Abstract
Introduction
In Europe and the United States, verteporfin (Visudyne; VP) is registered and used in treating macular degeneration. Research showed that VP decreased expression of fibrotic genes in fibroblasts collected from nodules of patients suffering from Dupuytren’s disease, plausibly by de-activating transcription in the Yes Activated Protein (YAP) pathway.
Aim
To analyze the effect of VP on myofibroblasts cultured from Peyronie’s disease (PD) plaques.
Methods
At surgery for PD we took biopsies from the plaques of 5 patients. By immunostaining, the presence of the pathologic myofibroblasts was determined. After culturing cells, VP was dispensed in starvation medium for 24 and 48 hours and messenger(m)RNA levels of COL1A1, ACTA2, COL5A1, EDA-FN, LOXL2, CCN2, SERPINH1, PLOD2, and YAP were quantified and compared with controls with real-time polymerase chain reaction.
Main Outcome Measure
mRNA-levels of COL1A1, ACTA2, COL5A1, EDA-FN, LOXL2, CCN2, SERPINH1, PLOD2, and YAP.
Results
The pathologic phenotype of cells isolated from PD plaques was confirmed with baseline immunofluorescent stainings that showed considerable levels of α-smooth muscle actin, being a marker for the presence of myofibroblasts. The mRNA ratios of all the genes related to fibrosis (COL1A1, etc.) except YAP decreased significantly after treatment with VP within 24 and 48 hours. These results suggest inhibition of fibrosis in the YAP cascade, downstream of YAP.
Conclusion
In our opinion, urologists must move the focus to disease before deformity, and the search for new oral or intralesional agents, well-tolerated and effective in both the acute and chronic phase of PD must continue. VP blocked the expression of genes related to fibrosis in the YAP cascade in myofibroblasts derived from PD plaque.
Mohede DCJ, de Jong IJ, Bank RA, et al. Verteporfin as a medical treatment in Peyronie’s disease. Sex Med 2018;6:302–308.
Key Words: Peyronie’s Disease, Verteporfin, Fibrosis, YAP Cascade
Introduction
In 1743, François Gigot de la Peyronie (1678–1747) first provided a comprehensive description of induratio penis plastic, now known as Peyronie’s disease (PD).1 PD is a benign fibroproliferative disorder of the penis, which causes the formation of a plaque in the tunica albuginea. Many pharmacologic options have been proposed, but until now none has received a grade-A recommendation. Currently, collagenase from Clostridium histolyticum is the only drug approved by the American and European authorities for intralesional injection in patients with dorsal or dorsolateral curvature >30° with a palpable plaque. In the Netherlands the prescription of oral drugs for PD, for example pentoxifylline, is only allowed if one informs the patient about its off-label use and possible adverse events.
Verteporfin (trade name Visudyne; VP) is registered in the United States and Europe as a sensitizer for photodynamic therapy to eradicate abnormal blood vessels in the eye associated with the wet form of macular degeneration. The first report on photodynamic therapy with VP for choroidal neovascularization was published in 1999.2 VP accumulates in the abnormal blood vessels. When stimulated by non-thermal red light (wavelength 693 nm) in the presence of oxygen, VP produces reactive short-lived singlet oxygen and other oxygen radicals, locally damaging the endothelium and resulting in blockage of these vessels. In its inactivated form (ie, VP that is not subjected to photo irradiation), VP is a small molecular inhibitor of the Hippo-Yes Activated Protein (YAP) pathway (Figure 1) and considered a promising drug in the treatment of pancreatic cancer.3 In that respect it is relevant to mention that Scandinavian studies showed that patients with Dupuytren’s disease (DD) in this part of the world have greater incidences of a variety of smoking-related diseases such as pancreatic, buccal, and lung cancer.4, 5, 6 DD and its related diseases are also a burden for societies in other parts of the world.7 Especially severe and younger-onset cases may be subject to increased cancer incidence and mortality.8, 9
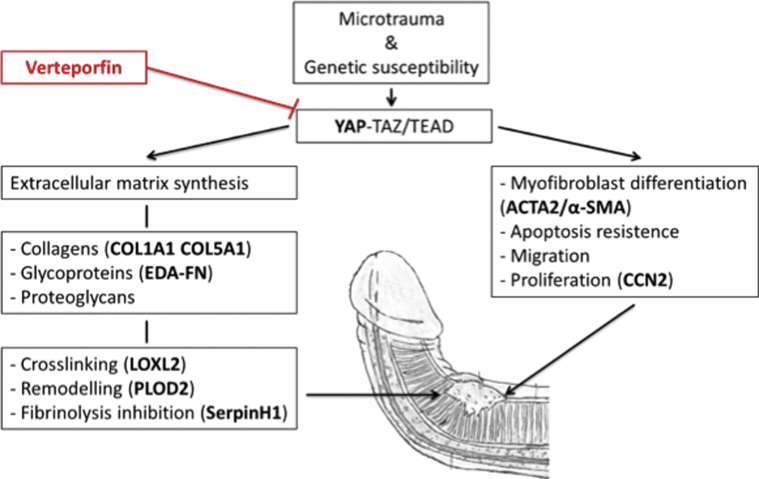
Figure 1.
