Abstract
Introduction
Female sexual dysfunction (FSD) is a common disease with serious potential hazards, but it has not received much attention. The pathogenesis of FSD is urgently needed for the diagnosis and treatment of FSD.
Aim
To investigate the role of microribonucleic acid (mRNA, miR)-137 in FSD.
Methods
Vaginal epithelium tissues from 15 women with lubrication disorder and 15 women with normal function were collected for this study. The expression level of miR-137 in lubrication disorder and normal function women were measured by microarray analysis and Real-time Quantitative Polymerase Chain Reaction (PCR, qPCR). miR-137 was overexpressed in vaginal epithelial cells VK2/E6E7 by lentivirus infection. The cell water permeability was measured using the calcein-quenching method. Cell apoptosis was analyzed by flow cytometry. The potential target of miR-137 was predicted by bioinformatic analysis, then verified by luciferase reporter assays.
Main Outcome Measure
The expression level of miR-137 and aquaporin-2 (AQP2), cell water permeability, cell apoptosis, and luciferase reporter assays were examined.
Results
miR-137 was found to be highly expressed in vaginal epithelial tissues of women with lubrication disorder. Additionally, functional in vitro studies suggested that overexpression of miR-137 leads to a decrease in cell permeability. By combining target prediction and examination, we identified AQP2 as the direct mechanistic target of miR-137 that affected the water permeability of vaginal epithelial cells.
Conclusion
Our results point to a novel role for miR-137 and its downstream effector AQP2 in vaginal lubrication, which can be manipulated as therapeutic targets against lubrication disorder and its related disorders.
Zhang H, Liu T, Zhou Z. miR-137 affects vaginal lubrication in female sexual dysfunction by targeting Aquaporin-2. Sex Med 2018;6:339–347.
Key Words: Female Sexual Dysfunction, miR-137, Vaginal Lubrication, Aquaporin-2
Introduction
Female sexual dysfunction (FSD) is a reproductive system disease defined as disturbances in sexual desire and psychophysiological changes that characterize the sexual response cycle and cause marked distress and interpersonal difficulty.1, 2 FSD can affect the physical and mental health and quality of life of women worldwide. Until now, FSD has not been given enough attention in China because China is a traditional country, and most women refuse to discuss sex-related issues in public.3, 4 However, according to the results of our previous studies, the incidence of FSD in China is up to 37.6%. Vaginal lubrication disorder is one of the most common FSDs, accounting for 97.9% of the total incidence.2, 5 It is well known that vaginal lubrication is an important physiological manifestation for the beginning and maintenance of sex. Lubrication disorder leads to a series of problems, such as pain, orgasm dysfunction, and other follow-up symptoms of FSD.6 An increasing number of women complain of lubrication disorder in the clinic; therefore, it is necessary to explore the pathogenesis of lubrication disorder and find effective treatment.
At present, the pathophysiological mechanism of vaginal lubrication is poorly understood. According to limited literature reports, the possible mechanism of vaginal lubrication is as follows: when the body is stimulated and the vaginal hemodynamics change, capillary liquid in the lower layer of the vaginal mucosa leaks into the surface of the vaginal mucosa through the vaginal epithelium to lubricate the vagina. In addition, mucus secretion from the glands around the cervix and urethra also participates in vaginal lubrication.7, 8, 9 The main physiological causes of the disorder are endocrine disorder, vascular endothelial injury, and nervous system abnormalities. The main pathologic tissues include nerve tissue, blood vessels, vaginal smooth muscle, and vaginal epithelium.10 As the last barrier of vaginal lubrication, epithelial tissue is responsible for the transport and rematching of ions, water, and other molecules that play an important role in the formation of vaginal lubricants. Therefore, the role of fluid transport in vaginal lubrication has attracted increasing attention.11 Estrogen, androgen, and progestin and the regulation of ion channel proteins, water channel proteins, P2Y2, and others12 have been found to be potentially related to the regulation of fluid transport in the vaginal epithelium, which provides a certain molecular basis and clue for understanding the mechanism of vaginal lubrication. However, the problem of clinical treatment has not been solved, and the regulatory mechanism of fluid transport in the vaginal epithelium needs to be further explored.
In recent years, highly conserved non-coding small single-stranded ribonucleic acids (RNAs, microRNAs, miRNAs) have provided a new target for disease mechanism research. Target genes are mainly regulated by miRNAs at the posttranscriptional level, which is closely related to the occurrence of diseases.13, 14 Functional research on miRNAs has been performed in many fields of life science, such as cancer, cardiovascular disease, and nervous system diseases.15, 16 Moreover, the role of miRNA in the regulation of fluid transport has also begun to be a topic of interest.11 For example, aldosterone regulates the transport of sodium ions in the renal cortical collecting duct through miR-335-3p, miR-290-5p, and miR-1983,17 and miR-802 regulates the renal outer medullary potassium channel pathway in the renal cortical collecting duct by inhibiting caveolin-1.18 These results suggest that miRNAs play an important role in the regulation of fluid transport. However, the specific action of miRNA and its possible targets in the function of fluid transport in vaginal epithelium and lubrication disorder need to be further studied.
