Abstract
Purpose
To report 3 cases of corneal protein deposition occurring in association with systemic disease, with one case demonstrating a novel technique for clearing corneal deposits.
Observations
Three patients presented with corneal deposits associated with systemic disease. Corneal involvement was noted prior to diagnosis of systemic disease in two patients, leading to subsequent diagnosis of multiple myeloma or monoclonal gammopathy of undetermined significance. OCT revealed protein deposition at various corneal levels, including at different stromal depths in the two cases of multiple myeloma. A novel technique of posterior endothelial scraping was performed in one case with deep stromal deposits, leading to significant corneal clearing.
Conclusions and Importance
This case series demonstrates that recognition of corneal deposits may precede the diagnosis of systemic disease. It incorporates the use of anterior segment OCT to characterize corneal deposits, and demonstrates a novel surgical technique for clearing certain types corneal deposits.
Keywords: Crystalline keratopathy, Deposition keratopathy, Paraproteinemia, Corneal pathology, Anterior segment optical coherence tomography, Multiple myeloma
Abbreviations and acronyms
- AS-OCT
anterior segment optical coherence tomography
- MGUS
monoclonal gammopathy of undetermined significance
- PAS
Periodic acid-Schiff
- BCVA
best corrected visual acuity
- SPEP
serum protein electrophoresis
- UPEP
urine protein electrophoresis
1. Introduction
Protein deposition keratopathy has been reported frequently in the ophthalmic literature, with the earliest case reports dating back to the early 1900s.1 Corneal deposits identified as immunoglobulin were reported by Klintworth et al. and have been shown to occur in the setting of multiple myeloma, monoclonal gammopathy of undetermined significance (MGUS), and less commonly lymphoma, leukemia, cryoglobulinemia, rheumatoid arthritis, or Waldenstrom's macroglobulinemia.1,2 Despite the many reported cases, corneal deposits in the setting of monoclonal gammopathies remain relatively rare, occurring in 1 in 100 patients in one series.3 Corneal deposits can also be seen in primary systemic amyloidosis.4 Importantly, corneal findings may be the presenting sign of an underlying paraproteinemia, reminding ophthalmologists to include these systemic diseases in the differential diagnosis of protein corneal deposits.
Herein, we describe three patients with corneal deposits in the setting of systemic paraproteinemias, with corneal findings preceding the diagnosis of systemic disease in 2 patients and signaling disease recurrence in one patient with a known history of multiple myeloma. We incorporate anterior segment OCT to further characterize corneal deposition in all three cases, and report on a novel surgical technique for clearing deposits in appropriate patients with a posterior corneal scraping technique.
2. Materials and methods
We conducted a retrospective chart review of three patients with corneal deposits seen in the cornea clinic at Oregon Health & Science University (Casey Eye Institute) in Portland, Oregon between January, 2014 and April, 2017. We collected demographic information as well as clinical and therapeutic history. Informed consent was obtained where relevant. IRB/Ethics Committee approval was not required for this chart review. Research was conducted in accordance with HIPAA requirements, and adhered to the tenets of the Declaration of Helsinki. All patients had slit lamp photographs and anterior segment OCT (Avanti, Optovue, Inc, Fremont, CA) performed to further characterize the corneal deposits. One patient had corneal tissues submitted for pathologic evaluation during penetrating keratoplasty. This tissue was fixed in 10% neutral buffered formalin, processed routinely, and embedded in paraffin. 5 μm sections were examined histologically with hematoxylin-eosin, PAS, Congo red, and Masson's trichrome stains. Immunohistochemical staining was performed with primary antibodies against heavy chains (immunoglobulin [Ig] G, IgM, IgA, and IgD) and κ and λ light chains. Samples used for transmission electron microscopy were processed using standard techniques: tissues were epoxy embedded in standard fashion without post-fixation. Ultrathin sections measuring 80 nm were stained with uranyl acetate and lead citrate and examined in a Zeiss EM900 transmission electron microscope at 80 kV (Carl Zeiss Microscopy, LLC, Peabody, MA).