Simplified fibrosis-related processes in the Yes Activated Protein (YAP) cascade. As a result of microtrauma and genetic susceptibility, the YAP cascade comes into force causing extracellular matrix synthesis, myofibroblast differentiation, apoptosis resistance, migration, and proliferation. These fibrotic processes lead up to plaque development and subsequent penile deformity. In red, verteporfin’s assumed point of engagement in the cascade. In boldface, markers of the different fibrotic processes.
Inactive VP attenuates renal fibrosis in mice subjected to unilateral ureteral obstruction, probably by blocking the transcriptional activation of targets in the YAP cascade involved in fibrosis-related processes.10 The objective of our study was to determine whether VP would have similar effects on (myo)fibroblasts derived from plaques in patients with PD (being the cells responsible for the production of fibrotic lesions).
Materials and Methods
Ethics Statement
Plaque tissue samples of PD patients were collected after obtaining informed written consent of the patients; our protocol was approved by the Medical Ethics Committee (METc) of the University Medical Center Groningen (2007/067; UMCG), in accordance with the Declaration of Helsinki.
Primary Tissues
Plaque tissues were obtained from 5 PD patients with primary disease that underwent plaque incision and grafting or a Nesbit procedure by a senior urologist (M.F.vD.) in the University Medical Center Groningen.
Cell Culture Conditions
(Myo)fibroblasts were obtained from dissected plaque tissue by culturing the small pieces in Dulbecco’s Modified Eagle’s Medium (DMEM; Lonza Group, Basel, Switzerland) containing 1% l-glutamine, 1% penicillin-streptomycin, and 10% fetal bovine serum (FBS) at 37oC in a 5% CO2 incubator. Experiments were performed on cells from the 8th passage to a new culturing-flask. To confirm the presence of myofibroblasts (ie, activated fibroblasts), immunofluorescent staining for an alpha-smooth muscle actin (α-SMA) was performed before starting the VP experiments.
VP Experiment
Starvation of the cells took place for 16 hours overnight in culture medium consisting of DMEM containing 1% penicillin/streptomycin, 1% l-glutamine, 0.5% FBS, and 17 mM ascorbic acid. Cells were kept at 37°C in 5% CO2. 1 day after starvation, the cells were exposed to VP (250 nM) in the previously mentioned culture medium containing 0.01% (volume/volume [v/v]) dimethyl sulfoxide (DMSO; this was used as solvation vehicle for VP). The control group was exposed to 0.01% (v/v) DMSO. Lights were off when working with either VP or DMSO. The treatment took place for 24 and 48 hours in an incubator at 37°C in 5% CO2.
Immunostaining
After treatment, cells were fixated in 1:1 acetone/methanol. The cells were then permeabilized with 0.5% Triton X-100 in phosphate-buffered saline (PBS), incubated with 10% serum of the secondary species and blocked with 1% bovine serum albumin (BSA) in PBS. Primary antibodies directed toward α (Dako M0851 IgG2a) were diluted in 1% BSA in PBS and incubated for 1 hour at room temperature. Secondary antibodies (GaMIgG2a-biotin) were diluted in 2% normal human serum in PBS and incubated for 30 min at room temperature, covered in tin foil. Cells were then incubated with 1:100 streptavidin-Cy3 in 1:5,000 4’,6-diamidino-2-phenylindole (DAPI) in tin foil for 30 minutes at room temperature. DAPI was used to stain the nuclei. After every incubation step a washing step was performed, incubating the cells in fresh PBS 3 times for 5 minutes. Pictures were taken under a fluorescence microscope.
