Abstract
Influenza viruses cause contagious respiratory infections, resulting in significant economic burdens to communities. Production of influenza-specific Igs, specifically IgGs, is one of the major protective immune mechanisms against influenza viruses. In humans, N-glycosylation of IgGs plays a critical role in antigen binding and effector functions. The ferret is the most commonly used animal model for studying influenza pathogenesis, virus transmission, and vaccine development, but its IgG structure and functions remain largely undefined. Here we show that ferret IgGs are N-glycosylated and that their N-glycan structures are diverse. Using a comprehensive strategy based on MS and ultra-HPLC analyses in combination with exoglycosidase digestions, we assigned 42 N-glycan structures in ferret IgGs. We observed that N-glycans of ferret IgGs consist mainly of complex-type glycans, including some high-mannose and hybrid glycans, similar to those observed in human IgG. The complex-type glycans of ferret IgGs were primarily core-fucosylated. Furthermore, a fraction of N-glycans carried bisecting GlcNAc. Ferret IgGs also had a minor fraction of glycans carrying α2–6Neu5Ac(s). We noted that, unlike human IgG, ferret IgGs have αGal epitopes on some N-glycans. Interestingly, influenza A infection caused prominent changes in the N-glycans of ferret IgG, mainly because of an increase in bisecting GlcNAc and F1A2G0 and a corresponding decrease in F1A2G1. This suggests that the glycosylation of virus-specific IgG may play a role in its functionality. Our study highlights the need to further elucidate the structure–function relationships of IgGs in universal influenza vaccine development.
Keywords: N-linked glycosylation, IgG, glycosylation, influenza, MS, ferret, fucosylation, immunity, UPLC, vaccine development
Introduction
Influenza epidemics are a major public health concern worldwide. Currently, seasonal influenza vaccination is the primary defense mechanism to prevent virus transmission in humans. However, influenza vaccine effectiveness varies significantly from season to season, with poor performance noted especially in recent years (1–4). For instance, the interim vaccine effectiveness was estimated as low as 33% for all influenza viruses and only as 10% for influenza A (H3N2) during the 2017 flu season in the southern hemisphere (4).
The ferret is the most commonly used animal model for studying influenza pathogenesis, virus transmission, and vaccine development. This is because the ferret has dominant expression of α2–6SA-Gal structure and a minor presence of α2–3–linked sialic acids in the respiratory tract (5), the receptors that are preferred by human-origin and avian-origin influenza viruses, respectively. Influenza-infected ferrets exhibit clinical signs and symptoms similar to those observed in humans (6). Because of this human-like respiratory physiology in ferrets, antisera collected from influenza-infected experimental ferrets have been used as reference standards to monitor virus antigenic changes for annual influenza vaccine strain selection (7–10). However, ferret antibodies have also been shown to target antigenic epitopes on influenza viruses differently than human antibodies (11–18). This difference between ferret immunity and human immunity is one of the factors responsible for influenza vaccine strain mismatch (7, 19). Our current knowledge regarding the ferret immune process on influenza viruses is limited because of a lack of systemic investigations of the ferret genome and immunity. Understanding ferret antibody structures and functionality is essential for influenza vaccine development and improvement of vaccine strain selection.
It has been recognized that glycosylation affects antibody affinity for Fc receptors and modulates antibody effector functions in humans (20–22). However, little is known about the glycosylation patterns of ferret antibodies. To fill this knowledge gap, we performed in-depth profiling of N-glycans on IgGs purified from ferrets with and without infection of H3N2 influenza A virus using MALDI-TOF/MS, hydrophilic interaction liquid chromatography (HILIC),4 ultra-performance liquid chromatography (UPLC), and nanoLC-MS/MS in combination with exoglycosidase treatment. Our study indicates that, despite having the overall N-glycan structures resembling human IgG, ferret IgG also shows different glycosylation patterns that can be modulated by influenza infections. These results are important not only to help optimize strategies to improve influenza vaccine strain selection but also to facilitate the development of new influenza vaccines.