In this work, we screened differentially expressed miRNAs in women with and without vaginal lubrication disorder by microarray analysis. Microarray analysis showed that miR-137 was highly expressed in the vaginal epithelial tissues of women with lubrication disorder. Additionally, functional studies in vitro validated the important role of miR-137 in the modulation of vaginal epithelial cell water permeability. By combining target prediction and examination, we identified aquaporin-2 (AQP2) as a direct target of miR-137 that affected the water permeability of vaginal epithelial cells, which can be manipulated as a therapeutic target against lubrication disorder and its related disorders.
Materials and Methods
Samples
Vaginal epithelium tissues from 15 women with lubrication disorder and 15 women with normal function were collected for this study at Nanjing Maternal and Child Health Hospital. The inclusion criteria were as follows: adult Han Chinese women with junior high school or higher education. The exclusion criteria included the following: lack of sexual history, partner with sexual dysfunction, inability to read or understand the study, unwillingness to participate, and diagnosis of a critical illness. The Female Sexual Function Index (FSFI) is a brief, multidimensional scale for assessing sexual function in women.19 The women were evaluated by FSFI before resection of the whole uterus and partial vagina (non-vaginal epithelial lesion), and the vaginal epithelial tissue was collected after operation. Informed verbal consent was obtained from the participants after explaining that the investigation involved women’s sexual health. The study was reviewed by the Ethics Committee of Nanjing Maternal and Child Health Hospital. Written informed consent was signed by all study subjects.
Cell Culture
The immortalized human vaginal epithelial cells VK2/E6E7 were purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Science. The VK2/E6E7 cell line was established from the normal vaginal mucosal tissue taken from a premenopausal woman undergoing anterior-posterior vaginal repair surgery. Cells were maintained in keratinocyte serum-free medium containing 10% fetal bovine serum and 2% streptomycin under a humidified atmosphere containing 5% CO2 at 37°C. Cultures were maintained by the replacement of fresh medium every 2–3 days. The logarithmic phase cells were used for the experiment.
Transfection of miR-137-Overexpressing Lentivirus in VK2/E6E7 Cells
The lentivirus overexpressing miR-137 was designed and built by Wuhan GeneCreate Biological Engineering Co, Ltd (Wuhan, Hubei, China). VK2/E6E7 cells were infected with the pCDH-CMV-MCS-EF1-copGFP lentivirus. After 48 hours, the cells were divided into 5 groups: miR-137 overexpression, AQP2 overexpression, miR-137 overexpression + AQP2 overexpression, unloaded virus, and the blank control group. The expression of fluorescent protein was observed under a fluorescence microscope to determine the efficiency of virus transfection.
Cell Permeability Measurements
VK2/E6E7 cells were seeded on black, clear-bottom plates (Corning Inc; Corning, NY, USA) and grown for 2 days. The water permeability was measured using the calcein-quenching method.20, 21 A 1-mmol/l calcein-acetoxymethyl (AM) stock solution was prepared by dissolving 1-mg calcein-AM with 1 mL of anhydrous dimethyl sulfoxide and then stored at –20 °C. The calcein-AM reserve solution was diluted to a 1:50 dyeing solution by aseptic 0.01-M phosphate-buffered saline (PBS) so that the final concentration of calcein-AM was 2 mol/L. The cells were rinsed with sterile 0.01-M PBS and washed several times until the medium was completely washed away. Calcein-AM staining solution was added to the cells and incubated for 30 minutes at 37°C in a 5% CO2 incubator. After removing the dye solution, the cells were rinsed 4 times for 5 minutes with 0.01-M PBS. The cells were incubated with 200-L isosmotic (300 mOsm) PBS solution, and after the liquid was stabilized, the confocal laser microscope was used to image cells. The maximum level of the cell section was selected as the focal plane and recorded with the microscope. The excitation wavelength and the emission wavelength were 490 nm and 515 nm, respectively. After adding an equal volume of 600-mOsm hypertonic solution, the water in the cell effluxed from the cell to the extracellular space and to the cytoplasm when the osmotic pressure increased. With an increase in the protein concentration, the quenching effect of albumin on calcein increased, and the fluorescence value decreased. The fluorescence values of cells were measured by confocal microscopy.
RNA Isolation and Quantitative Real-Time PCR
VK2/E6E7 cells were collected in TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Total RNA was extracted and purified by RNeasy Mini Kits (Qiagen, Hilden, Germany) in accordance with the manufacturer’s protocol. For miRNA analysis, reverse transcription was performed using a TaqMan MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA). qPCRs were performed with the SYBR green method (Applied Biosystems, Foster City, CA, USA) on a 386-well Applied Biosystems ViiA system (Applied Biosystems). Relative expression levels of miR-137 or mRNAs were normalized to the levels of U6 or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. The results were analyzed using the 2-△△Ct method. The primer sequences were as follows: mir-137 RT- CTC AAC TGG TGT CGT GGA GTC GGC AAT TCA GTT GAG CTA CGC GT; mir-137 F- ACA CTC CAG CTG GGT TAT TGC TTA AGA ATA C; mir-137 R- TGG TGT CGT GGA GTC G; AQP2 F- GCG TTT GGC TTG GGT ATT GGC; AQP2 R- CGT CGT GCT GTT GCT GAG AG; h-actin F: TGG ACT TCG AGC AAG AGA TG; h-actin R- GAA GGA AGG CTG GAA GAG TG; h-U6 F- CTC GCT TCG GCA GCA CA; h-U6 R- AAC GCT TCA CGA ATT GTG CGT.