3. Results
The clinical and pathologic findings, local and systemic disease management, and clinical course of all three patients were reviewed. There were two men and one woman. The average age at presentation was 67 years (range 65–70). Corneal findings included bilateral diffuse crystalline-like opacities in one patient and bilateral patch-like opacities in two patients, Corneal findings were the presenting sign of recurrent disease in a patient with a history of multiple myeloma (patient 1), and were the presenting sign of a previously undiagnosed paraproteinemia in two patients.
One patient underwent surgical intervention in both eyes, which led to complete and lasting clearance of the opacities. The other patients have not undergone surgical intervention.
4. Case 1
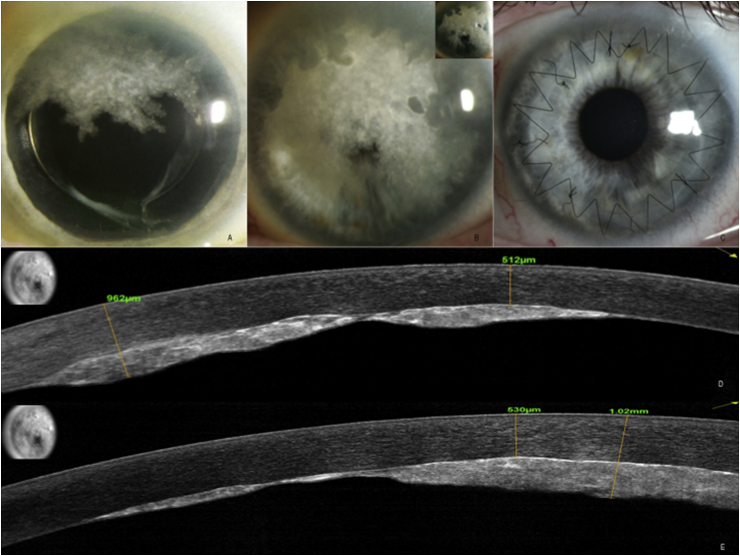
A 70 year old man with visual acuity (BCVA) of 20/20 OU and a 9-year history of IgG lambda multiple myeloma presented with blurred vision in the left eye of 1–2 months duration. He had been off all systemic therapy for three years prior to presentation, but labs at that time showed a two-fold increase in gamma globulin consistent with recurrence of multiple myeloma. His hematologist initiated daratunumab therapy (monoclonal antibody targeting CD38 which is over-expressed on multiple myeloma cells). Systemic therapy was continued throughout the course of his eye treatment. He had a patch-like pre-Descemet's plaque in the superior cornea of the left eye, while the right eye was uninvolved (Fig. 1, A). Left eye VA declined to 20/30 over 6 months with plaque growth (Fig. 1, B), and a new plaque formed in the right eye, despite systemic therapy. AS-OCT showed a highly reflective posterior stromal deposit measuring approximately 500 μm in some areas with significant distortion of the posterior corneal curvature (Fig. 1, D-E).
Fig. 1.