Results
Ferret IgG is N-glycosylated
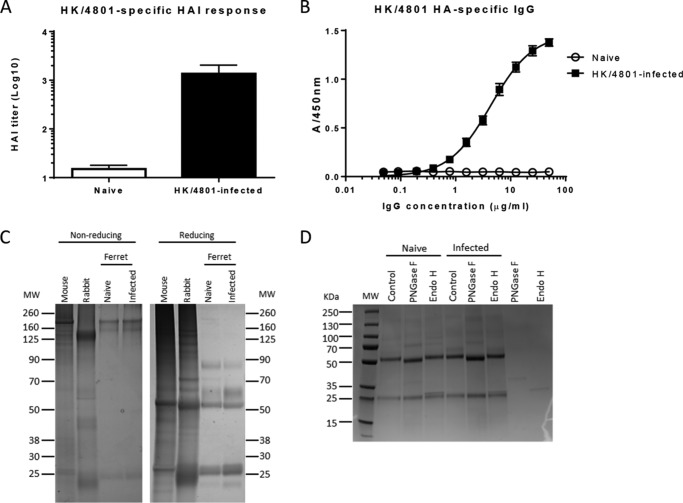
The ferret is a relevant animal model for influenza-related research. Understanding ferret antibody patterns and immune responses can also help to elucidate immune mechanisms in humans. Infection with seasonal H3N2 HK/4801 resulted in elevated antibody responses in ferrets (Fig. 1, A and B). In SDS-PAGE, IgGs purified from naïve or infected ferrets showed similar migration patterns as a nonspecific mouse mAb and rabbit polyclonal antibody, including heavy chains (50 kDa) and light chains (25 kDa) under reducing conditions (Fig. 1C). Although the full-length sequence of ferret IgG is unavailable in the database, one report (23) showed a partial sequence of ferret IgG with one sequon (potential N-glycosylation site) in its heavy chain. To experimentally confirm that ferret IgG is an N-glycosylated protein, we treated purified IgG samples from naïve and HK/4801-infected ferrets with the peptide-N-glycosidase F (PNGase F), which cleaves off all types of N-glycans except for the core α1,3-fucosylated ones, and endoglycosidase H (Endo H), which hydrolyzes the inner bichitose of high-mannose and hybrid N-glycans. On SDS-PAGE under reducing conditions, the ferret IgG heavy chains from both naïve and infected animals migrated at ∼ 52 kDa and shifted to a lower molecular weight band (∼50 kDa) after treatment with PNGase F but not with Endo H treatment (Fig. 1D). The light chains with ∼25 kDa from both samples remained unchanged on the SDS-PAGE gel before and after these enzyme treatments. These results demonstrate that the ferret IgG heavy chains, but not light chains, are N-glycosylated and that the N-glycans of ferret IgG are mainly complex-type. Thus, ferret IgG is an N-glycosylated protein.
Figure 1.
Influenza-specific antibody titers and SDS-PAGE analysis of IgGs purified from naïve and infected ferrets. A, influenza-specific hemagglutinin inhibition (HAI) titers in pooled sera collected from four ferrets before (naïve) or after infection (infected) with H3N2 A/Hong Kong/4801/2014 (HK/4801). B, HK/4801 HA-specific antibody titers in IgGs purified from pooled sera of four naïve or four infected ferrets. C, SDS-PAGE analysis of purified IgGs from naïve and infected ferrets along with nonspecific mouse monoclonal IgG and rabbit polyclonal IgG under both nonreducing and reducing conditions. MW, molecular weight. D, purified ferret IgGs were denatured, treated with PNGase F to remove all N-glycans or Endo H to cleave at the inner bichitose of high-mannose and hybrid N-glycans, and separated on SDS-PAGE under reducing conditions. As controls, the enzymes Endo F and Endo H were also loaded on SDS-PAGE, and electrophoresis was run to help with identification of bands in the treated samples.
