Graphical abstract
Keywords: Pulmonary hypertension, Scurvy, Echocardiography
Highlights
-
•
PH can sometimes be a challenging diagnosis.
-
•
Echocardiography can assess RV overload and evaluate sudden changing in PASP.
-
•
Although rare, scurvy should be part of an expanded differential diagnosis of PH.
Introduction
Etiologies of pulmonary hypertension (PH) include several conditions, such as left-sided and congenital heart disease, lung disease and hypoxia, diseases that affect directly the pulmonary vascular system, and thromboembolism.1 We describe the case of a patient who presented with dyspnea, tachycardia, and hypoxia. Transthoracic echocardiogram (TTE) revealed severe dilation of the right ventricle associated with elevated pulmonary pressures. The etiology of PH was unclear, and we administered different medical treatments considering the most common causes of PH. Despite these treatments and an initial alleviation of symptoms with normalization of pulmonary pressure, unexpected worsening of the clinical picture associated with waxing and waning of PH characterized the 6-month follow-up. The appearance of other systemic signs (dermatologic and ocular) led us to investigate rare causes of PH. A careful nutritional history revealed dietary deficiency in vitamin C, confirmed by an undetectable level of ascorbic acid, and a diagnosis of scurvy was supposed. Complete resolution of symptoms associated with pulmonary pressure normalization was obtained with daily vitamin C supplementation.
Case Presentation
A 66-year-old man was admitted to our hospital for increasing dyspnea, tachycardia, and hypotension. He had celiac disease and lactose intolerance and had no smoking history. He was well until 4 months previously, when dyspnea and asthenia occurred. On admission, blood pressure was 115/70 mm Hg. Electrocardiography showed sinus rhythm and mild ST-segment depression with inverted T waves in leads V1 to V4. Blood gas analysis showed pH of 7.49, pO2 of 57 mm Hg, pCO2 of 21 mm Hg, lactate of 2.4 mmol/L, HCO3− of 16 mmol/L, and O2 saturation of 91%. Results of chest radiography were normal. Blood test results were normal except for mild kidney failure (serum creatinine 1.80 mg/dL), mild increases in brain natriuretic peptide (298 pg/mL) and d-dimer (878 ng/mL), and normal values of C-Reactive Protein, total protein, serum protein electrophoresis, angiotensin-converting enzyme, and rheumatoid factor. Antinuclear antibody was 1:80 (speckled), with negative results on extractible nuclear antibody panel, including antibodies to Ribonucleoprotein, Anti Sjögren's syndrome related antigen-A, Anti Sjögren's syndrome related antigen-B, anti Smith, scleroderma antibody against topoisomerase 1, double-stranded deoxyribonucleic acid, mitochondrial, smooth muscle, antineutrophil cytoplasmic antibody, antineutrophil perinuclear antibody, and anticentromere antibody. Results of hypercoagulable workup were normal (prothrombin and thromboplastin time, fibrinogen, protein C and S, antilupus antibody, homocysteine, anticardiolipin antibody, and anti-B2 glycoprotein).
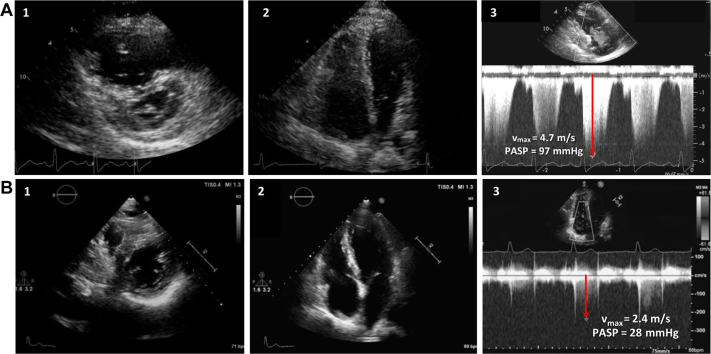
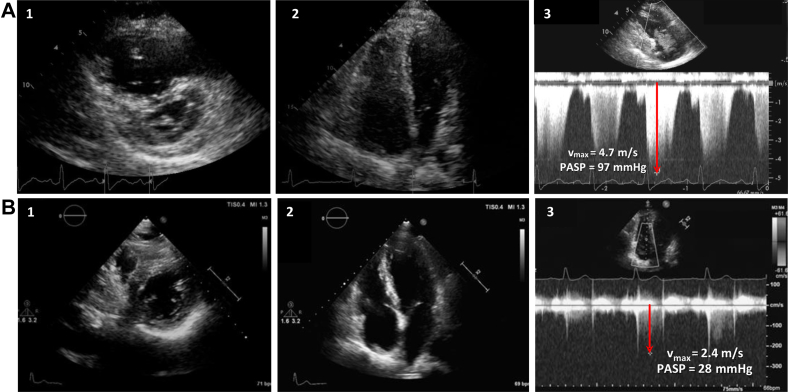