Slit lamp and AS-OCT photos of left eye, patient 1. A. Presenting slit lamp photo reveals a patch-like pre-Descemet's plaque in the superior cornea of the left eye. B. Slit lamp photos taken 6 months later with both direct and indirect illumination (inset) show growth of the plaque to obscure the central visual axis, with spared areas peripherally. C. Slit lamp photo following penetrating keratoplasty, with marked regression of the remaining peripheral plaque in the host cornea. D. and E. Corresponding AS-OCT taken in two diagonal directions through the cornea, respectively, showed a highly reflective posterior stromal deposit (inset shows line scan, with direction of scan shown by yellow arrows). Yellow calipers mark the anterior and posterior borders of the corneal plaque, respectively, with corresponding numerical values noted. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
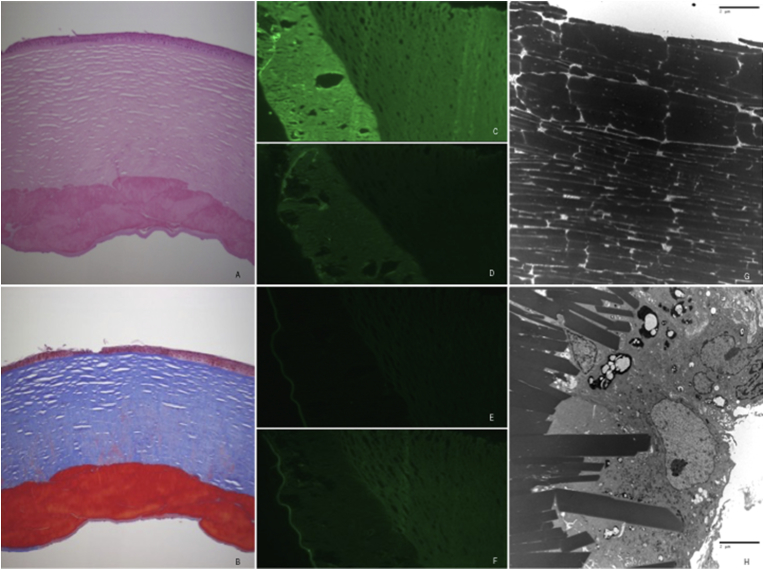
His visual acuity continued to decline over a 3 month period and he underwent femtosecond laser-assisted penetrating keratoplasty of the left eye. Corneal histology showed a dense, acellular material within the posterior stroma (Fig. 2, A). This material was acid fuscinophilic with Masson's trichrome (Fig. 2, B) and did not stain with Congo red. On immunohistochemistry, the material stained positively for IgG and lambda light chain, and negatively for IgM and kappa light chain (Fig. 2C–F). Electron microscopy revealed densely stacked, electron dense, trapezoidal, extracellular crystalline deposits measuring 0.25 - 3um in width (Fig. 2, G-H).
Fig. 2.
Pathologic findings of the corneal button following penetrating keratoplasty, left eye, patient 1. A. Light microscopy specimen showing dense, acellular material within the posterior stroma on hematoxylin-eosin. B. Light microscopy specimen stained with masson trichrome, with this acellular material appearing acid fuscinophilic. C. through F. On immunohistochemistry, the acellular material stained positively for IgG (C) and lambda light chain (D), and negatively for IgM (E) and kappa light chain (F). G. and H. Electron microscopy revealed densely stacked, electron dense, trapezoidal, extracellular crystalline deposits measuring 0.25 - 3um in width. (original magnification X5 [A, B], X10 [C-F]; direct magnification: 7100x/print magnification: 14000x @7.0 in. [G]; direct magnification: 2200x/print magnification: 4360x @ 7.0 in. [H]).
Surprisingly, we observed complete disappearance of the remaining peripheral plaque in the host cornea in the left eye within one month of surgery (Fig. 1C), suggesting that the injury of surgery and its post-operative inflammation promoted resorption of the immunoglobulin. The plaque in the right eye continued to enlarge (Fig. 3, A), arguing against a reduction in serum IgG from concurrent systemic therapy.
Fig. 3.
