Abstract
Objective
We compared the predictive value of the National Early Warning Score+Lactate (NEWS+L) score with those of other parameters such as the pre-endoscopic Rockall score (PERS), Glasgow-Blatchford score (GBS), and albumin, international normalized ratio, altered mental status, systolic blood pressure, age older than 65 years score (AIMS65) among patients with upper gastrointestinal bleeding (UGIB).
Methods
We conducted a retrospective study of patients with UGIB during 2 consecutive years. The primary outcome was the composite of in-hospital death, intensive care unit admission, and the need for ≥5 packs of red blood cell transfusion within 24 hours.
Results
Among 530 included patients, the composite outcome occurred in 59 patients (19 in-hospital deaths, 13 intensive care unit admissions, and 40 transfusions of ≥5 packs of red blood cells within 24 hours). The area under the receiver operating characteristic curve of the NEWS+L score for the composite outcome was 0.76 (95% confidence interval, 0.70 to 0.82), which demonstrated a significant difference compared to PERS (0.66, 0.59–0.73, P=0.004), but not to GBS (0.70, 0.64–0.77, P=0.141) and AIMS65 (0.76, 0.70–0.83, P=0.999). The sensitivities of NEWS+L scores of 3 (n=34, 6.4%), 4 (n=92, 17.4%), and 5 (n=171, 32.3%) were 100%, 98.3%, and 96.6%, respectively, while the sensitivity of an AIMS65 score of 0 (n=159, 30.0%) was 91.5%.
Conclusion
The NEWS+L score showed better discriminative performance than the PERS and comparable discriminative performance to the GBS and AIMS65. The NEWS+L score may be used to identify low-risk patients among patients with UGIB.
Keywords: Risk, Lactate, Mortality, Gastrointestinal, Bleeding
INTRODUCTION
Upper gastrointestinal bleeding (UGIB) is defined as bleeding that results from a source proximal to the ligament of Treitz and accounts for up to 85% of gastrointestinal bleeding episodes [1]. The annual incidence of UGIB is approximately 100 per 100,000 individuals [2,3]. UGIB is a potentially life-threatening condition, with a mortality rate of 6% to 10% [4-8]. To manage patients with UGIB, substantial medical resources, such as urgent endoscopy or blood transfusion, are often required; however, this may not always be the case. Therefore, the European Society of Gastrointestinal Endoscopy recently recommended stratifying patients with UGIB into high and low risk groups when physicians make decisions regarding the timing of endoscopy or hospital discharge [9]. High-risk patients have higher risks of requiring blood transfusions, admission to the intensive care unit (ICU), and death. To date, several scoring systems have been developed to predict outcomes in patients with UGIB such as the pre-endoscopic Rockall score (PERS) [10], Glasgow-Blatchford score (GBS) [11], and albumin, international normalized ratio, altered mental status, systolic blood pressure, age older than 65 years score (AIMS65) [12]. However, a recent study reported that the aforementioned three scoring systems showed only fair to poor discriminative values for 30-day mortality. Moreover, the PERS and AIMS65 showed poor discriminative values for the need for transfusions [13].
Recently, serum lactate has been identified as a significant factor associated with mortality in critically ill patients with UGIB [14-16]. However, the aforementioned risk scoring systems for patients with UGIB do not include the serum lactate level. To this end, the National Early Warning Score+Lactate (NEWS+L) score [17], which the authors originally designed, and which is composed of a physiologic component (NEWS) and a laboratory component (the serum lactate level), has been developed.
Herein, we hypothesized that the NEWS+L score, which includes the serum lactate level as a component, would have a better predictive value for clinically important outcomes than the PERS, GBS, and AIMS65 score among patients with UGIB. Accordingly, the primary aim of this study was to compare the predictive value of the NEWS+L score with those of the PERS, GBS, and AIMS65 score regarding the composite of mortality, ICU admission, and need for red blood cell (RBC) transfusions.
METHODS
Study design and setting
We performed a retrospective cohort study that included consecutive patients with UGIB between January 1, 2014 and December 31, 2015. This study was approved by the Institutional Review Board of the Chonbuk National University Hospital (2016-09-021), which waived the requirement of informed consent for all subjects in this study. The study hospital is a 1,200-bed urban academic, tertiary-care, university hospital.
Selection of participants
All patients 18 years of age or older who visited the study hospital with melena, hematochezia, hematemesis, and syncope were screened for eligibility in this study. For patients to be enrolled in the study, they needed to have confirmed UGIB by esophagogastroduodenoscopy (EGD) evaluation. The exclusion criteria were a lack of UGIB by EGD evaluation and/or an unavailable serum lactate level at the time of presentation to the emergency department (ED).
Measurement and data collection
A trained abstractor collected data from the charts using a structured data-collection form. The medical record review and data abstraction were performed by an emergency medicine resident who had 3 years of emergency medicine training. The resident underwent data-collection training that included how to define the eligibility criteria and other variables that were included in this study. After data collection was completed, random chart reviews were performed to ensure data accuracy. Screening was performed using a list that extracted symptoms of melena, hematochezia, hematemesis, or syncope from the electronic medical record.
The data points that were included were as follows: age, sex, comorbidities (chronic liver disease, chronic kidney disease, active malignancy, heart failure, or coronary artery disease), presenting symptoms (hematemesis, melena, hematochezia, or syncope), systolic blood pressure and heart rate at triage, National Early Warning Score (NEWS) at triage, lactate level, albumin, prothrombin time, INR, hemoglobin, blood urea nitrogen, endoscopic diagnosis (bleeding ulcer, bleeding varix, hemorrhagic gastritis, bleeding tumor, or Mallory-Weiss syndrome), endoscopic treatment (endoscopic variceal ligation, endoscopic variceal obturation, hemostasis [using hypertonic saline-epinephrine solution], or hemoclip), disposition (discharge, admission to the ICU, or ward), number of RBC transfusions within 24 hours, and survival status at hospital discharge. During the examination, comorbidities were checked based on the patients’ or guardians’ statements on the medical chart by ED physicians. At the study hospital, NEWS is automatically calculated using vital signs at triage and registered in the electronic medical record. The serum lactate level is a routine laboratory test at the study hospital ED.
The serum lactate level was primarily measured using arterial blood, but the venous blood lactate level was also permitted. The lactate level was measured using a Stat Profile Critical Care Xpress Analyzer (Nova Biomedical, Waltham, MA, USA). The measurement capacity for lactate in this instrument ranges from 0.3 to 20 mmol/L. This machine was periodically inspected by staff employed by the manufacturer.
