Abstract
Introduction
Non-curvature penile deformities associated with loss of erect penile volume are often overlooked and have not been thoroughly investigated.
Aim
To describe the prevalence and functional impact of penile volume-loss deformities in our cohort of men with Peyronie’s disease (PD).
Methods
We retrospectively examined medical records of patients with PD consecutively evaluated by a specialized urologist from December 2012 to June 2016. We excluded patients with prior surgical correction of PD, prior penile prosthesis, and inadequate erection during office examination. All patients underwent deformity assessment of the erect penis after intracavernosal injection. The assessment included measurement of penile curvature; evaluation for hourglass deformities, indentations, and distal tapering; and application of axial force to assess for penile buckling. Prior to the deformity assessment, each patient completed the Male Sexual Health Questionnaire and was asked if he experienced psychological distress and functional impairment related to his penile deformity.
Main Outcome Measure
The primary clinical parameters that we evaluated were presence or absence of axial instability, functional impairment, psychological distress, penile pain, erectile dysfunction, ejaculatory dysfunction, sexual dissatisfaction, decreased sexual activity, and decreased sexual desire.
Results
128 patients met criteria for inclusion. 83 patients (65%) had volume-loss deformities. Unilateral indentations, hourglass deformities, and distal tapering were present in 50 (39%), 30 (23%), and 16 (13%) patients, respectively. Penile curvature <10° degrees was present in 115 patients (90%). After controlling for angle of curvature, patients with volume-loss deformities had significantly higher rates of axial instability (odds ratio [OR] = 3.5, P = .01) and psychological distress (OR = 2.6, P = .03), as well as decreased sexual activity (OR = 2.7, P = .02), than patients with non–volume-loss deformities.
Conclusion
Volume-loss penile deformities are highly prevalent in men with PD. These deformities are associated with penile axial instability and psychological distress, which may contribute to decreased frequency of sexual activity.
Margolin EJ, Pagano MJ, Aisen CM, et al. Beyond curvature: prevalence and characteristics of penile volume-loss deformities in men with Peyronie's disease. Sex Med 2018;6:309–315.
Key Words: Peyronie’s Disease, Penile Deformity, Penile Volume, Penile Curvature
Introduction
Peyronie’s disease (PD) is an acquired fibrotic disease of the tunica albuginea that can cause penile pain, the development of palpable penile plaques, erectile dysfunction, and penile deformity.1 The prevalence of the disease is estimated at between 3% and 9%.2, 3 The cause is unknown, but trauma and genetic susceptibility are associated factors.4 Penile deformity in PD results from focal loss of tunical elasticity at involved areas of the tunica albuginea at sites of collagen deposition and fibrosis. This results in non-uniform tunical expansion during erection wherein uninvolved areas of the tunica albuginea stretch more than involved areas. When fibrosis results in focal loss of longitudinal tunical stretch, the resultant deformity is penile curvature.
Although curvature is the most commonly discussed and reported manifestation of PD, fibrosis in PD can also result in other deformities. Global loss of longitudinal tunical elasticity can result in global penile length loss.5, 6 Moreover, tunical fibrosis can also result loss of radial tunical elasticity that can manifest as focal or global loss of erect penile circumference. This results in penile indentations, hourglass deformities, and areas of proximal or distal girth loss.7
Non-curvature deformities associated with loss of erect penile volume, such as shortening, narrowing, and hourglass deformities, are prevalent and bothersome.8, 9 Such volume-loss deformities may interfere with an affected patient’s ability to engage in penetrative sexual intercourse by destabilizing the axial rigidity of the erect penis. In severe cases, penile length and girth loss may also result in functional impairment. In addition, penile volume-loss deformities caused by PD can cause psychological distress. For many patients, penile shortening and narrowing contribute more than curvature to depression and relationship problems.10, 11, 12
The prevalence and clinical importance of non-curvature deformities has not been thoroughly investigated. We sought to better characterize the prevalence and clinical importance of these deformities among adult men with PD. Specifically, we wanted to assess the impact that penile volume-loss deformities have on sexual, psychological, and physical function.