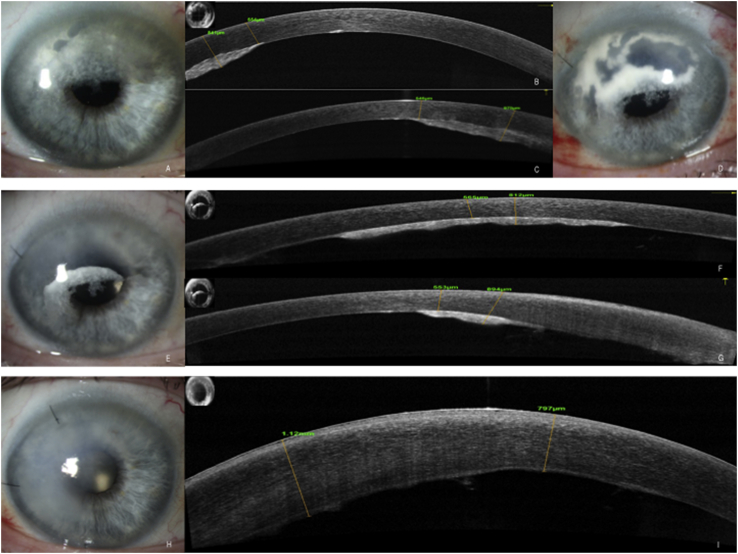
Slit lamp and AS-OCT photos of right eye, patient 1. A. Slit lamp photo showing a patch-like pre-Descemet's plaque in the superior cornea. B. and C. Corresponding AS-OCT taken horizontally and vertically through the cornea, respectively, showing a highly reflective posterior stromal deposit (inset shows line scan). D. Slit lamp photo taken 1 day post operatively following surgical removal of peripheral Descemet's membrane from the superior cornea with local surgical debridement of the plaque. E. Slit lamp photo taken 1 month post operatively showing marked clearing of the peripheral plaque. F and G. Corresponding AS-OCT taken horizontally and vertically through the cornea, respectively, showing a highly reflective residual posterior stromal deposit centrally with resorption of the plaque in the superior periphery (inset shows line scan). H. Slit lamp photo taken 1 day post operatively following central endothelial scraping of the right eye, showing absence of central corneal deposits with expected resultant sectoral corneal edema. G. Corresponding AS-OCT taken horizontally through the cornea showing absence of central plaque following surgical removal of central corneal deposits (inset shows line scan). For all AS-OCT images: Yellow calipers mark the anterior and posterior borders of the corneal plaque, respectively, with corresponding numerical values noted. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Subsequently, peripheral removal of Descemet's membrane and debridement of the right eye plaque was performed, leading to sectoral clearing of deposits by four weeks (Fig. 3D–G). Five months later he underwent additional endothelial scraping of the right eye for remaining central deposition, which resulted in complete clearing of the central corneal deposits (Fig. 3, H-I) with expected resultant geographic corneal edema. The patient underwent successful Descemet's stripping endothelial keratoplasty seven weeks later. At last follow up eleven months following this surgery his BCVA was 20/60 in the right eye (20/20 pinhole), with trace central interface haze, but no recurrence of immunoglobulin deposits in host or graft. His BCVA was 20/30 in the left eye (20/25 pinhole), with graft intact and minimal interface central haze.
5. Case 2
A 65 year old female was referred for evaluation after bilateral crystalline corneal deposits were noted on routine eye exam. Exam revealed diffuse fine needle-like crystalline deposits extending from limbus to limbus in both eyes. The deposits appeared to be located predominantly in the anterior stroma (Fig. 4A and B), involving the posterior stroma to a much lesser degree. BCVA was 20/20 in both eyes. She had a past medical history of psoriasis and unspecified arthritis, but was otherwise healthy, with no history of medication use associated with corneal crystalline deposition. Her posterior segment exam was noted to be normal with no evidence of retinal crystalline deposition. AS-OCT revealed very fine focal opacities in the anterior stroma with minimal disruption of the stromal architecture or thickness (Fig. 4, C). Laboratory testing revealed a mildly elevated total serum protein, as well as an elevated serum protein electrophoresis (SPEP) and urine protein electrophoresis (UPEP) with free kappa light chains present. The patient was referred to hematology, and subsequently diagnosed with multiple myeloma. Her disease was noted to be in an early stage without lytic lesions present, such that therapeutic intervention has been deferred. At follow up nine months after initial presentation, the appearance of her crystalline keratopathy remained similar, with acuity of 20/25 (20/20 pinhole) in the right eye and 20/20 in the left eye. Her systemic and corneal disease continue to be observed at this time.
Fig. 4.