The PERS, GBS, and AIMS65 score were calculated from the collected variables. The details of the NEWS+L score are shown in Table 1.
Table 1.
The NEWS+L score used in the present study
| 3 | 2 | 1 | 0 | 1 | 2 | 3 | Score | |
|---|---|---|---|---|---|---|---|---|
| Physiologic component | ||||||||
| Systolic blood pressure (mmHg) | ≤ 90 | 91–100 | 101–110 | 111–219 | ≥ 220 | ( ) | ||
| Pulse rate (bpm) | ≤ 40 | 41–50 | 51–90 | 91–110 | 111–130 | ≥ 131 | ( ) | |
| Respiratory rate (bpm) | ≤8 | 9–11 | 12–20 | 21–24 | ≥ 25 | ( ) | ||
| Temperature (°C) | ≤ 35.0 | 35.1–36.0 | 36.1–38.0 | 38.1–39.0 | ≥ 39.1 | ( ) | ||
| Oxygen saturation (%) | ≤ 91 | 92–93 | 94–95 | ≥ 96 | ( ) | |||
| Any supplemental oxygen | Yes | No | ||||||
| Level of consciousness | Alert | Voice | ||||||
| Pain | ||||||||
| Unresponsive | ||||||||
| Subtotal (NEWS) | ( ) | |||||||
| Laboratory component | + | |||||||
| Lactate level (mmol/L) | ( ) | |||||||
| NEWS+L score | ( ) |
NEWS+L, National Early Warning Score+Lactate; NEWS, National Early Warning Score.
Outcome measures
The primary outcome was the composite of in-hospital death, ICU admission, and RBC transfusion of ≥5 packs within 24 hours. Each component of the primary outcome was then set as a secondary outcome. All deaths during the hospital stay were counted, regardless of the cause. Five packs of RBCs were determined arbitrarily as the cut-off; however, it seemed to be a meaningful number considering the result of a previous randomized control trial that compared restrictive transfusion with liberal transfusion among patients with UGIB. In the liberal strategy group, the mean number of transfused RBC packs was 3.7±3.8 [18].
Statistical analysis
Continuous data are presented as the mean and standard deviation. Continuous data that were not normally distributed are presented as the median and interquartile range. Discrete data are presented as both counts and percentages.
Student’s t-test for independent samples was used to compare the means of normally distributed variables. The Mann-Whitney U-test was used for variables that were not normally distributed. For categorical data, the chi-square test or the chi-square test with a Fisher exact test for 2×2 tables was used. The results were considered significant at a threshold of P<0.05 (two-tailed).
Pearson’s correlation test or Spearman’s rank correlation was used to investigate the correlations between the NEWS+L score and other scores according to the variable distribution. Calibration was assessed using the Hosmer-Lemeshow goodness-of-fit test.
An area under the receiver operating characteristic curve (AUROC) analysis was used to determine the predictive value of the NEWS+L score and other risk scores including the NEWS. The standard error of the mean and P-values for the AUROC and comparisons between the NEWS+L and other risk scores were calculated using the method described by Hanley and McNeil [19]. Sensitivity and specificity analyses were also performed for various cut-off points of the NEWS+L score and for the specific points of the other risk scores that were calculated for the low-risk group.
The logistic regression analysis results are presented as odds ratios with 95% confidence intervals (CIs). To reveal the associations between the serum lactate level and outcomes, multivariable logistic regression analyses were performed after adjusting for other laboratory variables. Trend factors with P<0.1 were included in the multivariable logistic model.
We referred to the STARD (Standards for Reporting Diagnostic Accuracy) statement for the analysis of the results [20]. All analyses were conducted using Stata ver. 11.1 (StataCorp., College Station, TX, USA) and SAS ver. 9.1 (SAS Institute Inc., Cary, NC, USA).
RESULTS
A total of 780 patients who presented with melena, hematochezia, hematemesis, or syncope were screened. Among those, 95 patients were not examined using EGD evaluation. In addition, 111 patients had lower gastrointestinal bleeding, 26 patients did not have UGIB when the EGD evaluation was conducted, and 2 patients were examined before ED arrival. After exclusion of the aforementioned patients, 546 patients were eligible. Among those patients, 16 patients were excluded due to a lack of an initially recorded lactate level. thus, 530 patients were finally enrolled in the analysis (Fig. 1). Of those patients, the composite outcome occurred in 59 patients; in-hospital death occurred in 19 patients, 13 patients were admitted to the ICU, and 40 patients received ≥5 packs of RBC transfusions within 24 hours.
Fig. 1.
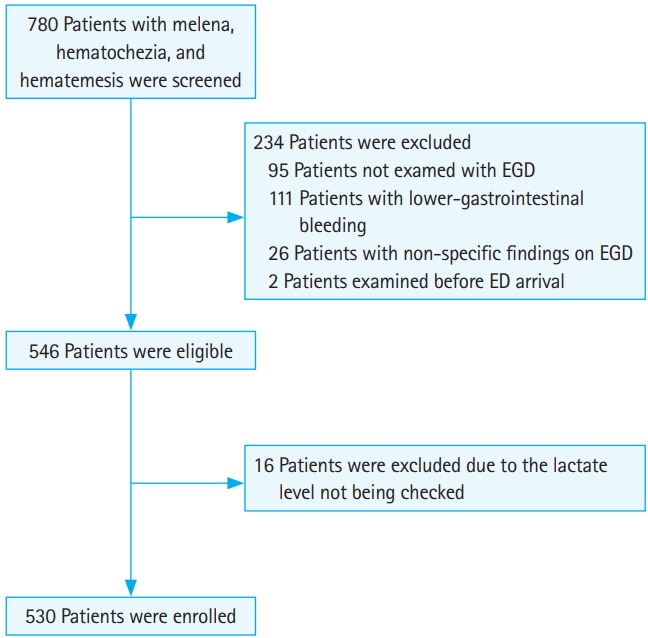
STARD (Standards for Reporting Diagnostic Accuracy) flow diagram for the study. EGD, esophagogastroduodenoscopy; ED, emergency department.
