Graphical abstract
Keywords: Left atrial appendage occlusion, WaveCrest device
Highlights
-
•
The WaveCrest device is a novel LAA occluder with unique features.
-
•
Two-dimensional and 3D TEE and fluoroscopy are essential for successful implantation.
-
•
Air between layers of WaveCrest ePTFE fabric may obscure visualization on TEE.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia, affecting >3 million people in the United States alone.1 AF impairs cardiac function, resulting in significant morbidity and mortality, and is a major risk factor for thromboembolic stroke.2 AF can be classified as either valvular (typically defined as developing in the context of rheumatic heart disease or valve replacement) or nonvalvular (developing in the absence of significant valvular heart disease).3
The left atrial appendage (LAA) is the most common site of thrombus formation in AF. Specifically, the LAA is the site of thrombus formation in approximately 91% of patients with nonvalvular AF and 57% of patients with valvular AF.4 Although exclusion of the LAA is frequently performed as an adjunct to other cardiac procedures, the mainstay of thromboembolism prophylaxis in AF has traditionally been systemic anticoagulation therapy using warfarin or the direct oral anticoagulants. As systemic therapies for a frequently local disease, all anticoagulants have significant bleeding risks and may be contraindicated in certain patients.
Percutaneous LAA occlusion has revolutionized the treatment of nonvalvular AF, using a local therapy for a local disease. At present, there are two categories of percutaneous LAA occlusion devices: endocardially delivered and epicardially delivered. In the United States, available endocardially delivered devices are the Watchman (Boston Scientific, Maple Grove, MN) and Amplatzer Amulet (St. Jude Medical, Minneapolis, MN). The Lariat device (Sentre-HEART, Palo Alto, CA) is epicardially delivered.
Two-dimensional and increasingly three-dimensional transesophageal echocardiography (TEE) is essential for preprocedural screening, device sizing, intraprocedural guidance, and assessment of procedural success.5
We describe the first US case of endocardial percutaneous LAA occlusion using the novel Coherex WaveCrest 1.3 device (Biosense Webster, Irvine, CA). This device is currently under investigation in the United States in the WaveCrest II trial, a noninferiority randomized controlled trial evaluating the safety and effectiveness of the WaveCrest device compared with the Watchman device.
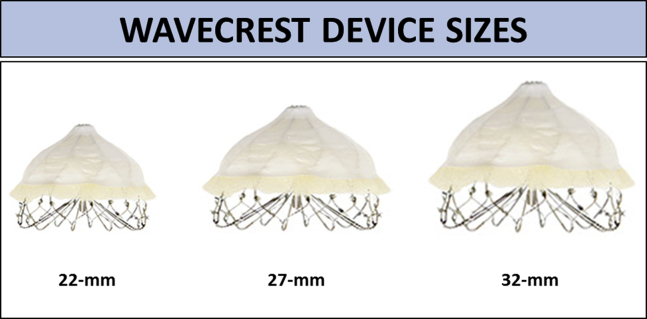
The WaveCrest 1.3 device consists of a self-expanding nitinol frame with 20 anchoring points, covered by an expanded polytetrafluoroethylene (ePTFE; also known as Gore-Tex) fabric. It comes in three sizes (22, 27, and 32 mm), as shown in Figure 1.
Figure 1.
WaveCrest device sizes. The WaveCrest LAA occluder device is available in three sizes: 22, 27, and 32 mm.
The following manufacturer's Web page provides an animated description of the WaveCrest procedure: https://coherex.com/the-wavecrest-option/.
This device has a number of unique features, which are listed in Table 1.
Table 1.
WaveCrest device features
| Possible advantages |
|
| Possible disadvantages |
|
Of note, the WaveCrest 1.3 device is an upgrade from the previous WaveCrest 1.2 device, which received a Conformité Européene mark in Europe. The major differences between the WaveCrest 1.2 and 1.3 devices are that the 1.3 device has more anchors and has an extended ePTFE cover.
Case Presentation
A 72-year-old woman with a history of hypertension was diagnosed with persistent nonvalvular AF. Her CHA2DS2-VASc score was calculated at 3 (age > 65 years, hypertension, female gender). She was originally started on systemic anticoagulation therapy with rivaroxaban. After experiencing severe bruising, she was referred to the electrophysiology department for consideration of percutaneous LAA occlusion therapy. She was then enrolled in the WaveCrest II trial.
On preprocedural TEE, LAA size and shape were deemed suitable for closure with a WaveCrest or Watchman device, and no echocardiographic exclusion criteria were found (Table 2).
Table 2.