Slit lamp and AS-OCT photos of patients 2 and 3. Patient 2: A. Presenting slit lamp photo reveals diffuse, fine crystalline deposits extending from limbus to limbus. B. These deposits were located predominantly in the anterior stroma. C. Corresponding AS-OCT shows very fine, scattered hyper-reflectivity within the anterior stroma, corresponding to the location of the corneal deposits (inset shows line scan). Patient 3: D. Presenting slit lamp photo reveals peripheral and mid-peripheral patchy corneal deposits with a shimmering, patchy appearance and associated haze in the anterior corneal stroma, with notable sparing of the central cornea. E. Slit beam photo illustrates that these deposits are located predominantly in the anterior stroma. F. Corresponding AS-OCT shows hyper-reflectivity within the anterior stroma, corresponding to the location of the corneal deposits (inset shows line scan).
6. Case 3
A 66 year old male was referred for evaluation of bilateral corneal opacities noted on routine eye exam two months prior. Exam revealed peripheral and mid-peripheral patchy anterior stromal deposits with a shimmering, fractal appearance and associated haze in the anterior corneal stroma, with notable sparing of the central cornea (Fig. 4D and E). BCVA was 20/20 in both eyes. He had a past medical history of prostate cancer s/p resection, and unspecified arthritis treated with weekly methotrexate. He had a normal posterior segment exam with no evidence of retinal crystalline deposition. AS-OCT revealed dense anterior stromal opacities with significant distortion of the anterior corneal contour and variable overlying epithelial thickness (Fig. 4, F). Laboratory testing including SPEP and UPEP was performed, revealing an elevated serum kappa light chain, after which the patient was referred to hematology where he underwent bone marrow biopsy consistent with MGUS. Further work up revealed a small lytic lesion in his left skull, not meeting criteria for diagnosis of multiple myeloma. He was seen back in follow up one, three and six months later, with a mild decrease in acuity to 20/25 in the right eye but maintained vision of 20/20 in the left eye, and a mild increase in central corneal deposits in both eyes. The patient still carries a diagnosis of MGUS, and does not meet criteria for multiple myeloma at this time.
7. Discussion
Cases of protein deposition keratopathy have been previously described in the literature, but to our knowledge we present for the first time a minimally invasive surgical approach to removing posterior corneal deposits. We characterized the deposits on all three patients with anterior segment OCT imaging. We discuss here the various patterns in this case series and how detection and selective treatment at the deepest stromal level in 1 patient allowed for clearance of the deposits.
Our 3 patients illustrate that the clinical and pathologic presentation of this entity is highly variable, which is in agreement previous reports.1,5 Protein deposition keratopathy has been found to present with multiple phenotypes, even resembling the deposition seen in lattice dystrophy, Schnyder corneal dystrophy, or Salzmann nodular degeneration. Lisch et al. proposed five categories to describe the varied presentation of these deposits: crystalline-like, lattice-like, peripheral granular-like, peripheral band-like, and peripheral patch-like.6 Two of the three cases (patients 1,3) presented here fall into the category of peripheral progressing to central patch-like opacities. Interestingly, these two patients had distinct distributions of deposits, with patient 1 demonstrating a rare pre-Descemet's location noted only in a few cases.1 Patient 2 had distinct crystalline-like deposition noted at the slit lamp, which could have been a manifestation of cystinosis.
Electron microscopy patterns of immunoglobulin deposition in paraproteinemic keratopathy have been previously described and vary considerable in patterns, however some of the patterns observed may occur as a result of the fixation process. The electron micrography findings of patient 1 in our series are similar to the angulated, geometric extracellular deposits described previously.7,8
The depth and distribution of corneal deposits in the setting of paraproteinemia is also variable.9 The depth of the deposits might be determined by the source, with deposited material entering the eye from the tear film, limbal vessels, or aqueous to reach these locations, respectively. Corneal stroma appears to be the most commonly reported location of such deposits, followed by the epithelium and subepithelium.1,5, 6, 7, 8, 9, 10, 11, 12, 13 There have been several reported cases of deposition occurring in all layers of the cornea as well as the conjunctiva, and rare reports of deposition in the lens, ciliary processes, pars plana, and choroid.1 The endothelium and Descemet membrane are relatively uncommon locations for such corneal deposition, reported in only 2 previous reports.1,14 All three cases in our series included deposits at the level of the corneal stroma, with case one having deposits isolated to the deep stroma/pre-Descemet zone, a location which made these particularly well suited to Descemet debridement and posterior corneal scraping as a surgical intervention.
