Abstract
Electrophiles and reactive oxygen species (ROS) play a major role in modulating cellular defense mechanisms as well as physiological functions, and intracellular signaling. However, excessive ROS generation (endogenous and exogenous) can create a state of redox imbalance leading to cellular and tissue damage (Ma and He, 2012) [1]. A growing body of research data strongly suggests that imbalanced ROS and electrophile overproduction are among the major prodromal factors in the onset and progression of several cerebrovascular and neurodegenerative disorders such as amyotrophic lateral sclerosis (ALS), stroke, Alzheimer's disease (AD), Parkinson's disease (PD), and aging (Ma and He, 2012; Ramsey et al., 2017; Salminen et al., 2012; Sandberg et al., 2014; Sarlette et al., 2008; Tanji et al., 2013) [1–6]. Cells offset oxidative stress by the action of housekeeping antioxidative enzymes (such as superoxide dismutase, catalase, glutathione peroxidase) as well direct and indirect antioxidants (Dinkova-Kostova and Talalay, 2010) [7]. The DNA sequence responsible for modulating the antioxidative and cytoprotective responses of the cells has been identified as the antioxidant response element (ARE), while the nuclear factor erythroid 2-related factor (NRF2) is the major regulator of the xenobiotic-activated receptor (XAR) responsible for activating the ARE-pathway, thus defined as the NRF2-ARE system (Ma and He, 2012) [1]. In addition, the interplay between the NRF2-ARE system and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ĸB, a protein complex that controls cytokine production and cell survival), has been further investigated in relation to neurodegenerative and neuroinflammatory disorders. On these premises, we provide a review analysis of current understanding of the NRF2-NF-ĸB interplay, their specific role in major CNS disorders, and consequent therapeutic implication for the treatment of neurodegenerative and cerebrovascular diseases.
Abbreviations: AMPK, AMP-activated protein kinase; ARE, Anti-Oxidant Response Element; BBB, Blood-Brain Barrier; CNC, cap‘n’collar; EGCG, Epigallocatechin gallate; Glut-1, Glucose transporter; HO-1, Heme Oxygenase 1; ICAM-1, Intercellular Adhesion Molecule-1; IKK, IκB kinase; KEAP1, Kelch Like ECH Associated Protein 1; MF, Metformin; NQO-1, NAD(P)H: Quinone reductase I; NTF2, Nuclear factor erythroid 2-related factor; PAMPs, Pathogen-associated molecular patterns; PGC-1α, Proliferator-activated receptor gamma coactivator 1-alpha; PKC, protein kinase C; ROS, Reactive Oxygen Species; SFN, Sulforaphane; TLRs, Toll-like receptors; TJ, Tight Junction; tBHQ, Terbutylhydroquinone; tMCAO, Transient middle artery occlusion; TS, Tobacco smoking; ZO-1, Zonulae occludentes-1
Keywords: Oxidative stress, Antioxidative, Nf-κB, Cerebrovascular, Cytoprotection Neurodegenerative, Inflammation, Alternative
Graphical abstract
Highlights
-
•
ROS overproduction promotes cerebrovascular and neurodegenerative disorders.
-
•
NRF2 activity is critical to maintain the redox balance against oxidative stress.
-
•
NF-κB modulates inflammatory/immune responses, apoptosis and cell growth.
-
•
NRF2 and NF-κB pathways interfere with one another at the transcription level.
-
•
NRF2 enhancing/NF-κB inhibitory substances could relieve CNS disorders.
1. Introduction
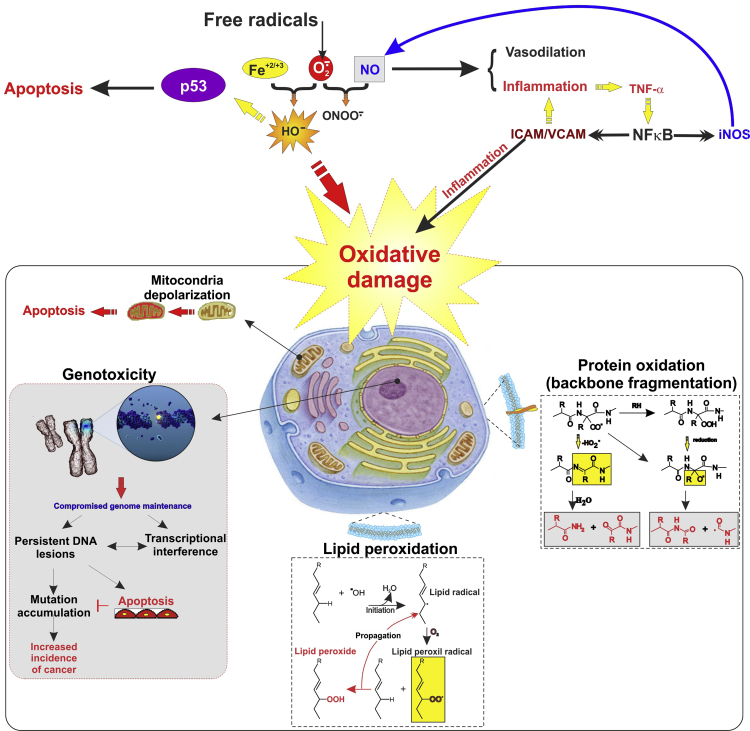
Production of reactive oxygen species (ROS) such as superoxide, hydrogen peroxide, and hydroxyl free radicals is triggered by aerobic respiration, radiation, ultraviolet light, tobacco smoke or metal among the most common sources [8]. Oxidative stress (OS), as one of the major sign of various pathologic processes, results from the unhindered production of ROS, promoting severe damage to the brain tissue [9]. It is not only due to excitotoxicity and depletion of the endogenous antioxidant resources, but also caused by the high susceptibility to lipid peroxidation and damaging interactions between free radical and polyunsaturated fatty acids of which the brain tissue is highly enriched [9], [10]. In addition to lipid peroxidation affecting the majority of the cell membranes, imbalanced OS also promotes proteins backbone fragmentation, genotoxicity and mitochondrial depolarization [11] (see Fig. 1). Not surprisingly, the majority of this highly reactive oxidative species are generated by oxidative phosphorylation reactions that occur in the mitochondria [12]. To circumvent the damaging effect of ROS, cells have developed several defense mechanisms with the scope of scavenging free radicals and reduce the risk for cellular damage induced by OS [4]. These involve transcription factors promoting the expression of antioxidative response elements to neutralize oxidative treats as well as inflammatory responses. Of these several transcription factors, our major focus for this review paper is on nuclear factor erythroid 2-related factor (NRF2) and nuclear factor binding near the k light-chain gene in B cells (NF-kB) promoting cellular responses to OS and immunity.
Fig. 1.
ROS promotes cellular inflammatory response and oxidative damage. Schematic illustration depicting major oxidative stress-activated pathways through which exposure to ROS (generated either endogenously and/or exogenously) promotes genotoxicity, lipid peroxidation, and protein degradation leading to cellular and tissue damage.
NRF2 belongs the cap‘n’collar (CNC) family of transcription factors and contains 605 amino acids. This ubiquitously expressed redox-sensitive transcription factor is the primary modulator of constitutive or inducible molecular systems regulating redox homeostasis. The process involves the activation of a network system [13] comprised of antioxidants (including glutathione, thioredoxin, and others), anti-inflammatory molecules, phase I & II drug metabolizing enzymes (including cytochrome P450s), efflux transporters (classified as Phase III enzymes), and free radical scavengers [4], [14], [15], [16], [17]. Conversely, down-regulation or suppression of NRF2 activity increases the cell susceptibility to the damaging effects of ROS and pro-inflammatory stimuli. Further, OS damage to mitochondrial function can collapse the cellular bioenergetics leading to cell apoptosis [18], [19], [20], [21].
In addition to antioxidative and detoxification responses, NRF2 also modulates the expression of other types of protective elements including the anti-apoptotic B-cell lymphoma 2, the ubiquitary iron exporter ferroportin 1 (also important for sustaining mitochondrial redox-metabolic functions), brain-derived neurotrophic factors, anti-inflammatory responses (such as the expression of interleukin (IL)-10), the mitochondrial nuclear respiratory factor 1 (Nrf1; involved in the regulation of mitochondrial biogenesis and bioenergetic functions), the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α; a key regulator of energy metabolism), and the autophagic proteins such as p62 [22], [23], [24], [25], [26], [27], [28]. NRF2 resides in the cytoplasm at a low basal level until a spike in cellular OS initiates a sequence of biological responses leading to its translocation into to the nucleus [29] where NRF2 binds to ARE sequence (core sequence: TGAG/CNNNGC) which contains the gene promoters associated with ROS detoxification (including glutathione synthesis). Following the transcriptional activation of ARE genes, NRF2 heterodimerizes into other basic leucine zipper proteins, including small Mafs (musculoaponeurotic fibrosarcoma oncogene homolog such as MafG, MafK, and MafF) and Jun (c-Jun, Jun-D, and Jun-B).
Moreover, the NRF2 gene (NFE2L2) contains two ARE-like sequences in its promoter so that NRF2 can control and sustain the expression of detoxification and antioxidant response elements but also autoregulate itself [29], [30].
NF-ĸB is a rapid response factor which is held in a ‘‘resting’’ state, through association with Inhibitor of ĸB (IĸB) proteins. Inducing stimuli such as OS, cytokines and pathogen-associated molecular patterns (PAMPs) stimulate cell surface receptors including toll-like receptors (TLRs) thereby triggering the activation of the IĸB kinase complex, leading to phosphorylation, ubiquitination, and degradation of IĸB proteins. Proteasomal degradation of IĸB, thus leads to release and translocation of NF-ĸB dimers to the nucleus, where it binds to specific DNA sequences and promotes the transcription of target genes to counteract OS and cell injury. Although NF-ĸB was first characterized in cells of the hematopoietic system, recent research validated its activation in most cell types including neurons, astrocytes, microglia, oligodendrocytes and endothelial cells of neurovascular and cerebrovascular units. Indeed, recent reports have demonstrated a key physiological role for the NF-ĸB signaling pathway in the central nervous system serving important functions in cellular responses to neuronal injury and synaptic plasticity [31], [32], [33], [34].
Considering these premises along with the rising of OS-related pathologies, it is understandable that current research has been increasingly focused on unraveling the underlying mechanisms controlling mitochondrial function, energy metabolism, and redox imbalances [30] whereas the interplay between redox metabolism and ROS generation is clearly evident at the mitochondrial level. Enhanced cellular defense and protection against oxidative damage by NRF2 activation occurs in response to acute injuries, inflammation, xenobiotics (such as drugs), and many other stimuli. Activation of NF-κB occurs in response to similar stimuli and current data clearly implies the existence of a NRF2-NF-κB interplay as an integral set of mechanism aimed at counterbalancing each other activity.
2. NRF2 regulation and signaling pathway in oxidative stress
The molecular structure of NRF2 includes seven functional domains with CNC homology (NRF2-ECH homology) named Neh1-Neh7 which regulates its stability as well as its transcriptional activity [35]. Neh1, as the first conserved domain, contains 3 bZIP motif, which allows for NRF2 binding to the ARE sequence [36], [37]. After NRF2 release from Kelch Like ECH Associated Protein 1 (KEAP1), the Neh1 domain exposes a nuclear localization signal necessary to NRF2 for translocating into the nucleus [38]. Moreover, Neh1 can regulate the NRF2 protein stability through interaction with the E2 ubiquitin-conjugating enzyme [39]. By contract, Neh2 (located in the N-terminal region) acts as a negative regulatory domain which enhances KEAP1-NRF2 binding, thus promoting NRF2 ubiquitination followed by its proteosomal degradation.
Neh3 (located in the carboxyl-terminal region of the protein) modulates the transcriptional activation of the ARE genes [35], [40] while the Neh4 and Neh5 domains act cooperatively to facilitate NRF2 transcription by binding to CBP (CREBBP - cAMP Responsive Element Binding protein) which is a transcriptional co-activator [41]. In addition to that, Neh4 and Neh5 can also enhance the NRF2-ARE gene expression by interfacing with the nuclear cofactor RAC3/AIB1/SRC-3 [42], [43]. Finally, both the Neh6 and Neh7 domains act as negative regulator of NRF2 activity. Specifically, based on the recognition of phosphorylated Neh6 by the E3 ligase adaptor beta-TrCP, a KEAP1-alternative pathway of NRF2 degradation occurs [44], [45], [46] and Neh7 represses NRF2 via interaction with retinoic X receptor α [47].
The cytoplasmic and nuclear disposition of NRF2 acts as the main step in detoxification [30]. Under basal conditions, NRF2 is rapidly polyubiquitinated through the KEAP1/cullin-3/Rbx-1 and degraded by the 26S proteasome [48]. However, cellular redox homeostasis is maintained by allowing for the basal accumulation of the NRF2 in the nucleus to mediate the normal expression of ARE-dependent genes.
KEAP1 is composed of 624 amino acids and is divided in three main domains: 1) Broad-complex, 2) Tramtrack, and 3) Bric-a-Brac (BTB; a cysteine-rich intervening region and a double glycine repeat -DGR- binding site between KEAP1 and NRF2) [35]. Within this region, several cysteine residues (Cys 151, Cys257, 273, 288, and 297) act as OS sensors and/or inducer ligands within the cell's environment. Among these cysteine residues C151, is distinctively required for sulforaphane and oxidative stress-induced inhibition of NRF2 KEAP1-dependent degradation as well as oxidative stress-induced posttranslational modification of KEAP1 [49]. The activity of the NRF2-ARE signaling pathway is controlled by degradation and sequestration of NRF2 in the cytoplasm via binding with KEAP1 [30]. The Neh2 domain of NRF2 binds to the KEAP1 region comprised between the BTB and DGR domains and act as an adaptor for the cullin3/ring box 1 (Cul3/Rbx1) E3 ubiquitin ligase complex, which promotes NRF2 polyubiquitination followed by degradation by the 26S proteasome [8], [30]. In addition to function as nuclear transcription factor, NRF2 can directly interact with the mitochondrial membrane in response to mitochondrial oxidative stressors [50]. It is also important to note that the NRF2 basal activity is also impacted by other factors including gene polymorphisms in the promoter region, post-transcriptional modifications, as well as protein-protein interactions [48], [51].
In response to stress stimuli or ARE inducers, the NRF2 DLG motif is released from the DGR domain within KEAP1; KEAP1 undergoes conformational changes and releases NRF2 which is now free to translocate into the nucleus [48] (see also Fig. 2). Phosphorylation of NRF2's serine in position 40 by AMP-activated protein kinase (AMPK), protein kinase C (PKC), and acetylation/deacetylation processes also promote dissociation of NRF2 from KEAP1 [30]. Independently from KEAP1 activity, NRF2 expression and activation are also regulated by glycogen synthase kinase-3β (GSK-3β) [52], [53]. Further, histone phosphorylation and acetylation, methylation of CpG islands (regions of DNA where a cytosine nucleotide is positioned next to a guanine nucleotide in the linear sequence of bases along its length) and synthesis of specific microRNAs (miRNAs; involving epigenetic mechanisms) are additional means to regulate NRF2 levels/activity [1], [4], [54].