MALDI-TOF/MS and nanoLC-MS/MS analyses revealed 42 different N-glycan structures in ferret IgGs
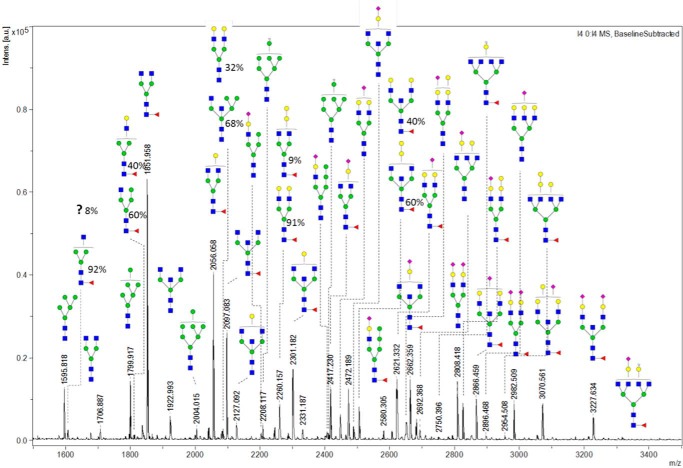
Our advanced analytics allow detection of multiple glycan species in the ferret antibodies, even those at low abundance. In MALDI-TOF/MS spectra (Fig. 2), the monoisotopic m/z values of (M + Na)+ ions of N-glycans were used to search for their corresponding compositions and possible structures. We found a great diversity in glycan profiles. In total, 37 unique m/z values from permethylated N-glycans from naive ferret IgG were identified. The 42 detailed structures and relative abundance of each N-glycan were determined by the following nanoLC-MS/MS analysis, which gave five more different N-glycans isomers (Fig. 2).
Figure 2.
MALDI-TOF/MS spectrum of the N-glycans released from IgGs of naïve ferrets. N-glycans released from the purified IgGs of pooled sera from four naïve ferrets were reduced, permethylated, and measured by MALDI-TOF-MS. The composition of the glycans was determined through a database search with GlycoMod. The corresponding detailed structures were investigated through HCD and CID fragmentation experiments. Among these structures, five pairs of isomers were identified by their differing retention times on C18 reverse-phase nanoLC, and their relative abundance was determined by the intensity of monoisotopic m/z peaks in LC-MS.
To determine the N-glycan structures, nanoLC-MS/MS analysis of reducing end–reduced and permethylated N-glycans was applied. In MS/MS measurements, both collision-induced dissociation (CID) and high-energy collisional dissociation (HCD) experiments were utilized to analyze the same samples (24). CID and HCD experiments are complementary. In general, heavier fragment ions from stepwise glycosidic cleavage are dominant in CID spectra, whereas monosaccharide oxonium ions and their dissociated fragments are dominant in HCD spectra. HCD has no low-mass cutoff as CID does, so HCD spectra are not limited by the “one-third rule” that applies to CID spectra. In HCD, terminal monosaccharide residues are easily assigned. In addition, fragment ions of reducing end–reduced and permethylated glycans produced in HCD are very informative to assign terminal structures. Nonfucosylated reducing end GlcNAc ions at m/z 294.1911 or fucosylated reducing end GlcNAc ions at m/z 280.1755, nonreducing end terminal GlcNAc ions at m/z 260.1492, and internal GlcNAc ions at m/z 246.1336 can be differentiated and assigned (Fig. S1). These fragmentation spectra can be termed “signatures of GlcNAc residues in glycans.”
The high resolving power of the Orbitrap MS allows unambiguous assignment. For instance, the ion at m/z of 280.1177 and the ion at m/z of 280.1739 with a difference of 0.06 were unambiguously separated (Fig. S2). The former ion at m/z 280.1177 resulted from the dissociation of the oxonium ion of permethylated sialic acid residue (25) and is slightly lighter than 280.1739, and the unique fragment ion resulted from core-fucosylated GlcNAc at the reducing end.
Some m/z values of the molecular ions indicated compositions with possible N-glycan structures containing bisecting GlcNAc. To confirm them, we performed HCD on those N-glycan molecular ions. The HCD spectra of two closely related structures with m/z 1829.97 ((M + H)+, composition (HexNAc)2 (deoxyhexose)1 + (Man)3(GlcNAc)2), and 2075.10 ((M + H)+, composition (HexNAc)3 (deoxyhexose)1 + (Man)3(GlcNAc)2) were compared (Fig. S3). The glycan with the (M + H)+ ion at m/z 1829.97 (Fig. S3, left panel) without bisecting GlcNAc has a specific fragment ion at m/z 640.3175, which can be further decomposed to a lighter fragment with the loss of HOCH3 (−32). In comparison, the glycan with bisecting GlcNAc with the (M + H)+ ion at m/z 2075.10 (Fig. S3, right panel) has a specific fragment ion at m/z of 681.3440, which can be further decomposed to a lighter fragment ion at m/z 649.3157 with the loss of HOCH3 (−32). This fragment ion was not seen in the CID spectrum, possibly because of the higher collisional energy of HCD than CID. Also, this fragment ion was verified and used to identify multiple bisecting GlcNAc-containing glycans and was proven to be very useful and specific for the identification of bisecting structures.
