ABSTRACT
Primary and secondary liver malignancies are common and associated with a poor prognosis. Surgical resection is the treatment of choice; however, many patients have unresectable disease. In these cases, several liver directed therapies are available, including selective internal radiation therapy (SIRT). SIRT is a multidisciplinary treatment involving nuclear medicine, interventional radiology and oncology. High doses of localised internal radiation are selectively delivered to liver tumour tissues, with relative sparing of adjacent normal liver parenchyma. Side effects are minimal and radiation protection measures following treatment are straightforward. In patients who have progressed following chemotherapy, clinical trials demonstrate prolonged liver progression-free survival. SIRT is offered at 10 centres in England via the NHS England Commissioning through Evaluation programme and is approved by the National Institute for Health and Care Excellence for certain liver malignancies. SIRT holds unique promise for personalised treatment of liver tumours.
KEYWORDS: Internal, liver, malignancy, radiation, selective, SIRT, therapy
Key points
Most patients with liver metastases are unsuitable for liver resection
SIRT is a truly multidisciplinary treatment involving nuclear medicine, interventional radiology and oncology, relying on targeted intra-arterial delivery of radioactive (usually90Y) labelled microspheres to the liver
There is established clinical benefit evidence and growing research evidence about the safety and efficacy of SIRT in patients refractory to chemotherapy
NICE have reviewed the evidence for SIRT in colorectal liver metastases, hepatocellular carcinoma and cholangiocarcinoma and NHS England funding via the Commissioning Through Evaluation programme is currently available
The two-part procedure is reasonably well tolerated, with minimal side effects
Introduction
Liver metastases are the commonest form of malignant liver disease and are associated with a poor prognosis.1 Metastases come most frequently from primary colorectal cancers (CRCs) and about 20% of CRC patients present with liver metastases. In Europe, primary liver tumours, including hepatocellular carcinoma (HCC) and cholangiocarcinoma are less common. Surgical resection is the treatment of choice, with 5-year overall survival rates of around 50%. However, only up to 20% of patients with liver metastases are candidates for surgical resection.2 For unresectable disease, non-surgical approaches include systemic chemotherapy, which has proven survival benefits3 but is associated with toxicity, short duration and poor response rates, leading to poor clinical outcomes; therefore, effective non-surgical liver directed therapeutic strategies are required.
For primary liver tumours or liver only/liver predominant metastases, local rather than systemic treatments should logically result in better efficacy and reduced systemic toxicity. Examples of these local treatments include microwave and radiofrequency ablation, trans-arterial embolisation without chemotherapy and with chemotherapy, stereotactic ablative body radiotherapy and selective internal radiation therapy (SIRT), also known as transarterial radioembolisation.
SIRT is a promising treatment modality in inoperable primary liver malignancies (mainly HCC) and liver metastases (mainly CRC) and also other malignancies such as intrahepatic cholangiocarcinoma, breast and neuroendocrine tumour metastases. SIRT is not yet included in liver management guidelines as outcomes of randomised controlled trials are awaited. The European Association for the Study of the Liver guidelines recommend trans-arterial embolisation with chemotherapy for intermediate stage HCC and the biological agent sorafenib for advanced stage disease.4 Published studies suggest outcomes following SIRT compare favourably with those following these conventional treatments. For CRC metastases, the role of SIRT in the therapeutic strategy remains to be defined, with current and future research anticipated to better define this.
Selective internal radiation therapy
SIRT relies on the delivery of a radioactive substance into the tumour vascular supply via an intra-arterial catheter placed under radiological guidance. Depending on the tumour site, this may be in the common hepatic artery, right or left hepatic arteries or smaller branches. This exploits the fact that both primary and secondary liver tumours derive 80–100% of their blood supply from the hepatic artery, whereas the hepatic artery supplies only up to 30% of the normal liver parenchyma. The aim is to deliver a tumouricidal dose of radiation (>100 Gy) to tumour tissues, with relative sparing of adjacent normal liver parenchyma. The most commonly used radionuclide is yttrium-90 (90Y) labelled to resin microspheres (SIR-Spheres) or embedded in glass microspheres (TheraSpheres). Holmium-166 labelled microspheres (QuiremSpheres) are also used (Table 1). A typical whole liver treatment activity using resin microspheres is 1.5-2 GBq, while a typical glass microsphere treatment is 5 GBq. The microspheres size range (20–60 μm) allows optimal peri-tumoral vascular deposition and prevents passage into the venous circulation and bypassing the liver. They remain permanently implanted in the liver and are not metabolised or excreted. The 90Y isotope is on the surface of resin microspheres and internally for glass microspheres. For a similar unit of activity, there are fewer glass microspheres, which contain more activity per sphere, compared with more resin microspheres, which contain less activity per sphere. The high number of resin microspheres most probably accounts for flow stasis during resin microspheres administration, stasis has never been reported with glass microspheres. The fewer glass microspheres give less uniform irradiation, which is associated with reduced toxicity and which also makes tolerable a higher absorbed dose.5
Table 1.