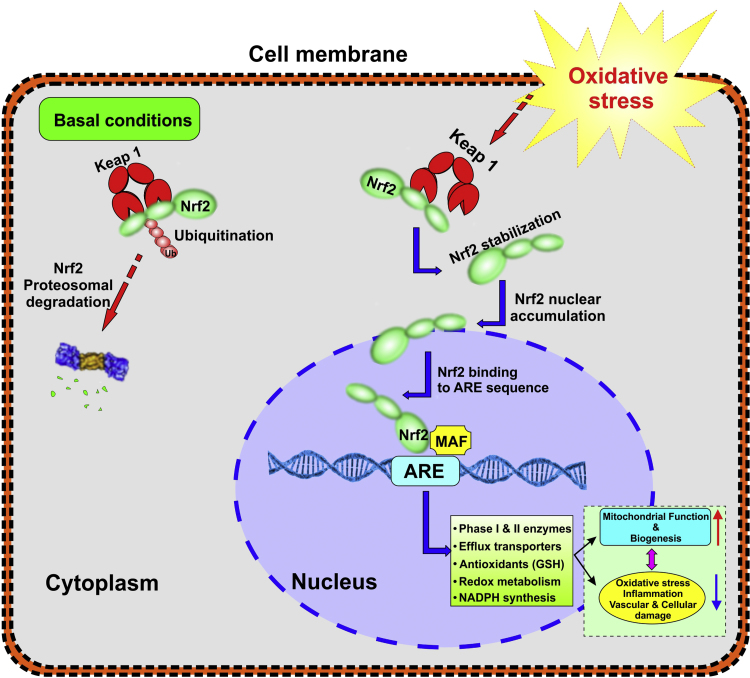
Fig. 2.
Cellular regulation of NRF2 under normal and stressed cellular conditions: NRF2 is recognized as master regulator of cellular redox homeostasis. Under basal conditions, NRF2 remains bound to an adaptor protein KEAP1 and is maintained at a low level in the cytoplasm through ubiquitination followed by proteosomal degradation. Under stressed conditions NRF2 is instead released from KEAP1 then translocate and accumulate in the nucleus where it binds to the ARE sequence. Activation of the NRF2-ARE pathway triggers the transcription of multiple genes involved in the expression of antioxidants, efflux transporters, phase I & II enzymes, glutathione and ATP synthesis, etc.).
Considering the pool of genes within the ARE sequence and the downstream transcriptional effectors, this cytoprotective pathway encompasses the following main categories: Phase I, II and III detoxification systems including oxidation/reduction elements, conjugation enzymes, and drug efflux transporters respectively [48], [54]. Phase I encompasses nearly 500 genes encoding proteins including redox balancing factors, detoxifying enzymes, stress response proteins and metabolic enzymes such as Nicotinamide adenine dinucleotide phosphate (NAD(P)H), Quinone reductase I (NQO1), glutathione S-transferase (GST), glutathione reductase (GSR), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), heme oxygenase-1 (HO-1), carbonyl reductase (CR), malondialdehyde (MDA), and glutamate-cysteine ligase (GCL) [55], [56]. Metabolites then enter Phase II where they are conjugated to bulky polar groups (such as glutathione, glucuronic acid, sulfate or glycine) to enhance their water solubility and facilitate excretion. In addition to these metabolic process, potentially harmful compounds (either endogenous or xenobiotics) are also removed or kept out of the cell by active efflux transporters whose expression levels are controlled/enhanced by NRF2 [48]. There is evidence confirming NRF2 nuclear accumulation can also have detrimental effects [37] which explains the need for autoregulating the cellular levels of NRF2 [40], [57]. This is evident in feedback autoregulatory loop between KEAP1 and NRF2 whereas KEAP1 controls NRF2 by facilitating its degradation and NRF2 modulating the transcription of KEAP1. Moreover, as recently reported by Niture et al., the complex KEAP1/Cul3/Rbx1 is not only present in the cytosol, but also in the nucleus, where it degrades NRF2 and controls the “switching off” of NRF2-activated gene expression [40].
Exogenous NRF2-ARE inducers derive from various sources ranging from natural phytochemical derivatives (e.g., oleanolic acid, sulforaphane, genistein and curcumin to name a few) to synthetic products such as acetaminophen, nimesulide, and metformin. In certain cases, these agents have been found to be more potent than endogenous inducers such as prostaglandins and others and are being heavily investigated to assessor their clinical viability as preventive and/or therapeutic treatments [1].
3. NF-ĸB regulation and signaling pathway in oxidative stress
NF-ĸB proteins contain an N-terminal Rel homology domain (RHD) that builds contact with DNA, supporting dimerization and binding to ĸB sites. There are five NF-ĸB family members in mammals: RelA/p65, RelB, c-Rel, p50 (NF-ĸB1), and p52 (NF-ĸB2). Among these five members, p65, c-Rel, and RelB contains C-terminal transactivation domains (TADs) that promotes initiation of transcription. p52 and p50 lack TADs, however they regulate transcription positively by interacting with TAD-containing NF-ĸB subunits, other non-Rel proteins and regulate transcription negatively by competing with TAD containing dimers for binding to ĸB sites [32].
The NF-ĸB signaling pathways have been largely categorized into two types: canonical (classical) and noncanonical pathways. In canonical pathway, the activating stimuli such as cytokines upon recognition by receptors such as the TNF receptor (TNFR), IL-1 receptor (IL-1R), TLRs and antigen receptors trigger signaling cascades that ultimately activates IĸB (inhibitor of ĸB) kinase β (IKKβ). Activated IKKβ phosphorylates IĸB proteins which sets the stage for ubiquitination and proteasomal degradation (see also Fig. 3). In the noncanonical NF-ĸB pathway, specific members of the cytokine family activate IKKα which leads to phosphorylation of p100 and the generation of p52/RelB complexes. Importantly, both IKKα and IKKβ can cross-talk with additional signaling pathways such as the p53, MAP kinase, and IRF pathways to directly regulate certain transcriptional changes [58], [59].
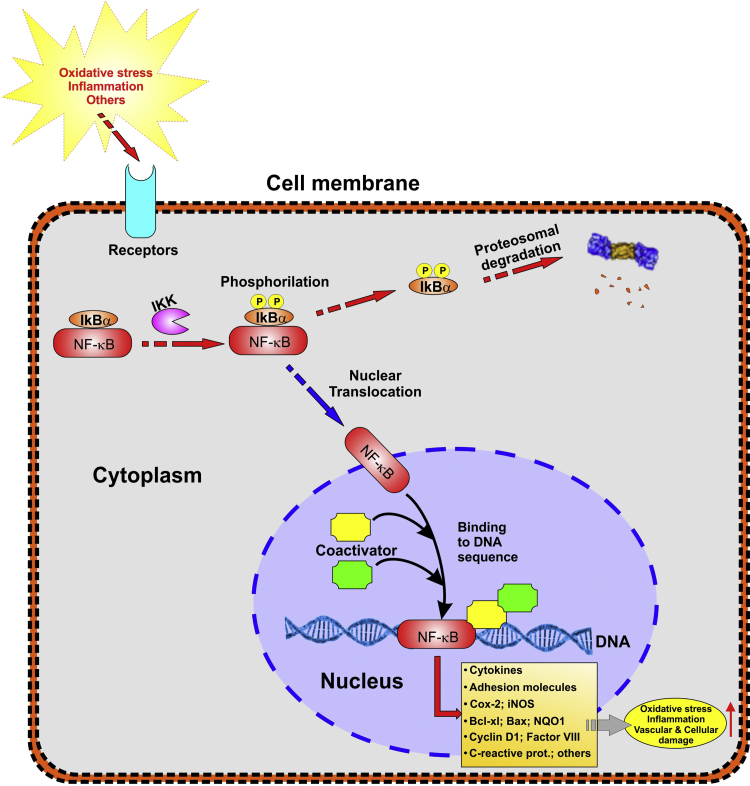
Fig. 3.
Cellular regulation of NF-κB under stressed cellular conditions: NF-κB is a redox regulated transcription factor, involved in the modulation of inflammation, immune function, cellular growth and apoptosis. Oxidative stress can cause the activation of IκB kinase (IKK) which phosphorylate NF-κB inhibitor. The resulting effect is polyubiquitination mediated proteasomal degradation of IκB and the release of NF-κB, which migrates into the nucleus, binds with its corresponding DNA responsive elements (the κ region of genome) and with the coadjuvant help of other coactivators promotes the transcription of proinflammatory mediators.
Several genes underlying pro-inflammatory, inflammatory, stress and growth responses have been shown to be transcriptionally regulated by NF-ĸB [60]. Indeed, one of the majorly studied roles of NF-ĸB in aging and different pathologies is the transcriptional regulation of cytokine expression such as TNFα, IL-1α/β and proteins involved in antigen presentation such as MHC class I and β2 microglobulin. Furthermore, to facilitate the recruitment and attachment of immune cells at the sites of inflammation, NF-κB also enhances/controls the expression of chemokines such as MCP-1, MIP-1 and adhesion molecules such as ICAM-1, E-selectin, and VCAM-1. In addition, NFκB also regulates pro-apoptotic (e.g. Bim, Bax), anti-apoptotic genes (XIAP, bcl-2), and growth factors (e.g. nerve growth factor, vascular endothelial growth factor) to promote cell proliferation and survival [34].
4. NRF2 and NF-ĸB interplay in oxidative stress
Recent studies have established a crosstalk between NRF2 and NF-ĸB signaling pathways under stress and a variety of pathophysiological conditions [61]. Deletion of NRF2 is associated with enhanced inflammation while its upregulation decreases pro-inflammatory and immune responses transcriptionally regulated by NF-ĸB. In a study by Innamorato et al. NRF2 knock out mice showed hypersensitiveness to the inflammation induced by LPS as evident by increase in the levels of inflammatory markers such as F4/80 (both mRNA and protein expression), inducible NO synthase, IL-6, and TNF-α, compared with the hippocampi of wild-type littermates [62]. Interestingly, these harmful changes were abrogated upon treatment with sulforaphane, a compound that induces NRF2-dependent gene expression [62]. This evidence has been further supported by other reports involving other cell types such as microglial cells [63], [64], [65], [66] and monocytes [67]. Furthermore, Kobayashi et al. have also shown that NRF2 can oppose transcriptional upregulation of proinflammatory genes independently from its redox control activity [68]. Silencing TNF induced NRF2 and acute inflammatory responses mediated by NF-ĸB in human monocytes. However, TNF induced prolonged activation of NRF2 resulted in its autocrine regulation [69], suggesting that the interplay between the NRF2 and NF-ĸB mediated inflammatory responses is a complex phenomenon. It is remarkable that NRF2 and NF-ĸB affect each other to coordinate anti-oxidative and inflammatory responses determining the fate of innate immune cells [70], but it is not completely known how this interconnection takes place.
In a study by Cuadrado et al., it was reported that modulation of Rho Family, GTP-binding Protein- RAC1 (a pleiotropic regulator of transcription factor NF-ĸB) activated inflammatory pathway through a cross-talk between NF-ĸB and NRF2 [71]. RAC1 participation in NADPH oxidase-dependent production superoxide has been well established [72] wherein LPS induced TNF-α secretion through IKK regulation of NF-ĸB was shown to be mediated through RAC1 dependent ROS formation. In the study by Cuadrado et al., NRF2 overexpression inhibited RAC1-dependent activation of NF-ĸB pathway, while NRF2 deletion enhanced the activation of NF-ĸB dependent inflammatory markers, suggesting that RAC1 induces NF-ĸB, which in turn affects NRF2. Furthermore, the study suggested that NRF2 inhibits NF-ĸB as a part of regulatory feedback loop [71] (see also Fig. 4).
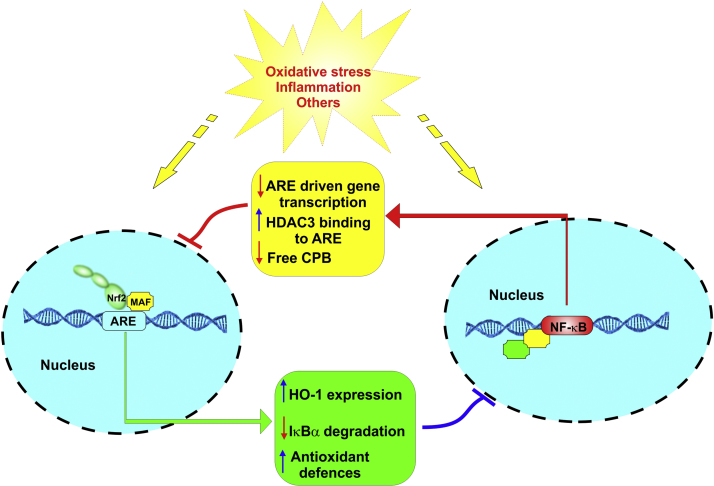
Fig. 4.
Crosstalk between the NRF2 and NF-κB pathways: The figure is a schematic illustration depicting the major point of reciprocal interference between NRF2 and NF-κB.
5. NRF2 and NF-ĸB in tobacco smoke-induced OS and hyperglycemia
The effect of tobacco smoking (TS) on resident macrophages, lung bronchial and alveolar epithelium and lung fibroblasts of chronic smokers is the main trigger of the NRF2-ARE signaling pathway and related downstream effectors [73]. In fact, NRF2 activation plays a major coping role to counteract the onset and progression of Chronic obstructive pulmonary disease (COPD); a chronic disorder which includes emphysema and chronic bronchitis and is strongly associated with smoking [74]. TS has also been established as a strong promoter of cerebrovascular and blood-brain barrier dysfunction and associated with the onset of major neurovascular and neurodegenerative diseases similarly linked to dysregulation of NRF2 activity (such as stroke, ALS, vascular dementia, Parkinson's and Alzheimer's diseases plus others) [48], [75], [76], [77].
The impact of the NRF2-ARE signaling pathway on the cerebrovascular system has been recently investigated in several preclinical studies as well as in vitro. The results strongly suggest that the use of NRF2 enhancers and/or antioxidant supplements (such as docosahexaenoic acid - DHA, resveratrol, lipoic acid, melatonin, and bicyclol) can counteract OS and possibly reduce the burden of neuropathologies including ischemic stroke cerebral stroke [75], [78], [79], [80], [81]. Recently published data by Prasad et al. have shown upregulation of NRF2 upon acute TS/e-Cig exposure which was followed up by investigating the impact of NRF2 on mitochondria biogenesis and bioenergetic functions at the BBB endothelial level. Their results strongly suggest that NRF2 positively modulates the redox metabolic interplay leading to increased ATP production while sustaining generation of antioxidative active elements (such as NADPH production [7]) and ultimately protecting the BBB from OS damage [77]. In addition, NRF2 was shown to play a cytoprotective role against TS exposure at the BBB whereas NRF2 nuclear translocation followed by increased transcription of detoxification enzymes and antioxidants provided an effective protection against chronic TS exposure [82]. In addition, their studies provided further insight into mechanism underlying TS-dependent oxidative damage of the BBB such as that while acute exposure to TS and vapors from electronic cigarette [17] (e-Cig) initially upregulate NRF2 expression and activation as a natural copying mechanism, chronic exposure instead impairs the activity of NRF2 i leading to a suboptimal antioxidative response and consequent cellular oxidative stress damage [75], [77], [82], [83], [84]. Moreover, similar results have been observed in lung mice tissue where acute exposure to TS promotes activation of the NRF2-ARE signaling pathway while NRF2 deficiency prompted by chronic TS exposure (6 months) lead to the development of extensive emphysemas [76]. Unfortunately, there is still a paucity of studies addressing this facet of chronic TS and e-cig exposure and their impact on the NRF2-ARE system which needs to be addressed. This especially in view of the fact that early stage former smokers remain at high risk of developing cerebrovascular disorders (including stroke) for years after quitting [48]. From a parallel toxicological aspect, TS has been strongly correlated with the release of cytokines, angiogenic factors, numerous chemokines, adhesion molecules, growth factors, and respiratory secretions which are produced in response to the solicitation of NF-κB-induced transcription factors. This aspect of smoking-induced physiological responses have been well demonstrated in TS-associated onset of asthma and chronic obstructive pulmonary disease (COPD) [85]. Several studies have in fact reported an increased NF-κB pathway activity in asthmatic tissues in humans. Protein expression analysis through western blot in peripheral blood mononuclear cells (PBMCs) of adult uncontrolled, severe and moderate asthmatic patients revealed higher levels of NF-κB p65, IκB phosphorylation and IKK-β levels than normal individuals [86]. Furthermore, bronchial biopsies in smokers with normal lung function and COPD showed increased expression of p65 protein (samples analyzed by immunohistochemistry and western blotting), with disease severity being associated with an increased epithelial expression of NF-ĸB [87]. Another study reported NF-ĸB to be activated in sputum macrophages upon exacerbation in smokers having COPD [88]. However, these findings remain controversial. Several other studies report unaltered levels and activity of NF-ĸB in smokers. The fact that studies on uncontrolled adult patients with severe and moderate asthmatics revealed higher levels of NF-κB p65, IKK-β and IκB phosphorylation in peripheral blood mononuclear cells (PBMCs) than normal individuals [86] and a similar pattern was also observed in PBMCs of children with moderate asthma when compared to normal individuals [89] suggests that perhaps NF-κB activation may not so stringently linked to TS per se. Unfortunately, none of these studies have investigated the concomitant involvement of NRF2 which may have affected the activation of the NF-κB system through feedback regulatory mechanisms of control.