Table 2 shows the characteristics of the entire cohort, the non-composite outcome group, and composite outcome group. The mean age of the entire cohort was 64.5±14.9 years, and 371 (70.0%) patients were male. There was no significant difference between the groups. Chronic liver disease and malignancy were the most frequent co-morbidities, and malignancies were found more often in the composite outcome group. Hematemesis was the most frequent presenting symptom, followed by melena, hematochezia, and syncope. Hematemesis occurred slightly more often in the composite outcome group, while melena occurred more frequently in the non-composite outcome group. The physiologic and laboratory values were significantly worse in the composite outcome group. Bleeding ulcer was the most frequent diagnosis, followed by bleeding varices, hemorrhagic gastritis, bleeding tumor, and Mallory-Weiss syndrome. Bleeding varices were the most frequent diagnosis in the composite outcome group, whereas bleeding ulcers were the most frequent diagnosis in the non-composite outcome group. Endoscopic variceal ligation was the most frequent endoscopic treatment.
Table 2.
Baseline characteristics of the enrolled patients
| All | Non-composite outcome group | Composite outcome group | P-value | |
|---|---|---|---|---|
| Demographics | 530 (100) | 471 (88.9) | 59 (11.1) | |
| Age (yr) | 64.5 ± 14.9 | 64.5 ± 15.1 | 64.6 ± 13.1 | 0.97 |
| < 65 | 237 (44.7) | 209 (44.4) | 28 (47.5) | 0.90 |
| 65–74 | 149 (26.4) | 125 (26.5) | 15 (25.4) | |
| ≥ 75 | 153 (28.9) | 137 (29.1) | 16 (27.1) | |
| Male sex | 371 (70.0) | 330 (70.1) | 41 (69.5) | 0.93 |
| Co-morbidity | ||||
| Chronic liver disease | 157 (29.6) | 134 (28.5) | 23 (39.0) | 0.10 |
| Chronic kidney disease | 31 (5.9) | 26 (5.5) | 5 (8.5) | 0.36 |
| Malignancy | 96 (18.1) | 71 (15.1) | 25 (42.4) | < 0.01 |
| Heart failure | 29 (5.5) | 26 (5.5) | 3 (5.1) | 0.89 |
| Coronary artery disease | 41 (7.7) | 39 (8.3) | 2 (3.4) | 0.19 |
| Presenting symptoms | ||||
| Hematemesis | 262 (49.4) | 227 (48.2) | 35 (59.3) | 0.11 |
| Melena | 259 (48.9) | 240 (51.0) | 19 (32.2) | < 0.01 |
| Hematochezia | 47 (8.9) | 38 (8.1) | 9 (15.3) | 0.07 |
| Syncope | 11 (2.1) | 6 (1.3) | 5 (8.5) | < 0.01 |
| Physiology | ||||
| SBP (mmHg) | 112.9 ± 23.9 | 114.2 ± 23.3 | 102.4 ± 25.8 | < 0.01 |
| ≥ 100, < 110 | 94 (17.7) | 80 (17.0) | 14 (23.7) | < 0.01 |
| ≥ 90, < 100 | 57 (10.8) | 54 (11.5) | 3 (5.1) | |
| < 90 | 61 (11.5) | 44 (9.3) | 17 (28.8) | |
| Pulse rate | 87.7 ± 18.1 | 86.6 ± 17.2 | 96.1 ± 22.6 | < 0.01 |
| < 100 | 120 (22.6) | 97 (20.6) | 23 (39.0) | < 0.01 |
| NEWS | 5.1 ± 1.8 | 5.0 ± 1.7 | 6.3 ± 2.2 | < 0.01 |
| Laboratory findings | ||||
| Lactate (mmol/L) | 2.5 ± 2.7 | 2.2 ± 2.3 | 4.8 ± 3.9 | < 0.01 |
| ≥ 2, < 4 | 122 (23.0) | 104 (22.1) | 18 (30.5) | < 0.01 |
| ≥ 4 | 88 (16.6) | 60 (12.7) | 28 (47.5) | |
| Albumin (g/dL) | 3.3 ± 0.7 | 3.4 ± 0.7 | 2.7 ± 0.6 | < 0.01 |
| < 3.0 | 165 (31.1) | 121 (25.7) | 44 (74.6) | < 0.01 |
| PT INR | 1.3 ± 0.7 | 1.3 ± 0.7 | 1.6 ± 1.0 | < 0.01 |
| > 1.5 | 77 (14.5) | 54 (11.5) | 23 (39.0) | < 0.01 |
| Hb (g/dL) | 9.2 ± 2.8 | 9.5 ± 2.8 | 7.1 ± 2.5 | < 0.01 |
| ≥ 10, < 12 | 100 (18.9) | 96 (20.4) | 4 (6.8) | < 0.01 |
| < 10 | 335 (63.2) | 283 (60.1) | 52 (88.1) | |
| BUN (mg/dL) | 35.3 ± 25.3 | 34.3 ± 23.9 | 43.9 ± 34.0 | <0.01 |
| ≥ 18.2, < 22.4 | 55 (10.4) | 47 (10.0) | 8 (13.6) | 0.03 |
| ≥ 22.4, < 28 | 60 (11.3) | 55 (11.7) | 5 (8.5) | |
| ≥ 28, < 70 | 244 (46.0) | 216 (45.9) | 28 (47.5) | |
| ≥ 70 | 43 (8.1) | 33 (7.0) | 10 (17.0) | |
| Endoscopy | ||||
| Diagnosis | < 0.01 | |||
| Ulcer bleeding | 253 (47.7) | 232 (49.3) | 21 (35.6) | |
| Varix bleeding | 127 (24.0) | 101 (21.4) | 26 (44.1) | |
| Hemorrhagic gastritis | 70 (13.2) | 68 (14.4) | 2 (3.4) | |
| Cancer bleeding | 49 (9.3) | 41 (8.7) | 8 (13.6) | |
| Mallory-Weiss syndrome | 31 (5.9) | 29 (6.2) | 2 (3.4) | |
| Treatment | < 0.01 | |||
| EVL | 87 (43.3) | 72 (42.6) | 15 (46.9) | |
| EVO | 22 (11.0) | 18 (10.7) | 4 (12.5) | |
| Hemostasis (H-E solution) | 71 (35.3) | 67 (39.6) | 4 (12.5) | |
| Hemoclip | 22 (10.5) | 12 (7.1) | 9 (28.1) | |
| Time to endoscopy (hr) | 11.5 ± 17.9 | 11.4 ± 17.8 | 12.4 ± 18.5 | 0.68 |
| Risk scores | ||||
| NEWS+L score | 7.1±3.9 | 6.6±3.4 | 10.7±5.2 | <0.01 |
| PERS | 3.0±1.9 | 2.9±1.8 | 3.9±1.8 | <0.01 |
| GBS | 9.0±4.0 | 8.7±3.9 | 11.4±3.2 | <0.01 |
| AIMS65 | 1.1±1.0 | 1.0±1.0 | 2.0±1.0 | <0.01 |
| Others | ||||
| RBC transfusion number in 24 hr | 1.5 ± 1.0 | 1.4 ± 0.9 | 2.6 ± 0.7 | < 0.01 |
| Ward admission | 396 (74.7) | 354 (75.2) | 42 (71.2) | 0.44 |
| Intervention | 33 (6.2) | 21 (4.5) | 12 (20.3) | < 0.01 |
| Time to intervention (hr) | 14.1 (7.4–67.2) | 15.6 (7.1–57.1) | 12.2 (7.5–69.0) | 0.94a) |
| Operation | 7 (1.3) | 6 (1.3) | 1 (1.7) | 0.56b) |
| Time to operation (day) | 7.2 (3.8–9.4) | 7.3 (7.2–9.4) | 0.9 | 0.13a) |
Values are presented as number (%), mean±standard deviation, or median (interquartile range).