Properties of compounds used in selective internal radiation therapy
| TheraSpheres | SIR-Spheres | QuiremSpheres | |
|---|---|---|---|
| Size, μm | 20–30 | 20–60 | 20–50 |
| Isotope | Yttrium-90 in glass matrix | Yttrium-90 adsorbed on resin surface | Holmium-166 on PLLA (poly-L-lactic acid) |
| Radiation emitted | beta (β) | beta (β) | beta (β) and low energy gamma (γ) |
| Physical half-life (T 1/2) | 64 hours | 64 hours | 26.8 hours |
| Activity per sphere, Bq | 2500 | 50 | 450 |
| Number of particles | ∼1–8×106 | ∼40–80×106 | 33×106 |
| Angiographic monitoring | No | Necessary (potential reflux) | Necessary (potential reflux) |
Evidence for efficacy
A phase I study demonstrated that SIRT could be added safely to oxaliplatin-based chemotherapy, with promising outcome data.6 Another clinical trial was carried out in Australia and included 21 patients with colorectal liver metastases, 11 of whom received SIRT plus chemotherapy. Median overall survival was 29 months with SIRT plus chemotherapy versus 13 months with chemotherapy alone.7 Another multicentre trial from Europe included 44 patients; median overall survival was 10 months with SIRT plus chemotherapy versus 7 months with chemotherapy alone. The shorter survival period in this trial was due to the greater liver metastatic burden.8
The SIRFLOX study compared the efficacy and safety of SIRT using SIR-Spheres in 530 chemotherapy naïve patients with colorectal liver metastases.9 The addition of SIRT to FOLFOX chemotherapy did not improve progression-free survival at any site. However, there was a median 7.9 months prolongation of liver progression-free survival in the SIRT arm. There has been criticism of the SIRFLOX trial, in that additional treatment, such as liver resection, was not pursued if SIRT was successful;10 however, this view may not be universally shared. The UK-led FOXFIRE study tested the hypothesis that addition of SIRT to chemotherapy for patients with colorectal liver metastases will show superiority compared with chemotherapy alone. FOXFIRE is now closed and outcome reports are due in 2017.11 The ongoing EPOCH trial is evaluating the efficacy and safety of TheraSpheres in patients with colorectal liver metastases scheduled to receive second-line chemotherapy.
In HCC, SIRT results in longer time-to-progression and reduced toxicity compared with chemoembolisation.12 Several ongoing phase III randomised trials are comparing the safety and efficacy of SIRT in advanced/unresectable HCC. STOP-HCC (NCT01556490, ClinicalTrials.gov) and YES-P (NCT01887717, ClinicalTrials.gov) are comparing SIRT with TheraSpheres versus standard of care with sorafenib. SIRVENIB (NCT01135056, ClinicalTrials.gov) and SARAH (NCT01482442, ClinicalTrials.gov) are comparing overall survival between SIRT with SIR-spheres and sorafenib. The SORAMIC study (NCT01126645, ClinicalTrials.gov) is comparing sorafenib versus sorafenib plus SIRT with SIR-spheres. Early results are due to be presented in 2017.
Practical aspects of SIRT
SIRT delivery requires a truly multidisciplinary team approach involving nuclear medicine, interventional radiology and oncology. Radiologists assess liver tumour and extra-hepatic disease using computerised tomography (CT) or magnetic resonance imaging (Fig 1) and interventional radiologists perform the required angiographies. Nuclear medicine and medical physics assist in SIRT dosimetry, delivery and radiation protection, while oncologists provide ongoing patient care. Pre-therapy imaging also assesses hepatic vasculature, including portal vein patency. Biochemical liver and renal function are evaluated. Good liver synthetic function is required and patients with irreversible elevated bilirubin level should be excluded. Patients with renal impairment may need to be excluded as high volumes of iodinated contrast media are used during angiography.