Real-Time Polymerase Chain Reaction
After treatment, cells were lysed and stored in 350 μl of a mix with dithiothreitol (DTT) and FARB Buffer (10 μl DTT per 1 ml FARB Buffer) at -80°C. RNA was isolated using an RNA isolation kit (FavorPrep mini kit, Favorgen Biotech Corp., Ping-Tung, Taiwan) according to the manufacturer’s instructions.
Complementary DNA (cDNA) was synthesized by priming with random hexamer primer (1 μl added to 11 μl concentrated RNA). After adding the primer, cells were incubated for 5 min in 65°C. A mixture of 4 μl reaction buffer 5x, 2 μl dNTP'S (Qiagen, Stokach, Germany) (10 mM) mixture, 0.5 μl RiboLock RNAse inhibitor (Thermo Fisher Scientific, Waltham, MA, USA), 0.5 μl RNAse free H2O, and 1 μl Reverse Transcriptase RevertAid M-MuLV (Thermo Fisher Scientific, Waltham, MA, USA) was then added to each sample, making a total volume of 20 μl. Cells were subsequently incubated according to the next program: 5 minutes at 25°C; 60 minutes at 42°C; 5 minutes at 70°C; and stored at -20oC. For real-time polymerase chain reaction, triplicates of each sample were used.
Primers (Table 1) were dissolved in Milli-Q water (Merck, Darmstadt, Germany), needing 0.25 μl of both forward and reverse primer per reaction (150 nM/primer). Mastermix (Thermo Fisher Scientific, Waltham, MA, USA) per gene contains 5 μl SYBRgreen (Rouche; 2x) and 0.5 μl Prime Mix (6 μM). 5 μl diluted cDNA sample (5 ng/μl) and 5 μl Mastermix were added to each well in a 384 well plate, making a total volume of 10 μl. Plates were run on the ViiA 7 Real-Time PCR system (Thermo Fisher Scientific, Waltham, MA, USA). The relative gene expression was calculated using the ΔΔCt method, normalizing for the average expression of the household gene YWHAZ.
Table 1.
Sequences of the used primers
| Primers | Sequence | |
|---|---|---|
| F (5' - 3') | R (5' - 3') | |
| ACTA2 | CTGTTCCAGCCATCCTTCAT | TCATGATGCTGTTGTAGGTGGT |
| CCN2 | AGCTGACCTGGAAGAGAACATT | GCTCGGTATGTCTTCATGCTG |
| COL1A1 | GCCTCAAGGTATTGCTGGAC | ACCTTGTTTGCCAGGTTCAC |
| COL5A1 | CCTGGATGAGGAGGTGTTTG | CGGTGGTCCGAGACAAAG |
| EDA-FN | AATCCAAGCGGAGAGAGTCA | GGAATCGACATCCACATCAG |
| LOXL2 | TGACCTGCTGAACCTCAATG | TGGCACACTCGTAATTCTTCTG |
| PLOD2 | GGGAGTTCATTGCACCAGTT | GAGGACGAAGAGAACGCTGT |
| SERPINH1 | TGGTGCTGATCTCATCCTTG | AGAAACCCAGCAGCAGATTC |
| YAP | AATCCCACTCCCGACAGG | GACTACTCCAGTGGGGGTCA |
| YWHAZ | GATCCCCAATGCTTCACAAG | TGCTTGTTGTGACTGATCGAC |
Sequences in 5’ to 3’ direction.
A = adenine; C = cytosine; F = forward; G = guanine; R = reversed; T = thymine.
Statistics
Experiments were performed in triplicate. Statistics were performed using GraphPad Prism Software version 7 (GraphPad Software, La Jolla, CA, USA). A Kruskal-Wallis test was used to determine whether there were differences in ranks between the 3 groups. 2-way ANOVA with Bonferroni posttest was used for statistics; P < .05 was considered significant.