In complementarity to HCD, CID experiments are useful to identify branching structures. The structure with the (M + H)+ ion at m/z 2558.36 (composition (Hex)3 (HexNAc)1 (deoxyhexose)1 (NeuAc)1 + (Man)3(GlcNAc)2) has no branching on the α1–6 Man arm of the trimannosyl core, as shown in both B ions (Fig. S4, top right panel) and B/Y ions (Fig. S4, bottom right panel). A similar fragmentation pattern was also observed for the nonfucosylated hybrid glycan with the (M + H)+ ion at m/z 2384.27 (composition (Hex)3 (HexNAc)1 (NeuAc)1 + (Man)3(GlcNAc)2) (Fig. S4B). These unusual hybrid structures were confirmed by α-mannosidase treatments.
Isomeric separation of permethylated glycans was partially achieved by C18 nanoLC, and the isomers were identified by MS/MS through nanoLC-MS/MS. The structures with (M + H)+ at m/z 2483.001 (composition (Hex)2 (HexNAc)3 (deoxyhexose)1 + (Man)3(GlcNAc)2) contain a possible Galα1–3Gal and/or its corresponding isomer with two separated terminal β1–3/4–linked galactoses on trimannosyl core α1–3 and α1–6 Man arms, respectively. In its HCD spectra, fragment ions at m/z 668 m/z 464 were used to assign terminal Galα1-3Galβ1–4GlcNAc and Galβ1–4GlcNAc, respectively (Fig. S5), and the linkages were verified by exoglycosidase experiments. According to the peak intensities, the relative abundance of each isomer was determined to be 40% for bF1A2G2 and 60% for bF1A2Galα1–3Gal.
Naïve ferret IgGs had 75% of N-glycans carrying core α1–6fucose (Fig. 2). It is known that core fucosylation of IgGs is important for its Fc-mediated effector functions (26); therefore, the fucosylated N-glycan structures were carefully examined. To determine the position of fucosylation, permethylated glycans were used because it has been reported that permethylation can make the structure stable and eliminate the migration of fucose residues of native and reducing end-labeled glycans during measurement by MS (27, 28). In addition, the reduced reducing ends of all N-glycans also provide more information to clarify the structures. The fragment ion at m/z 280.1755 was used exclusively to assign core-fucosylated reducing-end GlcNAc (Fig. S1). Without fucosylation, a fragment ion with the m/z shifted to 294.1911 was observed instead, a very distinctive feature in HCD spectra (Fig. S1). It turned out that only core- and not antenna-fucosylated N-glycans were found in ferret IgGs, the same as in human IgGs.
A minor fraction of ferret IgG carried N-glycans with either mono- or disialylation. Just as in humans, only N-acetylneuraminic acid (Neu5Ac) was detected on ferret IgGs; no N-glycolylneuraminic acid (Neu5Gc) was found. This concurs with the recent discovery that ferrets have a gene mutation of CMAH (CMP-N-acetylneuraminic acid hydroxylase), the same as humans, which inactivates the hydrolase converting Neu5Ac to Neu5Gc (29). A summary of ferret IgG N-glycans with their structures and corresponding relative abundances is shown in Table S1. As for the site(s) of N-glycans besides the one in the Fc region of ferret IgG, it is unknown whether there are additional N-glycans in the Fab region in a subpopulation of, if not in all, total IgG.
HILIC UPLC and LC-MS accompanied by the same HILIC column quantified relative abundances of each N-glycan of ferret IgG
MALDI-TOF/MS analysis of permethylated N-glycans measures the relative abundance of each glycan of ferret IgGs. For a precise quantification, ferret N-glycans were released by PNGase F, labeled by RapiFluor-MS, and then analyzed using HILIC UPLC equipped with a fluorescence detector. More than 26 peaks with different fluorescence intensities were detected, and in some peaks, more than one glycan was confirmed by LC-MS (Fig. 3 and Table S2). In addition, some isomers were separated. Interestingly, related isomers presented as an adjacent pair of peaks in the chromatogram, such as peak 6 and p6a, P7 and P8, and P9 and P10, respectively. Based on other studies (30, 31), the component Galβ1–4GlcNAc in P6 and glycan peaks P7 and P9 were assigned to the α1–6 Man arm of the trimannosyl core. Correspondingly, the component Galβ1–4GlcNAc in glycan peaks 6a, 8, and 10 was assigned to the α1–3 Man arm of the trimannosyl core. In addition, two of the isomers (P11 and P12) of the high-mannose-type glycan Man6 were separated and identified: the terminal α1–2 linked mannose to inner α1–6 Man for P11 and inner α1–3 Man for P12, respectively.