Fig 1.
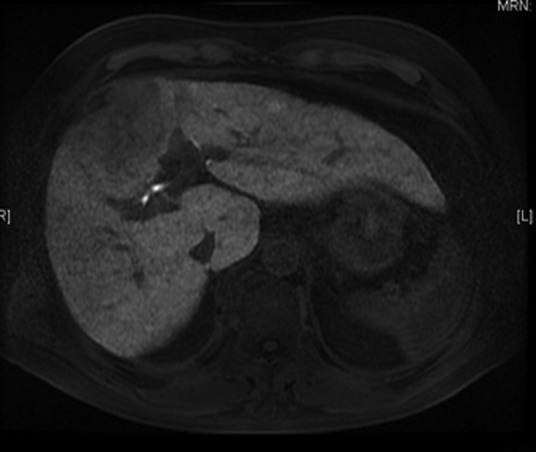
LAVA magnetic resonance image showing a hepatocellular carcinoma in segment 4 of the liver.
Procedure
SIRT is a two-part procedure and a pre-therapy angiography is initially performed. Vessels feeding the tumour are assessed, usually the common hepatic artery, lobar arteries or smaller branches. To prevent potential extrahepatic retrograde flow of radioactive material into the stomach, duodenum and pancreas, the gastroduodenal, right gastric and left gastric arteries may be embolised. The catheter is then placed at the site(s) of subsequent treatment delivery and technetium-99m labelled macroaggregated albumin (99mTc-MAA) is administered. MAA is similar in size to microspheres and acts as a surrogate marker of its distribution. MAA is used in lung perfusion scans for potential pulmonary emboli assessment.
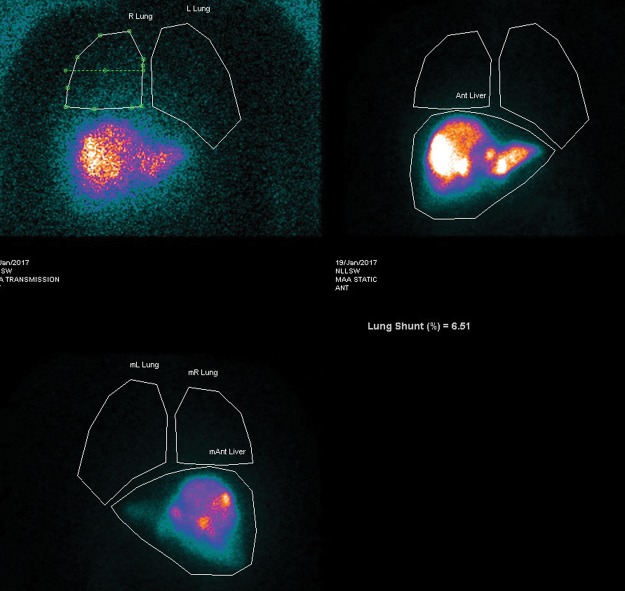
Following MAA administration, the patient is transferred to nuclear medicine for planar thorax and abdomen and single photon emission computerised tomography (SPECT)-CT abdomen imaging. This identifies hepatic and extrahepatic uptake. If there is extrahepatic activity (Fig 2), further vessel embolisation is needed before SIRT. Arteriovenous shunts within the tumour neovasculature can result in blood being shunted from the liver to the lungs, with risk of radiation-induced pneumonitis. From these images, the lung shunt fraction is calculated (Fig 3). This determines if SIRT is appropriate and also allows treatment dose adjustments.
Fig 2.
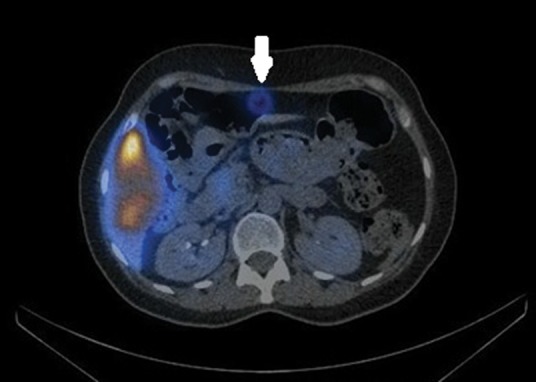
A technetium-99m macroaggregated albumin single photon emission computerised tomography-computerised tomography scan of the upper abdomen showing extra-hepatic activity in the falciform artery territory (arrowed). The falciform artery was embolised and the patient successfully treated 10 days later.