SBP, systolic blood pressure; NEWS, National Early Warning Score; PT INR, prothrombin time international normalized ratio; Hb, hemoglobin; BUN, blood urea nitrogen; EVL, endoscopic variceal ligation; EVO, endoscopic variceal obturation; H-E, hypertonic saline-epinephrine; NEWS+L score, National Early Warning Score+Lactate score; PERS, pre-endoscopic Rockall score; GBS, Glasgow-Blatchford score; AIMS65, albumin, INR, altered mental status, systolic blood pressure, age older than 65 years score; RBC, red blood cell.
Compared by Mann-Whitney U-test.
Compared by Fisher exact test.
The mean time to endoscopy was 11.5±17.9 hours, and there was no significant difference between the composite outcome and non-composite outcome groups. In the entire cohort, the mean values were 7.1±3.9 for the NEWS+L score, 3.0±1.9 for the PERS, 9.0±4.0 for the GBS, and 1.1±1.0 for the AIMS65 score. All scores were worse in the composite outcome group. The mean number of packs of RBCs used for transfusions was 1.5±1.0. The composite outcome group received more RBC transfusions (2.6±0.7) than the non-composite outcome group (1.4±0.9, P<0.001). In the entire cohort, 396 patients (74.7%) were admitted to the ward, and 42 (10.6%) of those patients were in the composite outcome group. Radiologic intervention was more frequent in the composite outcome group (20.3% vs. 6.2%, P<0.01). There were no significant differences in the time to intervention and time to operation.
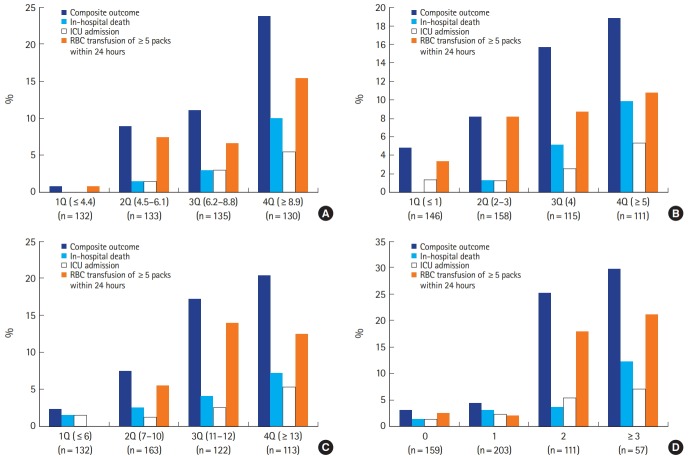
Quartile graphing was used for the easy presentation of the distribution and accordant composite outcomes of the risk scores, except the AIMS65 score (Fig. 2). It was difficult to divide the AIMS65 scores into quartiles due to the inherent nature of this narrow score spectrum. Instead of quartiles, the data presentation of the AIMS65 scores was performed as scores of 0, 1, 2, and ≥3. The proportion of the primary outcome (composite outcome) for the NEWS+L score were 0.8% for the 1st quartile (1Q), 9.0% for 2Q, 11.1% for 3Q, and 23.8% for 4Q. For the PERS, the corresponding proportions were 4.8%, 8.2%, 15.7%, and 18.9%, respectively. For the GBS, they were 2.3%, 7.4%, 17.2%, and 20.4%, respectively. For the AIMS65, the outcomes were 3.1% for 0 points, 4.4% for 1 points, 25.2% for 2 points, and 29.8% for ≥3 points. For patients classified as 1Q of the NEWS+L score, there were no in-hospital deaths or ICU admissions.
Fig. 2.
Distribution and accordant outcomes of (A) the National Early Warning Score+Lactate score, (B) Rockall score, (C) Glasgow-Blatchford score, and (D) albumin, international normalized ratio, altered mental status, systolic blood pressure, age older than 65 years score among patients with upper gastrointestinal bleeding. ICU, intensive care unit; RBC, red blood cell; Q, quartile.
The NEWS+L score showed a moderately (0.3 to 0.5) positive association with other risk scores (with PERS: Spearman’s correlation coefficient r=0.414, P<0.001; with GBS: r=0.407, P<0.001; and with AIMS65: r=0.300, P<0.001).
As measured by the Hosmer-Lemeshow chi-square test for assessment of the goodness of fit for the predicted composite outcome, the observed probability and predicted probability measured by the NEWS+L score were not significantly different (Hosmer-Lemeshow χ2=135.75, 10 degrees of freedom, P=0.538), and neither were those for the PERS (Hosmer-Lemeshow χ2=2.94, 10 degrees of freedom, P=0.916) and GBS (Hosmer-Lemeshow χ2=22.23, 10 degrees of freedom, P=0.176). Only the AIMS65 score showed a significant difference (Hosmer-Lemeshow χ2=11.30, 10 degrees of freedom, P=0.010).