Related to TS, development of hyperglycemia and then diabetes is another long-term side effect of smoking. Hyperglycemia or high blood sugar is defined as an abnormally high blood sugar level (fasting glucose levels > 130 mg/dL) and a hallmark sign of type 1 and type 2 diabetes. Recent studies have evaluated the effect of hyperglycemia on NRF2 expression [90]. The results showed that although hyperglycemia does not direct influence NRF2 expression, it modulates its cellular distribution promoting NRF2 translocation into the nucleus. As a matter of fact, TS exposure and hyperglycemia markedly elevated the NRF2 nuclear/cytoplasmic ratio which not only suggests the ongoing activation of antioxidant pathways (type-2 diabetes presents hallmark of OS damage similar to that promoted by TS) but also (as recently demonstrated) the activation of a copying vascular response aimed at facilitating glucose transport into the vascular endothelium (perhaps to facilitate its removal from the blood stream), production of ATP (aerobic respiration), and NADPH (antioxidative response) [11]. Sustaining this hypothesis is the fact that NRF2 activation by TS was further enhanced by hyperglycemia thus accounting for the existence of a cooperative effect in term of cellular response as well as data showing that hyperglycemia promotes the activation of endothelial pro-inflammatory responses. However, additional studies will be necessary to dissect out and validate the underlying mechanisms and determine (to its full extent) the corresponding pathophysiological implications in relation to the cerebrovascular system and the onset of neuroinflammatory disorders.
6. NRF2 and NF-ĸB in aging
Increased ROS production unmatched by effective antioxidative responses is one of the hallmark of advanced aging which results in the progressive accumulation of cell OS damage to proteins, nucleotides, and lipids thus compromising cellular functions [91]. This is also frequently coupled to changes in redox signaling which also plays a major role in modulating the adaptive oxidative responses [92]. Recent studies have shown that age-related OS damages encompass a substantive increase in ROS production, a decreases antioxidant response as well as a decreased efficiency of the proteasome and the mitochondrial ion proteases resulting in the accumulation of intracellular and intramitochondrial aggregates of oxidized and cross-linked proteins [93]. These studies strongly support the notion that the impaired antioxidant capacity (including diminished basal antioxidant concentrations) underlies how OS increases with aging and the role played by the NRF2-ARE system in maintaining the redox balance.
Level of antioxidant enzymes such as NADPH and GSH, not only decrease with aging because of a cellular decreased antioxidative capacity, but also as a result of a reduced intake of dietary antioxidants (which is negatively affected by age-dependent alteration of intestinal absorption). However, despite the consensus that production of antioxidants and detoxifying enzymes declines with age, whether the basal expression levels of these elements are similarly affected by age is not univocal. This decline of the antioxidative responses seems to be primarily triggered by a decreasing NRF2 activity [94] but this is not exclusively dependent on the expression level of NRF2. In fact, Zhang et al. recently demonstrated in vivo in the cerebellum of 21- month-old mice that the expression levels of NRF2 (both total NRF2 and nuclear NRF2) was significantly higher than that of much younger (6 months old) animals. However, despite the lower expression level of NRF2, the downstream effectors resulting from the activation of the NRF2-ARE (specifically phase 2 enzymes) were significantly upregulated compared to that of older animals exhibiting higher expression of NRF2 [95]. Furthermore, more recent studies have shown that changes of KEAP1 levels or activity as well as that of other molecular regulators such as GSK-3β, p53, Bach1,and E3 ubiquitin ligase (Hrd1) may negatively affect NRF2 activity [96]. In this respect, several studies reported that NF-κB activity increases with aging. NF-κB binding to DNA increases in skin, liver, kidney, cerebellum, cardiac muscle, and gastric mucosa of old rodents as compared to that in young rodents [60], [97], [98]. NF-κB was reported as the most strongly associated motif (among fourteen other motifs) with aging in an integrated analysis of 365 microarrays spanning nine tissue types in both human and mouse. Furthermore, the same study showed that genetic blockade of NF-κB for two weeks in the epidermis of chronologically aged mice reverted tissue characteristics and global gene expression profiles as of young mice [99]. Human fibroblasts from aged individuals and patients with Hutchinson-Gilford progeria syndrome have increased NF-κB activation [99], [100]. In a mouse model of XFE progeroid syndrome (a disease of accelerated aging), enhanced NF-κB activation was observed in progeroid model mice vs controls. Importantly, genetic depletion of one allele of the p65 subunit of NF-κB or biological treatment with a pharmacological inhibitor of IKK (NF-κB–activating kinase) delayed the development of age-related pathologies in progeroid mice [60]. Yet another preclinical study rescued the early lethality and degenerative syndrome of Sirt6-deficient mice (pathologically characterized by increased activity of NF-κB-driven gene expression) upon RelA haploinsufficiency confirming that hyperactive NF-κB signaling contributes to premature and normal aging [101]. In respect to NRF2- NF-κB interplay and feedback regulatory controls it is possible to compare the balance between these 2 opposing factors to anabolic and catabolic processes which are kept in check throughout our pre-middle age life. However, as age progresses the anabolic activities decrease substantially more than the catabolic ones rendering the system imbalanced. In our specific case the results can be summarized in a decreased NRF2 activity unable to balance the activity of the NF-kB system.
This complex interplay will definitely require more in-depth investigation since it appears that other modulatory factors (possibly unrelated to the NRF2 - NF-κB interplay) might be involved. For example, Rahman et al. found a decrease in antioxidative responses in the old flies, in spite of no significant change of NRF2 mRNA basal expression levels as well as its downstream effectors [102] although, the NF-κB activity was not investigated.
7. NRF2 and NF-ĸB in traumatic brain injury
Traumatic brain injury (TBI), is among the most common type of trauma and one of the major causes of death and disability in modern society caused the disruption in the normal brain function because of brain injury caused by a physical trauma (by external forces). TBI can lead to severe physical, emotional and cognitive impairment as well development of post-traumatic epilepsy [103], [104], [105]. Although the primary injury is the main factor to cause disorders, the secondary injury derived from OS, imbalanced calcium homeostasis, excitotoxicity, inflammation, increased vascular permeability and BBB disruption, exacerbates post-traumatic brain damage [106], [107]. In fact, there is the occurrence of a series of delayed secondary biochemical and metabolic changes at the cellular level that lead to increased ROS production, cell damage and eventual neuronal dysfunction [108]. The excessive ROS generation following TBI represent the primary pathological link to apoptosis and neuronal cell death. According to scientific literature, NRF2 plays a significant neuroprotective role in TBI and neurodegenerative disorders [106] whereas suppression of NRF2 activity exacerbates TBI-induced oxidative damage as well as post-traumatic neurological deficits. By contrast, NRF2 activation ameliorates TBI-induced brain damage suggesting that positive modulation of NRF2 activity could provide a viable strategy to treat post-traumatic brain injuries and improve clinical outcomes through reduction of oxidative stress and post-traumatic inflammatory responses [109]. By contrast, prolonged activation of NF-κB observed in glial cells and neurons in the same brain region undergoing atrophy following brain injury is consistent with the long-term inflammatory processes observed following TBI [110]. Further, repression of the NF-κB inhibitor system in an experimental model of closed-head injury promoted neuronal cell death, worsen the neurological outcome and increased posttraumatic mortality rate [111]. The study outlined a potential mechanism of action associated with the upregulation of proapoptotic mediators such as Bax and Bad. In the contest of Nrf2-NF-κB interplay, this recent finding fits well with the cytoprotective mechanisms of action associated to Nrf2 which has be shown to promote the expression of the anti-apoptotic Bcl-xL protein, thus leading to the downregulation of Bax, BAD, and other pro-apoptotic factors promoted by the activity of NF-κB and involved in cell death and tissue damage [112], [113].
8. Oxidative damage, NRF2 and NF-κB in ischemic stroke
Stroke is the main cause of permanent disability and the fifth leading cause of death in the United States and the only FDA-approved treatment currently available is tissue plasminogen activator (t-PA). Of consideration is also the fact that the effectiveness of the treatment is highly dependent upon the time interval before the occurrence of stroke and administration of t-PA with a window of clinical effectiveness in respect to the severity of post-ischemic brain injuries that is extremely narrow.
Stroke is defined as an abrupt interruption of blood supply into parts of the brain, caused by the blockage or bursting of blood vessels carrying oxygen and nutrients to that region. This triggers the onset of an anoxic and hypoglycemic state in the affected brain [12]. The neuronal cell membrane depolarizes triggering the release of the neurotransmitter glutamate which activates NMDA receptors. This results in the opening of this non selective ion channel with consequent calcium overload and neuronal cell death [114]. The cascade of events associated with ischemic stroke also includes NF-κB activation (although is contribution to post-ischemic injury is not yet fully understood), overproduction of ROS by the mitochondria (which contributes to overwhelm the antioxidant defenses), and post-ischemic inflammation. All together these events promotes additional tissue damage [115], [116], [117], [118]. Moreover, restoration of blood flow (post-ischemic reperfusion) enhances tissue oxygenation exacerbating ROS production, inflammatory responses and further OS damage. As a result, degradation of the structural proteins of the vascular wall and loss of BBB integrity by activated matrix metalloproteinases (MMPs) occur. In addition, the concurrent increased expression of vascular adhesion molecules on the luminal surface of the vascular walls facilitates adhesion and transmigration of leukocytes across the blood vessels and into the brain. This leads to inflammation with increase in ROS production and more brain tissue damage. Based on these premises it has been postulated that OS prevention and control of ROS levels in cerebral ischemia/reperfusion injury could afford a viable therapeutic approach to reduce post-ischemic secondary brain injury and improve stroke outcome [119].
Recent studies demonstrated that the interactions between p62 and the Nrf2–EpRE signaling pathway play an effective role in preventing OS injury during cerebral ischemia/ reperfusion in rat undergoing transient middle artery occlusion (tMCAO) [120]. In addition, enhancing Nrf2 activity has been shown to reduce the infarct volume and post-ischemic neurocognitive impairments in a mouse model of tMCAO [75]. NF-ĸB activation is reported in various experimental ischemic reperfusion animal models- myocardial ischemia-reperfusion [121], global ischemia [122] and focal ischemia [116]. These studies further report that inhibition of NF-kB activation confers protection from ischemic reperfusion injury [116], [121]. Moreover, NF-kB has been recently shown in vitro to form positive feedback loop with lncRNA functional intergenic repeating RNA element (FIRRE) to promote the transcription of NLRP3 inflammasome, thus contributing to stroke injury of cerebral microglial cells [123], [124]. Conversely, Nrf2 has been reported to play beneficial role in protecting brain against injury and its activation by using pharmacological agents resulted in increased neuronal cell viability, induction of cytoprotective genes and decrease in BBB permeability [125], [126]. Furthermore, Nrf2 knock out mice showed enhanced infarct size, inflammatory damages and neurological deficits in comparison to wild type mice [127]. These Nrf2 deficient mice also failed to show any beneficial effect of ursolic acid, a well-established Nrff2 enhancer [127]. Lately, the interplay between Nrf2 and NF-ĸB behind physiological and pathophysiological conditions is being evaluated to better understand the approach needed for targeted therapy. It was noted that activation of Nrf2 attenuated oxidative stress, neuronal apoptosis and neuroinflammation against I/R injury both in vitro and in vivo via Nrf2-mediated inhibition of the NOX4/ROS/NF-ĸB pathway [128], [129]. However, additional studies will be necessary to assess the effective viability of these treatments as well as dosing and optimal time window for intervention.
9. Oxidative damage in neurodegenerative diseases
Neurodegenerative diseases (NDDs) are a rapidly rising concern in public health. Recent discoveries have demonstrated that OS is a major prodromal factor for the onset of these disorders [12]. At a certain point chronic ROS generation overwhelms the antioxidative response system resulting in cellular damage (spanning from lipid peroxidation to genotoxicity) as commonly observed in neurodegenerative disorders including PD, AD, ALS and Huntington's disease (HD) [130]. In fact, increased levels of oxidative markers and damaged cell components have been diagnosed in patients with neurodegenerative disorders [130]. Although the underlying mechanisms linking NRF2 and NF-ĸB with the onset/progression of these various disorders is still poorly understood [4], one pathogenic hallmark shared between these disease is the fact that they are in essence proteinopathies. Another common feature of these neurodegenerative disorders rest on the impairment of mitochondrial function (more specifically redox metabolism) leading to increased ROS production (not effectively balanced by a symmetric response of the antioxidative system), inflammation and ATP depletion [1], [2], [3], [4], [5], [6]. This ultimately leads to ROS accumulation which can impact the proteins structure and render them more prone to form intracellular aggregates [130]. This could provide opportunities for interventions focused on restoring/normalizing the cellular antioxidative response (NRF2 activation has been shown to delays PD [131]) and decrease inflammation, thus reducing/preventing the progression of these disorders.
10. NRF2 and NF-ĸB in Parkinson's disease
PD is a progressive neurodegenerative disorder caused by a reduction of dopamine levels in the striatum (due to the loss of dopaminergic neurons located in the substantia nigra) which affects movement [130]. The initial symptoms sometime are barely noticeable (such as tremors affecting one hand or slowing of movement) but with the progression of the disease controls over movement id completely compromised and the effects can extend to neurocognitive functions dementia. The presence of Lewy bodies (LBs; abnormal protein aggregates developing inside nerve cells), is certain diagnosis of PD and have been found in both familial and sporadic PD patients. Alpha-synuclein (αSyn; a small protein with 140 amino acids abundant in presynaptic nerve terminals) is the main constituent of LBs and plays a role in synaptic transmission and dopamine levels adjustment. αSyn primarily affect tyrosine hydroxylase phosphorylation and activity and the expression level of dopamine transporter on the cell membrane. Recent studies strongly suggests that OS plays an major role in αSyn proteostasis [132], [133] and that NRF2 activity can counteract αSyn generation. It was also demonstrated that chemical induction of NRF2 in mice ameliorated the damage caused by alpha-synuclein [134]. The increased OS activity in PD patients has been associated with abnormal ROS production promoted by the metabolism of dopamine as well as excitatory amino acids [135], [136]. In neuroblastoma cells, inhibition of the NRF2-ARE signal by ferrous iron promotes αSyn aggregation whereas αSyn aggregation ends up exacerbating ferrous iron-induced, mitochondrial impairment leading to cell apoptosis [130]. In agreement with these findings, NRF2 overexpression has been shown to reduce the generation of αSyn aggregates in the CNS [137]. Furthermore, transgenic activation of NRF2 appeared to shelve the loss of dopaminergic neurons mediated by αSyn and the consequent impairment of motor functions [138]. On the same line mice bearing NRF2 deficiency and increased expression of αSyn experienced heighten loss of dopaminergic neuron, increased neuroinflammation and protein aggregation [139]. By contrast, increased expression level of NRF2 in a mutant αSyn transgenic mouse model, afforded neuroprotection [136]. Given the abnormal ROS production in Parkinson's disease, it is not surprising that inflammation is also part of the diseases the process and as such the involvement of NF-ĸB. In fact, evidence suggests that NF-κB is involved in PD [140]. In Parkinson's patients, Immunohistochemical analyses of brain sections revealed more than 70-fold increase in NF-κB activation, relative to control subject whereas the dopaminergic neurons in the substantia nigra exhibiting strong nuclear p65 immunoreactivity [141]. Consistent with the fact that OS o and mitochondrial dysfunction are increased in PD, Flood and et al., concluded that NF-κB, could be an ideal target for therapy due to its key role in the production of the inflammatory mediators known to exhibit neurotoxicity in models of PD [142]. It has been also shown that inhibition of NF-κB increases susceptibility of dopaminergic neurons to 6-hydroxydopamine in PD models [140]. Thus, NF-κB activity has been considered as a key target for controlling chronic inflammation (including microglia activity) [143]. Given the feedback control of NRF2 on NF-ĸB activity and vice versa, These findings well connect with previous data showing that loss of NRF2 activity aggravates neuroinflammation in PD while increased NRF2 expression affords neuroprotection.