Figure 3.
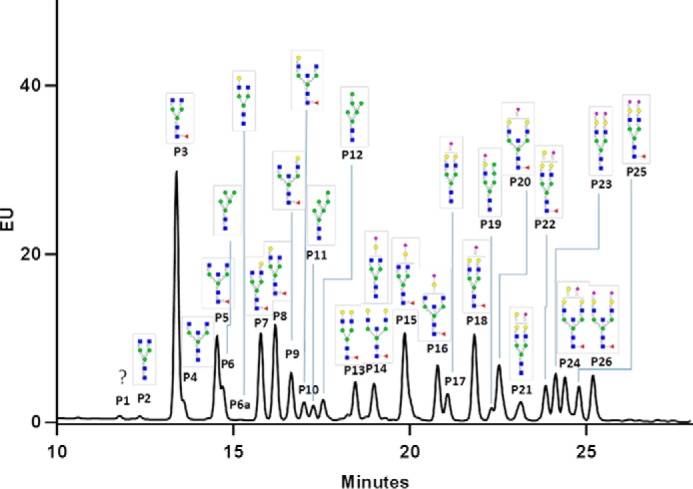
HILIC UPLC chromatogram of RapiFluor-MS–labeled N-glycans of naïve ferret IgG. N-glycans released from the purified IgGs pooled from four naïve ferrets were labeled with RapiFluor-MS and separated by HILIC UPLC monitored with fluorescence. The N-glycan structures in each peak were assigned according to the HILIC–nanoLC-MS/MS. EU, emission units.
A minor fraction of ferret IgG N-glycans contains an αGal epitope, whereas type II chain Galβ1–4GlcNAc occurs on all complex N-glycans
To determine the linkage of nonreducing end saccharide residues, exoglycosidases were used to selectively remove monosaccharides of specific linkages. The removal causes decreased abundance or even disappearance of specific glycans, accompanied by a shift and an increasing abundance of related lighter glycans or even the appearance of a new glycan on MALDI-TOF MS spectra. As demonstrated in an overview (Fig. S6), structures with terminal Gal-GlcNAc were susceptible to β1–4 galactosidase but not β1–3 or α1–3galactosidase, confirming β1–4 linked Gals. In contrary, structures with terminal Gal-Gal were susceptible to α1–3galactosidase, indicative of the presence of a Galα1–3Gal motif.
The presence of the α-galactosyl (αGal) epitope and β1–4 galactose was confirmed by galactosidase treatments, as β1–3galactosidase did not cause any change of the spectra, whereas β1–4 and α1–3 galactosidase treatments caused a decrease in structures with terminal Galβ1–4GlcNAc and Galα-3Gal, respectively. The N-glycan (m/z of 3070) disappeared after treatment with α1–3galactosidase (Fig. S7) but not with β1–3 or β1–4galactosidase, demonstrating that this glycan contained a Galα1–3Gal epitope. The subsequent hexose in the structure was not removed by this α-glycosidase, which resulted in the accumulation of a glycan (m/z of 2866). In contrast, the terminal Gal of the glycan (m/z of 2866) was cleaved by β1–4 galactosidase, demonstrating that it was a type II chain, Galβ-4GlcNAc motif. These principles were applied to the assignment of other N-glycans with relevant structures from ferret IgG. These data demonstrated that a small fraction of ferret IgG contains the αgalactosyl epitope.
To further identify the αGal-containing N-glycans, the RapiFluor-tagged N-glycans were also treated with α1–3galactosidase and analyzed using HILIC UPLC fluorescence. After the loss of one terminal α1–3–linked Gal, peaks 21, 22, and 24 disappeared, and the intensity of the corresponding peaks 17, 18, and 20 increased (Fig. 4). This result demonstrated that peaks 21, 22, and 24 were derived from α1–3galactosylated peaks 17, 18, and 20, respectively.