Methods
We retrospectively examined medical records of consecutive patients with PD who underwent comprehensive physical examination of the penis after pharmacologic erection by a specialized urologist (P.J.S.) at our institution from December 2012 to June 2016. Inclusion criteria included men older than age 18 who were presenting for an evaluation of PD. Exclusion criteria included prior surgical correction of PD, prior insertion of penile prosthesis, and failure to achieve an adequate erection during office examination.
After giving informed consent, all patients underwent intracavernosal injection of Trimix (alprostadil, papaverine, and phentolamine). Injections were given between 1 and 3 times with a dose of between 5 and 40 units per injection, as needed to achieve clinically maximum erection. The adequacy of erection was recorded subjectively on a scale from 1 to 10, and patients with erections <7 were excluded from further analysis. Penile curvature was measured with goniometer, and the presence of penile curvature was defined as an angle >10°. Penile volume-loss deformities were defined as hourglass deformities, unilateral indentations, or distal tapering on physical examination and were defined irrespective of the presence or absence of penile curvature. All goniometer measurements and deformity assessments were performed by a single investigator (P.J.S.).
At the time of pharmacologic erection, patients also underwent penile duplex Doppler ultrasound (PDDU) to assess penile vascular function and plaque characteristics. Images were obtained in the longitudinal and transverse planes from the base of the penis to the coronal sulcus with greyscale and Doppler mode ultrasonography at 12 to 18 MHz using a linear array transducer. After completion of the examination and ultrasound, all patients remained in the office until the erection subsided. If necessary, they received an intracavernosal injection of phenylephrine to reverse the erection.
The primary clinical parameters that we evaluated were presence or absence of axial instability, functional impairment, psychological distress, penile pain, erectile dysfunction, ejaculatory dysfunction, sexual dissatisfaction, decreased sexual activity, and decreased sexual desire. Axial instability was identified as penile buckling with application of axial force during physical examination. The parameters of functional impairment, psychological distress, and penile pain were obtained from the patient history, as recorded in the patient’s medical record before the examination under pharmacologic erection. The parameters of erectile dysfunction, ejaculatory dysfunction, sexual dissatisfaction, decreased sexual activity, and decreased sexual desire were obtained from the Male Sexual Health Questionnaire (MSHQ), which was filled out by each patient before the examination under pharmacologic erection. The MSHQ is a 25-question survey that assesses 5 domains: Erection Scale, Ejaculation Scale, Sexual Satisfaction, Sexual Activity, and Sexual Desire. Each item is measured on a scale from 1 to 5 or 0 to 5, with a score of 3 indicating a neutral response and lower numbers indicating worse function. Each of the 5 domains was considered a clinical parameter and was assessed by taking the average of the responses for each question in the domain. An average score <3 was considered a positive response.
Clinical parameters of patients with and without volume-loss deformities were compared using a chi-square test for univariate analysis and a multivariate logistic regression model that controlled for the degree of penile curvature as a continuous variable. P values <.05 were considered statistically significant. All statistical analysis was done using Stata (StataCorp, College Station, TX, USA). Institutional review board approval was obtained from our institution.
Results
We identified 128 patients who met criteria for inclusion. Baseline characteristics are shown in Table 1. The prevalence of volume-loss deformities in our cohort was 65% (83/128). Unilateral indentations were present in 39% of patients (50/128). Hourglass deformities were present in 23% of patients (30/128). Distal tapering was present in 13% of patients (16/128). Penile curvature >10° was present in 90% of patients (115/128). When indentation/hourglass and curvature were both present, 81% of indentations/hourglasses were at the site of maximal curvature. Additional characteristics of penile deformities are described in Table 2.
Table 1.