Fig 3.
Planar thorax and abdomen images following technetium-99m macroaggregated albumin intrahepatic administration. Lung shunt fraction calculated at 6.5%. This confirms no significant liver to lung shunting and selective internal radiation therapy would be appropriate.
Before treatment, the amount of radioactivity to be administered is calculated from the liver and tumour volumes and lung shunt fraction values. To ensure that no embolised vessels can recannulise, treatment should be performed within 14 days of the pre-therapy angiography. Despite embolisation, some particles may still reach the duodenum or stomach and therefore a 4–6 week course of proton pump inhibitors is prescribed. SIRT is then delivered under radiological control. After treatment, the patient returns to the ward and at 6–24 hours, 90Y Bremsstrahlung planar and SPECT CT or positron emission tomography CT imaging is performed to evaluate the distribution of the administered activity (Fig 4). Discharge the day after treatment is the norm.
Fig 4.
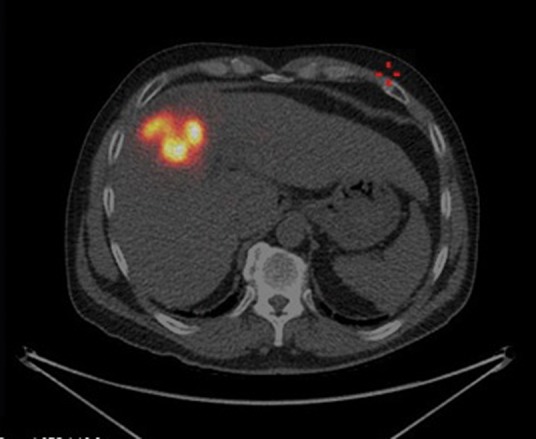
Post-selective internal radiation therapy (SIRT) positron emission tomography computerised tomography image shows intense concentration of SIRT within segment 4 of liver.
Aftercare
Following treatment, patients may have some liver pain and nausea, which can last for a few days. Therefore pain relief and anti-emetics are given. Radiation pneumonitis is rare and can manifest as dyspnoea and dry cough several weeks after treatment. Radioembolisation-induced liver disease is a late, usually 1–2 months post-treatment, and rare complication. It manifests as jaundice and ascites, without tumour progression or biliary obstruction.1390Y emits only β-particles, which cause DNA damage and cell death, with a range of about 2 cm. Bremsstrahlung radiation is also emitted as X-rays with a low yield but does allow imaging. The density of the human body is ideal for the absorption of β-radiation so minimal radioactivity is emitted post treatment. However, to minimise external radiation exposure to others, patients are advised to minimise prolonged close contact (<1 m) with children under 5 and pregnant women. The advice varies between different treatment centres and departments; however, in general, this would usually be for 1 week following therapy. As there is a potential risk of triggering radiation alarms, it is also usually advised to avoid international travel for 1 week post therapy.
National Institute for Health and Care Excellence guidelines
The National Institute for Health and Care Excellence (NICE) has reviewed the evidence for SIRT and published interventional procedures guidance for SIRT in unresectable colorectal liver metastases, primary intrahepatic cholangiocarcinoma and primary HCC.14–16 NICE has also published medtech innovation briefings about SIRT technology.17,18 These include SIR-spheres for treating inoperable HCC and TheraSpheres for treating operable and inoperable HCC.
Funding for SIRT in the NHS
In November 2013, NHS England announced a £4.8 million initiative as part of the time limited Commissioning through Evaluation (CTE) programme. This approach improves access to SIRT, enabling suitable patients to be treated. Ten centres in England were selected and provide SIRT to around 220 patients a year.19 Details for all patients undergoing SIRT are entered onto the UK SIRT register. The programme closed on 31 March 2017 and NHS England will make a funding decision based on the NICE report from the CTE programme, plus any other published evidence in the interim, including outcomes from ongoing and recently completed phase III trials.
