Abstract
Cryo-electron micrograph studies recently have identified a Ca2+-binding site in the 2,200-kDa ryanodine receptor ion channel (RyR1) in skeletal muscle. To clarify the role of this site in regulating RyR1 activity, here we applied mutational, electrophysiological, and computational methods. Three amino acid residues that interact directly with Ca2+ were replaced, and these RyR1 variants were expressed in HEK293 cells. Single-site RyR1-E3893Q, -E3893V, -E3967Q, -E3967V, and -T5001A variants and double-site RyR1-E3893Q/E3967Q and -E3893V/E3967V variants displayed cellular Ca2+ release in response to caffeine, which indicated that they retained functionality as caffeine-sensitive, Ca2+-conducting channels in the HEK293 cell system. Using [3H]ryanodine binding and single-channel measurements of membrane isolates, we found that single- and double-site RyR1-E3893 and -E3967 variants are not activated by Ca2+. We also noted that RyR1-E3893Q/E3967Q and -E3893V/E3967V variants maintain caffeine- and ATP-induced activation and that RyR1-E3893Q/E3967Q is inhibited by Mg2+ and elevated Ca2+. RyR1-T5001A exhibited decreased Ca2+ sensitivity compared with WT-RyR1 in single-channel measurements. Computational methods suggested that electrostatic interactions between Ca2+ and negatively charged glutamate residues have a critical role in transducing the functional effects of Ca2+ on RyR1. We conclude that the removal of negative charges in the recently identified RyR1 Ca2+-binding site impairs RyR1 activation by physiological Ca2+ concentrations and results in loss of binding to Ca2+ or reduced Ca2+ affinity of the binding site.
Keywords: ryanodine receptor, skeletal muscle, mutagenesis, computation, sarcoplasmic reticulum (SR), Ca2+ activation, Ca2+ release channel, calcium homeostasis, ion gating, structure-function analysis
Introduction
Ryanodine receptor ion channels (RyRs)2 release Ca2+ from intracellular Ca2+-storing compartments to regulate multiple cellular functions (1–4). There are three mammalian RyR isoforms. RyR1 is present in skeletal muscle, RyR2 is present in heart muscle, and RyR3 is present at low levels in many tissues, including brain and slow-twitch skeletal muscle. Calcium ions play a predominant role in the regulation of RyRs. Activation by micromolar Ca2+ and inhibition by millimolar Ca2+ suggest the presence of high-affinity Ca2+ activation and low-affinity Ca2+ inactivation sites. Additional regulation is mediated by ATP and caffeine, which increase Ca2+-gated RyR activities. Mg2+ inhibits Ca2+-activated RyRs by competing with Ca2+ for high-affinity Ca2+ activation sites and by binding to inhibitory low-affinity divalent cation sites (3).
Cryo-EM studies have provided detailed information about closed and open structures of the 2,200-kDa RyRs (5–12). Des Georges et al. (9) determined the location of RyR1-binding sites for endogenous channel activators Ca2+ and ATP and the exogenous activator caffeine (Fig. 1). Ca2+ or ATP/caffeine alone induced structural changes in the large cytosolic domain and primed RyR1 to nearly full open conformation in the presence of the three channel activators (9).
Figure 1.
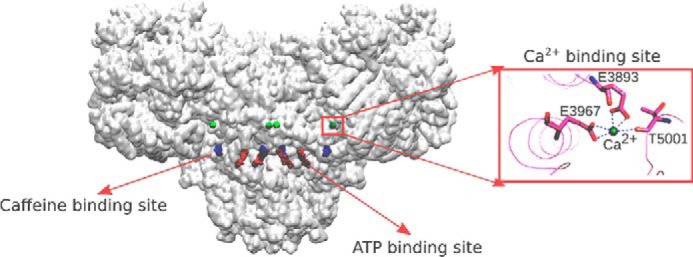
Location of Ca2+-, ATP-, and caffeine-binding sites of open RyR1 (PDB code 5TAL). The protein structure is shown as a transparent surface. Inset, structure of Ca2+-binding site of open RyR1 (PDB code 5TAL).
Three conserved amino acid residues contribute to the primary coordination sphere of bound Ca2+ in the large cytoplasmic domain of RyRs. Thr-5001 is conserved in RyRs, whereas RyR1 residues Glu-3893 and Glu-3967 are conserved in both RyRs and the related inositol trisphosphate receptor families. The RyRs and IP3 receptors are expressed in intracellular membrane compartments containing up to millimolar concentrations of bound and free Ca2+. The release of Ca2+ is triggered by a Ca2+-induced Ca2+ release mechanism for the cardiac and brain RyR isoforms and by the action of IP3 and Ca2+ for the IP3 receptors (3, 13). A distinguishing feature of skeletal muscle is that voltage-sensing L-type Ca2+ channels (DHPRs, Cav1.1s) open juxtaposed RyR1s through direct protein–protein interactions (14). Ca2+-induced Ca2+ release is regulated on a slow time scale in mammalian skeletal muscle compared with physiological rates of Ca2+ release (15, 16). This suggests that while having a predominant role in the regulation of cardiac and brain RyR isoforms, Ca2+ may have a more confined role in the activation of mammalian skeletal-muscle RyRs.
The present study tested the hypothesis that the Ca2+-binding site identified by cryo-EM (9) has a major role in transducing the functional effects of Ca2+ in RyR1. Five single-site RyR1 variants (E3893Q, E3893V, E3967Q, E3967V, and T5001A) and two double-site RyR1 variants (E3893Q/E3967Q and E3893V/E3967V) were expressed as caffeine-sensitive, Ca2+-conducting channels in HEK293 cells. Studies using membrane isolates suggested that Ca2+ did not significantly activate single- and double-site RyR1-E3893 and -E3967 variants, whereas RyR1-T5001A exhibited altered Ca2+-dependent regulation compared with WT. Computational methods using cryo-EM micrograph densities provided structural information on the interaction between Ca2+ and amino acid residues of the Ca2+-binding sites of RyR1-WT and variant channels.
Results
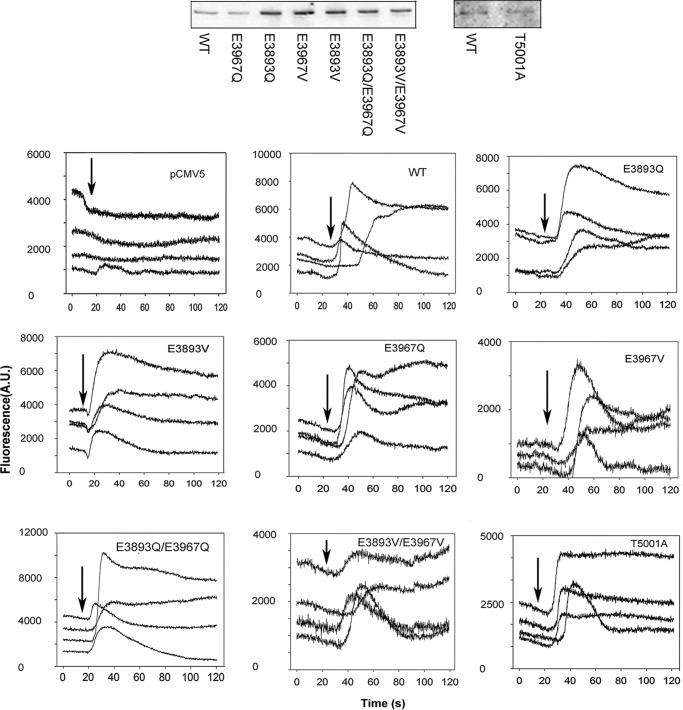
Three conserved amino acid residues (Glu-3893, Glu-3967, and Thr-5001) were shown previously to directly interact with Ca2+ in a Ca2+-binding site in RyR1 (Fig. 1) (9). In the present study, we focused on the role of the two negatively charged glutamates in regulating RyR1 activity. Negative charges were removed by replacing one or both Glu residues with Gln or Val (RyR1-E3893Q, -E3893V, -E3967Q, -E3967V, -E3893Q/E3967Q, and -E3893V/E3967V) while maintaining residue volumes (17). RyR1 Thr-5001 was mutated to Ala, reducing the size of the side chain at this position. SDS-PAGE and immunoblot analysis indicated that single- and double-site RyR1-E3893 and -E3967 variants were expressed at variable, elevated levels compared with WT and RyR1-T5001A at a level comparable with WT (Fig. 2, top and Table 1).
