Abstract
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are important causes of hospital admission and mortality. Pneumonia is a major contributor to hospitalization for AECOPD and has a close relationship with poor outcomes. We performed a prospective cohort study to evaluate the prognosis of AECOPD patients with or without community-acquired pneumonia (CAP) who hospitalized from January 2012 to December 2015. We investigated mortality and readmission rates within 6 months after the first admission between two groups and analyzed the difference of survival rate according to readmission duration (≤30 vs. >30 days) or intensive care unit (ICU) treatment. Total 308 AECOPD patients (134 with CAP and 174 without CAP) were enrolled. The mean age was 72.3 ± 9.5 years old, and 235 patients (76.3%) were male. The 180-day mortality was higher in AECOPD with CAP than without CAP (24.6% vs. 13.2%; hazard ratio (HR): 1.982; 95% CI: 1.164–3.375; p = 0.012). However, readmission rate showed no significant difference between two groups (51.5% vs. 46.6%; HR: 1.172; 95% CI: 0.850–1.616; p = 0.333). It showed a significantly lower survival rate in AECOPD with CAP rather than without CAP when were readmitted within 30 days (HR: 1.738; 95% CI:1.063–3.017; p = 0.031). According to ICU treatment, survival rate was not significantly different between two groups. Multivariate analysis revealed the readmission within 30 days (p < 0.001), serum hemoglobin concentration (p = 0.010), and albumin level (p = 0.049) were significantly associated with 180-day mortality of AECOPD with CAP. AECOPD with CAP showed lower survival rate than AECOPD without CAP during 6 months. Early readmission within 30 days was significantly associated with an increased risk of mortality.
Keywords: Acute exacerbation, chronic obstructive pulmonary disease, community-acquired pneumonia, mortality, readmission
Introduction
Chronic obstructive pulmonary disease (COPD) is an aggressive disease acknowledged as the fourth leading cause of death among chronic diseases, even though it is preventable and treatable.1 Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are major drivers of health status in COPD patients and are important causes of hospital admission and mortality.2,3 Exacerbations also accelerate the lung function decline and worsen the prognosis of the disease with elevated 1 year mortality and a 6-month relapse rate.4,5 Most exacerbations appear to be associated with infective triggers including bacterial or viral, although “noninfective” triggers such as air pollution may also be important.3 In a large observational cohort study, a history of COPD exacerbations was the most reliable predictor of future events, regardless of the severity of airflow limitation.6
Frequent exacerbators also have a worse health-related quality of life and accelerated lung function decline than infrequent exacerbators.7–9 Especially, it is known that many AECOPD patients required hospital admission had to readmit within 6 months after the treatment.8,9
Pneumonia is a major contributor to hospitalization for AECOPD and has a close relationship with poor outcomes.10 The patients with pneumonic exacerbation have been found to be admitted to intensive care units more often and stay there longer than those with non-pneumonic exacerbations.11 The use of corticosteroids has a beneficial effect on severe AECOPD patients and also increases the risk of pneumonia.11,12 In-hospital mortality in COPD complicated by community-acquired pneumonia has been reported to be 12.2%.13
Recent study showed there was no significant difference in the survival rate of AECOPD patients between with pneumonia and without pneumonia14 and others noted that mortality was higher in COPD patients combined pneumonia.15–17 However, many studies about AECOPD with community-acquired pneumonia (CAP) have not yet been published, and the difference of survival or readmission rate of AECOPD with or without CAP is still controversial.
Therefore, we performed a prospective cohort study to investigate the early mortality and readmission rates during 6 months of AECOPD with or without CAP. We investigated whether there was a difference in survival rate according to readmission duration or intensive care unit (ICU) treatment between two groups and according to microbiologic pathogens in AECOPD with CAP.
Methods
Study design and population
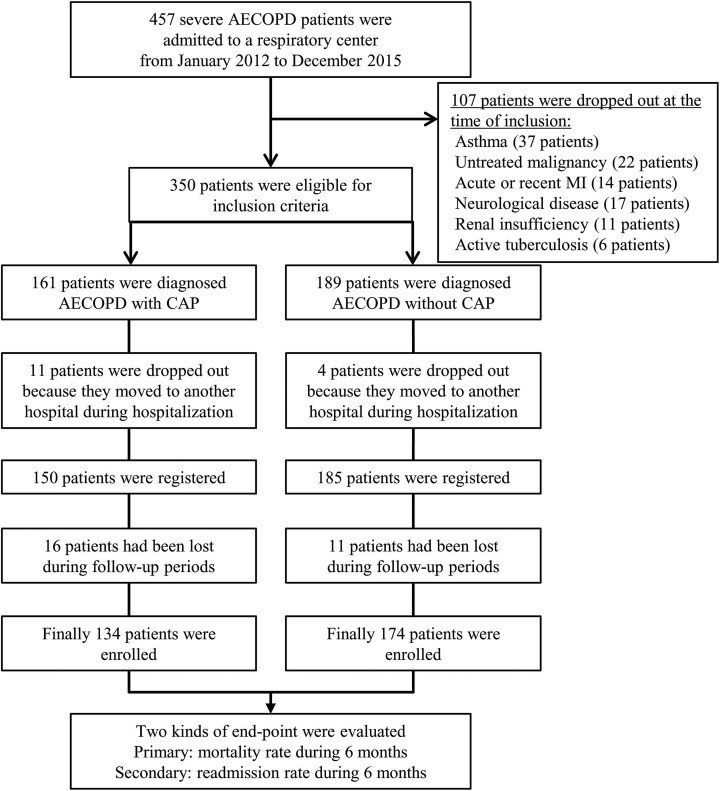
The prospective study was enrolled the patients (age ≥45 years old (y), smoking history of ≥10 pack-years (py)) who hospitalized with severe AECOPD in a respiratory center of Yonsei University Wonju Severance hospital (an 850-bed tertiary hospital) from January 2012 to December 2015 (Figure 1). All of the patients were diagnosed with COPD, according to the criteria established by the GOLD,1 and fulfilled the requirements of FEV1/forced vital capacity (FVC) <70% following inhalation of a bronchodilator.
