Abstract
Introduction
Phosphodiesterase type 5 inhibitors (PDE5i) are first-line therapy for most men with erectile dysfunction (ED). If ineffective, vacuum erection devices, intracavernous injections, and penile prosthesis implantation are suitable as second- or third-line therapies. However, very few patients select these therapies. It is critically important to improve erectile function with oral administration of effective agents. Administration of L-citrulline or transresveratrol in animal experiments has been reported to improve erectile function, but few such experiments have been performed on humans with ED.
Aim
We aimed to investigate the efficacy of combination therapy of L-citrulline and transresveratrol in patients with ED despite their use of PDE5i.
Methods
In this randomized, double-blind, placebo-controlled crossover pilot study, men with ED (Sexual Health Inventory for Men [SHIM] score below 16) despite on-demand use of PDE5i received a placebo for 1 month or the active treatment (L-citrulline 800 mg/day and transresveratrol 300 mg/day) for another month. Patients continued on-demand use of PDE5i.
Main Outcome Measure
The SHIM score, Erection Hardness Score (EHS), Aging Male Symptoms Scale-sexual domain (AMS-SD), and adverse events were examined.
Results
20 patients ages 29–78 years were enrolled, and after 6 men withdrew, 13 concluded the study without adverse events. Mean SHIM score for the active treatment increased significantly (10.96 ± 1.21) compared with baseline (8.32 ± 1.21) and placebo (8.31 ± 1.23) (both P < .05). Mean EHS score for the active treatment (2.56 ± 0.26) also increased from baseline (2.31 ± 0.26), but not significantly (P = .79). Mean AMS-SD score was not significantly different in either group.
Conclusion
To our knowledge, this is the first study to show that combination therapy of L-citrulline and transresveratrol is effective for ED treatment in men with added on-demand use of PDE5i. This combination supplement may be added if PDE5i is insufficient.
Shirai M, Hiramatsu I, Aoki Y, et al. Oral L-citrulline and Transresveratrol Supplementation Improves Erectile Function in Men With Phosphodiesterase 5 Inhibitors: A Randomized, Double-Blind, Placebo-Controlled Crossover Pilot Study. Sex Med 2018;6:291–296.
Key Words: L-Citrulline, Transresveratrol, PDE5i, Erectile Dysfunction
Introduction
Erectile dysfunction (ED) is defined as the “consistent or recurrent inability to attain and maintain penile erection sufficient for sexual satisfaction.”1 In a multinational survey of men ages 50–80 years, the prevalence of ED was 48.7%.2 Sexual activity is an important component of overall quality of life.2 Men with ED described lower rates of personal satisfaction on all quality of life attributes compared with men without ED.3 Thus, treatment for ED should be very valuable not only for the local regulation of penile status but also for improved quality of life. Phosphodiesterase type 5 inhibitors (PDE5i) are first-line therapy for most men with ED who do not have a specific contraindication to their use. (In Japan, 3 PDE5i, sildenafil, vardenafil, and tadalafil are approved and used clinically.4) Generally, it is estimated that the efficacy of PDE5i is 56% to 84% in ED patients.5 However, specific patient populations, such as those with severe diabetes mellitus, are hard to treat solely with PDE5i. If PDE5i therapy is not effective, vacuum erection devices, intracavernous injections, and surgical implantation of a penile prosthesis are suitable as second- or third-line therapies for ED treatment.5, 6 Although these therapies have been shown to be highly effective, the associated pain and inconvenience were the most important causes of dissatisfaction.4, 6, 7 Therefore, very few patients in Japan select these second- or third-line therapies.4 Under this situation, new oral medications or supplements with or without PDE5i have been expected for a long time. It is critically important to improve erectile function with the oral administration of effective agents. L-citrulline is abundantly contained in watermelon.8 Oral L-citrulline supplementation increases both serum L-arginine levels more efficiently than L-arginine supplementation alone and nitric oxide (NO) production.9 Oral L-arginine supplementation does not increase L-arginine blood levels significantly because of the hepatic first-pass effect or metabolization by intestinal bacteria.10, 11 However, L-citrulline is neither affected by the hepatic first-pass effect nor do intestinal bacteria metabolize it.11 L-citrulline is converted to L-arginine in the kidney, thus setting the rationale for oral L-citrulline supplementation as a donor for the L-arginine/NO/cGMP (cyclic guanosine monophosphate) pathway of penile erection.9 In animal experiments, administration of L-citrulline improved intracavernous pressure/mean arterial pressure (ICP/MAP), smooth muscle/collagen ratios, and serum levels of nitrogen oxides in arteriogenic ED12 and low-testosterone ED.13 However, few experiments have been performed on humans. 