Figure 2.
Immunoblots and caffeine-induced Ca2+ release in HEK293 cells transfected with WT and variant RyR1s. Top, proteins (20 μg/lane) were separated on 3–12% gradient SDS-PAGE, transferred to nitrocellulose membranes, and probed with primary rabbit anti-RyR1 antibody and peroxidase-conjugated anti-rabbit IgG. WT and variant 565-kDa RyR1 polypeptide bands are shown. Bottom, Ca2+ transients were determined in Ca2+-free Krebs–Ringer–Henseleit bath solution as changes of Fluo-4 fluorescence before and after the addition of ∼8 mm caffeine to the bath solution. Arrows, the position of caffeine addition to cells. Averaged data in Table 1 were corrected for number of pCMV5-transfected cells that showed a caffeine signal (∼10% of WT transfected cells). A.U., arbitrary units.
Table 1.
Properties of WT and variant channels
ND, not determined. Numbers in parentheses indicate the number of independent determinations.
| Immunoblot intensity | Caffeine-induced Ca2+ release | Single-channel properties |
||||
|---|---|---|---|---|---|---|
| Po at 0.01 μm Ca2+ | Po at 2 μm Ca2+ | γK+ | PCa/PK | |||
| % WTa | % WTb | pS | ||||
| WT | 100 | 100 | 0.0003 ± 0.0001 (7) | 0.10 ± 0.03 (10) | 766 ± 11 (12)c | 6.6 ± 0.2 (8)c |
| E3893Q | 540 ± 218 (9) | 119.0 ± 7.2 (4) | 0.0019 ± 0.0008 (4) | 0.0015 ± 0.0003 (4) | 810 ± 22 (4) | ND |
| E3893V | 772 ± 265 (9) | 101.2 ± 11.2 (3) | 0.0009 ± 0.0007 (4) | 0.0007 ± 0.0005 (4)d | 809 ± 13 (4) | ND |
| E3967Q | 298 ± 76 (8) | 77.6 ± 9.9 (4) | 0.0005 ± 0.0003 (3) | 0.0017 ± 0.0011 (4) | 780 ± 10 (4) | ND |
| E3967V | 494 ± 212 (9) | 46.5 ± 5.9 (5)e | 0.0005 ± 0.0003 (4) | 0.0011 ± 0.0004 (4) | 746 ± 18 (4) | ND |
| E3893Q/E3967Q | 207 ± 70 (10) | 105.6 ± 11.0 (4) | 0.020 ± 0.008 (10) | 0.016 ± 0.006 (10) | 790 ± 7 (5) | 6.2 ± 0.3 (4) |
| E3893V/E3967V | 728 ± 372 (9) | 48.9 ± 11.2 (4) | 0.0004 ± 0.0002 (7) | 0.0004 ± 0.0001 (8)d,f | 774 ± 24 (3) | ND |
| T5001A | 103 ± 40 (5) | 73.5 ± 11.5 (8) | 0.0004 ± 0.0003 (3) | 0.009 ± 0.007 (9) | 788 ± 14 (4) | 6.1 ± 0.3 (4) |
a Intensities of RyR1 variant bands on immunoblots were normalized to RyR1-WT intensities.
b Numbers of variant cells responding to 8 mm caffeine were normalized to WT cells showing a caffeine response; for each determination, 30–50 cells were examined.
c From Xu et al. (30).
d p < 0.05 compared with WT by Kruskal–Wallis one-way ANOVA followed by Dunn's method.
e p < 0.05 compared with E3893Q by Kruskal–Wallis one-way ANOVA followed by Dunn's method.
f p < 0.05 compared with E3893Q/E3967Q by Kruskal–Wallis one-way ANOVA followed by Dunn's method.
The expression of functional RyR1 variant channels was monitored in HEK293 cells using the RyR1 agonist caffeine and fluorescence Ca2+ indicator Fluo-4. Millimolar caffeine activates RyR1 (18) and has little effect on Fluo-4 fluorescence in HEK293 cells transfected with the pCMV5 plasmid (Fig. 2, bottom). A variable caffeine-induced Ca2+ release was observed in 30–60% of HEK293 cells transfected with WT-RyR1. The variable response may have resulted from uneven exposure to caffeine and/or removal of released Ca2+ by HEK293 cellular transport systems. The number of variant HEK293 cells showing caffeine-induced Ca2+ release ranged from 46.5% for RyR1-E3967V to 119% for RyR1-E3893Q compared with WT (Table 1). These observations suggest that all variants expressed caffeine-sensitive, Ca2+-conducting channels in HEK293 cells.
Two methods were used to determine WT and variant activities. Regulation by Ca2+ was directly measured using the lipid bilayer method (19) (see below). In a second widely used but less direct method, Ca2+-dependent RyR activity was determined using the RyR-specific plant alkaloid ryanodine (20).
Specific [3H]ryanodine binding to WT showed a bimodal Ca2+ activation/Ca2+ inhibition profile with peak activity at ∼70 μm Ca2+ (Fig. 3). [3H]Ryanodine binding to RyR1-T5001A was lower than WT. In contrast to RyR1-WT and -T5001A, RyR1-E3893 and -E3967 variants displayed the highest [3H]ryanodine binding levels at submicromolar Ca2+ (Fig. 3). RyR1-E3893Q/E3967Q bound the highest amount of [3H]ryanodine at <1 μm Ca2+ (∼275 fmol/mg protein), followed by E3967Q and E3893Q (∼55 and ∼40 fmol/mg protein, respectively), E3893V (∼20 fmol/mg protein), and E3967V and E3893V/E3967V variants (∼11 and 15 fmol/mg protein, respectively). Elevated Ca2+ concentrations inhibited [3H]ryanodine binding for all variants, with IC50 values ranging from 50 to 200 μm Ca2+.
Figure 3.