Figure 1.
Flowchart shows identification of severe AECOPD patients who were admitted to a respiratory center. AECOPD: acute exacerbation of chronic obstructive pulmonary disease; CAP: community-acquired pneumonia; MI: myocardial infarction.
The main exclusion criteria were a diagnosis of asthma, untreated malignancy including lung cancer, severe cardiovascular disorder including valvular heart disease, recent myocardial infarction (within 3 months), pacemaker insertion, renal insufficiency requiring hemodialysis, neurological diseases including cerebral infarction or hemorrhage, and active tuberculosis. Underlying comorbid conditions including cardiac, liver, renal, and diabetes mellitus (DM) were established using clinical history and examination findings during the admission, supported where appropriate with a review of available medical records.
The Korean language version of the COPD assessment test (CAT) questionnaire was used to assess the impact of COPD on the patient’s health.18 The treatments given for COPD before an acute exacerbation (inhaled corticosteroids, long-acting beta2 agonist, and long-acting muscarinic antagonists) were also investigated. The enrolled patients were observed during 6 months. This research was conducted in compliance with the requirements and under the authorization of the Yonsei University Wonju Severance Christian Hospital Institutional Review Board (IRB No. CR312009).
Definition of severe AECOPD and CAP
We enrolled the patients who diagnosed as COPD previously and treated more than a year. An exacerbation was defined as an increase in, or new onset of, ≥2 respiratory symptoms (cough, sputum, dyspnea, wheezing, and chest tightness) with ≥1 symptom lasting ≥3 days and leading the patient’s attending physician to initiate treatment with systemic corticosteroids and/or antibiotics.19,20
Severe AECOPD was defined as hospitalization due to exacerbation of COPD and the indications were as follows: severe underlying COPD with already receiving long-term oxygen therapy, marked increase in breathlessness, poor or deteriorating general condition with little activity, cyanosis or worsening peripheral edema, impaired level of consciousness or confusion, and oxygen saturation <90% despite initial oxygen therapy and medical treatment.21
CAP was defined according to the criteria of the US Centers for Disease Control and Prevention. The diagnosis required a new infiltrate on chest radiography and one or more of the following criteria to be met: fever increase of ≥1 °C or body temperature >38.3 °C; leukocytosis (25% increase and ≥10 × 109/L) or leukopenia (25% decrease and ≤5 × 109/L); and purulent tracheal secretion (>25% neutrophils per high-power field).22,23 A microorganism was defined as causing agent, if detected in respiratory specimens (sputum or bronchoalveolar lavage fluid), blood, or both, excluding normal flora. Only relevant microorganisms cultured from representative sputum specimens according to >25% leukocytes and <10% epithelial cells per high-power field.
Serologic tests were performed to detect antibodies against Mycoplasma pneumoniae and Chlamydophila pneumoniae.24,25 M. pneumoniae was diagnosed if there was a fourfold increase in antibodies in the paired serum samples and/or an antibody titer of ≥160 in at least one serum sample. A fourfold or greater increase in titer for any immunoglobulin class between paired serum samples, an IgG titer of ≥512 or an IgM titer of ≥16 was considered presumptive evidence that C. pneumoniae was the cause of pneumonia.24–26 Streptococcus pneumoniae and Legionella pneumophila serogroup 1 antigens in urine were tested by immunochromatography.27,28
Intensive care unit admission criteria
The ICU admission criteria for CAP were based on American Thoracic Society/Infectious Disease Society of America guidelines to identify two major criteria to determine ICU admission: septic shock requiring vasopressor support and requirement for mechanical ventilation.22 The presence of at least three other minor criteria was also used to determine ICU admission: respiratory rate ≥ 30 breaths/minutes, PaO2/FiO2 ratio ≤ 250, multilobar infiltrates, confusion, blood urea nitrogen (BUN) ≥20 mg/dL; hypotension requiring fluid support.22 The ICU admission criteria for AECOPD were as follows: unable to tolerate or fail to respond to noninvasive ventilator support, severe acidosis (pH < 7.25) and hypercapnia (PaCO2 > 60 mmHg), tachypnea > 35 breaths/minutes, respiratory arrest, cardiovascular instability, impaired mental status or inability to cooperate, and persistent incapacity to remove respiratory secretions.29
Lung function
Lung function was evaluated using the modified Medical Research Council (MMRC) dyspnea scale and most recent performed spirometry in the stable state before an admission, respectively. Spirometry including post-bronchodilator FEV1, FVC and FEV1/FVC was measured with a Vmax Spirometer (Vmax SensorMedics Viasys, type Encore 20/22). Spirometry was performed in accordance to the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines30 using the predicted values calculated with appropriate reference equations.31 Spirometry was accepted if FEV1, FVC, height, date of birth, and gender were available. Biologically implausible values of FEV1 or FVC were excluded. Patients whose spirometry met criteria for a diagnosis of COPD (FEV1/FVC ratio <70%) were categorized.