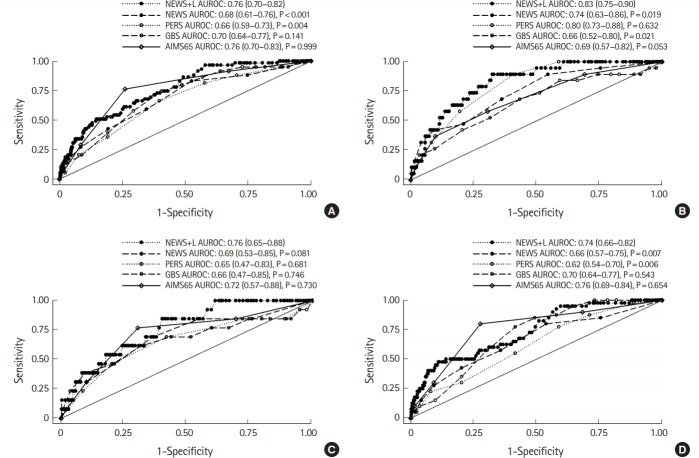
Fig. 3 shows the discriminative power of the risk scores evaluated by the AUROC analysis. For the composite outcome, the AUROC value of the NEWS+L score was the highest among the risk scores (0.76; 95% CI, 0.70 to 0.82), and was significantly higher than that of the PERS (0.66; 95% CI, 0.59 to 0.73; P=0.004); however, there was no significant difference compared to the GBS (0.70; 95% CI, 0.64 to 0.77; P=0.141) and AIMS65 score (0.76; 95% CI, 0.70 to 0.83; P=0.999). For the in-hospital death and ICU admission rates, the AUROC values of the NEWS+L score were also the highest and these were significantly higher or comparable to those of the other risk scores. For RBC transfusions ≥5 packs within 24 hours, the AUROC value of the AIMS65 was the highest, despite no significant difference being observed compared with that of the NEWS+L score. For all primary and secondary outcomes, the NEWS+L score showed a better discriminative power than the NEWS score alone.
Fig. 3.
Area under the receiver operating characteristic (AUROC) curves for the National Early Warning Score+Lactate (NEWS+L) score, pre-endoscopic Rockall score (PERS), Glasgow-Blatchford score (GBS), albumin, international normalized ratio, altered mental status, systolic blood pressure, age older than 65 years score (AIMS65), and NEWS among patients with upper gastrointestinal bleeding. (A) For composite outcome, (B) for in-hospital death, (C) for ICU admission, and (D) for RBC transfusion ≥5 packs within 24 hours. ICU, intensive care unit; RBC, red blood cell.
Table 3 shows the diagnostic performances of the risk scores, including the sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio. Generally, high sensitivity is required for the risk scores in order to identify the low-risk group for a certain outcome. The cut-off points for the PERS, GBS, and AIMS65 scores were determined according to the low-risk group point for each risk score. However, the NEWS+L score did not report any specific point for the low-risk group, only patients with a NEWS+L score of 7 or more were regarded as the high-risk group. Therefore, we tested various cut-off points that discriminated a similar number of patients for the low-risk group compared to other risk scores. The sensitivities of NEWS+L scores of 3, 4, and 5 for the composite outcome were 100%, 98.3%, and 96.6%, respectively. The sensitivity of a GBS of 0 was the same as the sensitivity of a NEWS+L score of 4, but the GBS discriminated only 13 patients (2.5%) while the NEWS+L score discriminated 92 patients (17.4%) as the low-risk group. The sensitivities of the PERS and AIMS65 score were lower than that of the NEWS+L score.
Table 3.
Diagnostic characteristics of the risk scores for the composite outcome among patients with upper gastrointestinal bleeding
| Risk scores | Cut-off point | No. of patients (%) | SN/SP | PPV/NPV | +LR/-LR |
|---|---|---|---|---|---|
| NEWS+L | 3 | 34 (6.4) | 100.0/7.2 | 11.9/100 | 1.08/- |
| 4 | 92 (17.4) | 98.3/19.3 | 13.2/98.9 | 1.22/0.09 | |
| 5 | 171 (32.3) | 96.6/35.9 | 15.9/98.8 | 1.51/0.09 | |
| PERS | 0 | 56 (10.6) | 94.9/11.3 | 11.8/94.6 | 1.07/0.45 |
| GBS | 0 | 13 (2.5) | 98.3/2.6 | 11.2/92.3 | 1.01/0.67 |
| AIMS65 | 0 | 159 (30.0) | 91.5/32.7 | 14.6/96.9 | 1.36/0.26 |
The cut-off points were chosen to identify the low-risk patient group. Composite outcome of in-hospital death, intensive care unit admission, and red blood cell transfusions requiring ≥5 packs within 24 hours.
SN, sensitivity; SP, specificity; PPV, positive predictive value; NPV, negative predictive value; +LR, positive likelihood ratio; -LR, negative likelihood ratio; NEWS+L, National Early Warning Score+Lactate; PERS, pre-endoscopic Rockall score; GBS, Glasgow-Blatchford score; AIMS65, albumin, international normalized ratio, altered mental status, systolic blood pressure, age older than 65 years score.
Logistic regression analyses were performed to investigate the associations between the laboratory variables and the primary and secondary outcomes (Table 4). When tested as a continuous variable, serum lactate was a powerful predictor for most outcomes (adjusted odds ratio [AOR] 1.22 [1.11 to 1.34], P<0.001 for the composite outcome; 1.16 [1.04 to 1.30], P=0.008 for in-hospital death; and 1.24 [1.12 to 1.37], P<0.001 for RBC transfusions requiring ≥5 packs within 24 hours) except for ICU admissions (1.13 [0.99 to 1.29], P=0.071). Only serum albumin was significantly associated with all outcomes. Similar results were obtained when tested as categorical variables (AOR of lactate ≥4 mmol/L, 6.60 [2.94 to 14.82], P<0.001 for the composite outcome; AOR of lactate ≥4 mmol/L, 11.10 [2.77 to 44.40], P=0.001 for in-hospital death; AOR of lactate ≥4 mmol/L, 5.09 [1.11 to 23.38], P=0.036 for the rate of ICU admission; and AOR of lactate ≥4 mmol/L, 5.98 [2.29 to 15.62], P<0.001 for RBC transfusions requiring ≥5 packs within 24 hours). No variable except lactate was significantly associated with the outcomes.
Table 4.