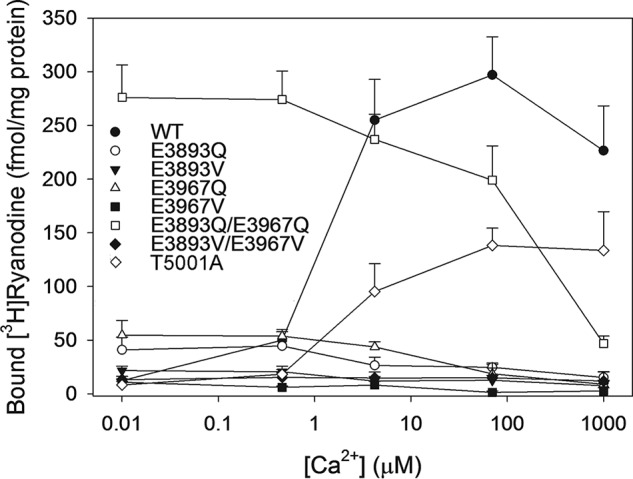
Effects of Ca2+ on [3H]ryanodine binding to RyR1-WT and variants. Specific [3H]ryanodine binding to RyR1-WT and RyR1-E3893Q, -E3893V, -E3967Q, -E3967V, -E3893Q/E3967Q, -E3893V/E3967V, and -T5001A variants was determined as described under “Experimental procedures” in the absence or presence of 10 μm unlabeled ryanodine in 0.25 m KCl, 20 mm imidazole, pH 7.0, containing 3 nm [3H]ryanodine, protease inhibitors, and the indicated concentrations of free Ca2+. Data are the mean ± S.E. (error bars) of 4–7 experiments.
We considered the possibility that the different [3H]ryanodine binding levels of the variants in Fig. 3 could be accounted for by varying levels of expression in HEK293 cells and/or recovery of functional variants in the membrane isolates. We assessed this in recording single channels using the lipid bilayer method, which directly compares WT and variant activities. RyR1s were recorded as K+-conducting channels using 0.25 m KCl on both sides of the bilayer at cytosolic Ca2+ ranging from 0.01 μm Ca2+ to 10 mm Ca2+.
RyR1-WT showed a biphasic Ca2+ activation/inhibition profile with a maximum averaged channel open probability (Po) of 0.16 at ∼20 μm cytosolic Ca2+ (Fig. 4). RyR1-T5001A resulted in a rightward shift of the Po–Ca2+ activation response curve and reduced peak Po at ∼100 μm Ca2+ compared with WT at 20 μm Ca2+.
Figure 4.
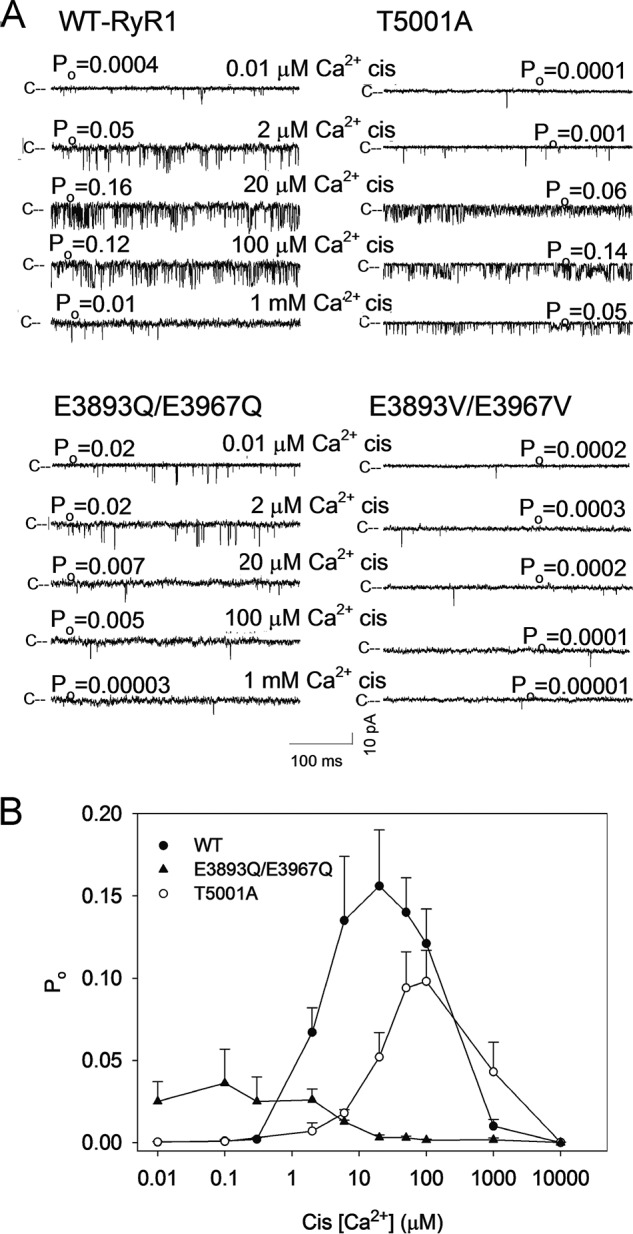
Single-channel measurements of RyR1-WT and RyR1-T5001A, E3893Q/E3967Q, and E3893V/E3967V variants. A, single-channel currents were recorded at a holding potential of −20 mV as downward deflections from the closed state (c−) in symmetrical 0.25 m KCl with 2 μm SR luminal Ca2+ and the indicated cytosolic Ca2+ concentrations. B, Ca2+ dependence of RyR1-WT and RyR1-T5001A and -E3893Q/E3967Q variant channel open probabilities. Data are the mean ± S.E. (error bars) of 3–21 recordings.
In agreement with the [3H]ryanodine-binding data of Fig. 3, RyR1-E3893Q/E3967Q was not significantly activated by Ca2+. Averaged Po was 0.02 at 0.01 μm Ca2+ (Table 1) and half-maximal levels at ∼6 μm Ca2+ (Fig. 4B) suggested that binding of elevated cytosolic Ca2+ had an inhibitory effect (p < 0.05). One possibility we could not rule out is that the presence of Ca2+-inhibitory sites interfered with the activation of RyR1-E3893Q/E3967Q by the binding of Ca2+ to low-affinity sites. RyR1-E3893Q, -E3893V, -E3967Q, -E3967V, and -E3893V/E3967V had very low averaged Po values of 0.002 and less at Ca2+ ranging from 0.01 to 2 μm (Table 1). The results suggest that the two negatively charged Glu-3893 and Glu-3967 residues have a critical role in transducing the functional effects of Ca2+ in RyR1.
The amino acid mutations could have potentially modified the RyR1 ion permeation properties, resulting in reduced K+ conductances and loss of Ca2+ conductances (3). However, measurement of the voltage dependence of variant channel currents indicated that K+ conductances were similar to WT (Table 1). In the presence of 10 mm luminal Ca2+, RyR1-E3893Q/E3967Q and T5001A variants conducted Ca2+ and maintained a Ca2+/K+ permeability ratio similar to WT. The permeability ratio of the remaining variants could not be determined due to low open channel probabilities.
Des Georges et al. (9) determined single-channel activities of the purified RyR1–FK506-binding protein 12.6 (FKBP12.6, Calstabin2) complex reconstituted in lipid bilayer vesicles. FKBP12.6 and the related FKBP12 bind with nanomolar affinity to RyRs and are considered constitutive members of RyR ion channel complexes. Dissociation of FKBP12 from the homotetrameric RyR1 complex increased channel open probability and induced substates in single-channel measurements (21). In other studies, full conductances were recorded (22, 23).