Treatments of AECOPD and antibiotics in CAP
According to GOLD guidelines,1 patients were treated with nebulized salbutamol, ipratropium bromide, and budesonide, intravenous prednisolone in a dosage of 30–40 mg daily. The duration of systemic corticosteroid treatment was 10–14 days as per our hospital guidelines. On day 4–7, patients were switched to an oral tapering schedule of prednisolone. Antibiotics were administered if bacterial infection including purulent sputum, consolidation on chest radiography is suspected and adjusted according to antimicrobial susceptibilities on sputum or blood culture analysis. Antibiotic therapy was initiated in basic accordance with the ATS/IDSA guidelines,22 but the detailed antibiotic regimen complied with the attending physician’s choice taking into consideration patient risk factors and the severity of the disease. Empirical antibiotic therapy was modulated according to the susceptibility test result for an identified pathogen or the physician’s decision in response to the patient’s condition.
Blood sampling and measurements
A peripheral venous blood sample was obtained from all patients on day 1 of hospitalization for the measurement of hemoglobin, albumin, high-sensitive C-reactive protein (hs-CRP), and blood urea nitrogen (BUN). Arterial gas analysis including oxygen saturation, B-type natriuretic peptide (BNP), and partial pressure of oxygen (PaO2) and carbon dioxide (PaCO2) was performed on day 1 as well. Blood samples were collected within the first hour of admission and before the administration of systemic corticosteroids.
End point
We investigated two kinds of end point including early mortality and readmission rates during 6 months after the first admission between AECOPD with CAP and AECOPD without CAP. Then we analyzed the difference of cumulative survival rate according to readmission duration (≤30 vs. >30 days) or ICU treatment and according to microbiologic pathogens in AECOPD with CAP.
Statistical analysis
SPSS 20.0 (SPSS Inc.; Chicago, Illinois, USA) were used for statistical analysis. Chi-square or Fisher’s exact test was used for categorical variables and Student t or Mann–Whitney U test used for continuous variables. The α (two-sided) and β (1– power) values were set at 0.05 and 0.20, respectively. Estimate required sample size to achieve 80% power to detect 30% reduction in a hazard of the survival rate using a two-sided 0.05 was 257 patients. Considering the dropout rate of 20%, minimum 309 patients were required for this study.
We used Cox proportional hazards regression model to estimate the survival and readmission rate during 6 months between two groups. Relative risks were expressed as hazard ratio (HR) and 95% confidence interval (CI). Cumulative survival and readmission rates were expressed using a Kaplan–Meier approach and the log-rank test. Univariate and multivariate analysis was performed to evaluate prognostic factors associated with 180-mortality in AECOPD with CAP. Descriptive statistics were expressed as mean value ± standard deviation for continuous data and number (%) for categorical data. The value of p < 0.05 was considered to be statistically significant.
Ethics
Research is based on administrative data treated anonymously in the analysis. Research was conducted in compliance with the requirements and under the authorization of the Yonsei University Wonju Severance Christian Hospital Institutional Review Board (IRB No. CR312009).
Results
Total subjects
During study period, 457 severe AECOPD patients were admitted to a respiratory center, and 350 patients were eligible for inclusion criteria. Fifteen (11 patients with CAP and 4 patients without CAP) patients were dropped out because they moved to another hospital during hospitalization. Twenty-seven (16 patients with CAP and 11 patients without CAP) patients had been lost during follow-up periods. Finally, total 308 severe AECOPD patients (134 patients with CAP and 174 patients without CAP) were enrolled (Figure 1). The mean age was 72.3 ± 9.5 years old, and 235 patients (76.3%) were male. The demographic characteristics between AECOPD patients with and without CAP are shown in Table 1. Smoking amounts between two groups were 40.5 ± 16.5 and 40.8 ± 18.5 pack-years, respectively. The regular inhaled medications and spirometric results before an admission were not different between two groups, but the patients had been prescribed systemic corticosteroids (20.1% vs. 10.3%; p = 0.016) or antibiotics (26.1% vs. 16.7%; p = 0.043) within 3 months were significantly higher in AECOPD with CAP (Table 1).
Table 1.
Characteristics of the patients between AECOPD with and without CAP.
| Characteristics | AECOPD with CAP | AECOPD without CAP | p-Value |
|---|---|---|---|
| Subjects (n) | 134 | 174 | |
| Age (y), mean (SD) | 72.8 ± 8.8 | 71.9 ± 10.0 | 0.388 |
| Male sex, n(%) | 102 (76.1) | 133 (76.4) | 0.948 |
| CAT score, mean (SD) | 23.2 ± 6.7 | 22.4 ± 7.1 | 0.336 |
| amMRC dyspnea scale, n(%) | |||
| 0–1 | 27 (20.1) | 39 (22.4) | |
| 2–4 | 107 (79.9) | 135 (77.6) | 0.631 |
| GOLD stage, A/B/C/D, n | 3/56/26/49 | 5/91/28/50 | 0.285 |
| GOLD C, D ratio, n(%) | 75 (56.0) | 77 (44.3) | 0.051 |
| Long-term oxygen therapy | 33 (24.6) | 33 (19.0) | 0.230 |
| Smoking amount, py, mean (SD) | 40.5 ± 16.5 | 40.8 ± 18.5 | 0.857 |
| BMI, kg/m2 | 21.4 ± 2.9 | 21.9 ± 3.7 | 0.237 |
| Underlying comorbid conditions, n(%) | |||
| Diabetes mellitus | 68 (50.7) | 79 (45.4) | 0.352 |
| Congestive heart failure | 37 (27.6) | 64 (36.8) | 0.089 |
| Chronic kidney disease | 6 (4.5) | 4 (2.3) | 0.340 |
| Hepatobiliary disease | 3 (2.2) | 4 (2.3) | 1.000 |
| Medications before an admission, n(%) | |||
| Inhaled corticosteroids | 81 (60.4) | 99 (56.9) | 0.531 |
| Long-acting muscarinic antagonists | 75 (56.0) | 88 (50.6) | 0.347 |
| Long-acting beta2 agonists | 72 (53.7) | 92 (52.9) | 0.881 |
| bSystemic corticosteroids | 27 (20.1) | 18 (10.3) | 0.016 |
| cAntibiotics use | 35 (26.1) | 29 (16.7) | 0.043 |
| Functional parameters, %, mean (SD) | |||
| Postbronchodilator FEV1/FVC | 49.3 ± 13.1 | 51.9 ± 12.9 | 0.081 |
| Postbronchodilator FEV1 | 55.0 ± 20.4 | 58.6 ± 23.0 | 0.150 |
| Postbronchodilator FVC | 73.3 ± 18.9 | 74.7 ± 21.1 | 0.548 |
AECOPD: acute exacerbations of chronic obstructive pulmonary disease; BMI: body mass index; CAP: community-acquired pneumonia; CAT: chronic obstructive pulmonary disease assessment test; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; mMRC: modified Medical Research Council; n: number; py: pack-years; SD: standard deviation; y: years old.