1 clinical study suggested that oral L-citrulline supplementation might improve the Erection Hardness Score (EHS) without adverse events in men with mild ED.14
Resveratrol is a polyphenol found in grapes and wine.15 Several studies have shown that resveratrol increases the expression of endothelial nitric oxide synthase (eNOS)16 and improves endothelial function17 by activation of sirtuin1 (SIRT1), which promotes endothelium-dependent vascular relaxation.17, 18 Resveratrol consumption increased plasma resveratrol concentrations and flow-mediated dilatation of the brachial artery, which is a biomarker of endothelial function and cardiovascular health, in a dose-related manner.19 In animal studies, resveratrol treatment leads to SIRT1 activation, and subsequently activated eNOS leads to enhancement of cGMP synthesis via the NO/cGMP pathway for penile erection.20 Resveratrol elevates the intracellular cGMP level in human corpus cavernosal smooth muscle cells.20 The combination treatment of resveratrol and PDE5i has a synergistic effect.20 Treatment with either resveratrol or PDE5i improved ICP/MAP ratios, and combination therapy with both had a synergistic effect in improving the ICP/MAP ratios in rats with diabetes mellitus.20 We speculated that the combination of L-citrulline and transresveratrol with PDE5i would have an even greater synergistic effect.
We hypothesized that the combination therapy of L-citrulline and transresveratrol would be effective for ED patients despite their on-demand use of PDE5i. Therefore, we investigated the efficacy of oral L-citrulline and transresveratrol supplementation in improving erections in patients with ED.
Materials and Methods
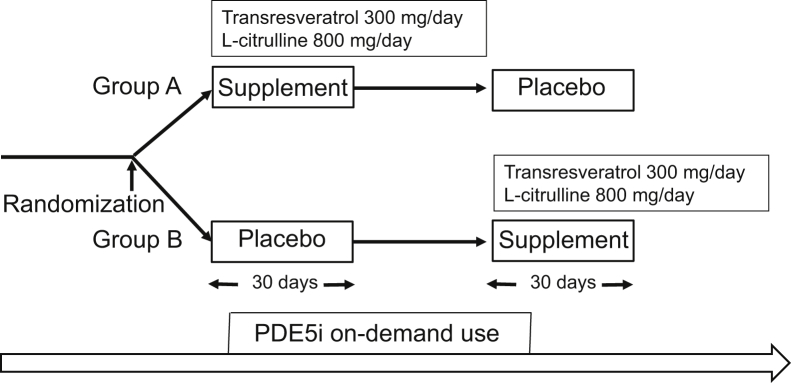
20 patients who visited our special clinics for sexual function were included in this study. In the present randomized, double-blind, placebo-controlled crossover pilot study, men with ED (Sexual Health Inventory for Men [SHIM] score below 16) despite the on-demand use of PDE5i received a placebo for 1 month or the active treatment (L-citrulline 800 mg/day and transresveratrol 300 mg/day) for another month without washout before crossover (Figure 1). The supplements or the placebo was taken once daily after a meal as a complex agent in capsule form. The patients were randomized into 2 groups (A, men who received the supplement first, and B, men who received the placebo first). Randomization was centralized at Juntendo University and done by the simple randomization method with a computed random number table. Patients continued their on-demand use of PDE5i. We asked Molecular Physiological Chemistry Laboratory, Inc (Tokyo, Japan) to prepare the supplement and placebo capsules. The chemical stability of the 2 supplements when used in combination was proven by the high-performance liquid chromatography measurement, and the amino acid automatic analyze method was proven at Japan Food Research Laboratories, Inc (Tokyo, Japan). The supplement was encapsulated in a hydroxypropyl methylcellulose capsule. The L-citrulline (800 mg) and transresveratrol (300 mg) were mixed with a diluting agent of crystalline cellulose and calcium stearate. The placebo was similarly encapsulated in a hydroxypropyl methylcellulose capsule, and the caramel placebo was also mixed with a diluting agent of crystalline cellulose and calcium stearate. We used the SHIM and EHS to measure erectile function. The SHIM and EHS have been shown to be simple, reliable, and valid tools for the assessment of erectile function in clinical trials research.21, 22 Both questionnaires were validated in Japanese.23 To measure the improvement in libido and sexual performance, we used the Aging Male Symptoms Scale-sexual domain (AMS-SD), which was also validated in Japan.24, 25 All patients were examined by a medical doctor for evaluation of their medical condition and adverse events, and all self-reported questionnaires were collected in the clinic.