11. NRF2 and NF-ĸB in Huntington's disease (HD)
HD is an inherited neurodegenerative disease characterized by the loss of GABAergic inhibitory spiny projection neurons in the striatum [130]. HD gene (huntingtin) presents an atypical expansion of cytosine, adenine, and guanine (CAG) trinucleotide repeats encoding for an abnormally elongated poly-glutamine (polyQ) stretch present at the N-terminal of the huntingtin protein (Htt). In vitro studies in the striatal cell line have shown that NRF2 signaling is compromised by the presence of this mutant Htt (mHtt) [144] while parallel studies have shown that NRF2 activation affords protection against mHtt-induced toxicity [145]. These data agree with previous observations showing that striatal cells in HD patients fail to initiate the NRF2-ARE system in response to OS stimuli due to the concurrent activation of the autophagy pathway. Specifically, while the expression levels of NRF2 (both the protein and the mRNA) remains unaltered, that of NRF2 modulators (including p62 and KEAP1) is instead affected thus leading to impaired NRF2 activity [146]. Furthermore, Hands et al. demonstrated that Htt aggregation directly enhanced ROS production worsening cell toxicity [147]. How NRF2 impacts the formation of Htt aggregates is still not fully understood and additional studies will be required to validate this hypothesis however, supporting data have shown that co-transfection of NRF2 with mHtt in primary striatal neurons reduced the mean lifetime of mHtt N-terminal fragments and improved cell viability. This suggests that NRF2 appear to be able to reduce the toxic impact of mHtt by negatively impacting its aggregation [148]. Moreover, Htt stimulates the transport of NF-κB out of dendritic spines and supports a high level of active NF-κB in neuronal nuclei in the HD model [149]. In a study conducted by Hsiao et al., enhanced activation of NF-κB observed in astrocytes of mice with HD suggest that these cells exhibited higher IκB kinase (IKK) activity that caused prolongation of NF-κB activation, thus upregulating proinflammatory factors during inflammation [150].
12. NRF2 and NF-ĸB in Alzheimer's disease (AD) and amyotrophic lateral sclerosis (ALS)
AD is the most common progressive neurodegenerative disorder and is characterized by the degeneration of synapses and loss of neurons in the hippocampus and neocortex, thus leading to the development of dementia and eventually death [130]. Formation of extracellular senile plaques (SPs), composed of a small peptide Aβ, and intracellular neurofibrillary tangles (NFTs), are neuropathological hallmarks of AD [151]. In this respect, several studies on AD patients have revealed activated NF-κB in cells involved in the neurodegenerative process [141] whereas upregulation of NF-κB activity induced by Aβ, not only promotes the expression of pro-inflammatory factors (including cytokines and chemokines responsible for the AD neurotoxicity), but also increases Aβ processing to exacerbate Aβ production [152], [153] and participate in the progression of AD [154]. NF-κB has been detected in degenerating neurons in the brains of AD patients (specifically in the cholinergic neurons in the basal forebrains [141]) and also in neuronal and glial nuclei proximal to early-stage plaques [140].
In respect to neuroprotection instead, parallel works have examined the neuroprotective effect of NRF2 through its ability to reduce ROS generation and ROS-induced toxicity mediated by Aβ [155], [156]. In fact, several NRF2 activators, such as Terbutylhydroquinone (tBHQ) and sulforaphane have been shown in vivo (in an acute AD mouse model) to decrease toxin-induced Aβ1–42 secretion, while increasing cell viability and improve cognitive function [157], [158]. However, it is not clear if NRF2 activation leads to the formation of Aβ aggregates or simply prevents the release of monomer/oligomeric Aβ from dead cells [159]. A recent study, suggests that overexpression of mitochondria catalase (which is also regulated by NRF2) in β-Amyloid precursor proteins (APP) transgenic mice (Tg2576), reduces the formation of full-length APPs and lowers soluble and insoluble Aβ levels, thus extending the lifespan of the patient while improving working memory [160]. It is also been found that NRF2 knockout APP/PS1 mice show increased accumulation of insoluble APP fragments and Aβ as well as mTOR activity. These data strongly suggest that NRF2 can facilitate autophagy and alter APP and Aβ processing. Furthermore, in the recent study using transgenic mouse models combining both tauopathy and amyloidopathy, Rojo et al. demonstrated the protective role of NRF2 against exacerbation of astrogliosis and microgliosis [159] and its relevance in the homeostatic responses which decline with ageing along with NRF2 activity leading to decreased protection against proteotoxic, inflammatory and oxidative stress stimuli [161].
As for ALS (also known as Lou Gehrig's disease), this is a progressive degenerative disorder caused by the loss of motor neurons in the ventral horn of the spinal cord and those in the motor cortex, leading to progressive motor weakness and loss of controls of voluntary movements [130]. Although for most of the last 2 decades mutation of Cu–Zn superoxide dismutase 1 (SOD1) was the only genetic aberration associated with the onset of familial ALS, recent studies have discovered additional abnormalities associated with the onset of sporadic and non-SOD1 familial ALS. These include a host of RNA/DNA-binding proteins such as the 43-kDa transactive response (TAR) DNA-binding protein (TDP-43) and the fused in sarcoma/translocated in liposarcoma (FUS/TLS). Recent studies by Frakes et al. have highlighted NF-κB as the highest-ranked regulator of inflammation by analysis of gene expression data from familial and sporadic post-mortem ALS astrocytes [162]. Interestingly NF-κB activation in astrocytes induced a Wnt-dependent microglial proliferation in the presymptomatic phase with neuroprotective effects on motoneurons, while in the later stage, the same process accelerated disease progression, thus suggesting that stage-dependent microglia modulation of NF-κB might be considered as a potential therapeutic strategy in ALS [163]. Also relevant is the fact that NRF2 activation protect against OS and cell death promoted by the SOD1 mutant protein [164], [165]. Using a transgenic mouse model of ALS recent studies have shown that glial NRF2 overexpression improves the survival of spinal cord's motor neurons and extends their viable lifespan. However, whether NRF2 can also affect other ALS-associated gene mutations (specifically TDP43 and FUS) as well as cellular proteostasis is not clear and needs to be further investigated. Additionally, the impact of NRF2 stimulation on late stage microglia activation as a means to suppress OS and control NF-κB activation should also be investigated as a potential complementary therapeutic strategy.
13. Role of NRF2 in BBB integrity and function
The BBB is a dynamic functional interface between the blood and the CNS, which strictly regulates the passage of substances (including nutrients, essential aminoacids, ions, etc) entering or leaving the brain. In essence the BBB maintains the brain homeostasis allowing for optimal neuronal activity and protects the brain from potentially harmful substances (endogenous and xenobiotics) and pathogens [103]. Related to cerebrovascular disorders, numerous studies have emphasized the neurovascular protective role of NRF2 signaling in respect to protecting the BBB and CNS [14], [17], [77], [166]. In respect to the BBB, and more specifically to the BBB endothelium, NRF2 has been shown to promote the expression of tight junctional proteins (TJ), enhance the redox metabolic functions, mitochondrial biogenesis, and ATP production [11], [17], [77], [82]. In fact, activation of the NRF2-ARE system has been proposed as a viable option to prevent the loss of BBB integrity and reduce post-ischemic brain damage and neurocognitive impairments [167]. Impairment of NRF2 activity has been linked to elevated risk of developing cerebrovascular and neurodegenerative disorders such as stroke, epilepsy, subarachnoid brain hemorrhage, ALS, multiple sclerosis (MS), AD, PD and type-2 diabetes [1], [4], [90], [166], [168], [169], [170], [171], [172]. This is not surprising since vascular endothelial dysfunction and consequent CNS injuries have been linked to ROS [173], [174], [175], and OS-driven inflammation [176] among the major prodromal factors whereas NRF2 activation has been shown to maintain ROS homeostasis and protect the BBB [167], [177], [178], [179], thus reducing the risk of cerebrovascular/ damage and CNS disorders [75].
14. NRF2 enhancers/NF-κB inhibitors for the treatment of cerebrovascular and neurodegenerative disorders
Recent studies have shown that some nutritional components and therapeutic agents currently approved for the treatment of pathologies unrelated to vascular and neurodegenerative disease could indeed offer some degree of protection against the onset/progression of neurodegeneration [8] and cerebrovascular disorders [77].
Sulforaphane (SFN), a well-known NRF2 promoter/activator, is an organic isothiocyanate (ITC) naturally found in cruciferous plants such as broccoli, brussels sprouts, cabbage, and cauliflower. SFN has been shown to possess neuroprotective properties in vitro as well as in vivo [167], [180], [181], [182], [183], [184], [185], [186]. Specifically, SFN has been demonstrated to activate the NRF2-KEAP1 pathway through direct modifications of KEAP1 cysteines and to promote the internalization of NRF2 mRNA in the ribosomes thus enhancing the corresponding protein synthesis [8], [187].
Recent reports have also outlined the ability of SFN to modulate mitochondrial dynamics also through both NRF2-dependent and independent mechanisms [188], [189] as well as the expression/activity of BBB efflux transporters [190]. Wang et al. have recently shown that activation NRF2-ARE signaling pathway with SFN in the hippocampus increased the expression of antioxidative enzymes (HO-1 and NQO1) and suppressed the progression of amygdala kindling. In addition, enhancement of NRF2 activity ameliorated OS and cognitive impairment induced by epileptic seizure [172]. In addition to the NRF2 enhancing activity SFN has been shown to inhibit (non-specifically) TNF-alpha-induced NF-kB activation through the inhibition of IkB-α phosphorylation, degradation, and p65 nuclear translocation thus preventing cell apoptosis [191] and reducing the burden associated with diabetic neuropathy [192]. In addition, SFN was shown to be able to block the direct interaction between NF-kB and its consensus sequence suppressing its pro-inflammatory activities in T cells [193]. These results strongly suggest that in addition to activation of NRF2 SFN can independently modulate (inhibit) NF-kB activity.
Metformin (MF) is a biguanide oral anti-hyperglycaemic agent derived from the plant Galega officinalis and used to treat patients with type-2 diabetes (non-insulin dependent). Metformin anti-hyperglycemic activity has been attributed to several reasons including increased insulin sensitivity and glucose uptake as well as inhibition of gluconeogenesis. Nevertheless, the molecular pathways responsible for these effects remain a subject of debate.
From a cerebrovascular perspective outside its primary scope of use, MF has been reported to promote neurogenesis and enhance spatial memory formation indicating its therapeutic value for the injured or degenerating neurovasculature [194]. Furthermore, MF has also been shown to attenuate BBB disruption and decrease/inhibit ischemic injury upon stroke via AMPK dependent and independent (NRF2 antioxidant pathway) mechanisms [195], [196]. These studies highlight additional MF therapeutic potential (outside the treatment of diabetes) encompassing in cardiovascular and cerebrovascular diseases, cancer and ageing [75], [197].
Prasad et al. provided recent evidence of NRF2 playing a role in TS-induced cerebrovascular/BBB impairments and showed for the first time a viable approach to reduce the burden of TS cerebrovascular toxicity based on MF treatments [77], [195]. The results showed that MF protective activity against OS-induced BBB damage by chronic TS exposure was firmly associated with activation of the NRF2-ARE pathway. These protective effects include decreased OS and OS-induced inflammation, normalization of TJ expression (from downregulated condition in response to chronic TS exposure), restoration of BBB integrity, renormalization of Glut-1 (a major glucose transporter at the BBB) and upregulation of the anticoagulant factor thrombomodulin (hence renormalization of blood-hemostasis from a TS-induced condition promoting blood-clot formation) [75], [77].
These results also support and emphasize the functional role of NRF2 and NRF2-ARE signaling pathways in preserving BBB integrity in human BBB microvascular endothelial cells chronically exposed to TS [17], [82], [195]. In addition, MF has been recently reported to promote neurogenesis and reduce memory impairment in neurodegenerative disorders and stroke [198], [199], [200] through similar mechanisms of action. Not surprisingly, MF has been also shown to concurrently inhibit NF-κB activation, thus preventing cytokine-induced expression of proinflammatory and adhesion molecule in vascular endothelial cells (supposedly through AMPK activation and blockade of the PI3K-Akt pathway) [201], [202], [203], [204]. These findings suggest that outside the currently approved scope of use (type 2 diabetes) MF could be repurposed for the treatment of vascular disorders associated with oxidative stress and inflammation. By wielding a dual effect as NRF2 activator and NF-κB inhibitor, MF could prove a useful therapeutic option to reduce the burden of cerebrovascular and neuroinflammatory disorders [77], [198], [199], [200].
Curcumin, a polyphenol derived from Curcuma longa rhizomes, has been shown to possess antioxidative, anti-inflammatory and anti-infective properties [106], [205], [206]. The ability to cross the BBB seems to extend Curcumin cytoprotection to the CNS whereas Curcumin has been shown to reduce the burden of several CNS disorders, including neurodegenerative diseases, global brain ischemia, intracerebral hemorrhage, and TBI [207], [208], [209] and these beneficial effects have been linked to the activation of NRF2 [68], [137] as well as superoxide dismutase and glutathione peroxidase [8], [210]. Furthermore, Curcumin has been shown to prevent NF-κB activation and suppress proinflammatory gene expression in microglial cells [211] as well as suppress NF-κB mediated IRAK-2 activity and mediate neuroprotection [212]. Curcumin has been also shown to reduce brain edema and neurological dysfunction after cerebral I/R by down-regulating NF-kB and elevating NRF2 [213]. Whether these are direct independent effects of curcumin or the end results of the enhancement of NRF2 and consequent suppression of NF-kB activity is still unclear and requires further investigation.
Epigallocatechin gallate (EGCG), is the most abundant catechin found in green tea. Experiments performed on human umbilical vein endothelial cells (HUVEC) have shown that EGCG possesses antioxidative properties which depend on its ability to up-regulate NRF2 via activation of p38 MAPK and ERK1/2 signaling pathways [214]. EGCG promotes the dissociation of NRF2 from KEAP1 and its nuclear translocation, thus promoting the activation of the ARE system [215]. In vivo experiments performed in tMCAO model confirmed the dependency of EGCG antioxidative activity from NRF2 activation whereas EGCG upregulated NRF2 expression in animals subjected to I/R injury when compared to I/R untreated animals and controls [216]. NRF2 upregulation was paralleled by that of its downstream effectors including heme oxygenase-1 (HO-1) and promoters of glutathione biosynthesis. Additionally, EGCG has been shown to inhibit the fibrillization of Αβ in vitro by interfering with IKKβ activation and consequently suppressing NF-κΒ mediated transactivation of β-secretase and release of soluble APP. Furthermore, EGCG-induced reduction of NF-κΒ mediated β- and γ-secretase activities (and consequently the extracellular Αβ levels) improved memory function in animal models of AD [217].