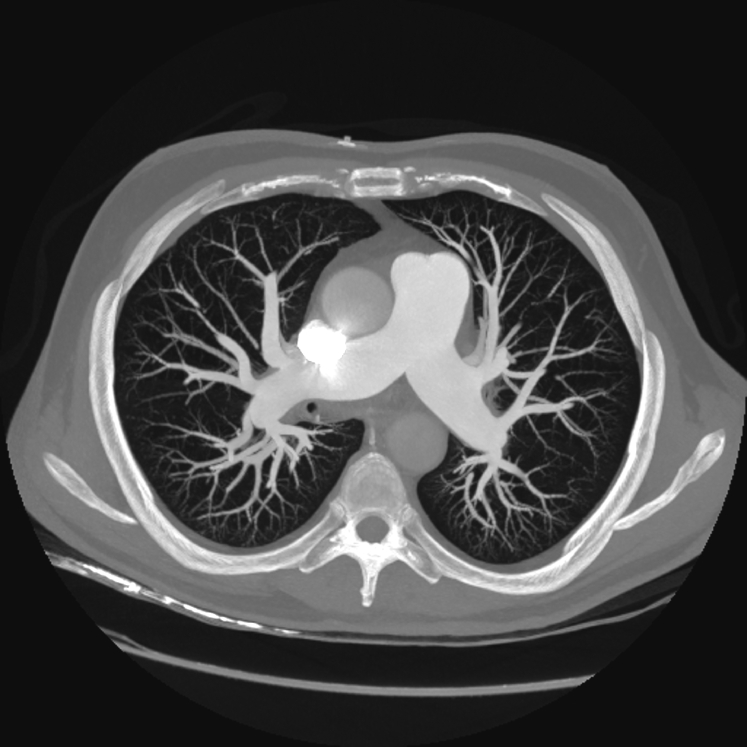
TTE showed right ventricular (RV) dilation (RV end-diastolic basal diameter 49 mm) and dysfunction (tricuspid annular plane systolic excursion 16 mm, fractional area change 25%) with moderate tricuspid regurgitation and severe pulmonary systolic hypertension (pulmonary artery systolic pressure [PASP] 97 mm Hg; Figure 1A, Video 1). Given suspicion for pulmonary thromboembolism, chest computed tomography and ventilation/perfusion lung scans (Figure 2) were performed, with normal results. Results of spirometry were normal except for a moderate depression of diffusing capacity for carbon monoxide. For further investigation, cardiac magnetic resonance was performed, confirming RV hypertrophy and dilatation and hypokinesis of the RV free wall (see Video 2). Results of lower extremity Doppler ultrasound were negative for deep vein thrombosis.
Figure 1.
(A) Severe dilation and pressure overload of the right ventricle on TTE in short-axis view (1) and four-chamber view (2) associated with high PASP derived by the maximum tricuspid regurgitation velocity (Vmax, red arrow) with continuous-wave Doppler (3). (B) Normalization of RV dimension in both short-axis view (1) and four-chamber view (2) with normal PASP (red arrow shows the tricuspid Vmax) (3) after vitamin C supplementation.
Figure 2.
Computed tomographic scan of the lungs showing normal filling of pulmonary arteries and of the entire pulmonary tree.
In the presence of an undetermined etiology of PH, treatment with diuretics and oral anticoagulation was started.1 The patient experienced temporary clinical improvement associated with near normalization of RV overload (PASP 30 mm Hg).
Six months later, the patient became increasingly dyspneic, and physical examination showed large, spontaneous ecchymosis along the left thigh and diffuse purpura over the upper and lower extremities.2, 3 Bilateral xerophthalmia was also observed.4 A new pulmonary computed tomographic scan was performed, with normal findings. Blood tests showed mild anemia (hemoglobin 10.4 g/dL), while the results of hypercoagulable and autoimmunity tests were negative. Soft tissue echography and computed tomography of the lower left leg showed a large hematoma with an adipose tissue effusion.5 New TTE showed RV overload and moderate PH (PASP 46 mm Hg). Anticoagulation was interrupted with mild clinical improvement and partial reabsorption of leg ecchymosis. The patient was discharged and 3 months later reported dyspnea and spontaneous onset of ecchymosis in the abdomen. He was readmitted and underwent a ventilation/perfusion lung scan that was negative for embolism sources, while TTE showed severe RV pressure overload (PASP 80 mm Hg). The hypothesis of primitive PH was raised, and bosentan was started,6 without any improvement. In the meantime an accurate dietary history revealed that for the past 8 months the patient had eaten mostly rice and chicken, with no fresh fruits or vegetables. Assays for vitamins revealed an undetectable level of vitamin C. A diagnosis of scurvy2, 3, 4, 5 was supposed, and oral vitamin C (1 g/d) supplementation was started.