a mMRC dyspnea scale consists in five statements that describe almost the entire range of dyspnea from none (grade 0) to almost complete incapacity (grade 4).
b Systemic corticosteroids uses were included when the patients have been prescribed within 3 months.
c Antibiotics uses were included when the patients have been prescribed within 3 months.
Laboratory and microbiologic findings between AECOPD with and without CAP
At the time of admission, oxygen saturation, PaO2, PaCO2, hemoglobin, creatinine, and BNP were not significantly different, but serum albumin (p < 0.001), hs-CRP (p < 0.001), and BUN (p = 0.022) showed significant differences between two groups (Table 2). We identified causative organisms in 76.1% (102of 134) of AECOPD patients with CAP. Streptococcus pneumoniae was the most frequently isolated pathogen (40.3%) and Staphylococcus aureus (14.2%), Pseudomonas aeruginosa (12.7%), Klebsiella pneumoniae (5.2%), and other pathogens (3.7%) were isolated, respectively (Table 2).
Table 2.
Laboratory and microbiologic findings between AECOPD with and without CAP.
| Variables (at the time of admission) | AECOPD with CAP | AECOPD without CAP | p-Value |
|---|---|---|---|
| Laboratory findings (mean ± SD) | |||
| Oxygen saturation (%) | 88.4 ± 9.9 | 90.1 ± 9.2 | 0.114 |
| PaO2 (mm Hg) | 62.6 ± 15.3 | 65.9 ± 14.6 | 0.051 |
| PaCO2 (mmHg) | 39.8 ± 16.5 | 40.5 ± 14.1 | 0.679 |
| Hemoglobin (g/dL) | 12.8 ± 1.8 | 13.1 ± 1.8 | 0.216 |
| Albumin (g/dL) | 3.5 ± 0.5 | 3.8 ± 0.5 | <0.001 |
| hs-CRP (mg/dL) | 16.2 ± 9.1 | 3.1 ± 5.5 | <0.001 |
| aProcalcitonin (mg/dL) | 4.3 ± 10.9 | 1.9 ± 5.9 | 0.083 |
| BUN (mg/dL) | 22.8 ± 15.1 | 19.2 ± 11.7 | 0.022 |
| Creatinine | 0.99 ± 0.63 | 0.88 ± 0.47 | 0.060 |
| BNP (pg/mL) | 231.0 ± 424.8 | 165.4 ± 306.6 | 0.132 |
| Microbiologic findings, n (%) | |||
| Streptococcus pneumoniae | 54 (40.3) | 3 (1.7) | <0.001 |
| Staphylococcus aureus | 19 (14.2) | 20 (11.5) | 0.579 |
| Pseudomonas aeruginosa | 17 (12.7) | 16 (9.2) | 0.397 |
| Klebsiella pneumoniae | 7 (5.2) | 11 (6.3) | 0.616 |
| bOthers | 5 (3.7) | 2 (1.1) | 0.249 |
AECOPD: acute exacerbations of chronic obstructive pulmonary disease; BNP: B-type natriuretic peptide; BUN: blood urea nitrogen; CAP: community-acquired pneumonia; hs-CRP: high sensitive C-reactive protein; n: number; MRSA: methicillin-resistant Staphylococcus aureus; PaCO2: arterial carbon dioxide partial pressure; PaO2: arterial oxygen partial pressure; SD: standard deviation.
a Initial procalcitonin results were available for 78 patients in pneumonic exacerbation group and 94 patients in nonpneumonic exacerbation group.
b Others were included Mycoplasma pneumoniae (2), Legionella pneumophila (1), Haemophilus influenzae (1) and Acinetobacter baumannii (1) in AECOPD with CAP, and Legionella pneumophila (1) and Haemophilus influenzae (1) in AECOPD without CAP, respectively.
The mortality and readmission rates during 6 months
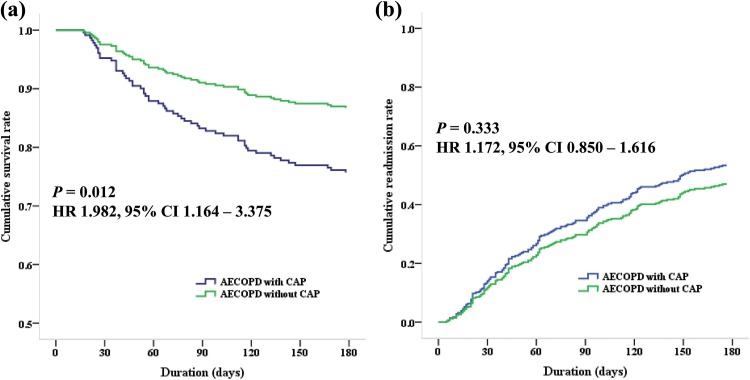
When we investigated the mortality and readmission rates during 6 months between AECOPD with and without CAP, the mortality rate during 6 months was higher in AECOPD patients with CAP than without CAP (24.6% vs. 13.2%; HR: 1.982; 95% CI:1.164–3.375; p = 0.012). The causes of mortality were as follows: 30 patients with AECOPD (18 patients with CAP vs. 12 patients without CAP), 18 pneumonia (13 vs. 5), 6 sepsis (2 vs. 4), and 2 pneumothorax (0 vs. 2).