Western Blotting
Proteins were extracted with RIPA lysis buffer (Beyotime Biotechnology, NanTong, China) containing phenylmethylsulfonyl fluoride (Beyotime) and protease inhibitor cocktail (Roche, Pleasanton, CA, USA). Proteins were loaded on a 10% SDS-PAGE gel for electrophoresis and electroblotted onto a polyvinylidene fluoride membrane. The membrane was blocked in 5% skim milk for 2 hours and then incubated with the anti-AQP2 antibody (ab15116) and the secondary antibodies for 1 hour sequentially. Developer was added to the membrane, and results were recorded using X-ray exposure.
Bioinformatic Predictions and Dual Luciferase Reporter Assays
The potential miRNA targets were from TargetScan 7.1 (Whitehead Institute, Cambridge, MA, USA). 293T cells were seeded in 48-well culture plates a day before transfection; the cell density of the transfected cells was 70–80%, and the medium used was DMEM+10% FBS. Then, 1 μL of transfection reagents and plasmids (total plasmid 0.2 μg) were diluted into 25 μL of medium and mixed and incubated at room temperature for 20 minutes. The culture medium was absorbed in the dish, and 50 μL of transfected compound was added into the 48-well culture plates and incubated at 37°C for 5 hours. Then, 0.2 mL of culture medium was added after the liquid was removed, followed by incubation at 37°C for 48 hours. Cell lysate (200 μL) was added to each well, incubated at room temperature for 10 minutes, and was collected by centrifugation (10,000 × g, 5 minutes). Then, 20 μL of supernatant was added to the 96-well luminescent plate, followed by the addition of 100 μL of firefly luciferase detection solution. After mixing, the luminescence value of the luciferase was measured, and 100 μL of sea kidney luciferase detection solution was added. Then, luminescence was redetected after mixing.
Flow Cytometry for Cell Apoptosis Analysis
Annexin V-FITC apoptosis was used to detect the apoptosis incidence. The cells were washed with 0.25% trypsin (without ethylenediaminetetraacetic acid), and trypsin was removed from the centrifuge tube. Then, 1 mL of trypsin was added to digest the cells. Cells were collected by centrifugation (5 minutes, 1,000 rpm). The cells were washed twice with cold PBS buffer solution and 1 × binding buffer to a final concentration of 1 × 106 cells/mL. Then, 5 μL of Annexin V-FITC was added to 100 μL of the cell suspension, which was then incubated at room temperature for 10 minutes in the dark. After the addition of 10 μL of propidium iodide, the samples were gently vortexed, and placed in the dark. After 1 hour, the samples were analyzed using flow cytometry.
Statistical Analysis
All experiments were repeated ≥3 times, and the data are presented as the mean ± standard deviation (SD). Student’s 2-tailed t-test was used to compare 2 groups, and 1-way ANOVA was used when >2 groups were compared. P < .05 was considered significant and is indicated in the figures.
Results
miR-137 Is Upregulated in Vaginal Epithelial Tissue
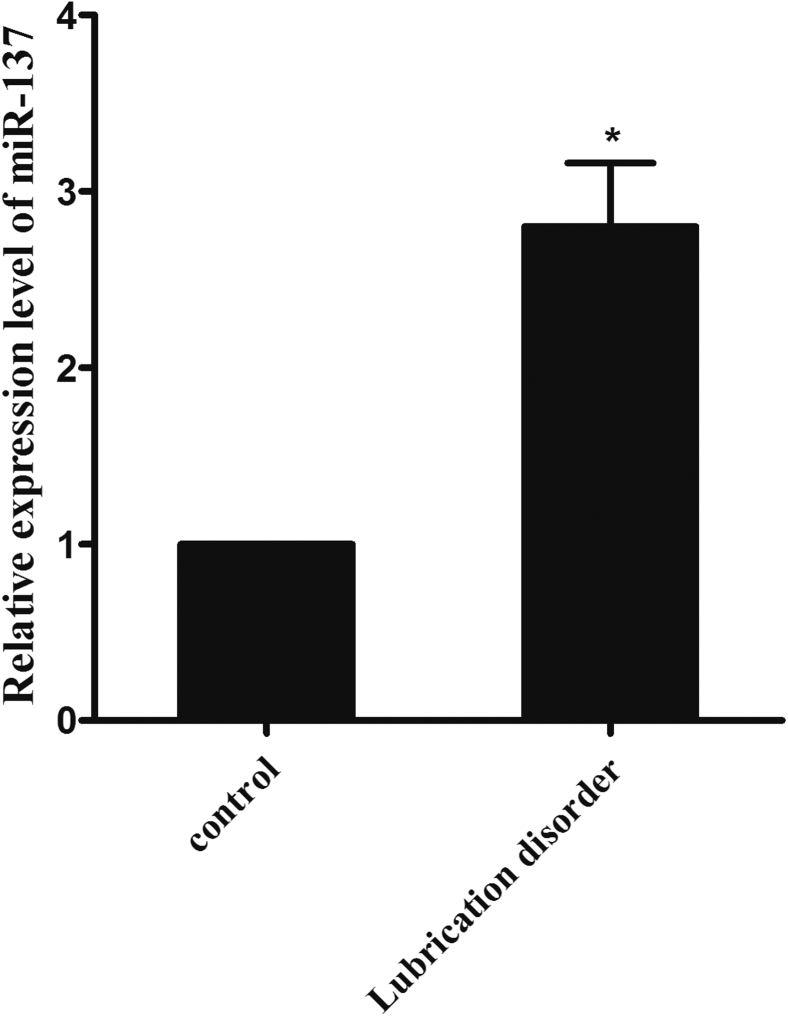
As shown in Table 1, the average ages were 56 and 35 for the lubrication disorder and control groups, respectively. The lubrication domain scores for the lubrication disorder group and the control group were 3.3 ± 0.28 and 4.63 ± 0.35, respectively. Compared with the control group, the lubrication disorder group had significantly lower lubrication, desire, arousal, and sexual pain scores. The expression level of miR-137 in the lubrication disorder group was 5.53 times higher than that in the normal control group (P < .05) based on microarray analysis and verified by qPCR (Figure 1).