15. Conclusion
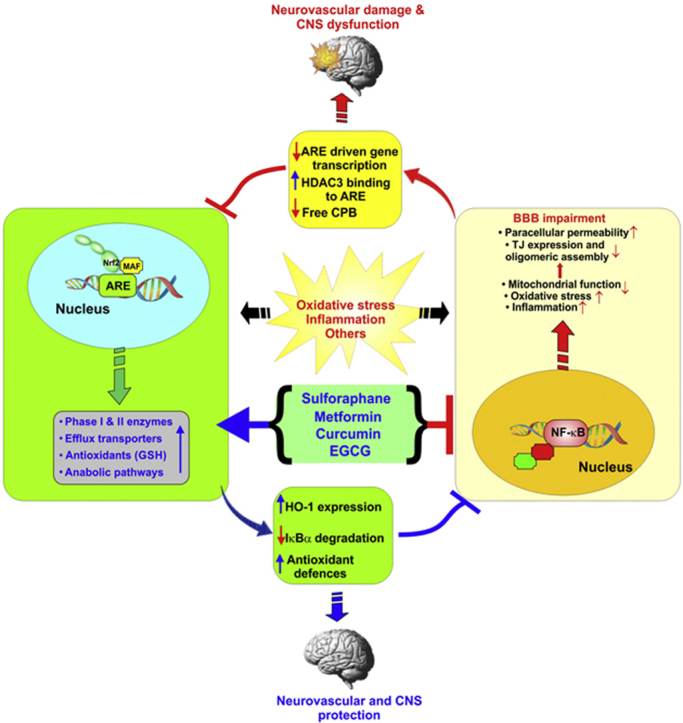
NRF2 is a master regulator of a complex biological network of molecules and enzymes implicated in the regulation of redox homeostasis. This involves the activation and modulation of anti-oxidant, drug metabolism, anti-inflammatory, detoxification and radical scavenging functions [54]. In this respect, the NRF2-ARE signaling is indeed a critical cytoprotective system for the cells to survive oxidative stress stimuli and maintain the appropriate redox balance in cells and tissues [218]. NF-κB is also a redox-regulated transcription factor, primarily involved in the modulation of inflammatory/immune responses, cell apoptosis as well as cellular growth [219]. OS promotes the activation of IκB kinase (IKK) which then mediates the phosphorylation of IκB (the NF-κB inhibitor) promoting its proteosomal degradation and the release of NF-κB. Free from its bound with IκB NF-κB migrates into the nucleus, binds with the κ region of genome and along with other cofactors and histone acetyl transferases (HAT), NF-κB initiates the transcription of proinflammatory molecules including cytokines (IL-1, IL-6, TNF-α), cyclooxygenase-2 (COX-2), vascular adhesion molecules, inducible nitric oxide synthase (iNOS) and others [219]. These 2 contraposing pathways however, can interfere with one another at their corresponding transcription level. A process mediated via protein–protein interactions or the effect of secondary messenger. Specifically, NRF2 inhibits the activation of NF-κB pathway by increasing antioxidant defenses neutralizing ROS, thus reduces ROS-mediated NF-κB activation [220]. In addition, NRF2 prevents the degradation of IκB-α, thus blocking NF-κB nuclear translocation and transcription of pro-inflammatory genes. By contrast, NF-κB can inhibit NRF2 activity through enhancement of the recruitment of histone deacetylase3 (HDAC3) to the ARE region thus preventing ARE gene transcription [70]. In addition to that, NF-κB decreases free CREB binding protein (CBP) by competing for CH1-KIX domain of CBP with NRF2 [221].
Several studies have attempted to dissect out the cerebrovascular protective role of NRF2 to preserve the functional integrity of the BBB and prevent the onset of neuroinflammatory and degenerative CNS disorders [19], [126], [190]. Not surprisingly, there is a rising interest in investigating the association between impairment of NRF2 signaling and the onset/progression of cerebrovascular and neurodegenerative disorders, thus the mounting research interest in the identification of novel approaches targeting NRF2 and/or NF-κB to prevent and/or reduce brain injury. From a translational and clinical point of view, current studies support the hypothesis that strategies aimed at restoring NRF2 activity (therefore inhibiting NF-κB activation) hold viable therapeutic potential to reduce the burden of major cerebrovascular and neurodegenerative diseases. However, more focused studies (both in vitro and in vivo) will be necessary to confirm these “preliminary” findings and assess their real therapeutic value. Today there are numerous over the counter nutritional dietary phytochemicals and therapeutic agents which possess NRF2 enhancing activities (see Table 1). These substances and additional derivates could be exploited for the treatment neurodegenerative and neurovascular diseases promoted by abnormal ROS generation. However, the feasibility of each treatment needs to be extensively confirmed and the beneficial effects needs to be proved and weighted against existing therapeutic options.
Table 1.
NRF2 enhancers for the treatment of oxidative stress-promoted cerebrovascular and neurodegenerative disorders.
| NRF2 enhancers | Category | The role in antioxidative systems | Ref. |
|---|---|---|---|
| Sulforaphane | Organic isothiocyanate |
|
[8], [19], [160], [172], [180], [181], [182], [183], [184], [185], [186], [187], [188], [189], [190] |
| |||
| Metformin | Biguanide |
|
[75], [77], [194], [195], [196], [197] |
| |||
| |||
| |||
| Astragaloside IV | Bioactive saponin |
|
[222], [223] |
| |||
| |||
| |||
| Curcumin | Polyphenol |
|
[106], [205], [206], [207], [208], [209], [210], [224] |
| |||
| |||
| EGCG | Polyphenol |
|
[214], [215], [216] |
| |||
| Genistein | Isoflavone |
|
[225], [226] |
| |||
| Fisetin | Flavonoid |
|
[227] |
| |||
| Melatonin | Hormone |
|
[194], [228], [229], [230], [231], [232], [233], [234] |
|
Acknowledgements
Funding
This work was supported by the National Institutes of Health/National Institute on Drug Abuse 2R01-DA029121-01A1 and ARDF to Dr. Luca Cucullo.
Acknowledgments
Competing interests
The authors declare no competing interests.
Contributor Information
Farzane Sivandzade, Email: farzane.sivandzade@ttuhsc.edu.
Shikha Prasad, Email: Shikha.Prasad@northwestern.edu.
Aditya Bhalerao, Email: aditya.bhalerao@ttuhsc.edu.
Luca Cucullo, Email: luca.cucullo@ttuhsc.edu.
References
- 1.Ma Q., He X. Molecular basis of electrophilic and oxidative defense: promises and perils of Nrf2. Pharmacol. Rev. 2012 doi: 10.1124/pr.110.004333. (pr. 110.004333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramsey C.P., Glass C.A., Montgomery M.B. Expression of Nrf2 in neurodegenerative diseases. J. Neuropathol. Exp. Neurol. 2007;66:75–85. doi: 10.1097/nen.0b013e31802d6da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salminen A., Kaarniranta K., Haapasalo A. Emerging role of p62/sequestosome-1 in the pathogenesis of Alzheimer's disease. Prog. Neurobiol. 2012;96:87–95. doi: 10.1016/j.pneurobio.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Sandberg M., Patil J., D'angelo B. NRF2-regulation in brain health and disease: implication of cerebral inflammation. Neuropharmacology. 2014;79:298–306. doi: 10.1016/j.neuropharm.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarlette A., Krampfl K., Grothe C. Nuclear erythroid 2-related factor 2-antioxidative response element signaling pathway in motor cortex and spinal cord in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2008;67:1055–1062. doi: 10.1097/NEN.0b013e31818b4906. [DOI] [PubMed] [Google Scholar]
- 6.Tanji K., Maruyama A., Odagiri S. Keap1 is localized in neuronal and glial cytoplasmic inclusions in various neurodegenerative diseases. J. Neuropathol. Exp. Neurol. 2013;72:18–28. doi: 10.1097/NEN.0b013e31827b5713. [DOI] [PubMed] [Google Scholar]
- 7.Dinkova-Kostova A.T., Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch. Biochem. Biophys. 2010;501:116–123. doi: 10.1016/j.abb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Y., Li W., Su Z.-y. The complexity of the Nrf2 pathway: beyond the antioxidant response. J. Nutr. Biochem. 2015;26:1401–1413. doi: 10.1016/j.jnutbio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J., Wang H., Ding K. Luteolin provides neuroprotection in models of traumatic brain injury via the Nrf2–ARE pathway. Free Radic. Biol. Med. 2014;71:186–195. doi: 10.1016/j.freeradbiomed.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Hall E.D., Vaishnav R.A., Mustafa A.G. Antioxidant therapies for traumatic brain injury. Neurotherapeutics. 2010;7:51–61. doi: 10.1016/j.nurt.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sajja R.K., Kaisar M.A., Vijay V. In vitro modulation of redox and metabolism interplay at the brain vascular endothelium: genomic and proteomic profiles of sulforaphane activity. Sci. Rep. 2018;8:12708. doi: 10.1038/s41598-018-31137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buendia I., Michalska P., Navarro E. Nrf2–ARE pathway: an emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol. Ther. 2016;157:84–104. doi: 10.1016/j.pharmthera.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Cuadrado A., Manda G., Hassan A. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol. Rev. 2018;70(348–383):2018/03/07. doi: 10.1124/pr.117.014753. [DOI] [PubMed] [Google Scholar]
- 14.Sajja R.K., Prasad S., Tang S. Blood-brain barrier disruption in diabetic mice is linked to Nrf2 signaling deficits: role of ABCB10? Neurosci. Lett. 2017;653:152–158. doi: 10.1016/j.neulet.2017.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowluru R.A., Mishra M. Epigenetic regulation of redox signaling in diabetic retinopathy: role of Nrf2. Free Radic. Biol. Med. 2017;103:155–164. doi: 10.1016/j.freeradbiomed.2016.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinkova-Kostova A.T., Abramov A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015;88:179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sajja R.K., Green K.N., Cucullo L. Altered nrf2 signaling mediates hypoglycemia-induced blood–brain barrier endothelial dysfunction in vitro. PLoS One. 2015;10:e0122358. doi: 10.1371/journal.pone.0122358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmström K.M., Baird L., Zhang Y. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol. Open. 2013;2:761–770. doi: 10.1242/bio.20134853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li T., Wang H., Ding Y. Genetic elimination of Nrf2 aggravates secondary complications except for vasospasm after experimental subarachnoid hemorrhage in mice. Brain Res. 2014;1558:90–99. doi: 10.1016/j.brainres.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 20.Mitsuishi Y., Taguchi K., Kawatani Y. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Lee J.M., Shih A.Y., Murphy T.H. NF-E2-related factor-2 mediates neuroprotection against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J. Biol. Chem. 2003;278:37948–37956. doi: 10.1074/jbc.M305204200. [DOI] [PubMed] [Google Scholar]
- 22.Cuadrado MPAIRA, P 087 - NRF2 controls proteostasis through the transcriptional regulation of autophagy, in: Proceedings of the OCC World Congress and Annual SFRR-E Conference 2017 Metabolic Stress and Redox Regulation Berlin, Germany, Free Radical Biology and Medicine, 2017.
- 23.Pajares M., Rojo A.I., Arias E. Transcription factor NFE2L2/NRF2 modulates chaperone-mediated autophagy through the regulation of LAMP2A. Autophagy. 2018;14:1310–1322. doi: 10.1080/15548627.2018.1474992. (2018/06/29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niture S.K., Jaiswal A.K. Nrf2 up-regulates anti-apoptotic protein Bcl-2 and prevents cellular apoptosis. J. Biol. Chem. 2012:312694. doi: 10.1074/jbc.M111.312694. (jbc. M111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harada N., Kanayama M., Maruyama A. Nrf2 regulates ferroportin 1-mediated iron efflux and counteracts lipopolysaccharide-induced ferroportin 1 mRNA suppression in macrophages. Arch. Biochem. Biophys. 2011;508:101–109. doi: 10.1016/j.abb.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Sakata H., Niizuma K., Yoshioka H. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J. Neurosci. 2012;32:3462–3473. doi: 10.1523/JNEUROSCI.5686-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komatsu M., Kurokawa H., Waguri S. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010;12:213. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 28.Piantadosi C.A., Withers C.M., Bartz R.R. Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J. Biol. Chem. 2011:207738. doi: 10.1074/jbc.M110.207738. (jbc. M110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi S., Krishnan J., Ruckmani K. Cigarette smoke and related risk factors in neurological disorders: an update. Biomed. Pharmacother. 2017;85:79–86. doi: 10.1016/j.biopha.2016.11.118. [DOI] [PubMed] [Google Scholar]
- 30.Vomhof-DeKrey E.E., Picklo Sr M.J. The Nrf2-antioxidant response element pathway: a target for regulating energy metabolism. J. Nutr. Biochem. 2012;23:1201–1206. doi: 10.1016/j.jnutbio.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Baker R.G., Hayden M.S., Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. (2011/01/05) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayden M.S., Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. (2012/02/04) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shih R.H., Wang C.Y., Yang C.M. NF-kappaB signaling pathways in neurological inflammation: a mini review. Front. Mol. Neurosci. 2015;8:77. doi: 10.3389/fnmol.2015.00077. (2016/01/07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Q., Lenardo M.J., Baltimore D. 30 Years of NF-kappaB: a blossoming of relevance to human pathobiology. Cell. 2017;168:37–57. doi: 10.1016/j.cell.2016.12.012. (2017/01/14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardozo L.F., Pedruzzi L.M., Stenvinkel P. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie. 2013;95:1525–1533. doi: 10.1016/j.biochi.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Baird L., Dinkova-Kostova A.T. The cytoprotective role of the Keap1–Nrf2 pathway. Arch. Toxicol. 2011;85:241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 37.Cominacini L., Mozzini C., Garbin U. Endoplasmic reticulum stress and Nrf2 signaling in cardiovascular diseases. Free Radic. Biol. Med. 2015;88:233–242. doi: 10.1016/j.freeradbiomed.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 38.Bellezza I., Giambanco I., Minelli A. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2018 doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Plafker K.S., Nguyen L., Barneche M. The ubiquitin conjugating enzyme, UbcM2, can regulate the stability and activity of the anti-oxidant transcription factor, Nrf2. J. Biol. Chem. 2010:121913. doi: 10.1074/jbc.M110.121913. (jbc. M110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niture S.K., Khatri R., Jaiswal A.K. Regulation of Nrf2—an update. Free Radic. Biol. Med. 2014;66:36–44. doi: 10.1016/j.freeradbiomed.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiang M., Namani A., Wu S. Nrf2: bane or blessing in cancer? J. Cancer Res. Clin. Oncol. 2014;140:1251–1259. doi: 10.1007/s00432-014-1627-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krajka-Kuźniak V., Paluszczak J., Baer-Dubowska W. The Nrf2-ARE signaling pathway: an update on its regulation and possible role in cancer prevention and treatment. Pharmacol. Rep. 2017;69:393–402. doi: 10.1016/j.pharep.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Namani A., Li Y., Wang X.J. Modulation of NRF2 signaling pathway by nuclear receptors: implications for cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014;1843:1875–1885. doi: 10.1016/j.bbamcr.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Rada P., Rojo A.I., Chowdhry S. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol. Cell. Biol. 2011;31:1121–1133. doi: 10.1128/MCB.01204-10. (2011/01/20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rada P., Rojo A.I., Evrard-Todeschi N. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/beta-TrCP axis. Mol. Cell. Biol. 2012;32:3486–3499. doi: 10.1128/MCB.00180-12. (2012/07/04) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chowdhry S., Zhang Y., McMahon M. Nrf2 is controlled by two distinct beta-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–3781. doi: 10.1038/onc.2012.388. (2012/09/12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bai X., Chen Y., Hou X. Emerging role of NRF2 in chemoresistance by regulating drug-metabolizing enzymes and efflux transporters. Drug Metab. Rev. 2016;48:541–567. doi: 10.1080/03602532.2016.1197239. [DOI] [PubMed] [Google Scholar]