However, the readmission rate was not significantly different between two groups (51.5% vs. 46.6%; HR: 1.172; 95% CI: 0.850–1.616; p = 0.333). The causes of readmission were as follows: 77 patients with AECOPD (28 vs. 49), 56 pneumonia (39 vs. 17), 12 congestive heart failure (0 vs. 12), 4 pneumothorax (1 vs. 3), and 1 sepsis (1 vs. 0). The cumulative survival and readmission rates are shown in Figure 2.
Figure 2.
Figure 2 shows cumulative survival and readmission rates during 6 months between AECOPD with and without CAP. (a) It shows lower cumulative survival rate in AECOPD with CAP than AECOPD without CAP (HR: 1.982; 95% CI: 1.164–3.375; p = 0.012). (b) Cumulative readmission rate is not significantly different between two groups (HR: 1.172; 95% CI: 0.850–1.616; p = 0.333). AECOPD: acute exacerbation of chronic obstructive pulmonary disease; CAP: community-acquired pneumonia; CI: confidence interval; HR: hazard ratio.
Cumulative survival rate according to readmission duration after discharge and ICU treatment
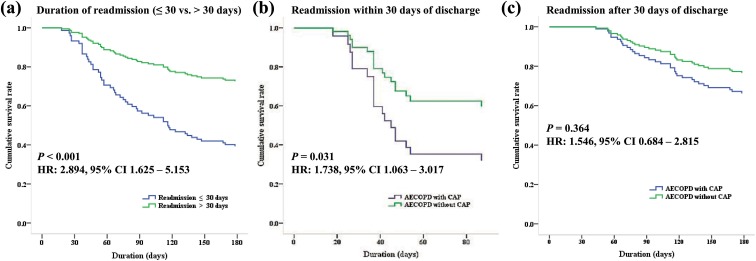
When we compared according to the duration of readmission after discharge (≤30 days vs. >30 days), patients who were readmitted within 30 days showed lower cumulative survival rate than those after 30 days (HR: 2.894; 95% CI: 1.625–5.153; p < 0.001) (Figure 3(a)). It showed a significantly lower survival rate in AECOPD with CAP rather than without CAP when were readmitted within 30 days (HR: 1.738; 95% CI: 1.063–3.017; p = 0.031) (Figure 3(b)), but there was no significant difference of survival rate when were readmitted after 30 days of discharge between two groups (HR: 1.387; 95% CI: 0.684–2.815; p = 0.364) (Figure 3(c)).
Figure 3.
Figure 3 shows cumulative survival rates according to the duration of readmission (≤30 days vs. >30 days). (a) AECOPD patients show a significantly lower survival rate when were early readmitted within 30 days after discharge than after 30 days (HR: 2.894; 95% CI: 1.625–5.153; p < 0.001). (b) AECOPD with CAP shows a significantly lower survival rate when were readmitted within 30 days than AECOPD without CAP (HR: 1.738; 95% CI:1.063–3.017; p = 0.031). (c) There is no significant difference of survival rate when were readmitted after 30 days after discharge between two groups (HR: 1.546; 95% CI: 0.684–2.815; p = 0.364). AECOPD: acute exacerbation of chronic obstructive pulmonary disease; CAP: community-acquired pneumonia; CI: confidence interval; HR: hazard ratio.
When we compared a cumulative survival rate according to ICU treatment, it did not show a significant difference between two groups either (HR: 1.337; 95% CI: 0.554–3.227; p = 0.518) (Online Supplemental Material 1A).
Cumulative survival rate according to microbiologic pathogens in AECOPD with CAP
We assessed the cumulative survival rate according to microbiologic pathogens in the group of AECOPD with CAP. There was a tendency to show a difference of survival rate according to causative pathogens, but there was no significant difference (Online Supplemental Material 1B)
Risk factors influencing on mortality in AECOPD with CAP
To identify risk factors associated with 180-day mortality in AECOPD with CAP, multivariate logistic regression analysis was performed using significant variables with p < 0.05 by univariate analysis. Multivariate analysis using factors that were found to be significant by univariate analysis revealed that the readmission within 30 days (HR:14.782; 95% CI: 6.264–34.882; p < 0.001), serum hemoglobin concentrations (HR: 0.759; 95% CI: 0.616–0.936; p = 0.010), and albumin levels (HR: 0.429; 95% CI: 0.185–0.995; p = 0.049) were significantly associated with 180-day mortality in AECOPD with CAP (Table 3).
Table 3.