Baseline characteristics of men with PD
| Characteristic | Volume-loss deformity (n = 83, 65%) | Non–volume-loss deformity (n = 45, 35%) | P value∗ |
|---|---|---|---|
| Age, median (IQR) | 55 (45–61) | 54 (45–63) | .82 |
| Race, frequency (%) | .28 | ||
| White | 39 (47) | 15 (33) | |
| Black | 4 (5) | 4 (9) | |
| Unknown/Other | 40 (48) | 26 (58) | |
| Ethnicity, frequency (%) | .05 | ||
| Hispanic | 4 (5) | 6 (13) | |
| Not Hispanic | 50 (60) | 18 (40) | |
| Unknown | 29 (35) | 21 (47) | |
| BMI, median (IQR) | 26 (24–29) | 27 (25–28) | .61 |
| Smoking history, frequency (%) | 26 (31) | 13 (29) | .78 |
| Penile trauma, frequency (%) | 16 (19) | 12 (27) | .33 |
| PD duration, months, median (IQR) | 14 (5–36) | 12 (6–36) | .91 |
| HTN, frequency (%) | 19 (23) | 14 (31) | .31 |
| DM, frequency (%) | 10 (12) | 5 (11) | .88 |
| Hypercholesterolemia, frequency (%) | 20 (24) | 8 (18) | .41 |
| BPH, frequency (%) | 8 (10) | 5 (11) | .79 |
| Prostate cancer, frequency (%) | 5 (6) | 3 (7) | .89 |
| Phosphodiesterase-5 inhibitors, frequency (%) | 34 (41) | 21 (47) | .53 |
| Prior non-surgical PD therapy, frequency (%) | 36 (43) | 18 (40) | .71 |
BMI = body mass index; BPH = benign prostatic hyperplasia; HTN = hypertension; IQR = interquartile range; PD = Peyronie’s disease.
P values were calculated using the chi-square test or Mann-Whitney U test.
Table 2.
Penile physical examination characteristics
| Characteristic | Volume-loss deformity (n = 83, 65%) | Non–volume-loss deformity (n = 45, 35%) | P value∗ |
|---|---|---|---|
| Penile length (cm), median (IQR)† | 12 (11–13) | 11 (11–14) | .91 |
| Angle of curvature (degrees), median (IQR) | 35 (25–50) | 40 (25–50) | .43 |
| Direction of curvature, n = 115, frequency (%) | .25 | ||
| Left | 17 (23) | 7 (17) | |
| Right | 4 (5) | 0 (0) | |
| Dorsal | 31 (42) | 22 (54) | |
| Ventral | 8 (11) | 7 (17) | |
| Left dorsal | 8 (11) | 1 (2) | |
| Right dorsal | 2 (3) | 3 (7) | |
| Left ventral | 2 (3) | 0 (0) | |
| Right ventral | 2 (3) | 1 (2) | |
| Curve location, n = 115, frequency (%) | .49 | ||
| Proximal | 20 (27) | 8 (20) | |
| Mid | 34 (46) | 18 (44) | |
| Distal | 20 (27) | 15 (37) | |
| Indent/hourglass location, n = 79, frequency (%) | |||
| Proximal | 31 (39) | — | |
| Mid | 34 (43) | — | |
| Distal | 14 (18) | — |
IQR = interquartile range.
P values were calculated using the chi-square test or Mann-Whitney U test.
Penile length was determined by measurement of the stretched dorsal length of the penis from the pubis to the coronal sulcus.
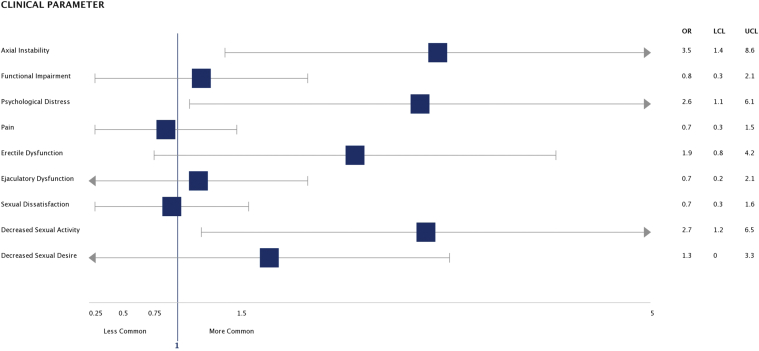
Compared with patients with non–volume-loss deformities, patients with volume-loss deformities had significantly higher rates of axial instability (42% vs 20%, P = .01); psychological distress (67% vs 44%, P = .03); and decreased sexual activity (53% vs 29%, P = .02) (Table 3). These results held true after controlling for angle of curvature using multivariate logistic regression; patients with volume-loss deformities had significantly higher rates of axial instability (OR = 3.5, P = .01) and psychological distress (OR = 2.6, P = .03), as well as decreased sexual activity (OR = 2.7, P = .02) (Figure 1).
Table 3.