Figure 1.
Study protocol. Men with erectile dysfunction received a placebo for 30 days or the active treatment for another 30 days without washout before crossover. Patients continued on-demand use of PDE5i. PDE5i = phosphodiesterase type 5 inhibitor.
Main Outcome Measure
The SHIM total score (SHIM-T), the 5 SHIM domain scores (SHIM-1–SHIM-5), the EHS, the AMS-SD, and adverse events were examined.
Ethical Approval and Informed Consent
The study protocol was in compliance with Good Clinical Practices and the Declaration of Helsinki (1996) and in accordance with applicable institutional review board regulations. We registered the protocol in the University Hospital Medical Information-Clinical Trials Registration (approval no. UMIN000028695). The protocol was also approved by the institutional review board of Juntendo University Urayasu Hospital, Chiba, Japan (approval no. 2014-061). The study participants gave informed consent before the initiation of any study-related procedures and medications.
Statistical Analysis
Data are presented as the mean ± standard error. Statistical significance was determined by a paired t-test. Then we used the linear mixed model for adjusted with age, which was the significant unique variable. Based on a power of 80% to detect a significant difference at a 2-sided level of 5%, 25 men were required for each study group. Initially, we planned to recruit a total of 50 men in this study. A P value less than .05 was considered statistically significant. We used IBM SPSS Statistics for Windows, Japanese version 20 (IBM Japan, Tokyo, Japan) for the statistical analysis.
Results
Initially, 20 men were enrolled and commenced treatment in the study (Table 1), with 13 men ages 29–78 (61.2 ± 4.1) years completing the study without adverse events (9 men in group A and 4 men in group B). Men with ED overlap used on-demand PDE5i, including 10 men taking tadalafil, 9 men taking sildenafil, and 7 men taking vardenafil. No men with ED took tadalafil once daily for lower urinary tract symptoms (LUTS) in this study. 6 men were withdrawn from this study (1 man in group A and 5 men in group B), along with 1 man in group A who could not complete the questionnaire.
Table 1.
Patient demographics at baseline∗
| Group A (n = 11)† | Group B (n = 9)‡ | P value | |
|---|---|---|---|
| Age (y) | 62.69 ± 3.41 | 55.00 ± 5.75 | .24 |
| SHIM-T | 7.22 ± 0.92 | 10.75 ± 2.02 | .09 |
| EHS | 2.22 ± 1.09 | 2.50 ± 0.58 | .65 |
| AMS-SD | 12.78 ± 4.41 | 14.00 ± 5.60 | .68 |
| Duration of ED (y) | 4.00 ± 1.41 | 4.00 ± 2.16 | 1.00 |
| On-demand PDE5i | T: 7, S: 5, V: 3 | T: 3, S: 4, V: 4 | – |
AMS-SD = Aging Male Symptoms Scale-sexual domain; ED = erectile dysfunction; EHS = Erection Hardness Score; PDE5i = phosphodiesterase type 5 inhibitor; SHIM-T = Sexual Health Inventory for Men total score; S = sildenafil; T = tadalafil; V = vardenafil.
Values are mean ± standard error.
Received supplementation first.
Received placebo first.
The mean SHIM-T score of the active treatment was significantly increased compared with baseline and placebo as shown in Table 2 (P = .035 and P = .040, respectively). The mean score of SHIM-1 active treatment was significantly increased compared with placebo (P = .026). The mean score of SHIM-5 active treatment was also significantly increased compared with SHIM-5 placebo (P = .049). The mean score of SHIM-2 active treatment was significantly increased compared with SHIM-2 baseline (P = .021). The mean scores of SHIM-3 and SHIM-4 active treatment were improved, but not significantly so, compared with placebo (P = .717 and P = .716, respectively).