Conclusions
There is well established clinical benefit evidence and growing research evidence regarding SIRT. Access to SIRT on the NHS is limited to a few expert centres that have the staff, equipment and experience to deliver the complex multiprofessional therapeutic pathway. Radiation protection concerns and high costs may be a drawback in considering SIRT as a therapeutic option. SIRT holds unique promise for a personalised therapeutic approach to liver tumours, which will enable us to administer the right treatment dose for the right patient at the right time.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Ananthakrishnan A. Gogineni V. Saeian K. Epidemiology of primary and secondary liver cancers. Semin Intervent Radiol. 2006;23:47–63. doi: 10.1055/s-2006-939841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Research UK Bowel cancer survival statistics. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/survival. [Accessed 29 August 2017]
- 3.Kopetz S. Chang GJ. Overman MJ. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–83. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Spreafico C. Maccauro M. Mazzaferro V. Chiesa C. The dosimetric importance of the number of 90Y microspheres in liver transarterial radioembolization (TARE) Eur J Nucl Med Mol Imaging. 2014;41:634–38. doi: 10.1007/s00259-013-2674-6. [DOI] [PubMed] [Google Scholar]
- 6.Sharma RA. Van Hazel GA. Morgan B. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol. 2007;25:1099–106. doi: 10.1200/JCO.2006.08.7916. [DOI] [PubMed] [Google Scholar]
- 7.Van Hazel G. Blackwell A. Anderson J. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol. 2004;88:78–85. doi: 10.1002/jso.20141. [DOI] [PubMed] [Google Scholar]
- 8.Hendlisz A. Van den Eynde M. Peeters M. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol. 2010;28:3687–94. doi: 10.1200/JCO.2010.28.5643. [DOI] [PubMed] [Google Scholar]
- 9.von Hazel GA. Heinemann V. Sharma NK. SIRFLOX: randomized phase III trial comparing first-line mFOLFOX6 (Plus or Minus Bevacizumab) versus mFOLFOX6 (plus or minus bevacizumab) plus selective internal radiation therapy in patients with metastatic colorectal cancer. J Clin Oncol. 2016;34:1723–31. doi: 10.1200/JCO.2015.66.1181. [DOI] [PubMed] [Google Scholar]
- 10.Buscombe JR. Selective internal radiation therapy in metastatic carcinoma of the colon: a story of nonintegrated care? World J Nucl Med. 2016;15:79–80. doi: 10.4103/1450-1147.178010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma RA. Wasan HS. Love SB. FOXFIRE: a phase III clinical trial of chemo-radio-embolisation as first-line treatment of liver metastases in patients with colorectal cancer. Clin Oncol (R Coll Radiol) 2008;20:261–3. doi: 10.1016/j.clon.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Salem R. Lewandowski RJ. Kulik L. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497–507. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winter H. Boardman P. Morgan D. Greenhalgh T. Sharma R. Advances in selective internal radiotherapy for primary and secondary liver cancer. RAD magazine. 2016;42(496):17–8. [Google Scholar]
- 14.National Institute for Health and Care Excellence Selective internal radiation therapy for non-resectable colorectal metastases in the liver. NICE interventional procedures guidance No 401. London: NICE; 2013. [Google Scholar]
- 15.National Institute for Health and Care Excellence Selective internal radiation therapy for primary hepatocellular carcinoma. NICE interventional procedures guidance No 460. London: NICE; 2013. [Google Scholar]
- 16.National Institute for Health and Care Excellence Selective internal radiation therapy for primary intrahepatic cholangiocarcinoma. NICE interventional procedures guidance No 459. London: NICE; 2013. [Google Scholar]
- 17.National Institute for Health and Care Excellence TheraSphere for treating operable and inoperable hepatocellular carcinoma. NICE medtech innovation briefing No 62. London: NICE; 2016. [Google Scholar]
- 18.National Institute for Health and Care Excellence SIR-Spheres for treating inoperable hepatocellular carcinoma. NICE medtech innovation briefing No 63. London: NICE; 2016. [Google Scholar]
- 19.NHS England NHS England announces hospitals chosen to take part in new initiative aimed at increasing access to radiotherapy. Leeds: NHS England; 2013. www.england.nhs.uk/2013/11/sirt-comm/ [Accessed 26 July 2017] [Google Scholar]