Prognostic factors associated with 180-mortality in AECOPD with CAP.
| Univariate (mortality) | Multivariate (mortality) | |||||
|---|---|---|---|---|---|---|
| Variables | 95% CI | HR | p-Value | 95% CI | HR | p-Value |
| Clinical | ||||||
| Age, y | 1.028, 1.130 | 1.077 | 0.002a | 0.990, 1.099 | 1.043 | 0.115 |
| GOLD group C-D | 0.804, 3.422 | 1.659 | 0.171 | |||
| Smoking amounts, py | 0.989, 1.030 | 1.009 | 0.370 | |||
| Long-term oxygen therapy | 1.115, 4.515 | 2.244 | 0.023a | 0.558, 3.028 | 1.300 | 0.542 |
| Readmission within 30 days | 6.064, 25.084 | 12.334 | <0.001a | 6.264, 34.882 | 14.782 | <0.001a |
| ICU treatment | 1.186, 5.049 | 2.304 | 0.016a | 0.304, 2.863 | 0.934 | 0.904 |
| Functional and laboratory | ||||||
| Post-bronchodilator FEV1 | 0.983, 1.017 | 1.000 | 0.962 | |||
| PaO2 (mmHg) | 0.966, 1.010 | 0.988 | 0.292 | |||
| PaCO2 (mmHg) | 1.067, 1.046 | 1.026 | 0.007a | 0.993, 1.044 | 1.018 | 0.157 |
| Hemoglobin (g/dL) | 0.570, 0.843 | 0.693 | <0.001a | 0.616, 0.936 | 0.759 | 0.010a |
| Albumin (g/dL) | 0.197, 0.703 | 0.372 | 0.002a | 0.185, 0.995 | 0.429 | 0.049a |
| hs-CRP (mg/dL) | 0.985, 1.057 | 1.020 | 0.263 | |||
| BNP (pg/mL) | 1.000, 1.001 | 1.001 | <0.001a | 1.000, 1.001 | 1.000 | 0.212 |
| BUN (mg/dL) | 1.012, 1.047 | 1.030 | 0.001a | 0.988, 1.033 | 1.011 | 0.352 |
AECOPD: acute exacerbations of chronic obstructive pulmonary disease; BNP: B-type natriuretic peptide; BUN: blood urea nitrogen; CAP: community-acquired pneumonia; CI: confidence interval; GOLD: Global Initiative for Chronic Obstructive Lung Disease; FEV1: forced expiratory volume in 1 second; hs-CRP: high sensitive C-reactive protein; HR: hazard ratio; ICU: intensive care unit; PaCO2: arterial carbon dioxide partial pressure; PaO2: arterial oxygen partial pressure; py: pack-years; y: years old. aSignificance in univariate and multivariate analysis (p < 0.05).
Discussion
This prospective observation study showed that the 180-day mortality was higher in AECOPD with CAP than AECOPD without CAP, but the readmission rate was not significantly different between two groups.
Until now, the paper by Huerta et al. was the main study on COPD with CAP and they found that mortality rates during hospitalization and follow-up period were similar between two groups despite higher COPD functional severity of AECOPD compared with COPD with CAP.14 However, other studies showed that in-hospital mortality rate of pneumonic AECOPD was significantly higher than non-pneumonic AECOPD.15–17
When we looked at the patients’ characteristics enrolled in the study by Huerta et al., patients with AECOPD at the time of registration showed significantly lower pulmonary function (FEV1 and FEV1/FVC) and experienced higher long-term oxygen therapy than patients with COPD combined CAP.14 As a result, it may have influenced the outcome of the study as more serious AECOPD patients were enrolled. On the other hand, the patients enrolled in our study showed no significant difference in pulmonary function between two groups and no difference in the rate of home oxygen therapy either. Therefore, we thought that there were differences in the results of the research because the underlying condition of enrolled subjects was differed from previous study. In our study, AECOPD with CAP showed lower survival rate during 6 months than AECOPD without CAP although it showed a similar readmission rate between two groups.
According to Steer et al., the mortality of AECOPD was significantly higher in pneumonic than in non-pneumonic exacerbations.15 They concluded that the severity of dyspnea was a strong predictor of mortality and pneumonic exacerbation of COPD was a clinical problem with overall worse outcome.15 In our study, the mortality rate also showed a tendency to increase according to mMRC dyspnea scale, but dyspnea severity was not an important prognostic factor and did not show a significant difference between two groups.
Although there was no difference of readmission between two groups, we found that the shorter duration of readmission within 30 days after discharge was strongly associated with the higher mortality rate in both groups, especially in AECOPD with CAP. There is a recent study that early readmission within 30 days of discharge was associated with a progressive increased long-term risk of death in AECOPD.32 In their study, they also reported that 6-month mortality was significantly higher in AECOPD patients with 30-day readmission. In general, it is known that approximately 25% of patients do not recover their lung function until 35 days after an AECOPD, and they therefore may require rehospitalization during the naturally high-risk 30-day time interval.33 The delay of pulmonary function recovery seems to be related to the deterioration of dyspnea and quality of life and therefore is likely to be associated with an increase of mortality in AECOPD. However, there is no clear evidence for this and more research will be needed. Our study also showed early readmission within 30-day after discharge in AECOPD, especially with CAP was significantly associated with 180-day mortality although there was no difference in readmission rate between AECOPD with and without CAP.
We also checked a difference of survival rate between the identified pathogens in AECOPD with CAP patients. In our study, S. pneumonia, S. aureus, and P. aeruginosa were commonly isolated pathogens in AECOPD with CAP similar to previous research.14 There was a tendency of differences in survival rates depending on identified pathogens but was not significantly different. Another study34 suggested that the proportion of isolated pathogens was slightly different depending on the severity of COPD, but this study does not mention the difference in the mortality rate according to isolated pathogens. As a result, pathogens did not affect the mortality rate when appropriate antibiotic treatments were given in AECOPD with CAP.
Finally, we investigated risk factors that affect mortality in AECOPD with CAP. In multivariate analysis, readmission within 30 days, serum hemoglobin concentrations and albumin levels were significantly associated with the mortality in AECOPD with CAP. As mentioned earlier, early readmission within 30 days after discharge showed a strong association with an increased risk of mortality in AECOPD with CAP.