Clinical parameters in patients with volume-loss and non–volume-loss penile deformities
| Clinical parameter | Volume-loss deformity, frequency (%) | Non–volume-loss deformity, frequency (%) | P value∗ |
|---|---|---|---|
| Axial instability, n = 128 | 35 (42) | 9 (20) | .01 |
| Functional impairment, n = 92 | 22 (38) | 15 (44) | .56 |
| Psychological distress, n = 97 | 42 (67) | 15 (44) | .03 |
| Pain, n = 125 | 28 (35) | 19 (42) | .42 |
| Erectile dysfunction, n = 107 | 32 (48) | 13 (33) | .12 |
| Ejaculatory dysfunction, n = 107 | 9 (13) | 7 (18) | .57 |
| Sexual dissatisfaction, n = 103 | 16 (25) | 13 (33) | .36 |
| Decreased sexual activity, n = 102 | 34 (53) | 11 (29) | .02 |
| Decreased sexual desire, n = 95 | 18 (31) | 9 (25) | .56 |
P values were calculated using the chi-square test.
Figure 1.
Associations of clinical outcomes with volume-loss deformities; logistic regression, controlling for angle of curvature; OR ± 95% confidence interval (478 × 220 mm [144 × 144 dots per inch]). N = number of patients; OR = odds ratio; LCL = lower confidence limit; UCL = upper confidence limit.
There were no significant differences between the 2 groups in the likelihoods of self-reported functional impairment or penile pain, and there were no significant differences between the 2 groups in MSHQ scores for erectile dysfunction, ejaculatory dysfunction, rates of sexual dissatisfaction, or decreased libido. Among the 121 patients who underwent PDDU, there were no significant differences in PDDU parameters between patients with and without volume-loss deformities (Table 4).
Table 4.
Penile duplex Doppler ultrasound measurements
| PDDU parameters | Volume-loss deformity (n = 79) | Non–volume-loss deformity (n = 42) | P value |
|---|---|---|---|
| Right PSV, median (IQR) | 44 (35–63) | 45 (36–55) | .81 |
| Right EDV, median (IQR) | 0 (−7–0) | 0 (−6–0) | .46 |
| Right RI, median (IQR) | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | .68 |
| Left PSV, median (IQR) | 43 (34–56) | 44 (35–54) | .80 |
| Left EDV, median (IQR) | 0 (−5–1) | 0 (−6–0) | .98 |
| Left RI, median (IQR) | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | .78 |
EDV = end diastolic velocity; PSV = peak systolic velocity; RI = resistive index; P-value calculated with Mann–Whitney test.
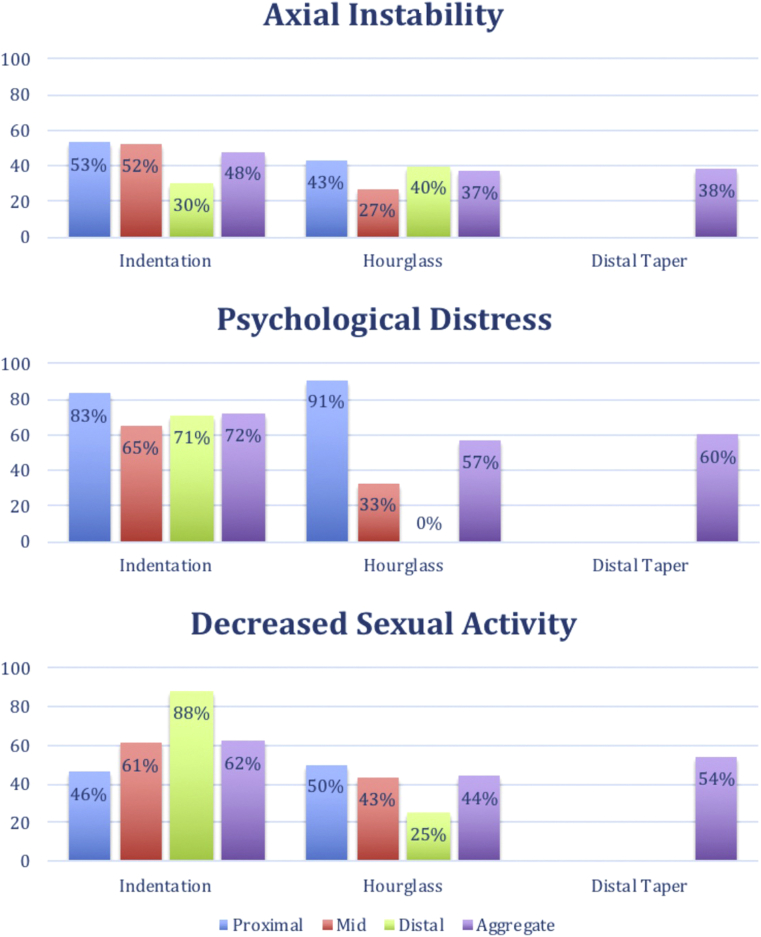
Based on the significantly higher rates of axial instability, psychological distress, and decreased sexual activity in patients with volume-loss deformities, we conducted a post hoc analysis of the variation in these clinical parameters based on the type of deformity and the location of deformity. Owing to the small numbers of data points at this level of detail, no statistical tests were done. The results are shown in Figure 2. Indentation appears to have the highest rates of these parameters (48% instability, 72% psychological distress, 62% decreased sexual activity) compared with hourglass and distal tapering. Additionally, proximal deformities (both indentation and hourglass) appear to have a more severe impact than mid or distal deformities.