- 48.Naik P., Cucullo L. Pathobiology of tobacco smoking and neurovascular disorders: untied strings and alternative products. Fluids Barriers CNS. 2015;12:25. doi: 10.1186/s12987-015-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang D.D., Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. (2003/10/31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strom J., Xu B., Tian X. Nrf2 protects mitochondrial decay by oxidative stress. FASEB J. 2016;30:66–80. doi: 10.1096/fj.14-268904. (2015/09/06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bryan H.K., Olayanju A., Goldring C.E. The Nrf2 cell defence pathway: Keap1-dependent and-independent mechanisms of regulation. Biochem. Pharmacol. 2013;85:705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 52.Salazar M., Rojo A.I., Velasco D. Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J. Biol. Chem. 2006;281:14841–14851. doi: 10.1074/jbc.M513737200. (2006/03/23) [DOI] [PubMed] [Google Scholar]
- 53.Espada S., Rojo A.I., Salinas M. The muscarinic M1 receptor activates Nrf2 through a signaling cascade that involves protein kinase C and inhibition of GSK-3beta: connecting neurotransmission with neuroprotection. J. Neurochem. 2009;110:1107–1119. doi: 10.1111/j.1471-4159.2009.06208.x. (2009/06/30) [DOI] [PubMed] [Google Scholar]
- 54.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 55.Yang L., Palliyaguru D.L., Kensler T.W. Frugal chemoprevention: targeting Nrf2 with foods rich in sulforaphane. Semin. Oncol. 2016:146–153. doi: 10.1053/j.seminoncol.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fuse Y., Kobayashi M. Conservation of the Keap1-Nrf2 system: an evolutionary journey through stressful space and time. Molecules. 2017;22:436. doi: 10.3390/molecules22030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niture S.K., Jain A.K., Shelton P.M. Src subfamily kinases regulate nuclear export and degradation of transcription factor Nrf2 to switch off Nrf2-mediated antioxidant activation of cytoprotective gene expression. J. Biol. Chem. 2017;292 doi: 10.1074/jbc.A117.255042. (2048-2048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mattson M.P., Camandola S. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J. Clin. Investig. 2001;107:247–254. doi: 10.1172/JCI11916. (2001/02/13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oeckinghaus A., Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009;1 doi: 10.1101/cshperspect.a000034. (2010/01/13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tilstra J.S., Robinson A.R., Wang J. NF-kappaB inhibition delays DNA damage-induced senescence and aging in mice. J. Clin. Investig. 2012;122:2601–2612. doi: 10.1172/JCI45785. (2012/06/19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wardyn Joanna D., Ponsford Amy H., Sanderson Christopher M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015;43:621–626. doi: 10.1042/BST20150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Innamorato N.G., Rojo A.I., Garcia-Yague A.J. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J. Immunol. (Baltim. Md: 1950) 2008;181:680–689. doi: 10.4049/jimmunol.181.1.680. (2008/06/21) [DOI] [PubMed] [Google Scholar]
- 63.Brandenburg L.O., Kipp M., Lucius R. Sulforaphane suppresses LPS-induced inflammation in primary rat microglia. Inflamm. Res.: Off. J. Eur. Histamine Res. Soc. 2010;59:443–450. doi: 10.1007/s00011-009-0116-5. (2009/11/20) [DOI] [PubMed] [Google Scholar]
- 64.Innamorato N.G., Lastres-Becker I., Cuadrado A. Role of microglial redox balance in modulation of neuroinflammation. Curr. Opin. Neurol. 2009;22:308–314. doi: 10.1097/WCO.0b013e32832a3225. (2009/04/11) [DOI] [PubMed] [Google Scholar]
- 65.Lastres-Becker I., Ulusoy A., Innamorato N.G. alpha-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson's disease. Hum. Mol. Genet. 2012;21:3173–3192. doi: 10.1093/hmg/dds143. (2012/04/20) [DOI] [PubMed] [Google Scholar]
- 66.Rojo A.I., Innamorato N.G., Martin-Moreno A.M. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson's disease. Glia. 2010;58:588–598. doi: 10.1002/glia.20947. (2009/11/13) [DOI] [PubMed] [Google Scholar]
- 67.Rushworth S.A., MacEwan D.J., O’Connell M.A. Lipopolysaccharide-induced expression of NAD(P)H:quinone oxidoreductase 1 and heme oxygenase-1 protects against excessive inflammatory responses in human monocytes. J. Immunol. (Baltim. Md: 1950) 2008;181:6730–6737. doi: 10.4049/jimmunol.181.10.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kobayashi E.H., Suzuki T., Funayama R. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016;7:11624. doi: 10.1038/ncomms11624. (2016/05/24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rushworth S.A., Shah S., MacEwan D.J. TNF mediates the sustained activation of Nrf2 in human monocytes. J. Immunol. (Baltim. Md: 1950) 2011;187:702–707. doi: 10.4049/jimmunol.1004117. (2011/06/15) [DOI] [PubMed] [Google Scholar]
- 70.Wakabayashi N., Slocum S.L., Skoko J.J. When NRF2 talks, who's listening? Antioxid. Redox Signal. 2010;13:1649–1663. doi: 10.1089/ars.2010.3216. (2010/04/07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cuadrado A., Martin-Moldes Z., Ye J. Transcription factors NRF2 and NF-kappaB are coordinated effectors of the Rho family, GTP-binding protein RAC1 during inflammation. J. Biol. Chem. 2014;289:15244–15258. doi: 10.1074/jbc.M113.540633. (2014/04/25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanlioglu S., Williams C.M., Samavati L. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-alpha secretion through IKK regulation of NF-kappa B. J. Biol. Chem. 2001;276:30188–30198. doi: 10.1074/jbc.M102061200. (2001/06/13) [DOI] [PubMed] [Google Scholar]
- 73.Zuo L., He F., Sergakis G.G. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014;307:L205–L218. doi: 10.1152/ajplung.00330.2013. [DOI] [PubMed] [Google Scholar]
- 74.Boutten A., Goven D., Artaud-Macari E. NRF2 targeting: a promising therapeutic strategy in chronic obstructive pulmonary disease. Trends Mol. Med. 2011;17:363–371. doi: 10.1016/j.molmed.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 75.Kaisar M.A., Villalba H., Prasad S. Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: is metformin a viable countermeasure? Redox Biol. 2017;13:353–362. doi: 10.1016/j.redox.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prasad S., Kaisar M.A., Cucullo L. Unhealthy smokers: scopes for prophylactic intervention and clinical treatment. BMC Neurosci. 2017;18:70. doi: 10.1186/s12868-017-0388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prasad S., Sajja R.K., Kaisar M.A. Role of Nrf2 and protective effects of Metformin against tobacco smoke-induced cerebrovascular toxicity. Redox Biol. 2017;12:58–69. doi: 10.1016/j.redox.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaisar M.A., Prasad S., Cucullo L. Protecting the BBB endothelium against cigarette smoke-induced oxidative stress using popular antioxidants: are they really beneficial? Brain Res. 2015;1627:90–100. doi: 10.1016/j.brainres.2015.09.018. (2015/09/28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang C.-Y., Kuan Y.-H., Li J.-R. Docosahexaenoic acid reduces cellular inflammatory response following permanent focal cerebral ischemia in rats. J. Nutr. Biochem. 2013;24:2127–2137. doi: 10.1016/j.jnutbio.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 80.Ren J., Fan C., Chen N. Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf2 and HO-1 in rats. Neurochem. Res. 2011;36:2352. doi: 10.1007/s11064-011-0561-8. [DOI] [PubMed] [Google Scholar]
- 81.Zhang J., Fu B., Zhang X. Bicyclol upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. Bull. 2014;100:38–43. doi: 10.1016/j.brainresbull.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 82.Naik P., Sajja R.K., Prasad S. Effect of full flavor and denicotinized cigarettes exposure on the brain microvascular endothelium: a microarray-based gene expression study using a human immortalized BBB endothelial cell line. BMC Neurosci. 2015;16:38. doi: 10.1186/s12868-015-0173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cantin A.M. Cellular response to cigarette smoke and oxidants: adapting to survive. Proc. Am. Thorac. Soc. 2010;7:368–375. doi: 10.1513/pats.201001-014AW. [DOI] [PubMed] [Google Scholar]
- 84.Tuder R.M., Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J. Clin. Investig. 2012;122:2749–2755. doi: 10.1172/JCI60324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Edwards M.R., Bartlett N.W., Clarke D. Targeting the NF-kappab pathway in asthma and chronic obstructive pulmonary disease. Pharmacol. Ther. 2009;121:1–13. doi: 10.1016/j.pharmthera.2008.09.003. (2008/10/28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gagliardo R., Chanez P., Mathieu M. Persistent activation of nuclear factor-kappaB signaling pathway in severe uncontrolled asthma. Am. J. Respir. Crit. Care Med. 2003;168:1190–1198. doi: 10.1164/rccm.200205-479OC. (2003/08/02) [DOI] [PubMed] [Google Scholar]
- 87.Di Stefano A., Caramori G., Oates T. Increased expression of nuclear factor-kappab in bronchial biopsies from smokers and patients with COPD. Eur. Respir. J. 2002;20:556–563. doi: 10.1183/09031936.02.00272002. (2002/10/03) [DOI] [PubMed] [Google Scholar]
- 88.Caramori G., Romagnoli M., Casolari P. Nuclear localisation of p65 in sputum macrophages but not in sputum neutrophils during COPD exacerbations. Thorax. 2003;58:348–351. doi: 10.1136/thorax.58.4.348. (2003/04/02) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.La Grutta S., Gagliardo R., Mirabella F. Clinical and biological heterogeneity in children with moderate asthma. Am. J. Respir. Crit. Care Med. 2003;167:1490–1495. doi: 10.1164/rccm.200206-549OC. (2003/02/08) [DOI] [PubMed] [Google Scholar]
- 90.Prasad S., Sajja R.K., Park J.H. Impact of cigarette smoke extract and hyperglycemic conditions on blood–brain barrier endothelial cells. Fluids Barriers CNS. 2015;12:18. doi: 10.1186/s12987-015-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang H., Davies K.J., Forman H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015;88:314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sohal R.S., Orr W.C. The redox stress hypothesis of aging. Free Radic. Biol. Med. 2012;52:539–555. doi: 10.1016/j.freeradbiomed.2011.10.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ngo J.K., Pomatto L.C., Davies K.J. Upregulation of the mitochondrial lon protease allows adaptation to acute oxidative stress but dysregulation is associated with chronic stress, disease, and aging. Redox Biol. 2013;1:258–264. doi: 10.1016/j.redox.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tarantini S., Valcarcel-Ares M.N., Yabluchanskiy A. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood-brain barrier disruption, neuroinflammation, amyloidogenic gene expression, and cognitive decline in mice, mimicking the aging phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73:853–863. doi: 10.1093/gerona/glx177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang H., Liu H., Davies K.J. Nrf2-regulated phase II enzymes are induced by chronic ambient nanoparticle exposure in young mice with age-related impairments. Free Radic. Biol. Med. 2012;52:2038–2046. doi: 10.1016/j.freeradbiomed.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Silva-Palacios A., Ostolga-Chavarria M., Zazueta C. Nrf2: molecular and epigenetic regulation during aging. Ageing Res. Rev. 2018;47:31–40. doi: 10.1016/j.arr.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 97.Helenius M., Hänninen M., Lehtinen S.K. Changes associated with aging and replicative senescence in the regulation of transcription factor nuclear factor-kappa B. Biochem. J. 1996;318:603–608. doi: 10.1042/bj3180603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Korhonen P., Helenius M., Salminen A. Age-related changes in the regulation of transcription factor NF-kappa B in rat brain. Neurosci. Lett. 1997;225:61–64. doi: 10.1016/s0304-3940(97)00190-0. (1997/03/28) [DOI] [PubMed] [Google Scholar]
- 99.Adler A.S., Sinha S., Kawahara T.L. Motif module map reveals enforcement of aging by continual NF-kappab activity. Genes Dev. 2007;21:3244–3257. doi: 10.1101/gad.1588507. (2007/12/07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kriete A., Mayo K.L., Yalamanchili N. Cell autonomous expression of inflammatory genes in biologically aged fibroblasts associated with elevated NF-kappab activity. Immun. Ageing.: I & A. 2008;5:5. doi: 10.1186/1742-4933-5-5. (2008/07/18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kawahara T.L., Michishita E., Adler A.S. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. (2009/01/13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rahman M.M., Sykiotis G.P., Nishimura M. Declining signal dependence of N rf2-M af S-regulated gene expression correlates with aging phenotypes. Aging Cell. 2013;12:554–562. doi: 10.1111/acel.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sivandzade F., Cucullo L. In-vitro blood–brain barrier modeling: a review of modern and fast-advancing technologies. J. Cereb. Blood Flow Metab. 2018 doi: 10.1177/0271678X18788769. (0271678X18788769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hasan A., Deeb G., Rahal R. Mesenchymal stem cells in the treatment of traumatic brain injury. Front. Neurol. 2017;8:28. doi: 10.3389/fneur.2017.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Semple B.D., Zamani A., Rayner G. Affective, neurocognitive and psychosocial disorders associated with traumatic brain injury and post-traumatic epilepsy. Neurobiol Dis. 2018 doi: 10.1016/j.nbd.2018.07.018. (2018/07/31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dong W., Yang B., Wang L. Curcumin plays neuroprotective roles against traumatic brain injury partly via Nrf2 signaling. Toxicol. Appl. Pharmacol. 2018;346:28–36. doi: 10.1016/j.taap.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 107.Angeloni C., Prata C., Vieceli Dalla Sega F. Traumatic brain injury and NADPH oxidase: a deep relationship. Oxid. Med. Cell. Longev. 2015:2015. doi: 10.1155/2015/370312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith J.A., Park S., Krause J.S. Oxidative stress, DNA damage, and the telomeric complex as therapeutic targets in acute neurodegeneration. Neurochem. Int. 2013;62:764–775. doi: 10.1016/j.neuint.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lu X.-Y., Wang H.-D., Xu J.-G. Deletion of Nrf2 exacerbates oxidative stress after traumatic brain injury in mice. Cell. Mol. Neurobiol. 2015;35:713–721. doi: 10.1007/s10571-015-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nonaka M., Chen X.H., Pierce J.E. Prolonged activation of NF-kappaB following traumatic brain injury in rats. J. Neurotrauma. 1999;16:1023–1034. doi: 10.1089/neu.1999.16.1023. (1999/12/14) [DOI] [PubMed] [Google Scholar]
- 111.Mettang M., Reichel S.N., Lattke M. IKK2/NF-kappaB signaling protects neurons after traumatic brain injury. FASEB J. 2018;32:1916–1932. doi: 10.1096/fj.201700826R. (2017/12/01) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.M. Nazir, T.M.H. Khan, Pleiotropic roles of Nrf2 as regulators of chondrocyte apoptosis, oxidative stress, inflammatory response and catabolic and anabolic pathways in osteoarthriti, in: Proceedings of the SfRBM's 24th Annual Meeting, Baltimore, MD, USA, Free Radical Biology and Medicine, 2017, p.191.