Results
Characterization of PD Plaque Cells
To confirm the pathologic phenotype of cells isolated from PD plaques, baseline immunofluorescent stainings were performed that showed considerable levels of α-SMA, being a marker for the presence of myofibroblasts (Figure 2). This finding confirmed the pathologic nature of the obtained plaque samples.
Figure 2.
Characterization of Peyronie’s Disease (PD) plaque cells (passage 8). Part of the fibroblasts showed features of myofibroblasts, indicating the presence of activated cells in the PD plaques; red α-smooth muscle actin, blue 6-diamidino-2-phenylindole. Black scale bar represents 50 μm.
Gene Expression of Fibrosis-Related Genes
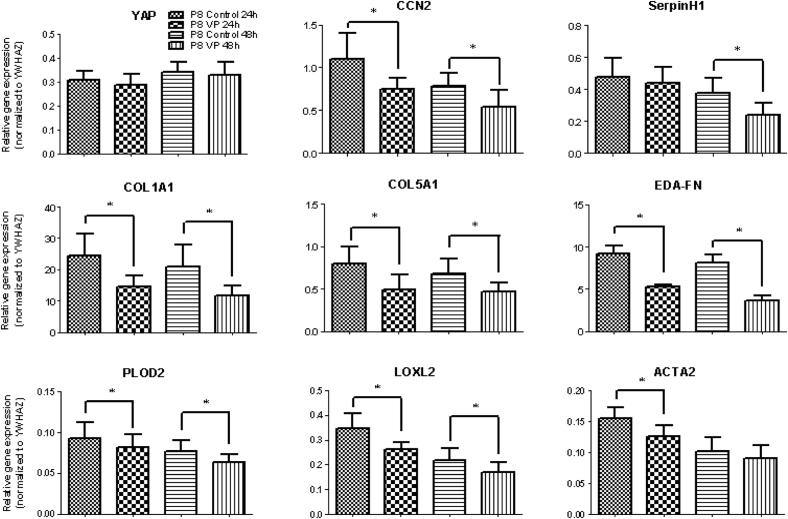
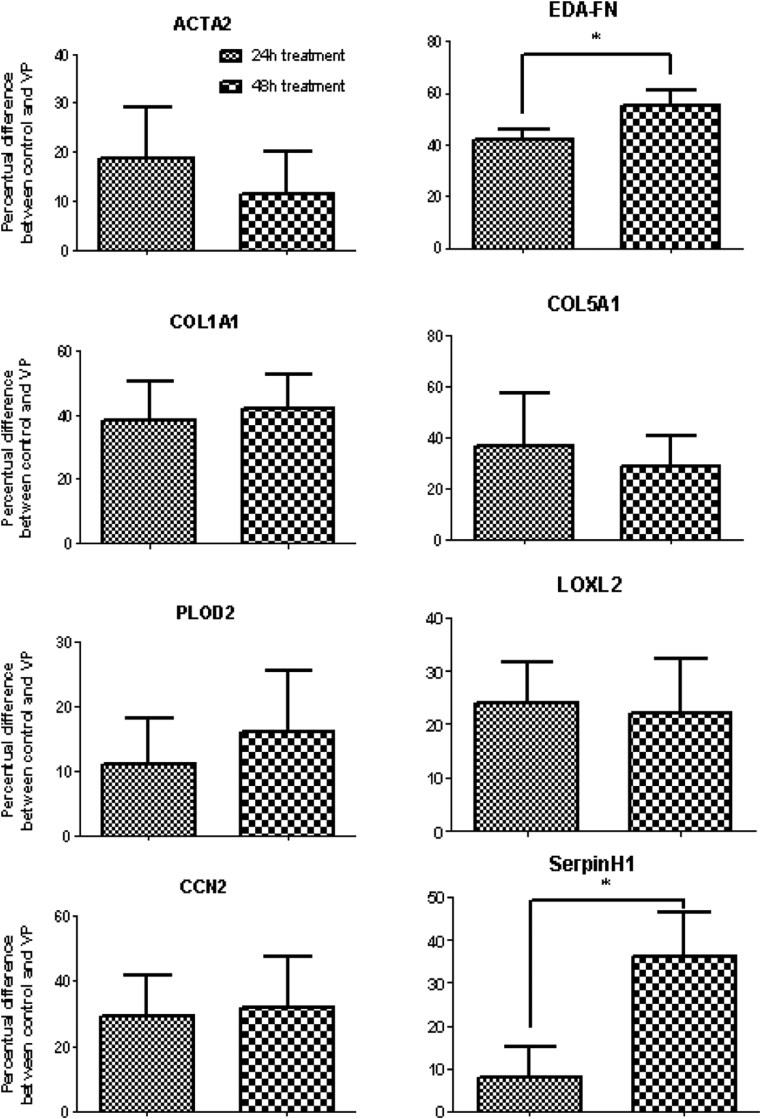
Myofibroblasts derived from the plaque tissue responded to VP treatment for both 24 and 48 hours, resulting in significant decreases in gene expression levels of CCN2, COL1A1, COL5A1, EDA-FN, PLOD2, and LOXL2 (Figure 3). The mRNA level of SERPINH1 was decreased only at 48 hours, whereas that of ACTA2 was reduced at 24 hours only (Figure 2). As expected, VP did not affect mRNA levels of YAP. The control group (DMSO only) did not differ from the control group without DMSO, which showed that DMSO did not affect the cultures (data not shown). Compared with 24 hours, the mRNA level of EDA-FN and SERPINH1 was even more decreased at 48 hours, showing that VP has long-lasting anti-fibrotic effects (Figure 4).
Figure 3.
Response of (myo)fibroblasts subjected to 250 nM verteporfin (VP) for 24 and 48 hours. mRNA levels of Yes Activated Protein did not change, whereas mRNA levels of extracellular matrix molecules (COL1A1, COL5A1, EDA-FN), collagen-modifying enzymes (SERPINH1, PLOD2, LOXL2), and the pro-fibrotic markers CCN2 and ACTA2 were attenuated by VP. * = P < .05.
Figure 4.
Response of (myo)fibroblasts subjected to 250 nM verteporfin (VP) as a percent difference after 24 and 48 hours. Compared with 24 hours, the mRNA level of EDA-FN and SERPINH1 was even more decreased at 48 hours, showing that VP has long-lasting anti-fibrotic effects. * = P < .05.