Table 1.
FSFI scores of female sexual function in the lubrication disorder and control groups
| Group | Average age | Lubrication | Desire | Arousal | Orgasm | Sexual pain | Satisfaction |
|---|---|---|---|---|---|---|---|
| Lubrication disorder | 56 | 3.3 ± 0.28∗ | 2.75 ± 0.33∗ | 3.00 ± 0.21∗ | 3.83 ± 0.45 | 3.71 ± 0.47∗ | 4.35 ± 0.66 |
| Control | 35 | 4.63 ± 0.35 | 3.38 ± 0.55 | 3.65 ± 0.41 | 4.22 ± 0.62 | 4.61 ± 0.58 | 4.73 ± 0.83 |
FSFI = Female Sexual Function Index.
Lubrication disorder vs Control, P < .05.
Figure 1.
miR-137 is differentially expressed between the lubrication disorder group and the control group, as validated by quantitative polymerase chain reaction. ∗P < .05.
Overexpression of miR-137 Results in Decreased Cell Permeability and Downregulation of AQP2 Expression
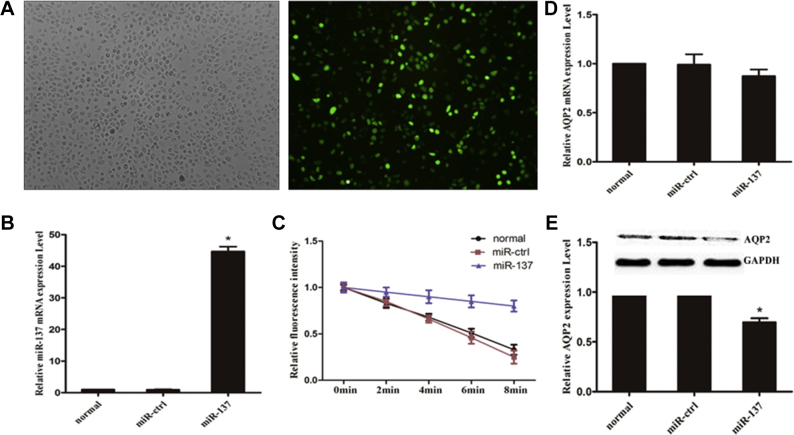
We next examined whether miR-137 functioned in cell permeability by using VK2/E6E7 cells. Transfection efficiency was detected after 48 hours of virus transfection. Microscopy revealed that green fluorescence intensity was high and cell growth was good (Figure 2A), which indicates that virus transfection was successful. At the same time, we detected high expression of miR-137 in VK2/E6E7 cells (Figure 2B). The cells presented yellowish-green fluorescence after incubation with calcein-AM. Preliminary studies demonstrated that the stimulation initiated by the confocal laser did not cause obvious quenching. The rate at which the fluorescence decreased was significantly lower in the miR-137 overexpression group than in the control groups (P < .05) (Figure 2C). The mRNA expression level of AQP2 in VK2/E6E7 cells was detected after overexpression of miR-137 by PCR. Compared with the control group, the miR-137 overexpression group expressed less AQP2 mRNA, but the difference was not statistically significant (Figure 2D). The protein expression level of AQP2 was detected by Western blot and normalized to the level of GAPDH. The AQP2 protein level in the miR-137 overexpression group was lower than that in the unloaded virus group and the blank control group (Figure 2E).
Figure 2.
Overexpression of miR-137 inhibits VK2/E6E7 cell permeability. (A) Transfection efficiency was detected after 48 hours of virus transfection. (B) The efficiency rate of miR-137 overexpression was evaluated by quantitative polymerase chain reaction (qPCR). (C) Overexpression of miR-137 inhibits cell permeability. (D) Relative expression level of aquaporin-2 (AQP2) mRNA was detected by qPCR. (E) Relative expression level of AQP2 protein was detected by Western blot.