- 113.Niture S.K., Jaiswal A.K. Nrf2-induced antiapoptotic Bcl-xL protein enhances cell survival and drug resistance. Free Radic. Biol. Med. 2013;57:119–131. doi: 10.1016/j.freeradbiomed.2012.12.014. (2013/01/01) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suvanish Kumar V., Gopalakrishnan A., Naziroglu M. Calcium ion–the key player in cerebral ischemia. Curr. Med. Chem. 2014;21:2065–2075. doi: 10.2174/0929867321666131228204246. [DOI] [PubMed] [Google Scholar]
- 115.Pradeep H., Diya J.B., Shashikumar S. Oxidative stress–assassin behind the ischemic stroke. Folia Neuropathol. 2012;50:219–230. doi: 10.5114/fn.2012.30522. [DOI] [PubMed] [Google Scholar]
- 116.Stephenson D., Yin T., Smalstig E.B. Transcription factor nuclear factor-kappa B is activated in neurons after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2000;20:592–603. doi: 10.1097/00004647-200003000-00017. (2000/03/21) [DOI] [PubMed] [Google Scholar]
- 117.Kunz A., Abe T., Hochrainer K. Nuclear factor-kappab activation and postischemic inflammation are suppressed in CD36-null mice after middle cerebral artery occlusion. J. Neurosci. 2008;28:1649–1658. doi: 10.1523/JNEUROSCI.5205-07.2008. (2008/02/15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Harari O.A., Liao J.K. NF-kappaB and innate immunity in ischemic stroke. Ann. N. Y. Acad. Sci. 2010;1207:32–40. doi: 10.1111/j.1749-6632.2010.05735.x. (2010/10/20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li L., Du J., Lian Y. Protective effects of coenzyme Q10 against hydrogen peroxide-induced oxidative stress in PC12 cell: the role of Nrf2 and antioxidant enzymes. Cell. Mol. Neurobiol. 2016;36:103–111. doi: 10.1007/s10571-015-0224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang W., Kang J., Li H. Regulation of endoplasmic reticulum stress in rat cortex by p62/ZIP through the Keap1-Nrf2-ARE signalling pathway after transient focal cerebral ischaemia. Brain Inj. 2013;27:924–933. doi: 10.3109/02699052.2013.793397. [DOI] [PubMed] [Google Scholar]
- 121.Morishita R., Sugimoto T., Aoki M. In vivo transfection of cis element "decoy" against nuclear factor-kappaB binding site prevents myocardial infarction. Nat. Med. 1997;3:894–899. doi: 10.1038/nm0897-894. (1997/08/01) [DOI] [PubMed] [Google Scholar]
- 122.Clemens J.A., Stephenson D.T., Smalstig E.B. Global ischemia activates nuclear factor-kappa B in forebrain neurons of rats. Stroke. 1997;28:1073–1080. doi: 10.1161/01.str.28.5.1073. (discussion1080-1071.1997/05/01) [DOI] [PubMed] [Google Scholar]
- 123.Lu Y., Liu X., Xie M. The NF-kappaB-responsive long noncoding RNA FIRRE regulates posttranscriptional regulation of inflammatory gene expression through interacting with hnRNPU. J. Immunol. (Baltim. Md: 1950) 2017;199:3571–3582. doi: 10.4049/jimmunol.1700091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zang Y., Zhou X., Wang Q. LncRNA FIRRE/NF-kB feedback loop contributes to OGD/R injury of cerebral microglial cells. Biochem. Biophys. Res. Commun. 2018;501:131–138. doi: 10.1016/j.bbrc.2018.04.194. (2018/05/02) [DOI] [PubMed] [Google Scholar]
- 125.Wang B., Zhu X., Kim Y. Histone deacetylase inhibition activates transcription factor Nrf2 and protects against cerebral ischemic damage. Free Radic. Biol. Med. 2012;52:928–936. doi: 10.1016/j.freeradbiomed.2011.12.006. (2012/01/10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao J., Moore A.N., Redell J.B. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J. Neurosci. 2007;27:10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li L., Zhang X., Cui L. Ursolic acid promotes the neuroprotection by activating Nrf2 pathway after cerebral ischemia in mice. Brain Res. 2013;1497:32–39. doi: 10.1016/j.brainres.2012.12.032. (2013/01/02) [DOI] [PubMed] [Google Scholar]
- 128.Dai Y., Zhang H., Zhang J. Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-kappaB pathway. Chem.-Biol. Interact. 2018;284:32–40. doi: 10.1016/j.cbi.2018.02.017. (2018/02/20) [DOI] [PubMed] [Google Scholar]
- 129.Yamauchi K., Nakano Y., Imai T. A novel nuclear factor erythroid 2-related factor 2 (Nrf2) activator RS9 attenuates brain injury after ischemia reperfusion in mice. Neuroscience. 2016;333:302–310. doi: 10.1016/j.neuroscience.2016.07.035. (2016/07/31) [DOI] [PubMed] [Google Scholar]
- 130.Gan L., Johnson J.A. Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014;1842:1208–1218. doi: 10.1016/j.bbadis.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 131.Lou H., Jing X., Wei X. Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the Nrf2/ARE signaling pathway. Neuropharmacology. 2014;79:380–388. doi: 10.1016/j.neuropharm.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 132.Bae E.-J., Ho D.-H., Park E. Lipid peroxidation product 4-hydroxy-2-nonenal promotes seeding-capable oligomer formation and cell-to-cell transfer of α-synuclein. Antioxid. Redox Signal. 2013;18:770–783. doi: 10.1089/ars.2011.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Näsström T., Fagerqvist T., Barbu M. The lipid peroxidation products 4-oxo-2-nonenal and 4-hydroxy-2-nonenal promote the formation of α-synuclein oligomers with distinct biochemical, morphological, and functional properties. Free Radic. Biol. Med. 2011;50:428–437. doi: 10.1016/j.freeradbiomed.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 134.Lastres-Becker I., Garcia-Yague A.J., Scannevin R.H. Repurposing the NRF2 activator dimethyl fumarate as therapy against synucleinopathy in Parkinson's disease. Antioxid. Redox Signal. 2016;25:61–77. doi: 10.1089/ars.2015.6549. (2016/03/25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Woo S.Y., Kim J.H., Moon M.K. Discovery of vinyl sulfones as a novel class of neuroprotective agents toward Parkinson's disease therapy. J. Med. Chem. 2014;57:1473–1487. doi: 10.1021/jm401788m. [DOI] [PubMed] [Google Scholar]
- 136.Gan L., Vargas M.R., Johnson D.A. Astrocyte-specific overexpression of Nrf2 delays motor pathology and synuclein aggregation throughout the CNS in the alpha-synuclein mutant (A53T) mouse model. J. Neurosci. 2012;32:17775–17787. doi: 10.1523/JNEUROSCI.3049-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.He Q., Song N., Jia F. Role of α-synuclein aggregation and the nuclear factor E2-related factor 2/heme oxygenase-1 pathway in iron-induced neurotoxicity. Int. J. Biochem. Cell Biol. 2013;45:1019–1030. doi: 10.1016/j.biocel.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 138.Barone M.C., Sykiotis G.P., Bohmann D. Genetic activation of Nrf2 signaling is sufficient to ameliorate neurodegenerative phenotypes in a Drosophila model of Parkinson's disease. Dis. Models Mech. 2011 doi: 10.1242/dmm.007575. (dmm. 007575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lastres-Becker I., Ulusoy A., Innamorato N.G. α-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson's disease. Hum. Mol. Genet. 2012;21:3173–3192. doi: 10.1093/hmg/dds143. [DOI] [PubMed] [Google Scholar]
- 140.Camandola S., Mattson M.P. NF-κB as a therapeutic target in neurodegenerative diseases. Expert Opin. Ther. Targets. 2007;11:123–132. doi: 10.1517/14728222.11.2.123. [DOI] [PubMed] [Google Scholar]
- 141.Mattson M.P., Camandola S. NF-κB in neuronal plasticity and neurodegenerative disorders. J. Clin. Investig. 2001;107:247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Flood P.M., Qian L., Peterson L.J. Transcriptional factor NF- Park.'s Dis. 2011:2011. doi: 10.4061/2011/216298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tiwari P.C., Pal R. The potential role of neuroinflammation and transcription factors in Parkinson disease. Dialog-. Clin. Neurosci. 2017;19:71. doi: 10.31887/DCNS.2017.19.1/rpal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jin Y.N., Yanxun V.Y., Gundemir S. Impaired mitochondrial dynamics and Nrf2 signaling contribute to compromised responses to oxidative stress in striatal cells expressing full-length mutant huntingtin. PLoS One. 2013;8:e57932. doi: 10.1371/journal.pone.0057932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stack C., Ho D., Wille E. Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington's disease. Free Radic. Biol. Med. 2010;49:147–158. doi: 10.1016/j.freeradbiomed.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Taguchi K., Fujikawa N., Komatsu M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc. Natl. Acad. Sci. USA. 2012;109:13561–13566. doi: 10.1073/pnas.1121572109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hands S., Sajjad M.U., Newton M.J. In vitro and in vivo aggregation of a fragment of huntingtin protein directly causes free radical production. J. Biol. Chem. 2011;286:44512–44520. doi: 10.1074/jbc.M111.307587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tsvetkov A.S., Arrasate M., Barmada S. Proteostasis of polyglutamine varies among neurons and predicts neurodegeneration. Nat. Chem. Biol. 2013;9:586. doi: 10.1038/nchembio.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Marcora E., Kennedy M.B. The Huntington's disease mutation impairs Huntingtin's role in the transport of NF-κB from the synapse to the nucleus. Hum. Mol. Genet. 2010;19:4373–4384. doi: 10.1093/hmg/ddq358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hsiao H.-Y., Chen Y.-C., Chen H.-M. A critical role of astrocyte-mediated nuclear factor-κB-dependent inflammation in Huntington's disease. Hum. Mol. Genet. 2013;22:1826–1842. doi: 10.1093/hmg/ddt036. [DOI] [PubMed] [Google Scholar]
- 151.De Strooper B. Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol. Rev. 2010;90:465–494. doi: 10.1152/physrev.00023.2009. [DOI] [PubMed] [Google Scholar]
- 152.Granic I., Dolga A.M., Nijholt I.M. Inflammation and NF-κB in Alzheimer's disease and diabetes. J. Alzheimer's Dis. 2009;16:809–821. doi: 10.3233/JAD-2009-0976. [DOI] [PubMed] [Google Scholar]
- 153.Chami L., Buggia-Prévot V., Duplan E. Nuclear factor-κB regulates βAPP and β-and γ-secretases differently at physiological and supraphysiological Aβ concentrations. J. Biol. Chem. 2012;287:24573–24584. doi: 10.1074/jbc.M111.333054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Valerio A., Boroni F., Benarese M. NF‐κB pathway: a target for preventing β‐amyloid (Aβ)‐induced neuronal damage and Aβ42 production. Eur. J. Neurosci. 2006;23:1711–1720. doi: 10.1111/j.1460-9568.2006.04722.x. [DOI] [PubMed] [Google Scholar]
- 155.Lee C., Park G.H., Lee S.-R. Attenuation of-amyloid-induced oxidative cell death by sulforaphane via activation of NF-E2-related factor 2. Oxid. Med. Cell. Longev. 2013:2013. doi: 10.1155/2013/313510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li X.-H., Li C.-Y., Lu J.-M. Allicin ameliorates cognitive deficits ageing-induced learning and memory deficits through enhancing of Nrf2 antioxidant signaling pathways. Neurosci. Lett. 2012;514:46–50. doi: 10.1016/j.neulet.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 157.Eftekharzadeh B., Maghsoudi N., Khodagholi F. Stabilization of transcription factor Nrf2 by tBHQ prevents oxidative stress-induced amyloid β formation in NT2N neurons. Biochimie. 2010;92:245–253. doi: 10.1016/j.biochi.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 158.Kim H.V., Kim H.Y., Ehrlich H.Y. Amelioration of Alzheimer's disease by neuroprotective effect of sulforaphane in animal model. Amyloid. 2013;20:7–12. doi: 10.3109/13506129.2012.751367. [DOI] [PubMed] [Google Scholar]
- 159.Rojo A.I., Pajares M., Garcia-Yague A.J. Deficiency in the transcription factor NRF2 worsens inflammatory parameters in a mouse model with combined tauopathy and amyloidopathy. Redox Biol. 2018;18:173–180. doi: 10.1016/j.redox.2018.07.006. (2018/07/22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mao P., Manczak M., Calkins M.J. Mitochondria-targeted catalase reduces abnormal APP processing, amyloid β production and BACE1 in a mouse model of Alzheimer's disease: implications for neuroprotection and lifespan extension. Hum. Mol. Genet. 2012;21:2973–2990. doi: 10.1093/hmg/dds128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rojo A.I., Pajares M., Rada P. NRF2 deficiency replicates transcriptomic changes in Alzheimer's patients and worsens APP and TAUpathology. Redox Biol. 2017;13:444–451. doi: 10.1016/j.redox.2017.07.006. (2017/07/14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Frakes A.E. The Role of Neuroinflammation in the Pathogenesis of Amyotrophic Lateral Sclerosis. Front. Immunol. 2014 The Ohio State University. [Google Scholar]
- 163.Alami N.O., Schurr C., Heuvel F.O. NF‐κB activation in astrocytes drives a stage‐specific beneficial neuroimmunological response in ALS. EMBO J. 2018:e98697. doi: 10.15252/embj.201798697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kanno T., Tanaka K., Yanagisawa Y. A novel small molecule, N-(4-(2-pyridyl)(1,3-thiazol-2-yl))-2-(2,4,6-trimethylphenoxy) acetamide, selectively protects against oxidative stress-induced cell death by activating the Nrf2–ARE pathway: therapeutic implications for ALS. Free Radic. Biol. Med. 2012;53:2028–2042. doi: 10.1016/j.freeradbiomed.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 165.Mead R.J., Higginbottom A., Allen S.P. S [+] apomorphine is a CNS penetrating activator of the Nrf2-ARE pathway with activity in mouse and patient fibroblast models of amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2013;61:438–452. doi: 10.1016/j.freeradbiomed.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Alfieri A., Srivastava S., Siow R.C. Targeting the Nrf2–Keap1 antioxidant defence pathway for neurovascular protection in stroke. J. Physiol. 2011;589:4125–4136. doi: 10.1113/jphysiol.2011.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Alfieri A., Srivastava S., Siow R.C. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood–brain barrier disruption and neurological deficits in stroke. Free Radic. Biol. Med. 2013;65:1012–1022. doi: 10.1016/j.freeradbiomed.2013.08.190. [DOI] [PubMed] [Google Scholar]
- 168.Chen G., Fang Q., Zhang J. Role of the Nrf2‐ARE pathway in early brain injury after experimental subarachnoid hemorrhage. J. Neurosci. Res. 2011;89:515–523. doi: 10.1002/jnr.22577. [DOI] [PubMed] [Google Scholar]
- 169.Petri S., Körner S., Kiaei M. Nrf2/ARE signaling pathway: key mediator in oxidative stress and potential therapeutic target in ALS. Neurol. Res. Int. 2012:2012. doi: 10.1155/2012/878030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lee D.-H., Gold R., Linker R.A. Mechanisms of oxidative damage in multiple sclerosis and neurodegenerative diseases: therapeutic modulation via fumaric acid esters. Int. J. Mol. Sci. 2012;13:11783–11803. doi: 10.3390/ijms130911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Prasad S., Sajja R.K., Naik P. Diabetes mellitus and blood-brain barrier dysfunction: an overview. J. Pharmacovigil. 2014;2:125. doi: 10.4172/2329-6887.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang W., Wu Y., Zhang G. Activation of Nrf2-ARE signal pathway protects the brain from damage induced by epileptic seizure. Brain Res. 2014;1544:54–61. doi: 10.1016/j.brainres.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 173.Patel M. Targeting oxidative stress in central nervous system disorders. Trends Pharmacol. Sci. 2016;37:768–778. doi: 10.1016/j.tips.2016.06.007. (2016/08/06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Salim S. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 2017;360:201–205. doi: 10.1124/jpet.116.237503. (2016/10/19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yaribeygi H., Panahi Y., Javadi B. The underlying role of oxidative stress in neurodegeneration: a mechanistic review. CNS Neurol. Disord. Drug Targets. 2018;17(207–215):2018/04/26. doi: 10.2174/1871527317666180425122557. [DOI] [PubMed] [Google Scholar]