Echocardiographic exclusion criteria
|
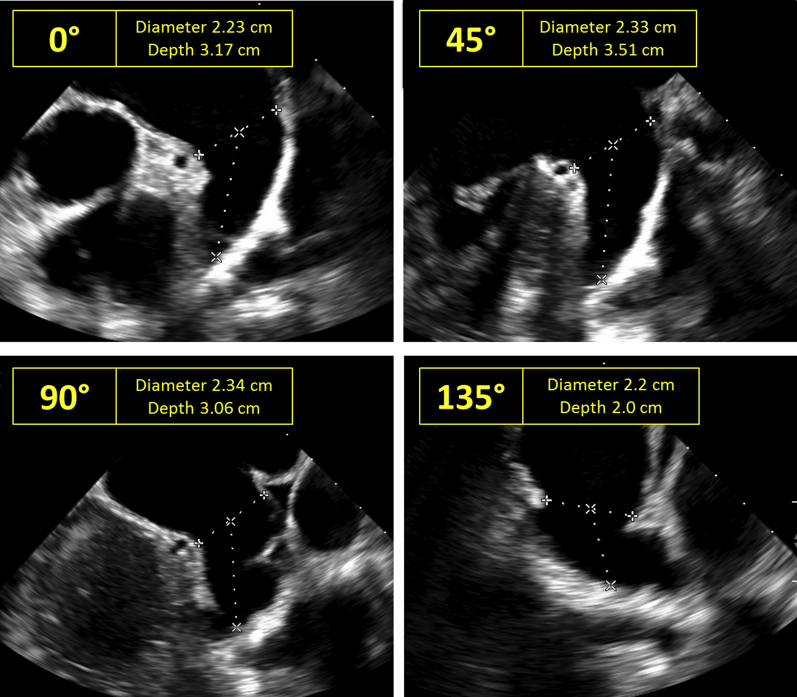
During the procedure, a comprehensive baseline assessment was performed using two-dimensional and three-dimensional TEE. LAA maximal orifice size and depth were imaged at 0°, 45°, 90°, and 135°, similar to the Watchman procedure (Figure 2).
Figure 2.
LAA device sizing for WaveCrest on two-dimensional TEE. To size the LAA for the WaveCrest occluder, LAA orifice diameter and depth are measured on two-dimensional TEE at 0°, 45°, 90°, and 135°, similar to the sizing done for the Watchman device.
The WaveCrest procedure begins with transfemoral venous access. Subsequently, a transseptal puncture is performed. Similar to other LAA occluder device designs, the ideal position for transseptal puncture during the WaveCrest procedure is within the inferior and posterior portion of the interatrial septum. This provides the most direct route to the LAA.
Thereafter, the delivery sheath is placed within the LAA, and the WaveCrest device is then opened within the LAA without anchoring. The device is positioned and tested for device seal and the absence of paradevice leak. This is achieved using color Doppler on echocardiography and contrast injections on fluoroscopy. Injections are performed both distal to the device (in the LAA) and proximal to the device (in the left atrium). Unlike the Watchman, in which deployment and proximal anchoring occur simultaneously, the WaveCrest is first positioned, and once adequate location and seal are confirmed, the device anchors are deployed on the distal, rather than proximal, edge of the device. Stability of the device is then assessed using a “tug test.” If excessive motion of the device is absent, it is released from the delivery sheath.