Logistic regression analyses for the primary and secondary outcomes among patients with upper gastrointestinal bleeding
| Primary outcome |
Secondary outcomes |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Composite outcome (n=59) |
In-hospital death (n=19) |
ICU admission (n=13) |
RBC transfusion ≥5 packs within 24 hr (n=40) |
||||||
| AOR | P-value | AOR | P-value | AOR | P-value | AOR | P-value | ||
| Continuous variables | |||||||||
| Lactate | 1.22 (1.11–1.34) | < 0.001 | 1.16 (1.04–1.30) | 0.008 | 1.13 (0.99–1.29) | 0.071 | 1.24 (1.12–1.37) | < 0.001 | |
| Albumin | 0.36 (0.21–0.62) | < 0.001 | 0.34 (0.17–0.69) | 0.003 | 0.42 (0.18–0.97) | 0.043 | 0.45 (0.24–0.83) | 0.011 | |
| PT INR | 1.03 (0.73–1.44) | 0.878 | 1.16 (0.83–1.63) | 0.385 | |||||
| Hb | 0.80 (0.68–0.93) | 0.003 | 0.62 (0.51–0.75) | < 0.001 | |||||
| BUN | 1.01 (1.00–1.02) | 0.112 | 1.01 (1.00–1.03) | 0.067 | 1.01 (1.00–1.03) | 0.111 | |||
| Categorical variables | |||||||||
| Lactate (mmol/L) | |||||||||
| < 2 | Reference | Reference | Reference | Reference | |||||
| ≥ 2, < 4 | 3.04 (1.37–6.74) | 0.006 | 5.00 (1.19–20.92) | 0.028 | 4.16 (0.97–17.88) | 0.056 | 2.60 (0.97–6.98) | 0.057 | |
| ≥ 4 | 6.60 (2.94–14.82) | < 0.001 | 11.10 (2.77–44.40) | 0.001 | 5.09 (1.11–23.38) | 0.036 | 5.98 (2.29–15.62) | < 0.001 | |
| Albumin (g/dL) | |||||||||
| ≥ 3.0 | Reference | Reference | Reference | Reference | |||||
| < 3.0 | 4.40 (2.12–9.12) | < 0.001 | 2.12 (0.75–6.02) | 0.156 | 1.76 (0.55–5.69) | 0.343 | 4.16 (1.69–10.25) | 0.002 | |
| PT INR | |||||||||
| ≤ 1.5 | Reference | Reference | |||||||
| > 1.5 | 1.13 (0.54–2.39) | 0.742 | 1.52 (0.66–3.50) | 0.322 | |||||
| Hb (g/dL) | |||||||||
| ≥ 12 | Reference | Reference | |||||||
| ≥ 10, < 12 | 0.84 (0.17–4.26) | 0.834 | 0.48 (0.13–1.78) | 0.270 | |||||
| < 10 | 1.92 (0.52–7.13) | 0.330 | |||||||
| BUN (mg/dL) | |||||||||
| < 18.2 | Reference | Reference | Reference | ||||||
| ≥ 18.2, < 22.4 | 2.44 (0.75–7.97) | 0.139 | 1.32 (0.28–6.18) | 0.725 | 3.29 (0.76–14.23) | 0.111 | |||
| ≥ 22.4, < 28 | 1.56 (0.42–5.79) | 0.508 | 0.51 (0.06–4.70) | 0.553 | 2.30 (0.48–11.13) | 0.299 | |||
| ≥ 28, < 70 | 1.45 (0.58–3.64) | 0.423 | 0.40 (0.11–1.48) | 0.170 | 2.11 (0.64–6.92) | 0.219 | |||
| ≥ 70 | 3.21 (1.01–10.19) | 0.048 | 2.72 (0.68–10.91) | 0.159 | 2.45 (0.55–10.92) | 0.239 | |||
Trend factors that showed a significance of P<0.1 in the univariate logistic regression analysis were entered into the multivariable logistic regression model. Only the following laboratory variables were included in the logistic regression analysis: lactate, albumin, PT INR, Hb, and BUN.
ICU, intensive care unit; RBC, red blood cell; AOR, adjusted odds ratio; PT INR, prothrombin time international normalized ratio; Hb, hemoglobin; BUN, blood urea nitrogen.
DISCUSSION
In this study, the NEWS+L score showed better predictive value for the composite outcome of in-hospital mortality, ICU admission, and RBC transfusions requiring ≥5 packs within 24 hours than the PERS, and comparable predictive value to the GBS and AIMS65 score among patients with UGIB. For the secondary outcomes, the NEWS+L score also showed better or comparable predictive values over the other risk scores. The sensitivities of NEWS+L scores of 3, 4, and 5 were 100%, 98.3%, and 96.6%, respectively, which were higher than those of the other risk scores. The serum lactate level was a significant predictor for outcomes even after adjusting for other laboratory variables.
The Rockall score was developed in 1996 to identify patients at low risk for further bleeding or death; this allowed those patients to be discharged without waiting for a diagnostic endoscopy [10]. The complete Rockall score includes age; the presence of shock, as measured by the pulse rate and systolic blood pressure; comorbidities; diagnosis; and endoscopic stigmata of recent hemorrhage, while the PERS only includes age, presence of shock, and comorbidities. In the original cohort, patients who scored 2 or less were regarded as being at low-risk for re-bleeding (4.3%) and death (0.1%). Of note, no laboratory markers are included in the Rockall score, which may be due to the limited use of laboratory machines in previous decades.
The GBS was developed in 2000 to identify a patient’s need for treatment [11]. However, the outcome is a mixture of a treatment portion, which includes any blood transfusion or any operative or endoscopic intervention, and another portion, which includes a substantial decrease in the hemoglobin level, re-bleeding, or death. In addition, the pulse rate, systolic blood pressure, comorbidities, presenting symptoms, and blood urea and hemoglobin levels are included in the GBS. This score showed an excellent discriminative performance (AUROC 0.92) in the original cohort [11]. In a previous meta-analysis, a GBS of 0 was associated with a low likelihood (0.02) of the need for urgent endoscopic intervention [21].
The AIMS65 score developed in 2011 was designed to provide an easy calculation for in-hospital mortality prediction [12]. The AIMS65 score includes albumin levels, the INR, (altered) mental status, systolic blood pressure, and age older than 65 years. In the original cohort, the AUROC was 0.80 for in-hospital mortality; among patients with an AIMS65 score of 0, 0.3% were deceased.