Table 2.
SHIM, EHS, and AMS-SD scores∗
| Baseline | Placebo | Supplement | |
|---|---|---|---|
| SHIM-T | 8.32 ± 1.21 | 8.31 ± 1.23 | 10.96 ± 1.21†,‡ |
| SHIM-1 | 1.69 ± 0.22 | 1.43 ± 0.22 | 2.08 ± 0.22† |
| SHIM-2 | 1.67 ± 0.26 | 1.80 ± 0.26 | 2.43 ± 0.27‡ |
| SHIM-3 | 1.62 ± 0.35 | 1.77 ± 0.35 | 2.08 ± 0.35 |
| SHIM-4 | 1.62 ± 0.40 | 1.87 ± 0.41 | 2.32 ± 0.40 |
| SHIM-5 | 1.85 ± 0.33 | 1.60 ± 0.33 | 2.24 ± 0.33† |
| EHS | 2.31 ± 0.26 | 2.18 ± 0.27 | 2.56 ± 0.26 |
| AMS-SD | 13.16 ± 1.29 | 12.56 ± 1.30 | 12.62 ± 1.29 |
AMS-SD = Aging Male Symptoms Scale-sexual domain; EHS = Erection Hardness Score; SHIM-T = Sexual Health Inventory for Men total score; SHIM-1 = Sexual Health Inventory for Men erection confidence; SHIM-2 = Sexual Health Inventory for Men erection firmness; SHIM-3 = Sexual Health Inventory for Men maintenance frequency; SHIM-4 = Sexual Health Inventory for Men maintenance ability; SHIM-5 = Sexual Health Inventory for Men intercourse satisfaction.
Values are mean ± standard error, and all scores are adjusted for age.
P < .05 compared with baseline.
P < .05 compared with placebo.
The mean EHS of active treatment was increased from baseline, but not significantly so (P = .794). There was also no significant change between the baseline and placebo EHS (P = .330). The mean score of AMS-SD was not significantly different in either group (P = 1.000). 13 patients continued on-demand PDE5i after completing the treatment period. We have no follow-up data on these patients following their completion of the study.
Discussion
We investigated the efficacy of oral L-citrulline and transresveratrol supplementation in improving erection in patients with ED. This is the first study, to our knowledge, to show that the combination therapy of L-citrulline and transresveratrol is effective for ED patients with unsatisfied efficacy from on-demand use of PDE5i. The data clearly showed that this supplementation can salvage the ED patient who fails treatment solely with PDE5i.
The present study revealed that SHIM-T, erection confidence (SHIM-1), erection firmness (SHIM-2), and intercourse satisfaction (SHIM-5) were improved significantly. Maintenance frequency (SHIM-3) and maintenance ability (SHIM-4) were also improved after the supplementation, but not significantly so. The present combination therapy might be more effective in improving confidence and satisfaction. There was no significant change in the AMS-SD after supplementation, indicating a possible limited effect on libido and sexual performance. 5 of the 6 men withdrawn from this study were from group B. We speculated that they may have been disappointed with the effect of the placebo used in group B.