Overexpression of AQP2 Rescued the Inhibitory Effect of miR-137 Overexpression on Cell Permeability
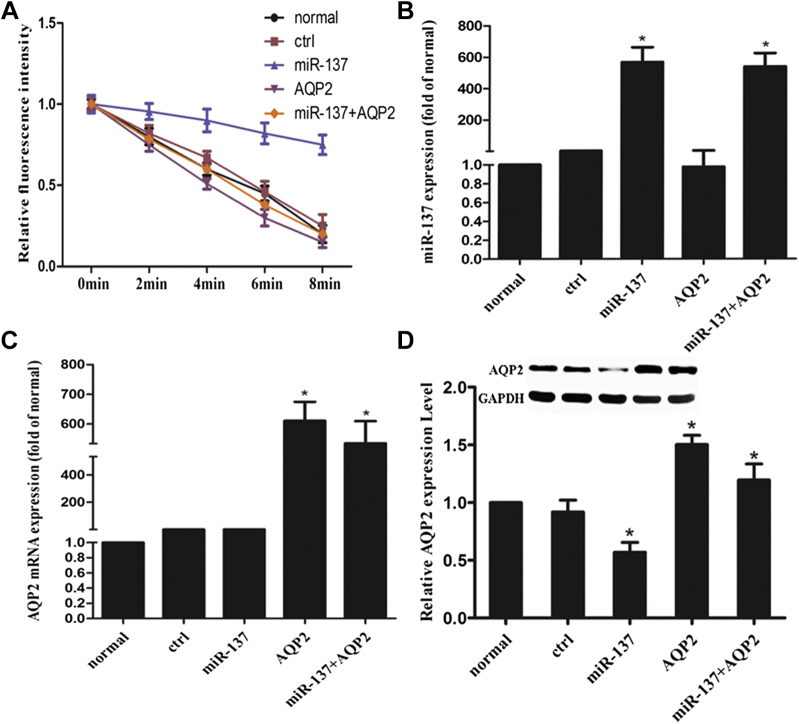
To further understand the function and possible mechanism of miR-137 in cell permeability, we simultaneously overexpressed miR-137 and AQP2. As shown in Figure 3A, miR-137 overexpression led to a decrease in cell permeability. However, the cell permeability of cells simultaneously overexpressing miR-137 and AQP2 was better than that of cells overexpressing miR-137 alone. The overexpression of miR-137 and AQP2 was verified by qPCR (Figures 3B and C). Additionally, overexpression of miR-137 significantly downregulated the protein expression level of AQP2, which was rescued by AQP2 overexpression (Figure 3D).
Figure 3.
Overexpression of aquaporin-2 (AQP2) rescued the inhibitory effect of miR-137 overexpression on cell permeability. (A) Cell permeability detected by the calcein-quenching method. (B) Relative expression levels of AQP2 microribonucleic acid (mRNA) were detected by quantitative polymerase chain reaction (qPCR). (C) Relative expression level of AQP2 mRNA was detected by qPCR. (D) Relative expression level of AQP2 protein was detected by Western blot.
Bioinformatic Analysis and Dual Luciferase Reporter Gene Assay
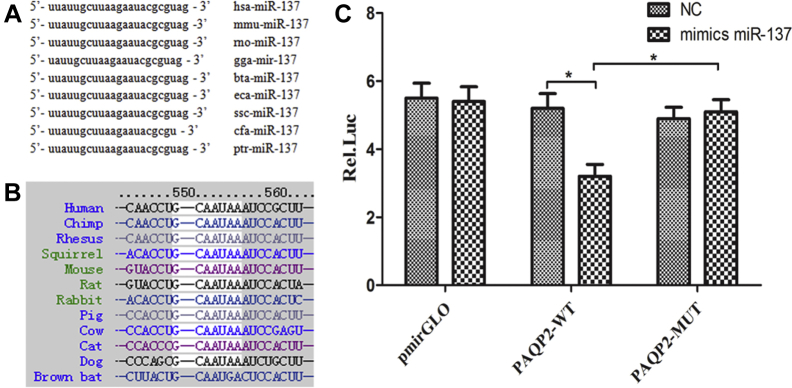
With the use of miRBase, TargetScan and miRDB, we selected AQP2, a well-known gene in fluid transport, for further investigation.22 Bioinformatic analysis showed that the miR-137 mature sequence and the miR-137 binding sequences on the AQP2 3′UTR were conserved among species, including humans, mice, and rats (Figures 4A and B).
Figure 4.
Target prediction and dual luciferase reporter gene assays identified the mechanistic target of aquaporin-2 (AQP2) as a direct target of miR-137. (A) The miR-137 mature sequence is conserved across species including mouse, human, and rat. (B) The miR-137 binding sequences on the AQP2 3′UTR are conserved among species, including humans, mice, and rats. (C) Dual luciferase reporter gene assay confirmed the direct interaction between A and B.
To further confirm the direct targeting of AQP2 by miR-137, we generated a luciferase reporter containing either the mutant 3′UTR of AQP2 (MUT) or the wild-type 3′UTR of AQP2 (WT). A dramatic reduction in luciferase activity was observed upon transient cotransfection of cells with the WT 3′UTR of AQP2 and miR-137 mimics, demonstrating a direct binding of miR-137 to the AQP2 3′UTR (Figure 4C).