We addressed the presence of RyR1–FKBP12 complex by incubating membrane isolates for 30 min with 5 μm FKBP12 before addition to the cis bilayer chamber (23). We found that treatment with FKBP12 did not significantly alter channel open probability at 2 μm cytosolic of RyR1-WT (Po = 0.07 ± 0.02 and 0.11 ± 0.02 with and without FKBP12, respectively, n = 8–9) and RyR1-E3893Q/E3967Q (Po = 0.022 ± 0.011 and 0.030 ± 0.019 with and without FKBP12, respectively, n = 6–7).
RyR1 is activated by ATP and caffeine and inhibited by Mg2+ (3). Single-channel traces (Fig. 5A) and averaged -fold changes of Po values (Fig. 5B) show that at 0.01 μm cytosolic Ca2+, the initial addition of 5 mm caffeine increased RyR1-WT, RyR1-E3893Q/E3967Q, and E3893V/E3967V channel activities from their low Po values (Table 1). The addition of caffeine significantly increased channel activity using Student's t test. The subsequent addition of 2 mm ATP further increased channel activities (Fig. 5A). One-way but not two-way ANOVA (except for WT) indicated significant increases of Po after the addition of 5 mm caffeine and 2 mm ATP compared with control (Fig. 5B). RyR1-E3893Q/E3967Q was inhibited ∼10-fold by 0.25 mm Mg2+ (Fig. 5, C and D), whereas inhibition of RyR1-WT and RyR1-E3893V/E3967V was not determined due to very low open channel probability. The results suggest that RyR1-E3893Q/E3967Q and E3893V/E3967V maintained the ability of WT to be activated by caffeine and ATP and of E3893Q/E3967Q to be inhibited by Mg2+.
Figure 5.
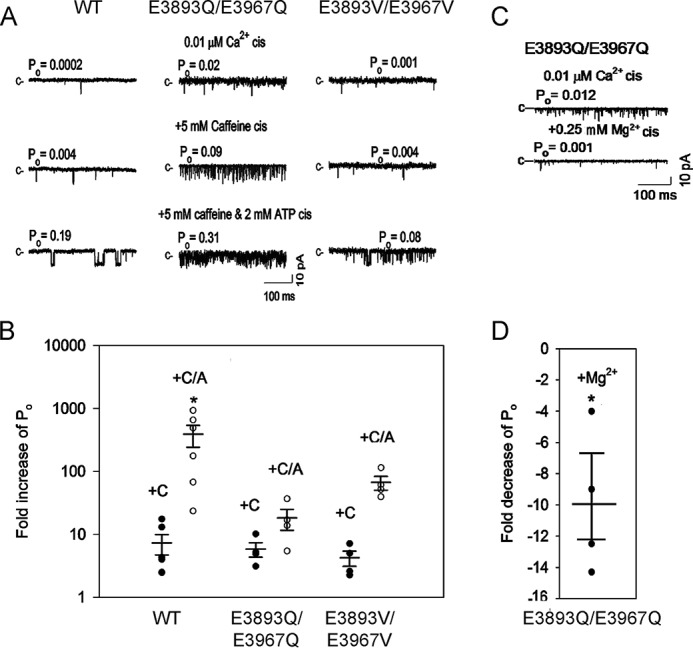
Effects of caffeine, ATP, and Mg2+ on RyR1-WT and E3893Q/E3967Q and E3893V/E3967V variant channel open probabilities in the absence of cytosolic Ca2+. A and C, representative single-channel currents are shown at −20 mV as downward deflections from the closed state (c−) in symmetrical 0.25 m KCl with 2 μm SR luminal Ca2+, 0.01 μm cytosolic Ca2+, and the indicated sequential additions of caffeine and ATP (A) and Mg2+ (C). B, -fold increase of normalized Po after the addition of caffeine (●, +C) and 5 mm caffeine plus 2 mm ATP (○, +C/A). D, -fold decrease of Po after the addition of 0.25 mm Mg2+. B and D, data are the mean ± S.E. (error bars) of 4–6 experiments. *, p < 0.05 compared with respective controls by two-way ANOVA followed by the Holm–Sidak method (B) and Student's t test (D).
Single RyR1-WT and RyR1-E3893Q/E3967Q, -E3893V/E3967V, and -T5001A variant channel activities were also determined under conditions comparable with those of open RyR1 channels in the presence of the three channel activators Ca2+, ATP, and caffeine (9). The addition of 2 mm ATP and 5 mm caffeine to cytosolic 30 μm Ca2+ raised single-channel activities of WT and variant channels (Fig. 6A). Averaged Po values of RyR1-WT increased 2.9-fold from 0.20 to 0.57, those of RyR1-E3893Q/E3967Q increased 70-fold from 0.003 to 0.207, those of RyR1-E3893V/E3967V increased 46-fold from 0.0003 to 0.014, and those of RyR1-T5001A increased 11-fold from 0.029 to 0.32 (Fig. 6B), confirming that all three variants retained ATP/caffeine activation.
Figure 6.
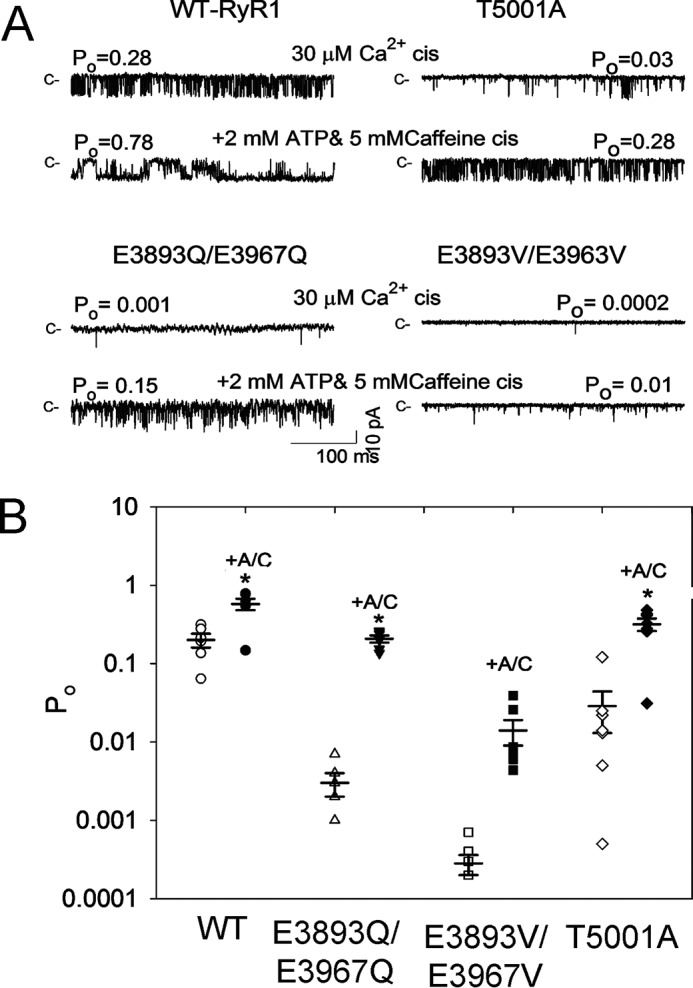
Effects of caffeine and ATP on RyR1-WT and RyR1-E3893Q/E3967Q, E3893V/E3967V, and T5001A variant channel open probabilities in presence of cytosolic Ca2+. A, representative single-channel currents are shown at −20 mV as downward deflections from the closed state (c−) in symmetrical 0.25 m KCl. Luminal Ca2+ was 2 μm. Free cytosolic Ca2+ was 30 μm (top traces) and 5 μm (as described under “Experimental procedures”) after the addition of 5 mm caffeine and 2 mm ATP (+A/C) (bottom traces). B, -fold increase of Po after the addition of 5 mm caffeine plus 2 mm ATP. Data are the mean ± S.E. (error bars) of six recordings. *, p < 0.05 compared with respective controls by two-way ANOVA followed by the Holm–Sidak method.