Oral L-citrulline supplementation acts as a donor for the L-arginine/NO/cGMP pathway of penile erection.9 It was reported that oral L-citrulline supplementation increased NO production in rats with ED and that administrations of L-citrulline improved the intracavernous pressure in rats with ED.12, 13 1 clinical study suggested that oral L-citrulline supplementation might improve the EHS in men with mild ED. In that study, a significant improvement in the EHS from 3–4 was reported by 8.3% of the men when taking the placebo and 50% of the men when taking L-citrulline. In addition, the mean number of intercourses per month increased significantly compared with baseline (2.3 ± 1.37 vs 1.37 ± 0.93). All patients reporting an improvement in the EHS from 3–4 were very satisfied.14
In animal studies, resveratrol treatment leads to SIRT1 activation, and subsequently activated eNOS leads to enhancement of cGMP synthesis via the NO/cGMP pathway for penile erection.20 Resveratrol elicited a concentration-dependent relaxing effect on the corpus cavernosum and an increase in blood testosterone concentration in a rabbit model.26 Resveratrol might be an effective treatment in the prevention of atherosclerotic changes in the corpus cavernosum of hypercholesterolemic rabbits.27
The intracellular cGMP level was elevated by resveratrol treatment in human corpus cavernosal smooth muscle cells.12 The combination treatment of resveratrol and PDE5i had a synergistic effect.20 Treatment with either resveratrol or PDE5i improved erectile function, and combination therapy with resveratrol and PDE5i had a synergistic effect in the improvement of erectile function in rats with ED.20
In our previous study, the mean score of the International Index of Erectile Function 5 items scale in 7 patients with ED was significantly improved (14.0 ± 5.8) compared with baseline (8.9 ± 5.8; P < .05), despite on-demand use of PDE5i, when they received 30 days of active treatment with 300 mg/day of transresveratrol (A. Tsujimura, unpublished data, 2013). The applied dosage of L-citrulline in the present study was 800 mg/day, which was the highest dosage available in Japan at the time of the study (Kyowa Hakko Bio, Ibaragi, Japan). This dosage was lower than that used in a previous study in which L-citrulline 1.5 g/day was administered in ED patients14 and 2% L-citrulline water (approximately 2 g/day) was used in a rat model.12, 13 Safety has been evaluated in humans and rats with ED.12, 13, 14 No side effects have been reported from citrulline administration as an oral supplement at doses up to 15 g.28
The applied dosage of transresveratrol was 300 mg/day in the present study, which is the same as that used in our previous study, in which we calculated a dose of 5 mg/kg in both the human study and rat study (A. Tsujimura, unpublished data, 2013).20 Safety was also evaluated in patients and rats with ED (A. Tsujimura, unpublished data, 2013).20 A previous study reported gastrointestinal side effects in a high proportion of participants taking 2,500 mg/day or more of transresveratrol, suggesting that doses at or above this level are unlikely to be tolerated chronically.29 That study also reported that 450 mg/day can be considered safe for a 60-kg individual.29 We believe that the dosage applied in our study was safe and effective.
Although prospective and placebo controlled, the present study has some limitations. First, this study did not include ED patients taking tadalafil once daily, which represents an effective and well-tolerated medical treatment for patients with LUTS and ED.30 It is possible that daily PDE5i is more effective for ED treatment with L-citrulline and transresveratrol. Second, this study did not include L-citrulline monotherapy. Because the participants had moderate to severe ED, monotherapy with L-citrulline was thought to be insufficient compared with the findings in a previous study.14 Third, we did not include the frequency of sexual intercourse in the study questionnaire. Men taking L-citrulline revealed that the frequency of sexual intercourse was significantly increased compared with baseline.14 It is possible that the frequency of sexual intercourse was increased with L-citrulline and transresveratrol owing to an improvement in confidence. Fourth, the duration of this study was short, only 1 month, and the sample size was small. We will be planning an additional study of longer duration that incorporates more institutions.
To our knowledge, this is the first study to show that combination therapy of L-citrulline and transresveratrol is effective for ED patients with unsatisfied efficacy from on-demand use of PDE5i. The present findings clearly showed that our supplementation therapy may be 1 possible treatment option for these patients.