However, a recent study reported that the aforementioned three scores showed only fair to poor discriminative value for 30-day mortality. Moreover, the PERS and AIMS65 score showed poor discriminative value for the need for transfusions [15]. Similarly, Robertson et al. [22] reported that the GBS and PERS showed fair discriminative values for mortality and that the three scores showed fair to poor discriminative values for ICU admission.
In addition to the components of the aforementioned risk scores, researchers have revealed other factors that are associated with mortality among patients with UGIB. Most of those factors are related to patient co-morbidities, including glucocorticosteroid use [23], smoking, alcohol consumption, chronic obstructive pulmonary disease [24], diabetes mellitus and malignancy [25]. Meanwhile, laboratory findings have not been vigorously tested to identify potential risk factors among patients with UGIB.
In 2014, Shah et al. [15] reported that hyperlactatemia (≥4 mmol/L) showed an odds ratio of 6.4 for in-hospital mortality among 1,644 patients with gastrointestinal bleeding. In addition, after controlling for age, initial hematocrit, and heart rate, every 1-point increase in lactate had an odds ratio of 1.4 for mortality. In 2015, El-Kersh et al. [16] reported that the AUROC of lactate was 0.802 to predict in-hospital mortality among 133 patients with UGIB who were admitted to the ICU. The AOR of lactate levels above 2.1 mmol/L was 8.2.
Consistent findings with previous report were confirmed in the present study. In the multivariable logistic regression analyses, the serum lactate level remained a significant predictive factor for in-hospital death, ICU admission, and the need for ≥5 packs of RBC transfusion. However, no other laboratory markers were identified as significant factors.
In this context, we investigated the predictive value of the NEWS+L score, which includes the serum lactate level, among patients with UGIB and compared the performance of the NEWS+L score to traditional risk scores, namely the PERS, GBS, and AIMS65 score. Originally, the NEWS+L score was designed from data of critically ill medical patients and showed comparable or better predictive performance for in-hospital mortality over the Acute Physiology And Chronic Health Evaluation II (APACHE II), Simplified Acute Physiology Score (SAPS) II, and SAPS III [17]. The NEWS+L score performed better than the Trauma and Injury Severity Score (TRISS) in blunt trauma patients [26] and was comparable to the Pneumonia Severity Index (PSI) in community-acquired pneumonia patients [27]. Additionally, the NEWS+L score showed an excellent discriminative value for predicting 2-day mortality in general ED patients [28]. As expected, the NEWS+L score showed better or comparable prediction of the primary and secondary outcomes among patients with UGIB than the other risk scores in the present study.
The serum lactate level is increased via anaerobic glycolysis, which is representative of tissue hypoxia. Decreased hemoglobin levels can lead to reduced arterial oxygen content. When the cardiac output is unable to compensate for the decreased arterial oxygen content, the patient will inevitably face tissue hypoxia. Furthermore, even normal ranges of hematocrit and vital signs do not convey the absence of tissue hypoxia among patients with UGIB. Actually, Shah et al. [15] reported the occurrence of hyperlactatemia despite normal hematocrit and heart rate values. This finding implied that hyperlactatemia may be a first presenting sign among clinically available parameters in UGIB. Other mechanisms for hyperlactatemia in sepsis, such as mitochondrial dysfunction, increased aerobic glycolysis via tissue cytokine-mediated glucose uptake, and catecholamine-enhanced Na-K skeletal-muscle pump activity, do not seem to be directly linked to UGIB; however, further detailed research may be needed.
Endoscopy is a major treatment for UGIB. However, endoscopy utilizes substantial hospital resources and tends to be limited after work hours. In the United Kingdom, only 52% of hospitals performed emergency endoscopy after work hours [29]. Therefore, for emergency physicians, it is important to discriminate patients who are at a low-risk for an adverse outcome and for whom the emergent call to a gastroenterologist can be postponed. To date, traditional clinical scores seem to play a given role for this purpose. However, 20 and 15 years have passed since the PERS [10] and GBS [11] were introduced, respectively. In the future, we expect that the NEWS+L score could be used to identify low-risk groups among patients with UGIB.
The present study has some limitations. First, this was a retrospective, observational, single center study. The inherent weaknesses relating to the study design should be addressed. Uncertainty for generalizability to other settings such as different hospitals, regions, or nations is a major concern that should be noted. However, while there is a report that patient-related factors are associated with poorer outcomes, surrounding factors such as country, the size of the hospital, or the profile of the team managing the event have not been shown to be associated with those poor outcomes [30]. This previous report favors the possibility that the results of this study could be replicated in another setting. The risk for impairments in data collection, biases, or a lack of randomly distributed exposure should also be considered. However, most of the variables in this study are objective and easy to retrieve, and possible biases were avoided whenever possible. Finally, it should be noted that the data abstractor was not blinded to the outcome.
In conclusion, the NEWS+L score showed better discriminative performance than the PERS, and comparable discriminative performance to both the GBS and AIMS65 values for the composite of in-hospital death, ICU admission, and requiring RBC transfusion of ≥5 packs within 24 hours. Thus, the NEWS+L score may be used to identify the low-risk group among patients with UGIB.
Capsule Summary
What is already known
Recently, the serum lactate level has been shown to be a significant predictor of mortality in upper gastrointestinal bleeding (UGIB). However, the traditional risk scores that are currently used for UGIB do not contain the serum lactate level as one of their components.