Discussion
Three amino acid residues that directly interact with Ca2+ in the high-affinity Ca2+-binding site were mutated and characterized. The results suggest that the high-affinity Ca2+-binding site plays a critical role in Ca2+-dependent activation of RyR1. Neutralization of negatively charged Glu-3893 and Glu-3967 resulted in loss of Ca2+-dependent activation of RyR1. Loss of activation appeared to be specific because other regulatory mechanisms, such as RyR1 ion permeation, inhibition by Mg2+ and elevated levels of Ca2+, and activation by ATP and caffeine, were maintained in single-channel measurements. RyR1-T5001A differentially affected RyR1 Ca2+ activation by shifting the Ca2+ activation/inactivation curve rightward relative to WT in single-channel measurements. Computational analysis using cryo-EM densities determined the structure of the Ca2+-binding sites of nominally Ca2+-free and Ca2+/ATP/caffeine-activated variant channels.
The Ca2+-binding site is located in the large cytoplasmic side of RyR1 ∼70 nm from the transmembrane effector channel sites. This suggests that transition from the nominally Ca2+-free closed to the Ca2+-activated open channel involves additional sites. RyR1-E4032A mutation outside the Ca2+-binding site (9) exhibited a reduced Ca2+-dependent channel activity in lipid bilayers (24) and depolarization-induced Ca2+ transients in myotubes (25). Activation of the RyR1-Δ183–4006 deletion variant by micromolar Ca2+ suggested a Ca2+-activation site different from the one identified by cryo-EM (26). Other regions shown to be involved in activation and inactivation of RyR1 by Ca2+ include residues in the transmembrane helix S2 (27), S4–S5 linker (28, 29), and pore-lining S6 helix of skeletal-muscle and cardiac-muscle RyR isoforms (30, 31).
Ca2+-induced Ca2+ release has been suggested to contribute little to the depolarization-induced Ca2+ release in adult mammalian skeletal muscle (15, 16). This raises the question of the physiological significance of Ca2+ activation of RyR1 seen with isolated RyR1s. We reported that the RyR1-G4941K mutation in the pore-lining S6 helix increased RyR1 sensitivity to luminal Ca2+ (30). This suggested that luminal Ca2+ activates RyR1 by accessing the cytosolic Ca2+-binding site in the open channel. Thus an intriguing possibility is that in skeletal muscle, voltage-dependent activation of Cav1.1 renders the Ca2+-binding site accessible to SR luminal Ca2+ passing through the channel and amplifies depolarization-induced Ca2+ release via a Ca2+-induced Ca2+ release mechanism.
Murayama et al. (32) reported results using RyR2 variants that correspond to RyR1-E3893 and -E3967 while this paper was under revision. Alanine substitution of glutamates caused loss of [3H]ryanodine binding to RyR2-E3847A and -E3921A. Decreasing size but not charge resulted in minimal [3H]ryanodine binding to RyR2-E3847D, whereas RyR2-E3921D had a Ca2+-binding profile similar to RyR2-WT. The results suggest that, as in RyR1, the corresponding Ca2+-binding site of RyR2 has a critical role in the regulation by Ca2+.
Replacement of negatively charged glutamate residues Glu-3893 and Glu-3967 with glutamine and valine resulted in loss of Ca2+-dependent activation of RyR1. Glu, Gln, and Val residues have a similar residue volume of 140, 147, and 139 Å3, respectively (17), suggesting that electrostatic interactions between Ca2+ and Glu residues contributed to transducing the functional effects of Ca2+.
We determined single-channel and structural properties of the Ca2+-binding site of RyR1-T5001A, -E3893Q/E3967Q, and -E3893V/E3967V variants under conditions similar to those reported by des Georges et al. (9). Single-channel activities and structure of the purified RyR1–FKBP12.6 (Calstabin2) complex were determined in the absence of Ca2+, the presence of Ca2+, the presence of ATP/caffeine, and the presence of Ca2+/ATP/caffeine (9). Cryo-EM data sets were divided into four classes in an attempt to account for conformational heterogeneity. Ca2+ or ATP/caffeine alone resulted in constricted closed-pore conformations comparable with the Ca2+-free closed states, even though a partial opening of channels was observed in the presence of 30 μm cytosolic Ca2+ or 2 mm ATP/5 mm caffeine in lipid bilayer studies. In contrast, in the combined presence of the three channel activators Ca2+, ATP, and caffeine, channels were nearly fully activated in single-channel recordings. Analysis of cryo-EM micrographs of Ca2+/ATP/caffeine-activated channels yielded two conformations with a dilated pore (class 1 and 2, PDB code 5TAL) and two with a constricted pore (class 3 and 4, PDB code 5TAQ), which suggested an equal number of open and closed channels. The data suggested that Ca2+ or ATP/caffeine alone primed RyR1 to open in the presence of the three channel activators (9). An alternative explanation was that in the presence of Ca2+ or ATP/caffeine, the number of open channels was too low to be scored as a separate class. In the combined presence of the three channel activators, the number of open channels was proposed to increase, as the equilibrium of channel openings/closings shifted toward the open state(s) (see Fig. S5 of des Georges et al. (9)).
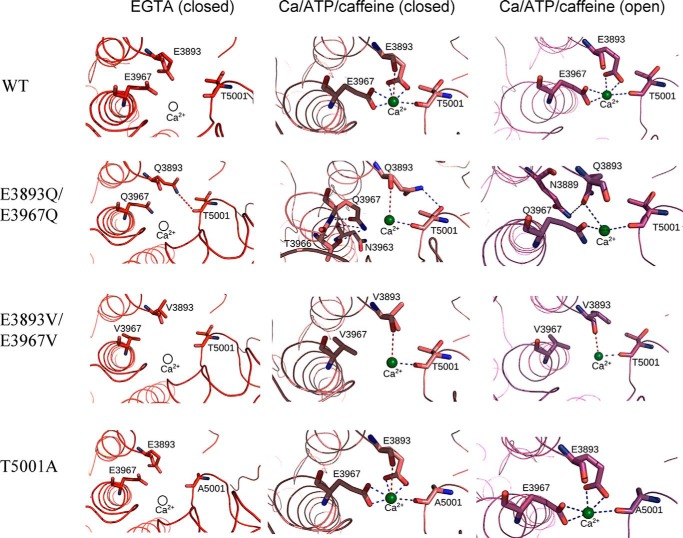
In our single-channel studies, membrane-bound RyR1s were incorporated into lipid bilayers. Fig. 7 shows the structure of the Ca2+-binding site of RyR1-WT and the in silico structures of RyR1-T5001A, -E3893Q/E3967Q, and -E3893V/E3967V variants. Low Po values and closely related Ca2+-binding structures were obtained for nominally Ca2+-free RyR1-WT and RyR1-E3893V/E3967V and -T5001A variants. A weak interaction between the Gln-3893 carboxyamide group and Thr-5001 carbonyl oxygen may have contributed to elevated Po of RyR1-E3893Q/E3967Q compared with WT and the two other variants (Fig. 7 and Table 2). The relative high Po values of RyR1-E3893Q/E3967Q compared with WT and single E3893Q and E3967Q variants also suggested the presence of distinct structural changes among the single- and double-site RyR1-E3893Q and -E3967Q variants.