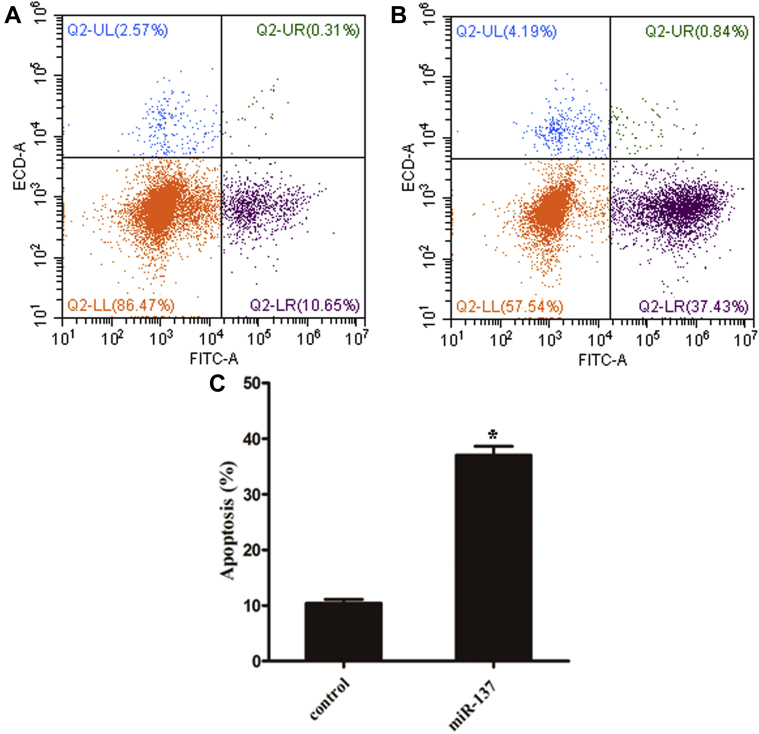
Overexpression of miR-137 Increased VK2/E6E7 Apoptosis
The flow cytometry results showed that the rate of apoptosis in the miR-137-overexpressing group was significantly higher than that in the control group. Among them, the early apoptosis rate was significantly higher, and the difference was statistically significant (Figure 5).
Figure 5.
Overexpression of miR-137 increased VK2/E6E7 apoptosis.
Discussion
With the development of social concepts and sexual medicine, an increasing number of women with FSD are willing to seek help from doctors. However, the study of vaginal lubrication disorders is still in an early stage. The existing treatment methods still have many shortcomings. For example, estrogen replacement therapy can alleviate vaginal lubrication owing to estrogen deficiency caused by perimenopausal or premature ovarian failure, but it is not effective for all patients. In addition, there are known adverse reactions and restrictions associated with hormone therapy.23 At the same time, many reports of other therapies, including phosphodiesterase type 5 inhibitors, antidepressants, and sexual psychotherapy, lack clear effectiveness and feasibility.24 Therefore, it is necessary to study the mechanism of FSD and seek new therapy methods.
Sex organs may be ideal targets for gene therapy because they are located on the surface of the body and have relatively slow blood circulation. Recently, researchers have found that miRNAs play an important role in the pathogenesis of cardiovascular diseases, nervous system diseases, reproductive system diseases, cancer, and other diseases.14, 15 Thus, it is necessary to explore the role of miRNA in vaginal lubrication.
In the current study, we screened human vaginal epithelial tissue by miRNA chip and observed high expression of miR-137 in vaginal lubrication tissue, which was verified by qPCR. These tissues were obtained from women who underwent a hysterectomy. These women were divided into the lubrication dysfunction group and the normal group after evaluation of the FSFI. As shown in Table 1, the average age of the lubrication dysfunction group was higher than that of the normal group. This observation is consistent with the literature that aging is one of the high risk factors for FSD.25, 26 Next, the cell experiments showed that the rate at which the cell fluorescence decreased in the miR-137 overexpression group was significantly lower than that in the control group, suggesting that the rate of cell permeability in the miR-137 overexpression group was significantly lower than that in the control group. That is, the transport capacity of the cell membrane for water in the miR-137 overexpression group was significantly lower than that in the control group. Thus, miR-137 can regulate the permeability of vaginal epithelial cells.
Our results also indicated that overexpression of miR-137 obviously downregulated the expression of AQP2 at the protein level. When we simultaneously overexpressed AQP2 and miR-137, the protein expression of AQP2 was significantly higher than that in the control group but was lower than that in the group overexpressing AQP2 alone. These results illustrated that miR-137 could downregulate the expression of AQP2 at the protein level. Previous studies have shown that miRNAs can play important regulatory roles in animals and plants by targeting mRNAs for cleavage or translational repression.27, 28 Because overexpression of miR-137 did not affect the mRNA level of AQP2, we speculate that miR-137 regulates AQP2 function by translational repression. AQPs are membrane proteins that facilitate water movement across biologic membranes and regulate water balance in cells and whole organisms. Previous studies have shown that 3 types of water channel proteins, AQP1, AQP2 and AQP3, are mainly expressed in the vaginal tissue. AQP1 is mainly expressed in the small vascular wall under the vaginal mucosa. AQP2 and AQP3 are mainly expressed in vaginal epithelial cells. However, during sexual excitement, AQP1 and AQP2 channels open to facilitate rapid transport of vaginal fluid, whereas AQP3 does not undergo a significant change. In summary, miR-137 may regulate permeability through AQP2, which is one of the key proteins in vaginal lubrication. Additionally, luciferase assays showed that miR-137 directly targets the 3’ UTR of the AQP2 gene. In accordance with these results, AQP2 activation can be induced by miR-137 overexpression in VK2/E6E7 cells.