- 176.Solleiro-Villavicencio H., Rivas-Arancibia S. Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4(+)T cells in neurodegenerative diseases. Front. Cell. Neurosci. 2018;12:114. doi: 10.3389/fncel.2018.00114. (2018/05/15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen Z., Mao X., Liu A. Osthole, a natural coumarin improves cognitive impairments and BBB dysfunction after transient global brain ischemia in C57 BL/6J mice: involvement of Nrf2 pathway. Neurochem. Res. 2015;40:186–194. doi: 10.1007/s11064-014-1483-z. [DOI] [PubMed] [Google Scholar]
- 178.Imai T., Takagi T., Kitashoji A. Nrf2 activator ameliorates hemorrhagic transformation in focal cerebral ischemia under warfarin anticoagulation. Neurobiol. Dis. 2016;89:136–146. doi: 10.1016/j.nbd.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 179.Li W., Suwanwela N.C., Patumraj S. Curcumin by down-regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc. Res. 2016;106:117–127. doi: 10.1016/j.mvr.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 180.Tarozzi A., Angeloni C., Malaguti M. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013:2013. doi: 10.1155/2013/415078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mao L., Yang T., Li X. Protective effects of sulforaphane in experimental vascular cognitive impairment: contribution of the Nrf2 pathway. J. Cereb. Blood Flow Metab. 2018 doi: 10.1177/0271678X18764083. (0271678X18764083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Soane L., Li Dai W., Fiskum G. Sulforaphane protects immature hippocampal neurons against death caused by exposure to hemin or to oxygen and glucose deprivation. J. Neurosci. Res. 2010;88:1355–1363. doi: 10.1002/jnr.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yu C., He Q., Zheng J. Sulforaphane improves outcomes and slows cerebral ischemic/reperfusion injury via inhibition of NLRP3 inflammasome activation in rats. Int. Immunopharmacol. 2017;45:74–78. doi: 10.1016/j.intimp.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 184.Holloway P.M., Gillespie S., Becker F. Sulforaphane induces neurovascular protection against a systemic inflammatory challenge via both Nrf2-dependent and independent pathways. Vasc. Pharmacol. 2016;85:29–38. doi: 10.1016/j.vph.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dinkova-Kostova A.T., Kostov R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012;18:337–347. doi: 10.1016/j.molmed.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 186.Zhao X., Wen L., Dong M. Sulforaphane activates the cerebral vascular Nrf2–ARE pathway and suppresses inflammation to attenuate cerebral vasospasm in rat with subarachnoid hemorrhage. Brain Res. 2016;1653:1–7. doi: 10.1016/j.brainres.2016.09.035. [DOI] [PubMed] [Google Scholar]
- 187.Takaya K., Suzuki T., Motohashi H. Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2012;53:817–827. doi: 10.1016/j.freeradbiomed.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.O'Mealey G.B., Berry W.L., Plafker S.M. Sulforaphane is a Nrf2-independent inhibitor of mitochondrial fission. Redox Biol. 2017;11:103–110. doi: 10.1016/j.redox.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.de Oliveira M.R., de Bittencourt Brasil F., Fürstenau C.R. Sulforaphane promotes mitochondrial protection in SH-SY5Y cells exposed to hydrogen peroxide by an Nrf2-dependent mechanism. Mol. Neurobiol. 2018;55:4777–4787. doi: 10.1007/s12035-017-0684-2. [DOI] [PubMed] [Google Scholar]
- 190.Wang X., Campos C.R., Peart J.C. Nrf2 upregulates ATP binding cassette transporter expression and activity at the blood–brain and blood–spinal cord barriers. J. Neurosci. 2014;34:8585–8593. doi: 10.1523/JNEUROSCI.2935-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Moon D.O., Kim M.O., Kang S.H. Sulforaphane suppresses TNF-alpha-mediated activation of NF-kappaB and induces apoptosis through activation of reactive oxygen species-dependent caspase-3. Cancer Lett. 2009;274:132–142. doi: 10.1016/j.canlet.2008.09.013. (2008/10/28) [DOI] [PubMed] [Google Scholar]
- 192.Negi G., Kumar A., Sharma S.S. Nrf2 and NF-kappab modulation by sulforaphane counteracts multiple manifestations of diabetic neuropathy in rats and high glucose-induced changes. Curr. Neurovasc. Res. 2011;8:294–304. doi: 10.2174/156720211798120972. (2011/10/26) [DOI] [PubMed] [Google Scholar]
- 193.Rahul Checker L.G., Maikho Thoh, Sharma Deepak, Sandu Santosh K. Sulforaphane, a naturally occurring isothiocyanate, exhibits anti-inflammatory effects by targeting GSK3β/Nrf-2 and NF-κB pathways in T cells. J. Funct. Foods. 2015;19:426–438. [Google Scholar]
- 194.Wang Z., Ma C., Meng C.J. Melatonin activates the Nrf2‐ARE pathway when it protects against early brain injury in a subarachnoid hemorrhage model. J. Pineal Res. 2012;53:129–137. doi: 10.1111/j.1600-079X.2012.00978.x. [DOI] [PubMed] [Google Scholar]
- 195.Ashabi G., Khalaj L., Khodagholi F. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab. Brain Dis. 2015;30:747–754. doi: 10.1007/s11011-014-9632-2. [DOI] [PubMed] [Google Scholar]
- 196.Liu Y., Tang G., Li Y. Metformin attenuates blood-brain barrier disruption in mice following middle cerebral artery occlusion. J. Neuroinflamm. 2014;11:177. doi: 10.1186/s12974-014-0177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pryor R., Cabreiro F. Repurposing metformin: an old drug with new tricks in its binding pockets. Biochem. J. 2015;471:307–322. doi: 10.1042/BJ20150497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jin Q., Cheng J., Liu Y. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav. Immun. 2014;40:131–142. doi: 10.1016/j.bbi.2014.03.003. (2014/03/19) [DOI] [PubMed] [Google Scholar]
- 199.Ou Z., Kong X., Sun X. Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav. Immun. 2018;69:351–363. doi: 10.1016/j.bbi.2017.12.009. (2017/12/19) [DOI] [PubMed] [Google Scholar]
- 200.Tanokashira D., Kurata E., Fukuokaya W. Metformin treatment ameliorates diabetes-associated decline in hippocampal neurogenesis and memory via phosphorylation of insulin receptor substrate 1. FEBS Open Bio. 2018;8:1104–1118. doi: 10.1002/2211-5463.12436. (2018/07/11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Isoda K., Young J.L., Zirlik A. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler. Thromb. Vasc. Biol. 2006;26:611–617. doi: 10.1161/01.ATV.0000201938.78044.75. (2005/12/31) [DOI] [PubMed] [Google Scholar]
- 202.Hattori Y., Suzuki K., Hattori S. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–1188. doi: 10.1161/01.HYP.0000221429.94591.72. (2006/04/26) [DOI] [PubMed] [Google Scholar]
- 203.Li S.N., Wang X., Zeng Q.T. Metformin inhibits nuclear factor kappaB activation and decreases serum high-sensitivity C-reactive protein level in experimental atherogenesis of rabbits. Heart Vessels. 2009;24:446–453. doi: 10.1007/s00380-008-1137-7. (2010/01/29) [DOI] [PubMed] [Google Scholar]
- 204.Kim H.G., Hien T.T., Han E.H. Metformin inhibits P-glycoprotein expression via the NF-kappaB pathway and CRE transcriptional activity through AMPK activation. Br. J. Pharmacol. 2011;162:1096–1108. doi: 10.1111/j.1476-5381.2010.01101.x. (2010/11/09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kakkar V., Kaur I.P. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem. Toxicol. 2011;49:2906–2913. doi: 10.1016/j.fct.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 206.Agarwal N.B., Jain S., Agarwal N.K. Modulation of pentylenetetrazole-induced kindling and oxidative stress by curcumin in mice. Phytomedicine. 2011;18:756–759. doi: 10.1016/j.phymed.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 207.Maiti P., Hall T.C., Paladugu L. A comparative study of dietary curcumin, nanocurcumin, and other classical amyloid-binding dyes for labeling and imaging of amyloid plaques in brain tissue of 5×-familial Alzheimer's disease mice. Histochem. Cell Biol. 2016;146:609–625. doi: 10.1007/s00418-016-1464-1. [DOI] [PubMed] [Google Scholar]
- 208.de Alcântara G.F.T., Simões-Neto E., da Cruz G.M.P. Curcumin reverses neurochemical, histological and immuno-histochemical alterations in the model of global brain ischemia. J. Tradit. Complement. Med. 2017;7:14–23. doi: 10.1016/j.jtcme.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang B.-f., Cui Z.-w., Zhong Z.-h. Curcumin attenuates brain edema in mice with intracerebral hemorrhage through inhibition of AQP4 and AQP9 expression. Acta Pharmacol. Sin. 2015;36:939. doi: 10.1038/aps.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Scapagnini G., Sonya V., Nader A.G. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol. Neurobiol. 2011;44:192–201. doi: 10.1007/s12035-011-8181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang L., Wu C., Zhao S. Demethoxycurcumin, a natural derivative of curcumin attenuates LPS-induced pro-inflammatory responses through down-regulation of intracellular ROS-related MAPK/NF-κB signaling pathways in N9 microglia induced by lipopolysaccharide. Int. Immunopharmacol. 2010;10:331–338. doi: 10.1016/j.intimp.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 212.Cui J.G., Li Y.Y., Zhao Y. Differential regulation of interleukin-1-receptor-associated kinase-1 (IRAK-1) and IRAK-2 by micro RNA-146a and NF-κB in stressed human astroglial cells and in Alzheimer's disease. J. Biol. Chem. 2010:178848. doi: 10.1074/jbc.M110.178848. (jbc. M110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Li W., Suwanwela N.C., Patumraj S. Curcumin by down-regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc. Res. 2016;106:117–127. doi: 10.1016/j.mvr.2015.12.008. (2015/12/22) [DOI] [PubMed] [Google Scholar]
- 214.Yang G.-Z., Wang Z.-J., Bai F. Epigallocatechin-3-gallate protects HUVECs from PM2. 5-induced oxidative stress injury by activating critical antioxidant pathways. Molecules. 2015;20:6626–6639. doi: 10.3390/molecules20046626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jiang J., Mo Z.-C., Yin K. Epigallocatechin-3-gallate prevents TNF-α-induced NF-κB activation thereby upregulating ABCA1 via the Nrf2/Keap1 pathway in macrophage foam cells. Int. J. Mol. Med. 2012;29:946–956. doi: 10.3892/ijmm.2012.924. [DOI] [PubMed] [Google Scholar]
- 216.Han J., Wang M., Jing X. (-)-Epigallocatechin gallate protects against cerebral ischemia-induced oxidative stress via Nrf2/ARE signaling. Neurochem. Res. 2014;39:1292–1299. doi: 10.1007/s11064-014-1311-5. [DOI] [PubMed] [Google Scholar]
- 217.Lee J.W., Lee Y.K., Ban J.O. Green tea (-)-epigallocatechin-3-gallate inhibits β-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-κ B pathways in mice. J. Nutr. 2009;139:1987–1993. doi: 10.3945/jn.109.109785. [DOI] [PubMed] [Google Scholar]
- 218.Kansanen E., Kuosmanen S.M., Leinonen H. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. doi: 10.1016/j.redox.2012.10.001. (2013/09/12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Karin M., Yamamoto Y., Wang Q.M. The IKK NF-kappa B system: a treasure trove for drug development. Nat. Rev. Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. (2004/01/07) [DOI] [PubMed] [Google Scholar]
- 220.Soares M.P., Seldon M.P., Gregoire I.P. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J. Immunol. (Baltim. Md: 1950) 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. (2004/03/09) [DOI] [PubMed] [Google Scholar]
- 221.Liu G.H., Qu J., Shen X. NF-kappab/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta. 2008;1783:713–727. doi: 10.1016/j.bbamcr.2008.01.002. (2008/02/05) [DOI] [PubMed] [Google Scholar]
- 222.Li H., Wang P., Huang F. Astragaloside IV protects blood-brain barrier integrity from LPS-induced disruption via activating Nrf2 antioxidant signaling pathway in mice. Toxicol. Appl. Pharmacol. 2018;340:58–66. doi: 10.1016/j.taap.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 223.Huang X.-P., Qiu Y.-Y., Wang B. Effects of Astragaloside IV combined with the active components of Panax notoginseng on oxidative stress injury and nuclear factor-erythroid 2-related factor 2/heme oxygenase-1 signaling pathway after cerebral ischemia-reperfusion in mice. Pharmacogn. Mag. 2014;10:402. doi: 10.4103/0973-1296.141765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Dai W., Wang H., Fang J. Curcumin provides neuroprotection in model of traumatic brain injury via the Nrf2-ARE signaling pathway. Brain Res. Bull. 2018;140:65–71. doi: 10.1016/j.brainresbull.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 225.Wang R., Tu J., Zhang Q. Genistein attenuates ischemic oxidative damage and behavioral deficits via eNOS/Nrf2/HO‐1 signaling. Hippocampus. 2013;23:634–647. doi: 10.1002/hipo.22126. [DOI] [PubMed] [Google Scholar]
- 226.Xi Y.-D., Yu H.-L., Ding J. Flavonoids protect cerebrovascular endothelial cells through Nrf2 and PI3K from β-amyloid peptide-induced oxidative damage. Curr. Neurovasc. Res. 2012;9:32–41. doi: 10.2174/156720212799297092. [DOI] [PubMed] [Google Scholar]
- 227.Zhang L., Wang H., Zhou Y. Fisetin alleviates oxidative stress after traumatic brain injury via the Nrf2-ARE pathway. Neurochem. Int. 2018 doi: 10.1016/j.neuint.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 228.Ding K., Wang H., Xu J. Melatonin stimulates antioxidant enzymes and reduces oxidative stress in experimental traumatic brain injury: the Nrf2–ARE signaling pathway as a potential mechanism. Free Radic. Biol. Med. 2014;73:1–11. doi: 10.1016/j.freeradbiomed.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 229.Tsai M.C., Chen W.J., Tsai M.S. Melatonin attenuates brain contusion‐induced oxidative insult, inactivation of signal transducers and activators of transcription 1, and upregulation of suppressor of cytokine signaling‐3 in rats. J. Pineal Res. 2011;51:233–245. doi: 10.1111/j.1600-079X.2011.00885.x. [DOI] [PubMed] [Google Scholar]
- 230.Park S., Lee S.K., Park K. Beneficial effects of endogenous and exogenous melatonin on neural reconstruction and functional recovery in an animal model of spinal cord injury. J. Pineal Res. 2012;52:107–119. doi: 10.1111/j.1600-079X.2011.00925.x. [DOI] [PubMed] [Google Scholar]
- 231.Koh P.O. Melatonin regulates the calcium‐buffering proteins, parvalbumin and hippocalcin, in ischemic brain injury. J. Pineal Res. 2012;53:358–365. doi: 10.1111/j.1600-079X.2012.01005.x. [DOI] [PubMed] [Google Scholar]
- 232.Ordoñez R., Carbajo‐Pescador S., Prieto‐Dominguez N. Inhibition of matrix metalloproteinase‐9 and nuclear factor kappa B contribute to melatonin prevention of motility and invasiveness in Hep G 2 liver cancer cells. J. Pineal Res. 2014;56:20–30. doi: 10.1111/jpi.12092. [DOI] [PubMed] [Google Scholar]
- 233.Kelso M.L., Scheff N.N., Scheff S.W. Melatonin and minocycline for combinatorial therapy to improve functional and histopathological deficits following traumatic brain injury. Neurosci. Lett. 2011;488:60–64. doi: 10.1016/j.neulet.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Buendia I., Parada E., Navarro E. Subthreshold concentrations of melatonin and galantamine improves pathological AD-hallmarks in hippocampal organotypic cultures. Mol. Neurobiol. 2016;53:3338–3348. doi: 10.1007/s12035-015-9272-5. [DOI] [PubMed] [Google Scholar]