Figure 7.
Interactions of Ca2+ with RyR1 residues in multiple functional states. Shown are the predicted interactions of Ca2+ with RyR1-WT and RyR1-E3893Q/E3967Q, E3893V/E3967V, and T5001A variants under nominally Ca2+-free (PDB code 5TB0) and Ca2+/ATP/caffeine (PDB code 5TAQ and 5TAL) conditions. Residues displaying electrostatic interactions with Ca2+ in the Ca2+/ATP/caffeine state are depicted in a stick representation, and the backbone of RyR1 is shown in a ribbon representation. Ca2+ is shown as a green sphere in Ca2+-bound states and as a hollow sphere in Ca2+-free states. Strong electrostatic interactions (d < 3.5 Å) are shown as blue dashed lines, and weak electrostatic interactions (3.5 < d < 4 Å) are shown as red dashed lines between Ca2+ and RyR1 residues. Distances less than 3.5 Å formed interresidue contacts and are shown as blue dashed lines.
Table 2.
Interactions between Ca2+ and amino acids
Strong electrostatic interactions (d < 3.5 Å) are shown in Fig. 7 as blue dashed lines and weak electrostatic interactions (3.5 < d < 4 Å) are shown as red dashed lines between Ca2+ and RyR1 residues. Distances less than 3.5 Å formed interresidue contacts and are shown as blue dashed lines.
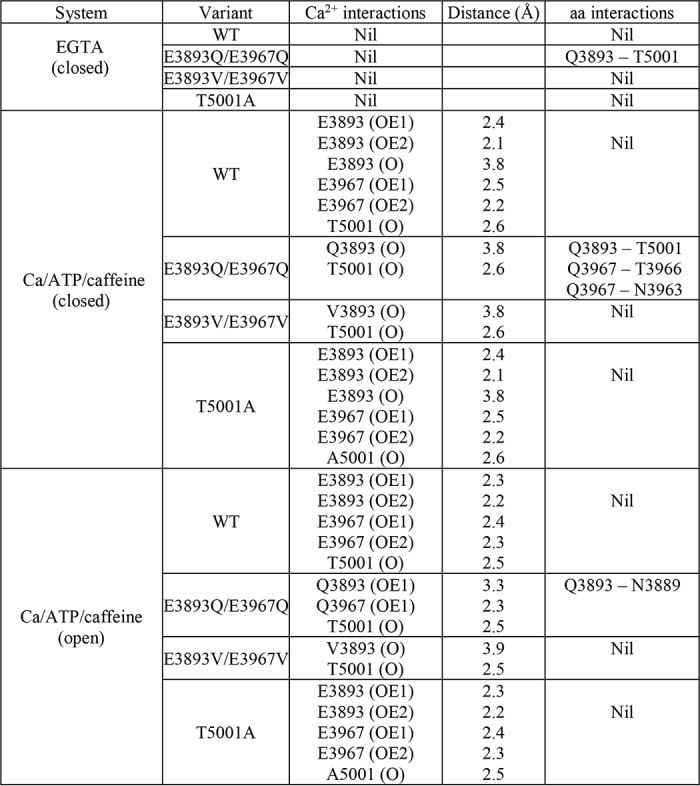
In the closed RyR1-WT channel in the presence of the three activating ligands Ca2+, ATP, and caffeine, Ca2+ strongly interacted with Glu-3893 and Glu-3967 carboxyl oxygens and Thr-5001 carbonyl oxygen and weakly with Glu-3893 carbonyl oxygen (Fig. 7 and Table 2). Small rearrangements in the interaction of Ca2+ with the three amino acid residues, including loss of weak interaction with Glu-3893 carbonyl oxygen, appeared to stabilize open WT channel states.
In RyR1-E3893V/E3967V closed- and open-channel structures, loss of a strong interaction between Ca2+ and Val resulted in a profound decrease of channel activity, reducing Po = 0.57 of WT to 0.014 for RyR1-E3893V/E3967V, which suggested a shift of the equilibrium of channel opening/closing toward the closed state(s). In the RyR1-E3893Q/E3967Q closed-channel structure, Ca2+ ceased to interact with the Gln carboxyamides. However, in contrast to E3893V/E3967V, a strong interaction with Gln carboxyamide oxygens was maintained in the open-channel states. Additionally, hydrogen bonds between three pairs of amino acid residues in the closed-channel state and one pair in the open-channel state were predicted (Fig. 7 and Table 2). We suggest that the additional interactions in RyR1-E3893Q/E3967Q stabilized the open state(s), decreasing Po = 0.57 of WT to 0.21 for RyR1-E3893Q/E3967Q rather than to 0.014 for RyR1-E3893V/E3967V (Fig. 6B, +A/C). Removal of the hydroxyl group and a change in side-chain volume of RyR1-T5001A did not noticeably alter the open and closed Ca2+-binding structures compared with WT. A shift of the Ca2+ activation/inactivation curve rightward from WT indicated that the T5001A mutation caused secondary structural changes in the presence of the three activating ligands Ca2+, ATP, and caffeine. Together, the results suggest that electrostatic interactions between Ca2+ and glutamate residues of the RyR1 Ca2+-binding site have a major role in transducing the functional effects of Ca2+ in RyR1.
In conclusion, our studies show that the removal of negative charges in a RyR1 Ca2+-binding site impairs activation of RyR1 by physiological concentrations of Ca2+ and suggests loss of binding to or reduced Ca2+ affinity of the site. Ca2+ binding to inhibitory sites is expected to interfere with any low-affinity Ca2+ activation of RyR1. Hence, a more detailed understanding of the mechanism(s) resulting in dysfunctional Ca2+ activation may depend on identifying and eliminating low-affinity RyR1 Ca2+-inactivation sites.
Experimental procedures
Materials
[3H]Ryanodine was obtained from PerkinElmer Life Sciences, protease and phosphatase inhibitors from Sigma-Aldrich, and phospholipids from Avanti Polar Lipids.
Preparation of variant channels
RyR1-E3893 and -E3967 single- and double-site variants were prepared using Pfu polymerase-based chain reaction, mutagenic oligonucleotides, and the QuikChange II site-directed mutagenesis kit (Agilent, Santa Clara, CA). RyR1-T5001A was prepared using a gene synthesis method (Genewiz, Inc., South Plainfield, NJ). WT and variant RyR1s were transiently expressed in HEK293 cells using jetPRIME (Polyplus, New York) according to the manufacturer's instructions. Transfected cells were harvested, and crude membrane isolates were prepared as described (33) in the presence of 1 mm GSSG.