Discussion
VP’s Role in Fibrosis
The lack of effective medical treatments for PD stems from a lack of understanding of its biology. However, today it is well known that tissue derived from PD plaques contains myofibroblasts, the cells responsible for the pathologic fibrotic processes. Indeed, we found considerable amounts of myofibroblasts in our cell cultures derived from plaques. Furthermore, our study shows that in vitro exposure of VP to (myo)fibroblasts inhibits the pathologic processes by means of the down-regulation of fibrosis-associated genes. Fibrosis is characterized by excessive production, deposition, and contraction of extracellular matrix (ECM), leading to significant organ dysfunction.11 Here we show that VP is able to reduce the expression of the 2 major fibrillary collagens, that is, collagen type I and type V, and the ECM component fibronectin (EDA-FN). This should lead to a less-excessive deposition of ECM. It also helps that VP is able to decrease SERPINH1 levels (also known as HSP47), a chaperone needed for the proper transportation of collagen. In fibrosis the ECM stiffens, even before scarring occurs, owing to an increased expression of enzymes involved in collagen cross-linking. In our study we found that the expression of at least 2 of these enzymes, LOXL2 and PLOD2, is inhibited by VP. This should lead to a less-stiff ECM. In addition, collagen cross-linked by hydroxylysine residues mediated by PLOD2 is more difficult to degrade by matrix metalloproteinases.12 Lower cross-links levels derived from PLOD2 most likely results in a faster degradation of collagen molecules, resulting in less ECM accumulation. Our findings suggest that VP might benefit patients mostly in the acute phase of PD, but possibly also in the chronic phase. However important some of these mechanisms in certain malignancies are, our main foci for this study were PD and fibrosis-related processes.