Moreover, apoptosis of VK2/E6E7 cells was detected by flow cytometry. The results showed that miR-137 overexpression increased the apoptosis of VK2/E6E7 cells. A change in cell volume has been considered to be a prerequisite for the initiation of cell apoptosis. Rivarola et al29 proposed that AQP2 promotes cell swelling or shrinkage and leads to the activation of channels that require the control of these processes. Flamenco et al30 showed that AQP2 participates in and regulates the rapid activation of potassium and sodium channels during the apoptosis of renal collecting duct cells, thereby decreasing cell permeability and increasing cell apoptosis. However, up until now, the mechanism by which miR-137 promotes cell apoptosis was not completely clear.
The preceding results and analyses suggest that the overexpression of miR-137 may downregulate the expression of AQP2, a key protein in fluid transport in vaginal epithelial cells. Decreased expression of AQP2 in vaginal epithelial cells may lead to impaired vaginal epithelial fluid transport capacity, thus inhibiting the secretion of vaginal lubricant, resulting in vaginal lubrication disorders. However, some limitations of this study must be noted. The cell line used in this study was immortalized and differs from human cells. Thus, these studies are not sufficient to clarify the association between real human vaginal epithelium and cells in vitro, and the lack of an in vivo study documenting the role of miR-137 and its relationship with AQP2 is a major limitation of this study. Follow-up studies at the animal level and even at the human level are needed to verify the function of miR-137. In addition, age differences between groups could be a confounding factor of the results. Thus, studies with larger sample sizes are necessary to eliminate age interference.
Conclusion
In this study, we found that miR-137 can inhibit the cell permeability of vaginal epithelial cells by downregulating the expression of AQP2, which may be the pathogenesis of vaginal lubrication disorder. This study may provide a new direction for the diagnosis and treatment of FSD. However, further research is needed to apply miR-137 as a new target for the treatment of lubrication disorder.
Statement of authorship
Category 1
-
(a)Conception and Design
- Jiehua Ma; Lianjun Pan
-
(b)Acquisition of Data
- Hepeng Zhang; Tianjiao Liu; Aixia Zhang
-
(c)Analysis and Interpretation of Data
- Jiehua Ma; Lianjun Pan; Jing Zhang;Yuan Zhu; Ziyun Zhou
Category 2
-
(a)Drafting the Article
- Hepeng Zhang; Tianjiao Liu
-
(b)Revising It for Intellectual Content
- Jiehua Ma; Lianjun Pan
Category 3
-
(a)Final Approval of the Completed Article
- Jiehua Ma; Lianjun Pan
Footnotes
Conflict of Interest: The authors report no conflict of interest.
Funding: The study was carried out under the support of the National Natural Science Foundation of China (81501239, 81771572); Nanjing science and technology project (201715051); Nanjing health youth talent training project (QRX17158); Jiangsu maternal and child health research project (F201530); Natural science research project of Jiangsu higher education institutions (18KJB320002); Natural Science Foundation of Jiangsu (BK20181122).