SDS-PAGE and immunoblot analyses
Proteins in crude membrane isolates (20 μg of protein/lane) were separated using 3–12% acrylamide gradient SDS-PAGE, transferred overnight to nitrocellulose membranes, and probed using primary rabbit anti-RyR1 polyclonal antibody 6425 (30). Immunoblots were developed using peroxidase-conjugated anti-rabbit IgG, enhanced chemiluminescence, and quantified using the Bio-Rad ChemiDoc MP Imaging System and ImageQuantTL analysis software. Intensity of RyR1 variant bands on immunoblots was normalized to RyR1-WT intensities.
Cellular Ca2+ release
Release of stored Ca2+ was determined as described (34). Briefly, Ca2+ transients in HEK293 cells grown on coverslips were monitored with the fluorescence Ca2+ indicator Fluo-4. Cellular Ca2+ release was induced by the addition of ∼8 mm caffeine and monitored in individual cells using the EasyRatioPro algorithm (Photon Technology International, Lawrenceville, NJ).
[3H]Ryanodine binding
Ryanodine binds with high specificity to RyR1 and is widely used to probe RyR activity and content (20). [3H]Ryanodine binding was measured by incubating crude membrane isolates for 20–24 h at 24 °C in 20 mm imidazole, pH 7.0, 0.25 m KCl, 3 nm [3H]ryanodine, and protease inhibitors at the indicated free Ca2+ concentrations. Nonspecific binding was determined in the presence of 10 μm unlabeled ryanodine. Amounts of bound [3H]ryanodine were determined using a filtration assay (30).
Single-channel recordings
Membrane isolates were added to the cis (cytosolic) chamber of the bilayer apparatus (35). Single-channel recordings took advantage of the impermeability of RyRs to Cl− and high conductance of K+ relative to Ca2+. Channel activities were recorded using 0.25 m KCl, 20 mm KHEPES, pH 7.4, on both sides of the bilayer, 2 μm trans (SR luminal), and the indicated cis (cytosolic) Ca2+ concentrations. The trans side of the bilayer was defined as ground. Electrical signals were filtered at 2 kHz, digitized at 10 kHz, and analyzed at 50% threshold setting (35). Data acquisition and analysis of 2-min recordings were performed using commercially available software (pClamp, Axon Instruments). Channel activities were also recorded in symmetrical 0.25 m KCl solution with 10 mm Ca2+ in the trans bilayer chamber. The reversal potential was measured to determine Ca2+/K+ permeability ratios using a modified form of the Goldman–Hodgkin–Katz equation (35).
Computational methods
The effects of RyR1 mutations on Ca2+ binding were modeled by performing in silico amino acid substitutions at chosen positions in three RyR1 cryo-EM structures solved under different physiological conditions (PDB entries 5TB0, 5TAQ, and 5TAL) (9). The position of Ca2+ in the Ca2+-free RyR1 structure (PDB code 5TB0) was simulated by superposing the closed RyR1 structure (PDB code 5TAQ). In silico mutations at chosen residue positions in RyR1 structures were performed using the Mutagenesis tool in the PyMOL molecular visualization suite (36). We chose side-chain rotamers for substituted amino acids at selected positions based on backbone dependence and minimum clash score. For further refinement, side-chain optimizations were executed on mutated RyR1 structures using GROMACS version 4 (37). Optimized structures were visualized and analyzed for loss or gain of Ca2+ interactions with surrounding residues using PyMOL (36).
Biochemical assays and data analysis
Free Ca2+ concentrations were obtained by including in the solutions the appropriate amounts of Ca2+ and EGTA using a Ca2+-selective electrode. Free Ca2+ concentrations following the addition of 2 mm ATP (Fig. 6) were calculated using MaxChelator and constants from Theo Schoenmakers' Chelator. Differences between samples were analyzed using SigmaPlot 11 Statistics. Comparison of two groups was determined by Student's t test or Mann–Whitney rank sum test (when data failed the normality test). Three sample groups or more were determined by one-way ANOVA with Tukey's test, Kruskal–Wallis one-way ANOVA on ranks followed by Dunn's method (when the normality test failed in one-way ANOVA), or two-way ANOVA using the Holm–Sidak method, where p < 0.05 was considered significant.
Author contributions
L. X., N. Y., and G. M. designed the experiments; L. X., J. S. C., and D. A. P. performed the experiments, V. R. C. and N. V. D. performed computational analysis of data, and N. Y. and G. M. wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
This work was supported by National Institutes of Health Grants AR018687 (to G. M.) and P20GM103499 and UL1TR001450 (to N. Y.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- RyR
- ryanodine receptor ion channel
- FKBP
- FK506-binding protein
- ANOVA
- analysis of variance
- PDB
- Protein Data Bank
- IP3
- inositol 1,4,5-trisphosphate.
References
- 1. Franzini-Armstrong C., and Protasi F. (1997) Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol. Rev. 77, 699–729 10.1152/physrev.1997.77.3.699 [DOI] [PubMed] [Google Scholar]
- 2. Lanner J. T., Georgiou D. K., Joshi A. D., and Hamilton S. L. (2010) Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2, a003996 10.1101/cshperspect.a003996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meissner G. (2017) The structural basis of ryanodine receptor ion channel function. J. Gen. Physiol. 149, 1065–1089 10.1085/jgp.201711878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zalk R., Lehnart S. E., and Marks A. R. (2007) Modulation of the ryanodine receptor and intracellular calcium. Annu. Rev. Biochem. 76, 367–385 10.1146/annurev.biochem.76.053105.094237 [DOI] [PubMed] [Google Scholar]
- 5. Yan Z., Bai X., Yan C., Wu J., Li Z., Xie T., Peng W., Yin C., Li X., Scheres S. H., Shi Y., and Yan N. (2015) Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature 517, 50–55 10.1038/nature14063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zalk R., Clarke O. B., des Georges A., Grassucci R. A., Reiken S., Mancia F., Hendrickson W. A., Frank J., and Marks A. R. (2015) Structure of a mammalian ryanodine receptor. Nature 517, 44–49 10.1038/nature13950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peng W., Shen H., Wu J., Guo W., Pan X., Wang R., Chen S. R., and Yan N. (2016) Structural basis for the gating mechanism of the type 2 ryanodine receptor RyR2. Science 354, aah5324 10.1126/science.aah5324 [DOI] [PubMed] [Google Scholar]
- 8. Efremov R. G., Leitner A., Aebersold R., and Raunser S. (2015) Architecture and conformational switch mechanism of the ryanodine receptor. Nature 517, 39–43 10.1038/nature13916 [DOI] [PubMed] [Google Scholar]