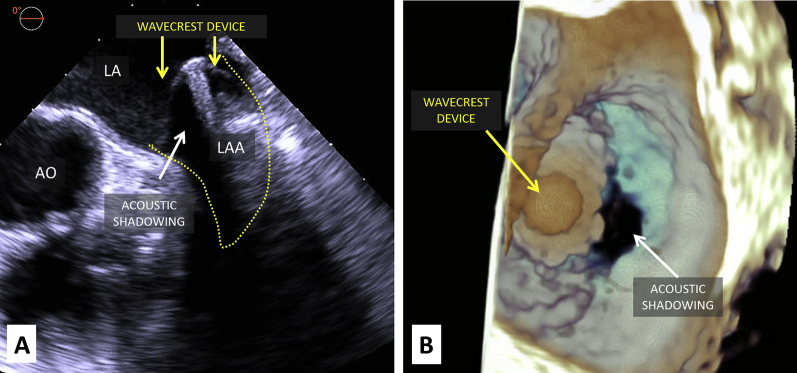
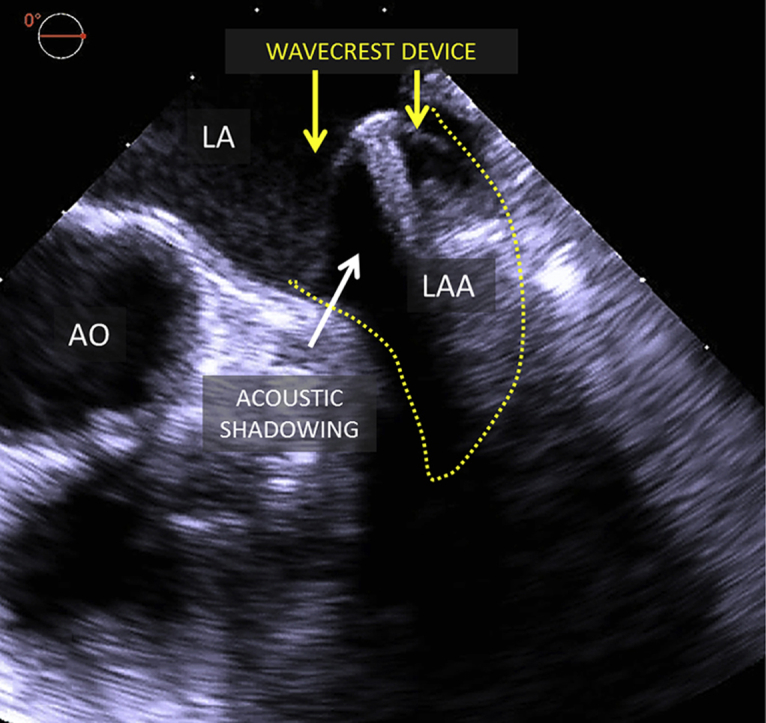
Although TEE is essential for transseptal puncture guidance, LAA sizing, and device deployment, acoustic shadowing due to the presence of air between layers of WaveCrest ePTFE fabric may partly hinder echocardiographic assessment of device seal (Figure 3, Video 1). However, this limitation is overcome by complementary use of fluoroscopy with injections in both the left atrium and LAA (Figure 4, Video 2).
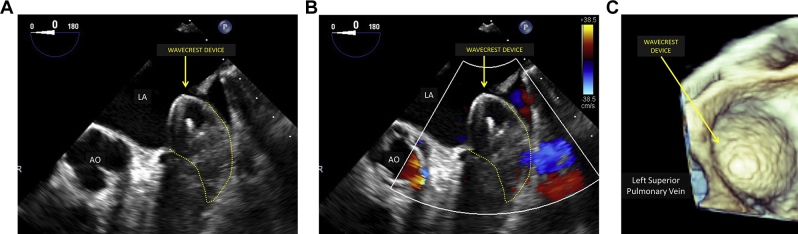
Figure 3.
Intraprocedural imaging of WaveCrest device on two-dimensional and three-dimensional TEE. Intraprocedural TEE of the WaveCrest device after deployment (midesophageal LAA view at 0°). Yellow arrows point to the WaveCrest device, and white arrows point to acoustic shadowing caused by air between device fabric layers on two-dimensional (A) and three-dimensional (B) TEE. AO, Aorta; LA, left atrium.
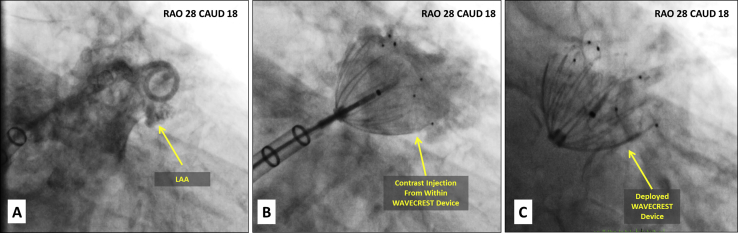
Figure 4.
Intraprocedural imaging of WaveCrest device on fluoroscopy. Contrast fluoroscopy of the LAA before (A), during (B), and after (C) WaveCrest device deployment. Contrast injection from within the partially deployed LAA occluder shown in (B) is a unique feature of the WaveCrest device. CAUD, Caudal; RAO, right anterior oblique.
On 45-day follow up, TEE is performed to reassess device seal and to confirm the absence of device-associated thrombus. At this time point, endothelialization typically has begun, which decreases the amount of air between the layers of ePTFE fabric. This improves the quality of echocardiographic imaging (Figure 5, Videos 3 and 4).
Figure 5.