Pharmacokinetics and Safety
For its current indication (ie, macular degeneration), VP is administered parenterally in the largest possible arm vein in a dose of 6 mg/m2 and light-activated. This can be repeated yearly 4 times. No drug–drug interactions were ever described in patients treated with VP, but simultaneous use of photosensibilizing drugs such as tetracyclines and thiazide diuretics may increase the risk of light-sensitivity reactions. Porphyria is an absolute contraindication. During 48 hours, patients are photosensitive and advised not to come in contact with direct bright (sun)light and to cover themselves with sunglasses and clothes.13 In animal studies, mild extravascular hemolysis and hematopoiesis were noted in inactivated daily doses 32–70 times (in dogs and rats) as high as advised in humans when administered for 4 weeks.14 Fast admission of 2.0 mg/kg in pigs under general anesthesia led to hemodynamic instability and diphenhydramine decreased these effects, suggesting histamine-related processes. Animal studies ruled out ocular toxicity. There was no teratogenicity reported in rabid fetuses that received 67 times the advised dosage in humans and no toxicity was seen in general genotoxic tests. In mice, beneficial effects were reported in immune-mediated diseases. Normal immune reactions in the skin were decreased without causing skin reactivity or generalized non-specific immune suppression. An overview of preliminary clinical studies showed that VP was safe in humans with only minimal side effects.15 Because VP is already FDA approved, testing it as an off-label treatment for PD may be an interesting step, but future research can better be focused on optimal administration and dosage schemes for possible treatment of PD. Until now orally administered VP has not been tested, because parenteral administration is preferred for its current indication.
None of the options depicted in the European guidelines carry a grade-A recommendation, but their assumed working mechanisms are worth taking into account considering the quest for new treatment strategies.16 Combination therapy with historically proposed medical methods and up-and-coming ones such as verteporfin inhibiting disease-related processes on different levels, can be considered for future trials. In that respect, we strongly recommend a close collaboration with biologists and experts in PD-related fibrotic diseases.
Recommendations and Conclusion
Regarding PD, urologists should focus on the disease before deformity, preferably in an internationally collaborative fashion. The search for new oral and intralesional agents, well tolerated and effective in both the acute and chronic phase of PD, should be encouraged. As in DD, new PD lab models (eg, 3-dimensional cell cultures) may be used to find novel therapeutic targets. To achieve these models, one obviously needs basic researchers. Human tissue-engineered models have already begun replacing animal models for preclinical drug testing.17 Urologists with special interest in PD should organize brainstorming symposia with non-urologists such as biologists and geneticists. Improved international collaboration will pave the way for funding and finding optimal research paths.
In the end, the management of patients with PD undoubtedly will improve, with fewer needing surgery. In this respect verteporfin is one of the drugs that needs further investigation, because it apparently displays a wide spectrum of anti-fibrotic properties.
Statement of Authorship
Category 1
-
(a)Conception and Design
- Daan C. J. Mohede; Igle Jan de Jong; Ruud A. Bank; Mels F. van Driel
-
(b)Acquisition of Data
- Daan C. J. Mohede; Ruud A. Bank
-
(c)Analysis and Interpretation of Data
- Daan C. J. Mohede; Igle Jan de Jong; Ruud A. Bank; Mels F. van Driel
Category 2
-
(a)Drafting the Article
- Daan C. J. Mohede
-
(b)Revising It for Intellectual Content
- Daan C. J. Mohede; Igle Jan de Jong; Ruud A. Bank; Mels F. van Driel
Category 3
-
(a)Final Approval of the Completed Article
- Daan C. J. Mohede; Igle Jan de Jong; Ruud A. Bank; Mels F. van Driel
Acknowledgments
We would like to thank Theo Borghuis for his help and support concerning laboratory testing and analysis.
Footnotes
Conflicts of Interest: M.F. van Driel is a speaker for GSK and Lilly.
Funding: None.