- 9. des Georges A., Clarke O. B., Zalk R., Yuan Q., Condon K. J., Grassucci R. A., Hendrickson W. A., Marks A. R., and Frank J. (2016) Structural basis for gating and activation of RyR1. Cell 167, 145–157.e17 10.1016/j.cell.2016.08.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Samsó M., Feng W., Pessah I. N., and Allen P. D. (2009) Coordinated movement of cytoplasmic and transmembrane domains of RyR1 upon gating. PLoS Biol. 7, e85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei R., Wang X., Zhang Y., Mukherjee S., Zhang L., Chen Q., Huang X., Jing S., Liu C., Li S., Wang G., Xu Y., Zhu S., Williams A. J., Sun F., and Yin C. C. (2016) Structural insights into Ca2+-activated long-range allosteric channel gating of RyR1. Cell Res. 26, 977–994 10.1038/cr.2016.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bai X. C., Yan Z., Wu J., Li Z., and Yan N. (2016) The central domain of RyR1 is the transducer for long-range allosteric gating of channel opening. Cell Res. 26, 995–1006 10.1038/cr.2016.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patterson R. L., Boehning D., and Snyder S. H. (2004) Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu. Rev. Biochem. 73, 437–465 10.1146/annurev.biochem.73.071403.161303 [DOI] [PubMed] [Google Scholar]
- 14. Schneider M. F., and Chandler W. K. (1973) Voltage dependent charge movement in skeletal muscle: a possible step in excitation-contraction coupling. Nature 242, 244–246 10.1038/242244a0 [DOI] [PubMed] [Google Scholar]
- 15. Endo M. (2009) Calcium-induced calcium release in skeletal muscle. Physiol. Rev. 89, 1153–1176 10.1152/physrev.00040.2008 [DOI] [PubMed] [Google Scholar]
- 16. Ríos E. (2018) Calcium-induced release of calcium in muscle: 50 years of work and the emerging consensus. J. Gen. Physiol. 150, 521–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsai J., Taylor R., Chothia C., and Gerstein M. (1999) The packing density in proteins: standard radii and volumes. J. Mol. Biol. 290, 253–266 10.1006/jmbi.1999.2829 [DOI] [PubMed] [Google Scholar]
- 18. Rousseau E., Ladine J., Liu Q. Y., and Meissner G. (1988) Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch. Biochem. Biophys. 267, 75–86 10.1016/0003-9861(88)90010-0 [DOI] [PubMed] [Google Scholar]
- 19. Smith J. S., Coronado R., and Meissner G. (1985) Sarcoplasmic reticulum contains adenine nucleotide-activated calcium channels. Nature 316, 446–449 10.1038/316446a0 [DOI] [PubMed] [Google Scholar]
- 20. Sutko J. L., and Airey J. A. (1996) Ryanodine receptor Ca2+ release channels: does diversity in form equal diversity in function? Physiol. Rev. 76, 1027–1071 10.1152/physrev.1996.76.4.1027 [DOI] [PubMed] [Google Scholar]
- 21. Brillantes A. B., Ondrias K., Scott A., Kobrinsky E., Ondriasová E., Moschella M. C., Jayaraman T., Landers M., Ehrlich B. E., and Marks A. R. (1994) Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell 77, 513–523 10.1016/0092-8674(94)90214-3 [DOI] [PubMed] [Google Scholar]
- 22. Barg S., Copello J. A., and Fleischer S. (1997) Different interactions of cardiac and skeletal muscle ryanodine receptors with FK-506 binding protein isoforms. Am. J. Physiol. 272, C1726–C1733 10.1152/ajpcell.1997.272.5.C1726 [DOI] [PubMed] [Google Scholar]
- 23. Mei Y., Xu L., Kramer H. F., Tomberlin G. H., Townsend C., and Meissner G. (2013) Stabilization of the skeletal muscle ryanodine receptor ion channel-FKBP12 complex by the 1,4-benzothiazepine derivative S107. PLoS One 8, e54208 10.1371/journal.pone.0054208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fessenden J. D., Chen L., Wang Y., Paolini C., Franzini-Armstrong C., Allen P. D., and Pessah I. N. (2001) Ryanodine receptor point mutant E4032A reveals an allosteric interaction with ryanodine. Proc. Natl. Acad. Sci. U.S.A. 98, 2865–2870 10.1073/pnas.041608898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Brien J. J., Feng W., Allen P. D., Chen S. R., Pessah I. N., and Beam K. G. (2002) Ca2+ activation of RyR1 is not necessary for the initiation of skeletal-type excitation-contraction coupling. Biophys. J. 82, 2428–2435 10.1016/S0006-3495(02)75586-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhat M. B., Zhao J., Takeshima H., and Ma J. (1997) Functional calcium release channel formed by the carboxyl-terminal portion of ryanodine receptor. Biophys. J. 73, 1329–1336 10.1016/S0006-3495(97)78166-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gomez A. C., and Yamaguchi N. (2014) Two regions of the ryanodine receptor calcium channel are involved in Ca2+-dependent inactivation. Biochemistry 53, 1373–1379 10.1021/bi401586h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murayama T., Kurebayashi N., Oba T., Oyamada H., Oguchi K., Sakurai T., and Ogawa Y. (2011) Role of amino-terminal half of the S4-S5 linker in type 1 ryanodine receptor (RyR1) channel gating. J. Biol. Chem. 286, 35571–35577 10.1074/jbc.M111.255240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramachandran S., Chakraborty A., Xu L., Mei Y., Samsó M., Dokholyan N. V., and Meissner G. (2013) Structural determinants of skeletal muscle ryanodine receptor gating. J. Biol. Chem. 288, 6154–6165 10.1074/jbc.M112.433789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu L., Mowrey D. D., Chirasani V. R., Wang Y., Pasek D. A., Dokholyan N. V., and Meissner G. (2018) G4941K substitution in the pore-lining S6 helix of the skeletal muscle ryanodine receptor increases RyR1 sensitivity to cytosolic and luminal Ca2+. J. Biol. Chem. 293, 2015–2028 10.1074/jbc.M117.803247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun B., Guo W., Tian X., Yao J., Zhang L., Wang R., and Chen S. R. (2016) The cytoplasmic region of inner helix S6 is an important determinant of cardiac ryanodine receptor channel gating. J. Biol. Chem. 291, 26024–26034 10.1074/jbc.M116.758821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murayama T., Ogawa H., Kurebayashi N., Ohno S., Horie M., and Sakurai T. (2018) A tryotophan residue in the caffeine-binding site of the ryanodine receptor regulates Ca2+ sensitivity. Commun. Biol. 1, 98 10.1038/s42003-018-0103-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gao L., Tripathy A., Lu X., and Meissner G. (1997) Evidence for a role of C-terminal amino acid residues in skeletal muscle Ca2+ release channel (ryanodine receptor) function. FEBS Lett. 412, 223–226 10.1016/S0014-5793(97)00781-3 [DOI] [PubMed] [Google Scholar]
- 34. Wang Y., Xu L., Duan H., Pasek D. A., Eu J. P., and Meissner G. (2006) Knocking down type 2 but not type 1 calsequestrin reduces calcium sequestration and release in C2C12 skeletal muscle myotubes. J. Biol. Chem. 281, 15572–15581 10.1074/jbc.M600090200 [DOI] [PubMed] [Google Scholar]
- 35. Xu L., Wang Y., Gillespie D., and Meissner G. (2006) Two rings of negative charges in the cytosolic vestibule of type-1 ryanodine receptor modulate ion fluxes. Biophys. J. 90, 443–453 10.1529/biophysj.105.072538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. DeLano W. L. (2011) The PyMOL Molecular Graphics System, version 1.5.0.4, Schroedinger, LLC, New York [Google Scholar]
- 37. Hess B., Kutzner C., van der Spoel D., and Lindahl E. (2008) GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comp. 4, 435–447 10.1021/ct700301q [DOI] [PubMed] [Google Scholar]