Figure 2.
Variation in clinical parameters based on type and location of deformity (204 × 251 mm [144 × 144 dots per inch]).
Discussion
In this study, we characterize the prevalence and clinical importance of penile deformities that decrease erect penile volume among adult men with PD. In our sample, 65% of patients had evidence of focal or global erect penile volume loss on physical examination. The most common type of volume-loss deformity was unilateral indentation, followed by hourglass deformity and distal tapering. The prevalence of volume-loss deformities did not vary significantly based on age, race, body mass index, smoking status, history of penile trauma, duration of disease, or comorbidities, although volume-loss deformities were less common among people of Hispanic ethnicity. The majority of patients had penile curvature in addition to volume loss, although 10% of patients in our sample did not have curvature >10°.
Our findings are consistent with results of previous studies. Farrell et al8 collected data on penile deformities subjectively from a cohort of 200 men with PD, and they found prevalences of penile shortening, erect penile narrowing, and penile hourglass deformities were reported to be 70%, 27%, 26%, respectively. In their study sample, 11% of patients had no penile curvature. Cakan et al9 evaluated a cohort of 614 patients with PD and noted a 13% prevalence of “pure notching deformity” with no penile curvature. Among the patients with pure notching deformity, 63% had unilateral notching, 35% had hourglass deformity, and 2% had both unilateral notching and hourglass deformities. Although Cakan et al9 described patients with “pure notching” and no curvature, we found that the relative rates of unilateral notching and hourglass deformities remained consistent when including patients with coexistent curvature and volume-loss deformities.
Despite their relatively high prevalence, the functional impact and clinical relevance of penile volume-loss deformities have not been adequately studied. Walsh et al13 reported that severe penile curvature was the only type of deformity independently predictive of sexual disability, but this study does not convincingly rule out a relationship between volume-loss deformities and sexual disability. In fact, Walsh et al13 reported that on bivariate analysis, penile shortening was functionally impairing and that there was a non-significant association of hourglass deformity with sexual disability. Although their multivariable analysis failed to report an association between specific volume-loss deformities and functional impairment, the results are difficult to interpret owing to the abundance of variables in the multivariable model (17) compared with the small number of patients with sexual disability (42) in the study.
Other studies have demonstrated the clinical significance of penile volume-loss deformities. Several groups have reported that penile length loss in PD is associated with emotional problems and depression.10, 12 A qualitative study found that some men consider penile shortening and narrowing to be more concerning than other deformities.11 Non-curvature deformities have also been associated with higher rates of erectile dysfunction, distal flaccidity, and pivoting of the penis during intercourse.9
This study supports and enhances the evidence that non-curvature deformities that cause loss of erect penile volume have important clinical implications. Men with volume-loss deformities in our cohort were 4 times more likely to have axial instability during erection, 5 times more likely to report psychological distress related to their condition, and twice as likely to report decreased sexual activity than men with the same degree of penile curvature.