References
- 1.De la Peyronie F. Sur quelques obstacles qui s’opposent à l’éjaculation naturelle de la semence. Mém Acad Roy Chir. 1743;1:318–333. [Google Scholar]
- 2.Miller J.W., Schmidt-Erfurth U., Sickenberg M. Photodynamic therapy with verteporfin for choroidal neovascularization caused by age-related macular degeneration: Results of a single treatment in a phase 1 and 2 study. Arch Ophthalmol. 1999;117(9):1161–1173. doi: 10.1001/archopht.117.9.1161. [DOI] [PubMed] [Google Scholar]
- 3.Wei H., Wang F., Wang Y. Verteporfin suppresses cell survival, angiogenesis and vasculogenic mimicry of pancreatic ductal adenocarcinoma via disrupting the YAP-TEAD complex. Cancer Sci. 2017;108(3):478–487. doi: 10.1111/cas.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilbrand S., Ekbom A., Gerdin B. Dupuytren’s contracture and sarcoma. J Hand Surg Br. 2002;27(1):50–52. doi: 10.1054/jhsb.2001.0652. [DOI] [PubMed] [Google Scholar]
- 5.Wilbrand S., Ekbom A., Gerdin B. A cohort study linked increased mortality in patients treated surgically for Dupuytren’s contracture. J Clin Epidemiol. 2005;58(1):68–74. doi: 10.1016/j.jclinepi.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Wilbrand S., Ekbom A., Gerdin B. Cancer incidence in patients treated surgically for Dupuytren’s contracture. J Hand Surg Br. 2000;25(3):283–287. doi: 10.1054/jhsb.2000.0382. [DOI] [PubMed] [Google Scholar]
- 7.Macaulay D., Ivanova J., Birnbaum H. Direct and indirect costs associated with Dupuytren’s contracture. J Med Econ. 2012;15(4):664–671. doi: 10.3111/13696998.2012.670678. [DOI] [PubMed] [Google Scholar]
- 8.Gudmundsson K.G., Arngrimsson R., Sigfusson N. Increased total mortality and cancer mortality in men with Dupuytren’s disease. A 15-year follow-up study. J Clin Epidemiol. 2002;55(1):5–10. doi: 10.1016/s0895-4356(01)00413-9. [DOI] [PubMed] [Google Scholar]
- 9.Mikkelsen O.A., Høyeraal H.M., Sandvik L. Increased mortality in Dupuytren’s disease. J Hand Surg Br. 1999;24(5):515–518. doi: 10.1054/jhsb.1999.0229. [DOI] [PubMed] [Google Scholar]
- 10.Szeto S.G., Narimatsu M., Lu M. YAP/TAZ are mechanoregulators of TGF-β-smad signaling and renal fibrogenesis. J Am Soc Nephrol. 2016;27(10):3117–3128. doi: 10.1681/ASN.2015050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Sakka A.I., Salabas E., Dinçer M. The pathophysiology of Peyronie’s disease. Arab Journal of Urology. 2013;11:272–277. doi: 10.1016/j.aju.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Der Slot-Verhoeven A.J., Van Dura E.A., Attema J. The type of collagen cross-link determines the reversibility of experimental skin fibrosis. BBA-Mol Basis Dis. 2005;1740(1):60–67. doi: 10.1016/j.bbadis.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Verteporfin Roundtable Participants Guidelines for using verteporfin (Visudyne) in photodynamic therapy for choroidal neovascularization due to age-related macular degeneration and other causes: Update. Retina. 2002;25(2):119–134. doi: 10.1097/00006982-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Adili F., Statius van Eps R.G., Flotte T.J. Photodynamic therapy with local photosensitizer delivery inhibits experimental intimal hyperplasia. Lasers Surg Med. 1998;23(5):263–273. doi: 10.1002/(sici)1096-9101(1998)23:5<263::aid-lsm6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 15.Dougherty T. Photodynamic therapy for the treatment of cancer: Current status and advances. In: Kessel D., editor. Photodynamic therapy of neoplastic disease. CRC Press; Boca Raton, FL: 1989. pp. 1–19. 1997;39:81. [Google Scholar]
- 16.Hatzimouratidis K., Eardley I., Giuliano F. EAU guidelines on penile curvature. Eur Urol. 2012;62:543–552. doi: 10.1016/j.eururo.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 17.O’Gorman D. The future of basic Dupuytren disease research. In: Werker P., editor. Dupuytren disease and related diseases—The cutting edge. Springer International Publishing; Basel, Switzerland: 1989. pp. 1–19. [Google Scholar]