The patient experienced relief of dyspnea and normalization of oxygen saturation within 1 week. After 2 weeks, TTE showed only mild RV dilation and normalization of PASP (31 mm Hg). To confirm that the hypothesis of scurvy was correct, we discontinued bosentan, maintaining only vitamin C implementation. The patient felt progressively better throughout 1-year follow-up, with normal exercise capacity and normalization of vitamin C level. TTE confirmed PASP normalization (28 mm Hg; Figure 1B, Video 3).
Discussion
Scurvy is rarely seen nowadays in developed countries. However, poor diets devoid of fresh fruits and vegetables and low food variety can still cause this forgotten disease. Classical signs of vitamin C deficiency include weakness, anemia, tooth loss, gum bleeding, bruises, petechiae, and dyspnea. In our patient the clinical presentation was very unusual, beginning with pure respiratory symptoms (i.e., dyspnea) thus orienting toward more common etiologies, such as pulmonary thromboembolism and rheumatologic or congenital or chronic heart disease. Subsequently the onset of dermatologic lesions and the absence of response to oral anticoagulation and to bosentan led us to consider other causes of PH. Unexpected worsening and alleviation of symptoms associated with waxing and waning of RV pressure overload would have also been signs that a common “organic” (i.e., thromboembolism or primary PH) etiology of PH was incoherent. In scurvy, PH refers to pulmonary vasoconstriction induced by the lack of endothelial nitric oxide (NO), which modulates vasodilatory capacity7, 8 and to changes in the collagen matrix.9
NO is a mediator of vascular smooth muscle relaxation and is generated by the action of the enzyme NO synthetase, which promotes the conversion of L-arginine to L-citrulline. Ascorbic acid has been shown to increase the production of NO by endothelial cells by the degradation of the NO synthetase inhibitor asymmetric dimethyl L-arginine, stabilizing and increasing NO synthetase cofactors and inhibiting the arginase pathway that competes for arginine. Thus, deficiency of vitamin C decreases NO production, leading to an increased vascular tone and resulting in PH. Vitamin C is also an essential cofactor of prolyl-hydroxylase, which regulates the activity of the hypoxia-inducible family of transcription factors, responsible for the body's response to hypoxia. An inappropriate response to hypoxia due to vitamin C lack is the other trigger mechanism involved in pulmonary vasoconstriction.10
Conclusion
Because scurvy presents with clinical features similar to many other common conditions, it must be considered as part of an expanded differential diagnosis of PH. Simple vitamin C supplementation therapy is indicated, leading to complete normalization of the clinical picture.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2018.07.010.
Supplementary Data
Short-axis view on TTE showing dilation and pressure overload (interventricular septal flattening).
Long-axis view of right ventricle in steady-state free precession.
Short-axis view on TTE showing normal dimension and function of both left and right ventricles.
References
- 1.Galiè N., Humbert M., Vachiery J.L., Gibbs S., Lang I., Torbicki A. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 2.Pimentel L. Scurvy: historical review and current diagnostic approach. Am J Emerg Med. 2003;21:328–332. doi: 10.1016/s0735-6757(03)00083-4. [DOI] [PubMed] [Google Scholar]
- 3.Hirschmann J.V., Raugi G.J. Adult scurvy. J Am Acad Dermatol. 1999;41:895–906. doi: 10.1016/s0190-9622(99)70244-6. [DOI] [PubMed] [Google Scholar]
- 4.Hood J., Burns C.A., Hodges R.E. Sjögren's syndrome in scurvy. N Engl J Med. 1970;282:1120. doi: 10.1056/NEJM197005142822003. [DOI] [PubMed] [Google Scholar]
- 5.Fain O. Musculoskeletal manifestations of scurvy. Joint Bone Spine. 2005;72:124–128. doi: 10.1016/j.jbspin.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Rubin L.J., Badesch D.B., Barst R.J., Galiè N., Black C.M., Keogh A. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 7.Smith T.G., Robbins P.A., Ratcliffe P.J. The human side of hypoxia-inducible factor. Br J Haematol. 2008;141:325–334. doi: 10.1111/j.1365-2141.2008.07029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holowatz L.A. Ascorbic acid: what do we really NO? J Appl Physiol. 2011;111:1542–1543. doi: 10.1152/japplphysiol.01187.2011. [DOI] [PubMed] [Google Scholar]
- 9.Peterkofsky B. Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy. Am J Clin Nutr. 1991;54:1135S–1140S. doi: 10.1093/ajcn/54.6.1135s. [DOI] [PubMed] [Google Scholar]
- 10.Duvall M.G., Pikman Y., Kantor D.B., Ariagno K., Summers L., Sectish T.C. Pulmonary hypertension associated with scurvy and vitamin deficiencies in an autistic child. Pediatrics. 2013;132:e1699–e1703. doi: 10.1542/peds.2012-3054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Short-axis view on TTE showing dilation and pressure overload (interventricular septal flattening).
Long-axis view of right ventricle in steady-state free precession.
Short-axis view on TTE showing normal dimension and function of both left and right ventricles.