Unfortunately, despite the clinical significance of volume-loss deformities, researchers often ignore these deformities because they are difficult to quantify. The majority of clinical trials of PD therapies use the angle of curvature as the primary end point.14, 15, 16, 17, 18, 19 Although some trials have assessed plaque size, this is difficult to measure and has questionable clinical importance.16, 17, 18, 19 Metrics that are associated with volume-loss deformities, such as length and circumference, have been studied to a limited extent, but the use of these parameters has been limited by difficulty in reproducing and interpreting the measurements.20, 21 A descriptive and intuitive metric to assess the severity of indentations, hourglass deformities, and narrowing deformities is erect penile volume. The ability to measure penile volume, such as using 3-dimensional photography, has the potential to improve the quantitative characterization of these deformities, thereby empowering clinical researchers to evaluate the effect of therapies on volume-loss deformities.22
This study is limited by the subjectivity of the clinical parameters, as well as by the systematic exclusion of patients with severe erectile dysfunction. Because a formal deformity assessment requires an adequate erection, patients who are unable to achieve an erection after Trimix injection were not included, potentially introducing bias. Our study was also unable to evaluate penile shortening, another type of volume-loss deformity, because this is a metric that requires a baseline measurement for objective assessment. Thus, we likely underestimate the prevalence of volume-loss deformities. Further, we did not use the validated Peyronie’s Disease Questionnaire, which has been validated as a tool to assess the impact and severity of PD symptoms, because this tool was not available until 2013.
To our knowledge, this is the first study to report significant clinical symptoms associated with objective findings of penile volume-loss deformities in men with PD, independent of angle of curvature. Additional studies are needed to improve the quantitative assessment of volume-loss deformities to develop clinical trials for volume-restoring therapies.
Conclusion
Volume-loss deformities are highly prevalent in men with PD. These non-curvature deformities are associated with axial instability, psychological distress, and decreased sexual activity. Volume loss in PD is clinically significant independent of penile curvature and merits further research.
Statement of Authorship
Category 1
-
(a)
Conception and Design
Ezra J. Margolin; Peter J. Stahl
-
(b)
Acquisition of Data
Ezra J. Margolin; Peter J. Stahl
-
(c)
Analysis and Interpretation of Data
Ezra J. Margolin; Matthew J. Pagano; Carrie M. Aisen; Ifeanyi C. Onyeji; Peter J. Stahl
Category 2
-
(a)
Drafting the Article
Peter J. Stahl
-
(b)
Revising It for Intellectual Content
Ezra J. Margolin; Carrie M. Aisen; Peter J. Stahl
Category 3
-
(a)
Final Approval of the Completed Article
Peter J. Stahl
References
- 1.Bella A.J., Perelman M.A., Brant W.O. Peyronie's disease (CME) J Sex Med. 2007;4:1527–1538. doi: 10.1111/j.1743-6109.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- 2.Mulhall J.P., Creech S.D., Boorjian S.A. Subjective and objective analysis of the prevalence of Peyronie’s disease in a population of men presenting for prostate cancer screening. J Urology. 2004;171:2350–2353. doi: 10.1097/01.ju.0000127744.18878.f1. [DOI] [PubMed] [Google Scholar]
- 3.Schwarzer U., Sommer F., Klotz T. The prevalence of Peyronie's disease: results of a large survey. BJU Int. 2001;88:727–730. doi: 10.1046/j.1464-4096.2001.02436.x. [DOI] [PubMed] [Google Scholar]
- 4.Chung E., Ralph D., Kagioglu A. Evidence-based management guidelines on Peyronie's disease. J Sex Med. 2016;13:905–923. doi: 10.1016/j.jsxm.2016.04.062. [DOI] [PubMed] [Google Scholar]