References
- 1.Rosen R., Brown C., Heiman J. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 2.Ma J., Pan L., Lei Y. Prevalence of female sexual dysfunction in urban Chinese women based on cutoff scores of the Chinese version of the Female Sexual Function Index: A preliminary study. J Sex Med. 2014;11:909–919. doi: 10.1111/jsm.12451. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y. On the relationship between traditional culture and population in China. Chin J Popul Sci. 1994;6:129–141. [PubMed] [Google Scholar]
- 4.So H.W., Cheung F.M. Review of Chinese sex attitudes and applicability of sex therapy for Chinese couples with sexual dysfunction. J Sex Res. 2005;42:93–101. doi: 10.1080/00224490509552262. [DOI] [PubMed] [Google Scholar]
- 5.Ma J., Kan Y., Zhang A. Female sexual dysfunction in women with non-malignant cervical diseases: A study from an urban Chinese sample. PloS One. 2015;10:e0141004. doi: 10.1371/journal.pone.0141004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munarriz R., Kim N.N., Goldstein I. Biology of female sexual function. Urol Clin N Am. 2002;29:685–693. doi: 10.1016/s0094-0143(02)00069-1. [DOI] [PubMed] [Google Scholar]
- 7.Levin R.J. The physiology of sexual function in women. Clin Obstet Gynaecol. 1980;7:213–252. [PubMed] [Google Scholar]
- 8.D'Amati G., di Gioia C.R., Proietti Pannunzi L. Functional anatomy of the human vagina. J Endocrinol Invest. 2003;26:92–96. [PubMed] [Google Scholar]
- 9.Shabsigh A., Buttyan R., Burchardt T. The microvascular architecture of the rat vagina revealed by image analysis of vascular corrosion casts. Int J Impot Res. 1999;11(Suppl 1):S23–S30. doi: 10.1038/sj.ijir.3900467. [DOI] [PubMed] [Google Scholar]
- 10.Traish A.M., Botchevar E., Kim N.N. Biochemical factors modulating female genital sexual arousal physiology. J Sex Med. 2010;7:2925–2946. doi: 10.1111/j.1743-6109.2010.01903.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z. miRNA in the regulation of ion channel/transporter expression. Compr Physiol. 2013;3:599–653. doi: 10.1002/cphy.c110002. [DOI] [PubMed] [Google Scholar]
- 12.Sun Q., Huang J., Yang D.L. Activation of beta-adrenergic receptors during sexual arousal facilitates vaginal lubrication by regulating vaginal epithelial Cl(-) secretion. J Sex Med. 2014;11:1936–1948. doi: 10.1111/jsm.12583. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Segura L., Perez-Andrade M., Miranda-Rios J. The emerging role of microRNAs in the regulation of gene expression by nutrients. J Nutrigenet Nutrigenomics. 2013;6:16–31. doi: 10.1159/000345826. [DOI] [PubMed] [Google Scholar]
- 14.Chiu H., Alqadah A., Chang C. The role of microRNAs in regulating neuronal connectivity. Front Cell Neurosci. 2014;7:283. doi: 10.3389/fncel.2013.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J., Lyu H., Wang J. MicroRNA regulation and therapeutic targeting of survivin in cancer. Am J Cancer Res. 2015;5:20–31. [PMC free article] [PubMed] [Google Scholar]
- 16.Ji F., Lu X., Jiao J. The role of microRNAs in neural stem cells and neurogenesis. J Genet Genomics. 2013;40:61–66. doi: 10.1016/j.jgg.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Edinger R.S., Coronnello C., Bodnar A.J. Aldosterone regulates microRNAs in the cortical collecting duct to alter sodium transport. J Am Soc Nephrol. 2014;25:2445–2457. doi: 10.1681/ASN.2013090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin D.H., Yue P., Pan C. MicroRNA 802 stimulates ROMK channels by suppressing caveolin-1. J Am Soc Nephrol. 2011;22:1087–1098. doi: 10.1681/ASN.2010090927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiegel M., Meston C., Rosen R. The Female Sexual Function Index (FSFI): Cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31:1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 20.Solenov E., Watanabe H., Manley G.T. Sevenfold-reduced osmotic water permeability in primary astrocyte cultures from AQP-4-deficient mice, measured by a fluorescence quenching method. Am J Physiol-Cell Ph. 2004;286:C426–C432. doi: 10.1152/ajpcell.00298.2003. [DOI] [PubMed] [Google Scholar]
- 21.Fenton R.A., Moeller H.B., Nielsen S. A plate reader-based method for cell water permeability measurement. Am J Physiol-Renal. 2010;298:F224–F230. doi: 10.1152/ajprenal.00463.2009. [DOI] [PubMed] [Google Scholar]
- 22.Radin M.J., Yu M.J., Stoedkilde L. Aquaporin-2 regulation in health and disease. Vet Clin Path. 2012;41:455–470. doi: 10.1111/j.1939-165x.2012.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hickey M., Elliott J., Davison S.L. Hormone replacement therapy. BMJ. 2012;344:e763. doi: 10.1136/bmj.e763. [DOI] [PubMed] [Google Scholar]
- 24.Nappi R.E., Cucinella L. Advances in pharmacotherapy for treating female sexual dysfunction. Expert Opin Pharmaco. 2015;16:875–887. doi: 10.1517/14656566.2015.1020791. [DOI] [PubMed] [Google Scholar]
- 25.Lianjun P., Aixia Z., Zhong W. Risk factors for low sexual function among urban Chinese women: A hospital-based investigation. J Sex Med. 2011;8:2299–2304. doi: 10.1111/j.1743-6109.2011.02313.x. [DOI] [PubMed] [Google Scholar]
- 26.Hayes R.D., Dennerstein L., Bennett C.M. Relationship between hypoactive sexual desire disorder and aging. Fertil Steril. 2007;87:107–112. doi: 10.1016/j.fertnstert.2006.05.071. [DOI] [PubMed] [Google Scholar]
- 27.Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 28.Shukla G.C., Singh J., Barik S. MicroRNAs: Processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3:83–92. [PMC free article] [PubMed] [Google Scholar]
- 29.Rivarola V., Flamenco P., Melamud L. Adaptation to alkalosis induces cell cycle delay and apoptosis in cortical collecting duct cells: Role of aquaporin-2. J Cell Physiol. 2010;224:405–413. doi: 10.1002/jcp.22136. [DOI] [PubMed] [Google Scholar]
- 30.Flamenco P., Galizia L., Rivarola V. Role of AQP2 during apoptosis in cortical collecting duct cells. Biol Cell. 2009;101:237–250. doi: 10.1042/BC20080050. [DOI] [PubMed] [Google Scholar]