What is new in the current study
The National Early Warning Score+Lactate score showed better discriminative performance than the pre-endoscopic Rockall score and comparable discriminative performance to the Glasgow-Blatchford score and albumin, international normalized ratio, altered mental status, systolic blood pressure, age older than 65 years score for the composite of in-hospital death, intensive care unit admission, and requiring red blood cell transfusion of ≥5 packs within 24 hours.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Lirio RA. Management of upper gastrointestinal bleeding in children: variceal and nonvariceal. Gastrointest Endosc Clin N Am. 2016;26:63–73. doi: 10.1016/j.giec.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth GF. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. 1995;90:206–10. [PubMed] [Google Scholar]
- 3.Lanas A, Perez-Aisa MA, Feu F, et al. A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal anti-inflammatory drug use. Am J Gastroenterol. 2005;100:1685–93. doi: 10.1111/j.1572-0241.2005.41833.x. [DOI] [PubMed] [Google Scholar]
- 4.Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med. 2008;359:928–37. doi: 10.1056/NEJMra0706113. [DOI] [PubMed] [Google Scholar]
- 5.Silverstein FE, Gilbert DA, Tedesco FJ, Buenger NK, Persing J. The national ASGE survey on upper gastrointestinal bleeding. II. Clinical prognostic factors. Gastrointest Endosc. 1981;27:80–93. doi: 10.1016/s0016-5107(81)73156-0. [DOI] [PubMed] [Google Scholar]
- 6.Lin HJ, Wang K, Perng CL, Lee CH, Lee SD. Heater probe thermocoagulation and multipolar electrocoagulation for arrest of peptic ulcer bleeding: a prospective, randomized comparative trial. J Clin Gastroenterol. 1995;21:99–102. doi: 10.1097/00004836-199509000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Kankaria AG, Fleischer DE. The critical care management of nonvariceal upper gastrointestinal bleeding. Crit Care Clin. 1995;11:347–68. [PubMed] [Google Scholar]
- 8.Choi YJ, Kim KS, Suh GJ, Kwon WY. Diagnostic accuracy and implementation of computed tomography angiography for gastrointestinal hemorrhage according to clinical severity. Clin Exp Emerg Med. 2016;3:69–74. doi: 10.15441/ceem.15.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gralnek IM, Dumonceau JM, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1–46. doi: 10.1055/s-0034-1393172. [DOI] [PubMed] [Google Scholar]
- 10.Rockall TA, Logan RF, Devlin HB, Northfield TC. Selection of patients for early discharge or outpatient care after acute upper gastrointestinal haemorrhage. National Audit of Acute Upper Gastrointestinal Haemorrhage. Lancet. 1996;347:1138–40. doi: 10.1016/s0140-6736(96)90607-8. [DOI] [PubMed] [Google Scholar]
- 11.Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356:1318–21. doi: 10.1016/S0140-6736(00)02816-6. [DOI] [PubMed] [Google Scholar]
- 12.Saltzman JR, Tabak YP, Hyett BH, Sun X, Travis AC, Johannes RS. A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest Endosc. 2011;74:1215–24. doi: 10.1016/j.gie.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Park SM, Yeum SC, Kim BW, et al. Comparison of AIMS65 score and other scoring systems for predicting clinical outcomes in Koreans with nonvariceal upper gastrointestinal bleeding. Gut Liver. 2016;10:526–31. doi: 10.5009/gnl15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch A, Buendgens L, Duckers H, et al. Bleeding origin, patient-related risk factors, and prognostic indicators in patients with acute gastrointestinal hemorrhages requiring intensive care treatment: a retrospective analysis from 1999 to 2010. Med Klin Intensivmed Notfmed. 2013;108:214–22. doi: 10.1007/s00063-013-0226-2. [DOI] [PubMed] [Google Scholar]
- 15.Shah A, Chisolm-Straker M, Alexander A, Rattu M, Dikdan S, Manini AF. Prognostic use of lactate to predict inpatient mortality in acute gastrointestinal hemorrhage. Am J Emerg Med. 2014;32:752–5. doi: 10.1016/j.ajem.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 16.El-Kersh K, Chaddha U, Sinha RS, Saad M, Guardiola J, Cavallazzi R. Predictive role of admission lactate level in critically Ill patients with acute upper gastrointestinal bleeding. J Emerg Med. 2015;49:318–25. doi: 10.1016/j.jemermed.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Jo S, Lee JB, Jin YH, et al. Modified early warning score with rapid lactate level in critically ill medical patients: the ViEWS-L score. Emerg Med J. 2013;30:123–9. doi: 10.1136/emermed-2011-200760. [DOI] [PubMed] [Google Scholar]
- 18.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 19.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–43. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 20.Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. doi: 10.1136/bmj.h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srygley FD, Gerardo CJ, Tran T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307:1072–9. doi: 10.1001/jama.2012.253. [DOI] [PubMed] [Google Scholar]
- 22.Robertson M, Majumdar A, Boyapati R, et al. Risk stratification in acute upper GI bleeding: comparison of the AIMS65 score with the Glasgow-Blatchford and Rockall scoring systems. Gastrointest Endosc. 2016;83:1151–60. doi: 10.1016/j.gie.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Klebl F, Bregenzer N, Schofer L, et al. Risk factors for mortality in severe upper gastrointestinal bleeding. Int J Colorectal Dis. 2005;20:49–56. doi: 10.1007/s00384-004-0624-2. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Hernandez H, Rodriguez-Moran M, Gonzalez JL, et al. Risk factors associated with upper gastrointestinal bleeding and with mortality. Rev Med Inst Mex Seguro Soc. 2009;47:179–84. [PubMed] [Google Scholar]
- 25.Lee YJ, Min BR, Kim ES, et al. Predictive factors of mortality within 30 days in patients with nonvariceal upper gastrointestinal bleeding. Korean J Intern Med. 2016;31:54–64. doi: 10.3904/kjim.2016.31.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jo S, Lee JB, Jin YH, et al. Comparison of the trauma and injury severity score and modified early warning score with rapid lactate level (the ViEWS-L score) in blunt trauma patients. Eur J Emerg Med. 2014;21:199–205. doi: 10.1097/MEJ.0b013e32836192d6. [DOI] [PubMed] [Google Scholar]
- 27.Jo S, Jeong T, Lee JB, Jin Y, Yoon J, Park B. Validation of modified early warning score using serum lactate level in community-acquired pneumonia patients: the National Early Warning Score-Lactate score. Am J Emerg Med. 2016;34:536–41. doi: 10.1016/j.ajem.2015.12.067. [DOI] [PubMed] [Google Scholar]
- 28.Jo S, Yoon J, Lee JB, Jin Y, Jeong T, Park B. Predictive value of the National Early Warning Score-Lactate for mortality and the need for critical care among general emergency department patients. J Crit Care. 2016;36:60–8. doi: 10.1016/j.jcrc.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Use of endoscopy for management of acute upper gastrointestinal bleeding in the UK: results of a nationwide audit. Gut. 2010;59:1022–9. doi: 10.1136/gut.2008.174599. [DOI] [PubMed] [Google Scholar]
- 30.Lanas A, Aabakken L, Fonseca J, et al. Clinical predictors of poor outcomes among patients with nonvariceal upper gastrointestinal bleeding in Europe. Aliment Pharmacol Ther. 2011;33:1225–33. doi: 10.1111/j.1365-2036.2011.04651.x. [DOI] [PubMed] [Google Scholar]