- 5.Davoudzadeh E.P., Davoudzadeh N.P., Margolin E. Penile length: measurement technique and applications. Sex Med Rev. 2018;6:261–271. doi: 10.1016/j.sxmr.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Ziegelmann M., Bole R., Avant R. Conservatively managed Peyronie's disease-long-term survey results from patients undergoing nonsurgical and noninjection therapies. Urology. 2018;113:99–104. doi: 10.1016/j.urology.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Taylor F.L., Levine L.A. Peyronie's disease. Urol Clin North Am. 2007;34:517–534. doi: 10.1016/j.ucl.2007.08.017. vi. [DOI] [PubMed] [Google Scholar]
- 8.Farrell M.R., Corder C.J., Levine L.A. Peyronie's disease among men who have sex with men: characteristics, treatment, and psychosocial factors. J Sex Med. 2013;10:2077–2083. doi: 10.1111/jsm.12202. [DOI] [PubMed] [Google Scholar]
- 9.Cakan M., Akman T., Oktar T. The clinical characteristics of Peyronie's patients with notching deformity. J Sex Med. 2007;4:1174–1178. doi: 10.1111/j.1743-6109.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- 10.Nelson C.J., Diblasio C., Kendirci M. The chronology of depression and distress in men with Peyronie's disease. J Sex Med. 2008;5:1985–1990. doi: 10.1111/j.1743-6109.2008.00895.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosen R., Catania J., Lue T. Impact of Peyronie's disease on sexual and psychosocial functioning: qualitative findings in patients and controls. J Sex Med. 2008;5:1977–1984. doi: 10.1111/j.1743-6109.2008.00883.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith J.F., Walsh T.J., Conti S.L. Risk factors for emotional and relationship problems in Peyronie's disease. J Sex Med. 2008;5:2179–2184. doi: 10.1111/j.1743-6109.2008.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh T.J., Hotaling J.M., Lue T.F. How curved is too curved? The severity of penile deformity may predict sexual disability among men with Peyronie's disease. Int J Impot Res. 2013;25:109–112. doi: 10.1038/ijir.2012.48. [DOI] [PubMed] [Google Scholar]
- 14.Cormio L., Zucchi A., Lorusso F. Surgical treatment of Peyronie's disease by plaque incision and grafting with buccal mucosa. Eur Urol. 2009;55:1469–1475. doi: 10.1016/j.eururo.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 15.Gelbard M., Goldstein I., Hellstrom W.J. Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of Peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol. 2013;190:199–207. doi: 10.1016/j.juro.2013.01.087. [DOI] [PubMed] [Google Scholar]
- 16.Safarinejad M.R. Therapeutic effects of colchicine in the management of Peyronie's disease: a randomized double-blind, placebo-controlled study. Int J Impot Res. 2004;16:238–243. doi: 10.1038/sj.ijir.3901185. [DOI] [PubMed] [Google Scholar]
- 17.Safarinejad M.R., Hosseini S.Y., Kolahi A.A. Comparison of vitamin E and propionyl-L-carnitine, separately or in combination, in patients with early chronic Peyronie's disease: a double-blind, placebo controlled, randomized study. J Urol. 2007;178:1398–1403. doi: 10.1016/j.juro.2007.05.162. discussion 403. [DOI] [PubMed] [Google Scholar]
- 18.Teloken C., Rhoden E.L., Grazziotin T.M. Tamoxifen versus placebo in the treatment of Peyronie's disease. J Urol. 1999;162:2003–2005. doi: 10.1016/S0022-5347(05)68087-1. [DOI] [PubMed] [Google Scholar]
- 19.Weidner W., Hauck E.W., Schnitker J. Potassium paraaminobenzoate (POTABA) in the treatment of Peyronie's disease: a prospective, placebo-controlled, randomized study. Eur Urol. 2005;47:530–535. doi: 10.1016/j.eururo.2004.12.022. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 20.Greenfield J.M., Lucas S., Levine L.A. Factors affecting the loss of length associated with tunica albuginea plication for correction of penile curvature. J Urology. 2006;175:238–241. doi: 10.1016/S0022-5347(05)00063-7. [DOI] [PubMed] [Google Scholar]
- 21.Rybak J., Papagiannopoulos D., Levine L. A retrospective comparative study of traction therapy vs. no traction following tunica albuginea plication or partial excision and grafting for Peyronie's disease: measured lengths and patient perceptions. J Sex Med. 2012;9:2396–2403. doi: 10.1111/j.1743-6109.2012.02849.x. [DOI] [PubMed] [Google Scholar]
- 22.Margolin E.J., Mlynarczyk C.M., Mulhall J.P. Three-dimensional photography for quantitative assessment of penile volume-loss deformities in Peyronie's disease. J Sex Med. 2017;14:829–833. doi: 10.1016/j.jsxm.2017.03.257. [DOI] [PubMed] [Google Scholar]