Statement of Authorship
Category 1
-
(a)Conception and Design
- Masato Shirai; Ippei Hiramatsu; Yusuke Aoki; Hirofumi Shimoyama; Taiki Mizuno; Taiji Nozaki; Shinichiro Fukuhara; Atsushi Iwasa; Shinji Kageyama; Shiego Horie; Akira Tsujimura
-
(b)Acquisition of Data
- Masato Shirai; Ippei Hiramatsu; Yusuke Aoki; Hirofumi Shimoyama; Taiki Mizuno; Taiji Nozaki; Shinichiro Fukuhara; Atsushi Iwasa; Shinji Kageyama; Shiego Horie; Akira Tsujimura
-
(c)Analysis and Interpretation of Data
- Masato Shirai; Ippei Hiramatsu; Yusuke Aoki; Hirofumi Shimoyama; Taiki Mizuno; Taiji Nozaki; Shinichiro Fukuhara; Atsushi Iwasa; Shinji Kageyama; Shiego Horie; Akira Tsujimura
Category 2
-
(a)Drafting the Article
- Masato Shirai; Ippei Hiramatsu; Yusuke Aoki; Hirofumi Shimoyama; Taiki Mizuno; Taiji Nozaki; Shinichiro Fukuhara; Atsushi Iwasa; Shinji Kageyama; Shiego Horie; Akira Tsujimura
-
(b)Revising It for Intellectual Content
- Masato Shirai; Ippei Hiramatsu; Yusuke Aoki; Hirofumi Shimoyama; Taiki Mizuno; Taiji Nozaki; Shinichiro Fukuhara; Atsushi Iwasa; Shinji Kageyama; Shiego Horie; Akira Tsujimura
Category 3
-
(a)Final Approval of the Completed Article
- Masato Shirai; Ippei Hiramatsu; Yusuke Aoki; Hirofumi Shimoyama; Taiki Mizuno; Taiji Nozaki; Shinichiro Fukuhara; Atsushi Iwasa; Shinji Kageyama; Shiego Horie; Akira Tsujimura
Acknowledgments
Research dataset: The datasets generated and analyzed during the current study are available in the figshare repository at https://figshare.com/s/95936bccedd3b26e8aef.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
Funding: None.
References
- 1.McCabe M.P., Sharlip I.D., Atalla E. Definitions of sexual dysfunctions in women and men: a consensus statement from the Fourth International Consultation on Sexual Medicine 2015. J Sex Med. 2016;13:135–143. doi: 10.1016/j.jsxm.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 2.Rosen R., Altwein J., Boyle P. Lower urinary tract symptoms and male sexual dysfunction: the Multinational Survey of the Aging Male (MSAM-7) Eur Urol. 2003;44:637–649. doi: 10.1016/j.eururo.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Sand M.S., Fisher W., Rosen R. Erectile dysfunction and constructs of masculinity and quality of life in the multinational Men's Attitudes to Life Events and Sexuality (MALES) study. J Sex Med. 2008;5:583–594. doi: 10.1111/j.1743-6109.2007.00720.x. [DOI] [PubMed] [Google Scholar]
- 4.Tsujimura A., Kiuchi H., Soda T. Sexual life of Japanese patients with erectile dysfunction taking phosphodiesterase type 5 inhibitors: an Internet survey using the Psychological and Interpersonal Relationship Scales-Short Form questionnaire. Int J Urol. 2014;21:821–825. doi: 10.1111/iju.12429. [DOI] [PubMed] [Google Scholar]
- 5.Hatzimouratidis K., Salonia A., Adaikan G. Pharmacotherapy for erectile dysfunction: recommendations from the Fourth International Consultation for Sexual Medicine (ICSM 2015) J Sex Med. 2016;13:465–488. doi: 10.1016/j.jsxm.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Hatzimouratidis K., Eardley I., Giuliano F. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. http://uroweb.org/wp-content/uploads/14-Male-Sexual-Dysfunction_LR1.pdf Available at: [DOI] [PubMed]
- 7.Khayyamfar F., Forootan S.K., Ghasemi H. Evaluating the efficacy of vacuum constrictive device and causes of its failure in impotent patients. Urol J. 2014;10:1072–1078. [PubMed] [Google Scholar]
- 8.Rimando A.M., Perkins-Veazie P.M. Determination of citrulline in watermelon rind. J Chromatogr A. 2005;1078:196–200. doi: 10.1016/j.chroma.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Schwedhelm E., Maas R., Freese R. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. Br J Clin Pharmacol. 2008;65:51–59. doi: 10.1111/j.1365-2125.2007.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curis E., Nicolis I., Moinard C. Almost all about citrulline in mammals. Amino Acids. 2005;29:177–205. doi: 10.1007/s00726-005-0235-4. [DOI] [PubMed] [Google Scholar]
- 11.Smith E.A., Macfarlane G.T. Dissimilatory amino acid metabolism in human colonic bacteria. Anaerobe. 1997;3:327–337. doi: 10.1006/anae.1997.0121. [DOI] [PubMed] [Google Scholar]