Comorbid anemia in patients with COPD reported deleterious effects from on quality of life, mortality, and healthcare utilization.35,36 The causes of anemia in COPD are multifactorial, including nutritional deficits, stress ulcer due to steroids use, and carboxyhemoglobin effects of cigarette smoking. Anemia can compromise oxygen deliveries to tissues and cause dyspnea. This is likely to contribute to functional limitations and decreased exercise tolerance in COPD patients who have already oxygen deficiencies due to diminished lung function.35,37 Systematic review reported that serum albumin level was also significantly associated with the long-term in-hospital mortality in AECOPD.38 Serum albumin level within 24 hours of admission is a good prognostic marker in CAP.39 The mechanisms underlying the cause of hypoalbuminemia in hospitalized patients are diverse and infection including pneumonia been associated with hypoalbuminemia. Albumin may represent an underlying poor nutritional state prior to admission, and observational studies suggested that low albumin conferred a higher risk of mortality in AECOPD.4,40
The results of the present study may be limited by several factors. First, the present study was performed in a single institution, selection bias may have influenced the significance of the present findings and the possibility of recording errors related to baseline clinical data cannot be excluded, thus a multicenter study is required to validate the results. Second, follow-up period during 6 months may be relatively short, but we focused on early readmission and mortality of AECOPD patients with and without CAP thus the short-term monitoring may be useful in identifying variables associated with risk factor of AECOPD. Third, as the number of patients to be sampled is relatively small and more severe, AECOPD patients might be enrolled in our study although the criteria of hospitalization were established, readmission rate was relatively high and these patients could exert a prognostic influence at the results. Finally, we could not routinely perform viral testing including swabs or polymerase chain reaction so could not have assessed the rate of viral pneumonia and its effect on prognosis. Despite these limitations, this study is meaningful to investigate the mortality and readmission rates between AECOPD with and without CAP and whether a difference in survival rate according to early readmission.
In conclusion, AECOPD with CAP showed lower survival rate than AECOPD without CAP during 6 months. Early readmission within 30 days after discharge was significantly associated with an increased risk of mortality in AECOPD, especially with CAP.
Supplemental material
Supple_1 for Early readmission and mortality in acute exacerbation of chronic obstructive pulmonary disease with community-acquired pneumonia by Beomsu Shin, Sang-Ha Kim, Suk Joong Yong, Won-Yeon Lee, Sunmin Park, Sang Jun Lee, Seok Jeong Lee, and Myoung Kyu Lee in Chronic Respiratory Disease
Acknowledgments
The authors thank all members of the department of Internal Medicine, Yonsei University Wonju College of Medicine.
Footnotes
Author contributions: Beomsu Shin designed this investigation and wrote the manuscript. Sang-Ha Kim, Suk Joong Yong, and Won-Yeon Lee contributed to study design and investigation. Sunmin Park and Sang Jun Lee collected and analyzed the data. Seok Jeong Lee conducted statistical analysis and revision of the manuscript. Myoung Kyu Lee designed this investigation and reviewed the manuscript. The cohort study was performed at Yonsei University Wonju College of Medicine.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Sang-Ha Kim  https://orcid.org/0000-0002-1763-5366
https://orcid.org/0000-0002-1763-5366
Myoung Kyu Lee  https://orcid.org/0000-0002-2450-4882
https://orcid.org/0000-0002-2450-4882
Supplemental material: Supplemental material for this article is available online.
References
- 1. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347–365. [DOI] [PubMed] [Google Scholar]
- 2. Wedzicha JA, Seemungal TAR. COPD exacerbations: defining their cause and prevention. Lancet 2007; 370: 786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wedzicha JA, Brill SE, Allinson JP, et al. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med 2013; 11: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Connors AF, Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbations of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996; 154: 959–967. [DOI] [PubMed] [Google Scholar]
- 5. Wedzicha JA, Wilkinson T. Impact of chronic obstructive pulmonary diseasse exacerbations on patients and payers. Proc Am Thoracic Soc 2006; 3: 218–221. [DOI] [PubMed] [Google Scholar]
- 6. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363: 1128–1138. [DOI] [PubMed] [Google Scholar]
- 7. Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeniety in the ECLIPSE cohort. Respir Res 2010; 11: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seemungal TA, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157: 1418–1422. [DOI] [PubMed] [Google Scholar]
- 9. Donaldson GC, Seemungal TAR, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57: 847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Søgaard M, Madsen M, Løkke A, et al. Incidence and outcomes of patients hospitalized with COPD exacerbation with and without pneumonia. Int J Chron Obstruct Pulmon Dis 2016; 11: 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu Z, Cheng Y, Tu X, et al. Community-acquired pneumonia and survival of critically ill acute exacerbation of COPD patients in respiratory intensive care units. Int J Chron Obstruct Pulmon Dis 2016; 11: 1867–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Festic E, Scanlon PD. Incident pneumonia and mortality in patients with chronic obstructive pulmonary disease. A double effect of inhaled corticosteroids? Am J Respir Crit Care Med 2015; 191: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamauchi Y, Yasunaga H, Matsui H, et al. Comparison of clinical characteristics and outcomes between aspiration pneumonia and community-acquired pneumonia in patients with chronic obstructive pulmonary disease. BMC Pulm Med 2015; 15: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huerta A, Crisafulli E, Menéndez R, et al. Pneumonic and nonpneumonic exacerbations of COPD: inflammatory response and clinical characteristics. Chest 2013; 144: 1134–1142. [DOI] [PubMed] [Google Scholar]