Forty-five-day follow-up two-dimensional TEE in the midesophageal LAA view at 0° (A) demonstrates a better visualized WaveCrest device compared with intraprocedural TEE, as air between fabric layers has receded. Color Doppler shows no significant paradevice leak (B). Three-dimensional TEE also improves on 45-day follow-up compared with intraprocedural study (C). AO, Aorta; LA, left atrium.
Discussion
Enthusiasm for percutaneous exclusion of the LAA from systemic circulation has been increasing with the emergence of multiple new occluder designs. Because two-dimensional and three-dimensional TEE is an integral component of these procedures, echocardiographers must be aware of specific characteristics of each individual device.
We describe the first case in the United States of the novel WaveCrest LAA occlusion device. Overall, the echocardiographic guidance of the WaveCrest is very similar to other endocardially delivered LAA occluders, such as the Watchman; unique features of the WaveCrest device design and imaging guidance are shown in Table 1.
Conclusion
This case demonstrates the first US use of a novel percutaneous LAA occluder, the WaveCrest 1.3 device, which is currently under clinical investigation. The WaveCrest device is similar to the Watchman in that it is endocardially delivered using a transseptal puncture but has unique features such as separation of positioning from anchoring, seal along the distal rather than proximal margin of the device, and Gore-Tex fabric.
Although TEE is essential for transseptal puncture guidance, LAA sizing and device deployment, acoustic shadowing due to the presence of air between layers of WaveCrest ePTFE fabric may partly hinder intraprocedural echocardiographic assessment of device seal. This limitation is overcome by complementary use of fluoroscopy with injections in both the left atrium and LAA. Acoustic shadowing diminishes and echocardiographic imaging improves after device endothelialization.
Footnotes
Conflicts of interest: Dr. Saric is a member of the speakers bureaus of Philips and Medtronic and the advisory board of Siemens. The other authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2018.07.005.
Supplementary Data
Intraprocedural TEE of the WaveCrest device using simultaneous biplane imaging of the midesophageal LAA at 45° and 135° views as well as volume-rendered three-dimensional TEE. LA, Left atrium; MV, mitral valve.
Contrast fluoroscopy of the LAA during the WaveCrest procedure. Contrast injections are initially performed before WaveCrest deployment to define LAA anatomy. After the WaveCrest is partially deployed, contrast injections are performed on both the LAA and left atrial sides of the LAA occluder. LA, Left atrium.
Forty-five-day follow-up two-dimensional TEE in the midesophageal LAA view at 45° demonstrates the WaveCrest device. Color Doppler analysis shows no significant paradevice leak. LA, Left atrium; MV, mitral valve.
Forty-five-day follow-up three-dimensional TEE demonstrates the WaveCrest device within the LAA orifice. Three-dimensional TEE with color Doppler shows no significant paradevice leak. The basal portion of the WaveCrest device is anchored in the LAA orifice, while the mid and apical portion of the device protrudes like a cone into the left atrium.
References
- 1.Naccarelli G.V., Varker H., Lin J., Schulman K.L. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V., Rydén L.E., Cannom D.S., Crijns H.J., Curtis A.B., Ellenbogen K.A. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57:e101–e198. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 3.January C.T., Wann L.S., Alpert J.S., Calkins H., Cigarroa J.E., Cleveland J.C., Jr. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Blackshear J.L., Odell J.A. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 5.Vainrib A.F., Harb S.C., Jaber W., Benenstein R.J., Aizer A., Chinitz L.A. Left atrial appendage occlusion/exclusion: procedural image guidance with transesophageal echocardiography. J Am Soc Echocardiogr. 2018;31:454–474. doi: 10.1016/j.echo.2017.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intraprocedural TEE of the WaveCrest device using simultaneous biplane imaging of the midesophageal LAA at 45° and 135° views as well as volume-rendered three-dimensional TEE. LA, Left atrium; MV, mitral valve.
Contrast fluoroscopy of the LAA during the WaveCrest procedure. Contrast injections are initially performed before WaveCrest deployment to define LAA anatomy. After the WaveCrest is partially deployed, contrast injections are performed on both the LAA and left atrial sides of the LAA occluder. LA, Left atrium.
Forty-five-day follow-up two-dimensional TEE in the midesophageal LAA view at 45° demonstrates the WaveCrest device. Color Doppler analysis shows no significant paradevice leak. LA, Left atrium; MV, mitral valve.
Forty-five-day follow-up three-dimensional TEE demonstrates the WaveCrest device within the LAA orifice. Three-dimensional TEE with color Doppler shows no significant paradevice leak. The basal portion of the WaveCrest device is anchored in the LAA orifice, while the mid and apical portion of the device protrudes like a cone into the left atrium.