 Epub PubMed PMID: 24289127. doi: 10.3171/2013.8.focus13301. [DOI] [PubMed] [Google Scholar]
- 9.Sabang RL, Gandhiraj D, Fanucchi M, Epelbaum O. Role of bevacizumab in the management of the patient with malignant pleural effusion: more questions than answers. Expert Rev Respir Med. 2018;12(2):87–94. Epub [2017 December14]. PubMed PMID: 29235400. doi: 10.1080/17476348.2018.1417042. [DOI] [PubMed] [Google Scholar]
- 10.Yang GL, Zhao `Z, Qin TT, Wang D, Chen L, Xiang R, Xi Z, Jiang R, Zhang ZS, Zhang J, et al. TNFSF15 inhibits VEGF-stimulated vascular hyperpermeability by inducing VEGFR2 dephosphorylation. FASEB J. 2017. February 12;31(5):2001–2012. Epub PubMed PMID: 28183800. doi: 10.1096/fj.201600800R. [DOI] [PubMed] [Google Scholar]
- 11.Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007. July 31;19(10):2003–2012. Epub PubMed PMID: 17658244. doi: 10.1016/j.cellsig.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Ivy SP, Wick JY, Kaufman BM. An overview of small-molecule inhibitors of VEGFR signaling. Nat Rev Clin Oncol. 2009. September 09;6(10):569–579. Epub PubMed PMID: 19736552. doi: 10.1038/nrclinonc.2009.130. [DOI] [PubMed] [Google Scholar]
- 13.Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999. August 07;274(33):23463–23467. Epub PubMed PMID: 10438525. [DOI] [PubMed] [Google Scholar]
- 14.De Bonis P, Marziali G, Vigo V, Peraio S, Pompucci A, Anile C, Mangiola A. Antiangiogenic therapy for high-grade gliomas: current concepts and limitations. Expert Rev Neurother. 2013. November 02;13(11):1263–1270. Epub PubMed PMID: 24175724. doi: 10.1586/14737175.2013.856264. [DOI] [PubMed] [Google Scholar]
- 15.Glitza IC, Guha-Thakurta N, D’Souza NM, Amaria RN, McGovern SL, Rao G, Li J. Bevacizumab as an effective treatment for radiation necrosis after radiotherapy for melanoma brain metastases. Melanoma Res. 2017. August 18;27(6):580–584. Epub PubMed PMID: 28817446. doi: 10.1097/cmr.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Zheng C, Feng Y, Xu Q. Bevacizumab for the Treatment of Gammaknife Radiosurgery-Induced Brain Radiation Necrosis. J Craniofac Surg. 2017. July 28;28(6):e569–e71. Epub PubMed PMID: 28749838. doi: 10.1097/scs.0000000000003874. [DOI] [PubMed] [Google Scholar]
- 17.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014. February 21;370(8):709–722. Epub PubMed PMID: 24552318. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Zhao X, Chen L, Guo H, Lv F, Jia K, Yv K, Wang F, Li C, Qian J, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer. 2010. October 07;10:529 Epub PubMed PMID: 20923544; PubMed Central PMCID: PMCPMC2984425. doi: 10.1186/1471-2407-10-529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 64(19):7099–7109. Epub PubMed PMID: 15466206. doi: 10.1158/0008-5472.can-04-1443. [DOI] [PubMed] [Google Scholar]
- 20.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9(1):327–337. Epub 2003/ 01/23.PubMed PMID: 12538485. [PubMed] [Google Scholar]
- 21.Harris PA, Boloor A, Cheung M, Kumar R, Crosby RM, Davis-Ward RG, Epperly AH, Hinkle KW, Hunter RN 3rd, Johnson JH, et al. Discovery of 5-[[4-[(2,3-dimethyl-2H-indazol −6-yl)methylamino]-2-pyrimidinyl]amino]-2-methyl-b enzenesulfonamide (Pazopanib), a novel and potent vascular endothelial growth factor receptor inhibitor. J Med Chem. 2008. July 16;51(15):4632–4640. Epub PubMed PMID: 18620382. doi: 10.1021/jm800566m. [DOI] [PubMed] [Google Scholar]