- 12.Shiota A., Hotta Y., Kataoka T. Oral L-citrulline supplementation improves erectile function in rats with acute arteriogenic erectile dysfunction. J Sex Med. 2013;10:2423–2429. doi: 10.1111/jsm.12260. [DOI] [PubMed] [Google Scholar]
- 13.Hotta Y., Shiota A., Kataoka T. Oral L-citrulline supplementation improves erectile function and penile structure in castrated rats. Int J Urol. 2014;21:608–612. doi: 10.1111/iju.12362. [DOI] [PubMed] [Google Scholar]
- 14.Cormio L., De Siati M., Lorusso F. Oral L-citrulline supplementation improves erection hardness in men with mild erectile dysfunction. Urology. 2011;77:119–122. doi: 10.1016/j.urology.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 15.Hao H.D., He L.R. Mechanisms of cardiovascular protection by resveratrol. J Med Food. 2004;7:290–298. doi: 10.1089/jmf.2004.7.290. [DOI] [PubMed] [Google Scholar]
- 16.Wallerath T., Deckert G., Ternes T. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–1658. doi: 10.1161/01.cir.0000029925.18593.5c. [DOI] [PubMed] [Google Scholar]
- 17.Mattagajasingh I., Kim C.S., Naqvi A. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borra M.T., Smith B.C., Denu J.M. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 19.Wong R.H., Howe P.R., Buckley J.D. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis. 2011;21:851–856. doi: 10.1016/j.numecd.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Fukuhara S., Tsujimura A., Okuda H. Vardenafil and resveratrol synergistically enhance the nitric oxide/cyclic guanosine monophosphate pathway in corpus cavernosal smooth muscle cells and its therapeutic potential for erectile dysfunction in the streptozotocin-induced diabetic rat: preliminary findings. J Sex Med. 2011;8:1061–1071. doi: 10.1111/j.1743-6109.2010.02193.x. [DOI] [PubMed] [Google Scholar]
- 21.Mulhall J.P., Goldstein I., Bushmakin A.G. Validation of the erection hardness score. J Sex Med. 2007;4:1626–1634. doi: 10.1111/j.1743-6109.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 22.Cappelleri J.C., Rosen R.C. The Sexual Health Inventory for Men (SHIM): a 5-year review of research and clinical experience. Int J Impot Res. 2005;17:307–319. doi: 10.1038/sj.ijir.3901327. [DOI] [PubMed] [Google Scholar]
- 23.Kimoto Y., Ishikura F., Uchida Y. RichHill Medical Inc; Tokyo: 2012. JSSM guidelines for erectile dysfunction. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi K., Hashimoto K., Kato R. The Aging Males' Symptoms scale for Japanese men: reliability and applicability of the Japanese version. Int J Impot Res. 2008;20:544–548. doi: 10.1038/ijir.2008.27. [DOI] [PubMed] [Google Scholar]
- 25.Daig I., Heinemann L.A., Kim S. The Aging Males' Symptoms (AMS) scale: review of its methodological characteristics. Health Qual Life Outcomes. 2003;1:1–12. doi: 10.1186/1477-7525-1-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin S., Jeon J.H., Park D. Trans-resveratrol relaxes the corpus cavernosum ex vivo and enhances testosterone levels and sperm quality in vivo. Arch Pharm Res. 2008;31:83–87. doi: 10.1007/s12272-008-1124-7. [DOI] [PubMed] [Google Scholar]
- 27.Soner B.C., Murat N., Demir O. Evaluation of vascular smooth muscle and corpus cavernosum on hypercholesterolemia. Is resveratrol promising on erectile dysfunction? Int J Impot Res. 2010;22:227–233. doi: 10.1038/ijir.2010.8. [DOI] [PubMed] [Google Scholar]
- 28.Moinard C., Nicolis I., Neveux N. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: the Citrudose pharmacokinetic study. Br J Nutr. 2008;99:855–862. doi: 10.1017/S0007114507841110. [DOI] [PubMed] [Google Scholar]
- 29.Smoliga J.M., Vang O., Baur J.A. Challenges of translating basic research into therapeutics: resveratrol as an example. J Gerontol A Biol Sci Med Sci. 2012;67:158–167. doi: 10.1093/gerona/glr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokoyama O., Yoshida M., Kim S.C. Tadalafil once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a randomized placebo- and tamsulosin-controlled 12-week study in Asian men. Int J Urol. 2013;20:193–201. doi: 10.1111/j.1442-2042.2012.03130.x. [DOI] [PubMed] [Google Scholar]