Anterior segment OCT was useful and descriptive in these cases to confirm discreteness and location of corneal deposits and assist in planning of surgical approaches. To our knowledge, there is only one other report in the current literature on paraproteinemic keratopathy that incorporates anterior segment OCT findings, which features a single patient with anterior corneal stromal deposits who did not undergo surgical intervention.15 Our series imaged three patients with protein deposition keratopathy, expanding the literature on this topic, and is the first example of utilizing anterior segment OCT to assist in planning of minimally invasive surgical treatments for one appropriate case. OCT also highlighted the variability of corneal deposition, depths, and patterns among patients.
There have been numerous reported cases of corneal crystalline opacity as the initial presentation of underlying systemic disease.5,11,13 In our series, corneal findings preceded the diagnosis of systemic disease in 2 patients and was an indicator of disease recurrence in one patient with a known history of multiple myeloma, emphasizing the significance of such corneal findings.
Systemic therapy of paraproteinemia can result in resolution of the corneal opacity.11 In some cases, a combination of systemic therapy and surgical intervention (either penetrating keratoplasty or superficial keratectomy depending on the depth of disease) has been necessary before the deposits resolved.11,12 To our knowledge, case one in our series is the first to demonstrate that posterior lamellar scraping is a viable option to reduce the deposits, both by physically removing them and presumably inciting an inflammatory/healing response to clear the remainder of the deposits. We think that inflammation/wound healing mechanism must be at play as the patients first eye had clearing of posterior deposits outside the zone of surgery after penetrating keratoplasty. Injury to tissue could recruit phagocytic cells such as macrophages that may clear remnant deposits. Inflammatory mediators may further disrupt local tissue architecture and chemical environment after injury making dissolution of corneal crystals more likely. Further investigation of this phenomenon at other tissue levels in the cornea may lead to less invasive therapies than keratoplasty.
8. Conclusions
In summary, we add to the literature on protein deposition keratopathy by incorporating the use of anterior segment OCT, an imaging tool that can help further characterize corneal protein deposition depth. We also demonstrate a novel technique for clearing the deposits in appropriate patients with posterior corneal scraping. Lastly, this series reminds providers of the importance of recognizing corneal deposits as a possible presenting feature of underlying systemic disease.
Patient consent
Informed consent was obtained where relevant. Specific patient consent to publish the case report was not obtained, as this report does not contain personal information that could lead to the identification of the patients. IRB/Ethics Committee approval was not required for this chart review. Research was conducted in accordance with HIPAA requirements, and adhered to the tenets of the Declaration of Helsinki.
Funding
This research is supported by grant P30 EY010572 from the National Institutes of Health (Bethesda, MD) and by unrestricted departmental funding to Casey Eye Institute from Research to Prevent Blindness (New York, NY). The sponsor or funding organization had no role in the design or conduct of this research.
Conflicts of interest
The following authors have no financial disclosures: H.S., R.S., A.M., D.W., W.C.