- 15. Steer J, Norman EM, Afolabi OA, et al. Dyspnoea severity and pneumonia as predictors of in-hospital mortality and early readmission in acute exacerbations of COPD. Thorax 2012; 67: 117–121. [DOI] [PubMed] [Google Scholar]
- 16. Myint PK, Lowe D, Stone RA, et al. U.K. National COPD Resources and Outcomes Project 2008: patients with chronic obstructive pulmonary disease exacerbations who present with radiological pneumonia have worse outcome compared to those with non-pneumonic chronic obstructive pulmonary disease exacerbations. Respiration 2011; 82: 320–327. [DOI] [PubMed] [Google Scholar]
- 17. Lieberman D, Lieberman D, Gelfer Y, et al. Pneumonic vs nonpneumonic acute exacerbations of COPD. Chest 2002; 122: 1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee S, Lee JS, Song JW, et al. Validation of the Korean version of Chronic Obstructive Pulmonary Disease Assessment Test (CAT) and Dyspnea-12 Questionnaire. Tuberc Respir Dis 2010; 69: 171–176. [Google Scholar]
- 19. Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011; 364: 1093–1103. [DOI] [PubMed] [Google Scholar]
- 20. Wedzicha JA, Decramer M, Seemungal TAR. The role of bronchodilator treatment in the prevention of exacerbations of COPD. Eur Respir J 2012; 40: 1545–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. MacIntyre N, Huang YC. Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008; 5: 530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious diseases society of America/American thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44: S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Watkins RR, Lemonovich TL. Diagnosis and management of community-acquired pneumonia in adults. Am Fam Physician 2011; 83: 1299–1306. [PubMed] [Google Scholar]
- 24. Ishida T, Hashimoto T, Arita M, et al. Etiology of community-acquired pneumonia in hospitalized patients: a 3-year prospective study in Japan. Chest 1998; 114: 1588–1593. [DOI] [PubMed] [Google Scholar]
- 25. Miyashita N, Ouchi K, Kawasaki K, et al. Comparison of serological tests for detection of immunoglobulin M antibodies to Chlamydophila pneumoniae . Respirology 2008; 13: 427–431. [DOI] [PubMed] [Google Scholar]
- 26. Lieberman D, Schlaeffer F, Boldur I, et al. Multiple pathogens in adult patients admitted with community-acquired pneumonia: a one year prospective study of 346 consecutive patients. Thorax 1996; 51: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosón B, Fernández-Sabé N, Carratalà J, et al. Contribution of a urinary antigen assay (Binax NOW) to the early diagnosis of pneumococcal pneumonia. Clin Infect Dis 2004; 38: 222–226. [DOI] [PubMed] [Google Scholar]
- 28. Helbig JH, Uldum SA, Lück PC, et al. Detection of Legionella pneumophila antigen in urine samples by the BinaxNOW immunochromatographic assay and comparison with both Binax Legionella Urinary Enzyme Immunoassay (EIA) and Biotest Legionella Urin Antigen EIA. J Med Microbiol 2001; 50: 509–516. [DOI] [PubMed] [Google Scholar]
- 29. Dixit D, Bridgeman MB, Andrews LB, et al. Acute exacerbations of chronic obstructive pulmonary disease: diagnosis, management, and prevention in critically ill patients. Pharmacotherapy 2015; 35: 631–648. [DOI] [PubMed] [Google Scholar]
- 30. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 31. Hankinson JL, Kawut SM, Shahar E, et al. Performance of american thoracic society-recommended spirometry reference values in a multiethnic sample of adults: the multi-ethnic study of atherosclerosis (MESA) lung study. Chest 2010; 137: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guerrero M, Crisafulli E, Liapikou A, et al. Readmission for acute exacerbation within 30 days of discharge is associated with a subsequent progressive increase in mortality risk in COPD patients: a long-term observational study. PLoS One 2016; 11: e0150737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anecchino C, Rossi E, Fanizza C, et al. Prevalence of chronic obstructive pulmonary disease and pattern of comorbidities in a general population. Int J Chronic Obstruct Pulmon Dis 2007; 2: 567–574. [PMC free article] [PubMed] [Google Scholar]
- 34. Li XJ, Li Q, Si LY, et al. Bacteriological differences between COPD exacerbation and community-acquired pneumonia. Respir Care 2011; 56:1818–1824. [DOI] [PubMed] [Google Scholar]
- 35. Yohannes AM, Ershler WB. Anemia in COPD: a systematic review of the prevalence, quality of life, and mortality. Respir Care 2011; 56: 644–652. [DOI] [PubMed] [Google Scholar]
- 36. Toft-Petersen AP, Torp-Pedersen C, Weinreich UM, et al. Association between hemoglobin and prognosis in patients admitted to hospital for COPD. Int J Chron Obstruct Pulmon Dis 2016; 11: 2813–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet 2007; 370: 797–799. [DOI] [PubMed] [Google Scholar]
- 38. Singanayagam A, Schembri S, Chalmers JD. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc 2013; 10: 81–89. [DOI] [PubMed] [Google Scholar]
- 39. Viasus D, Garcia-Vidal C, Simonetti A, et al. Prognostic value of serum albumin levels in hospitalized adults with community-acquired pneumonia. J Infect 2013; 66: 415–423. [DOI] [PubMed] [Google Scholar]
- 40. Asiimwe AC, Brims FJ, Andrews NP, et al. Routine laboratory tests can predict in-hospital mortality in acute exacerbations of COPD. Lung 2011; 189: 225–232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supple_1 for Early readmission and mortality in acute exacerbation of chronic obstructive pulmonary disease with community-acquired pneumonia by Beomsu Shin, Sang-Ha Kim, Suk Joong Yong, Won-Yeon Lee, Sunmin Park, Sang Jun Lee, Seok Jeong Lee, and Myoung Kyu Lee in Chronic Respiratory Disease