Authorship
All authors attest that they meet current ICMJE criteria for Authorship.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2018.12.010.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Garibaldi D.C., Gottsch J., De la Cruz Z. Immunotactoid keratopathy: a clinicopathologic case report and a review of reports of corneal involvement in systemic paraproteinemias. Surv Ophthalmol. 2005;50(1):61–80. doi: 10.1016/j.survophthal.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Klintworth G.K., Bredehoeft S.J., Reed J.W. Analysis of corneal crystalline deposits in multiple myeloma. Am J Ophthalmol. 1978;86:303–313. doi: 10.1016/0002-9394(78)90230-1. [DOI] [PubMed] [Google Scholar]
- 3.Bourne W.M., Kyle R.A., Brubaker R.F. Incidence of corneal crystals in the monoclonal gammopathies. Am J Ophthalmol. 1989;107(2):192–193. doi: 10.1016/0002-9394(89)90225-0. [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan S., Shehadeh-Mashor R., Slomovic A. In: Part VII: Diseases of the Cornea: Corneal Manifestations of Metabolic Diseases. third ed. Krachmer J., Mannis M., Cornea Holland E., editors. Mosby Elsevier; 2011. pp. 665–689. [Google Scholar]
- 5.Milman T., Kao A.A., Chu D. Paraproteinemic keratopathy: the expanding diversity of clinical and pathologic manifestations. Ophthalmology. 2015;122(9):1748–1756. doi: 10.1016/j.ophtha.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Lisch W., Saikia P., Pitz S. Chameleon-like appearance of immunotactoid keratopathy. Cornea. 2012;31(1):55–58. doi: 10.1097/ICO.0b013e31821ddd0c. [DOI] [PubMed] [Google Scholar]; [a] Stirling J.W., Henderson D.W., Rozenbilds M.A. Crystalloidal paraprotein deposits in the cornea: an ultrastructural study of two new cases with tubular crystalloids that contain IgG kappa light chains and IgG gamma heavy chains. Ultrastruct Pathol. 1997;21(4):337–344. doi: 10.3109/01913129709021931. [DOI] [PubMed] [Google Scholar]
- 7.Font R.L., Matoba A.Y., Prabhakaran V.C. IgG-kappa immunoglobulin deposits involving the predescemetic region in a patient with multiple myeloma. Cornea. 2006;25:1237–1239. doi: 10.1097/01.ico.0000240094.78095.f5. [DOI] [PubMed] [Google Scholar]
- 8.Ormerod D.L., Collin B., Dohlman C.H. Paraproteinemic crystalline keratopathy. Ophthalmology. 1988;95:202–212. doi: 10.1016/s0161-6420(88)33200-8. [DOI] [PubMed] [Google Scholar]
- 9.Aragona P., Allegra A., Postorino E. Corneal structural changes in nonneoplastic and neoplastic monoclonal gammopathies. Invest Ophthalmol Vis Sci. 2016;57(6):2657–2665. doi: 10.1167/iovs.15-18594. [DOI] [PubMed] [Google Scholar]
- 10.Wang T.P., Safran S.G., Richter J.R. Subepithelial corneal immunoglobulin deposition as a manifestation of multiple myeloma: a case report and literature review. Clin Lymphoma Myeloma Leuk. 2014;14(1):39–42. doi: 10.1016/j.clml.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Nakatsukasa M., Sotozono C., Tanioka H.M. Diagnosis of multiple myeloma in a patient with atypical corneal findings. Cornea. 2008;27(2):249–251. doi: 10.1097/ICO.0b013e31815b82cd. [DOI] [PubMed] [Google Scholar]
- 12.Firkin F.C., Lee N., Ramsay R. Visual loss caused by corneal crystals in myeloma: rapid improvement and plasma exchange and chemotherapy. Med J Aust. 1979;2(13):677–678. doi: 10.5694/j.1326-5377.1979.tb104268.x. [DOI] [PubMed] [Google Scholar]
- 13.Edmunds M.R., Cikatricis P., Mukherji S. Ophthalmic manifestations of atypical IgD multiple myeloma. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr-2012-006486. bcr2012006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mannis T.E., Mannis G.N., Waterhouse E.G. Paraproteinemic keratopathy as the presenting sign of hematologic malignancy. Am J Hematol. 2016;91(9):961–962. doi: 10.1002/ajh.24327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.