Figure 4.
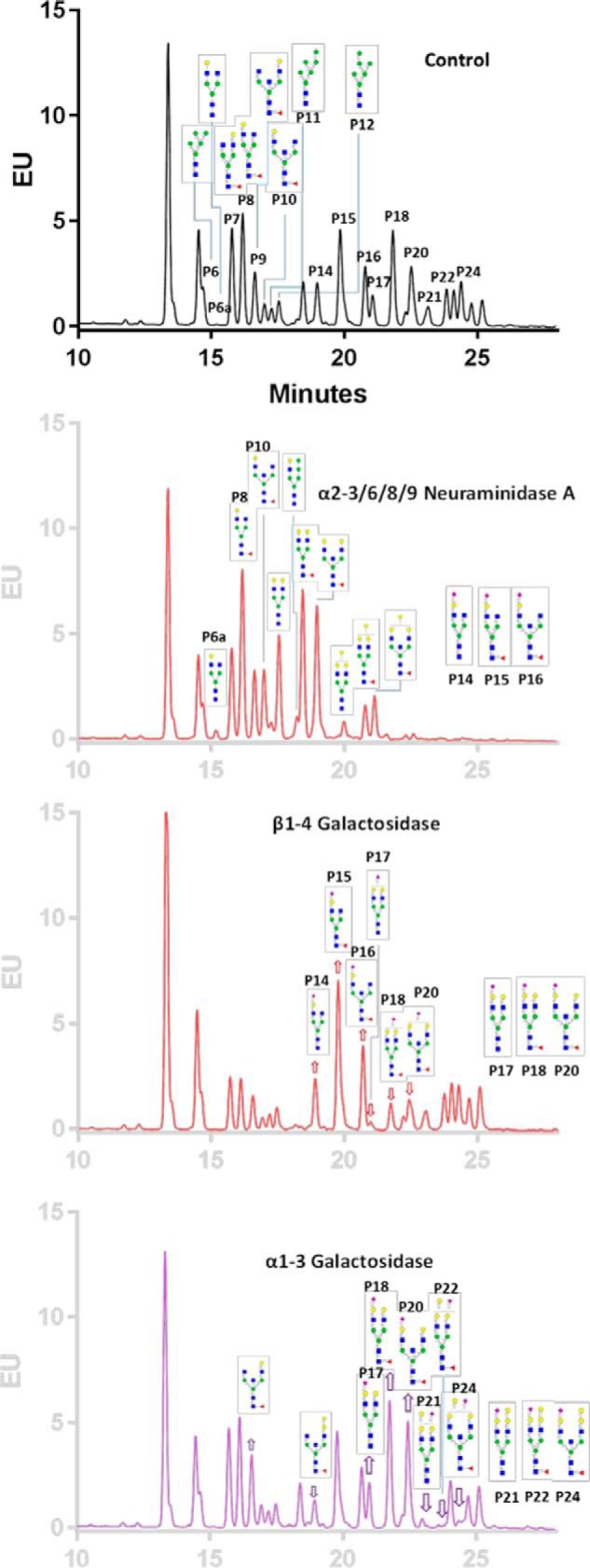
HILIC UPLC chromatograms of RapiFluor-MS–labeled N-glycans after exoglycosidase treatments. RapiFluor-MS–labeled N-glycans of naïve ferrets were treated with α2–3/6/8/9 neuraminidase A, β1–4galactosidase, and α1–3galactosidase and separated by HILIC UPLC monitored by fluorescence. EU, emission units.
Glycans with (M + Na)+ at m/z 2260 had two isomers, and F1A2G2 was dominant at 91%, as determined previously by nanoLC-MS/MS (Fig. S8). Upon treatment with different galactosidases, only β1–4 galactosidase significantly shifted the glycan profile. It caused two Gal residues to be removed by β1–4 galactosidase, with no accumulation of the glycan with one single terminal galactose (m/z of 2056). This result further confirmed that the structure determined previously by nanoLC-MS/MS had two terminal Galβ1–4GlcNAc.
A minor fraction of ferret IgG N-glycans is mono- and disialylated exclusively with Neu5Ac
A fraction of sialylated N-glycans were found in ferret IgG samples. When N-glycans were treated with α2–3/6/8/9 neuramidase A and α2–3neuraminidase S, drastically different end products were observed between the two different neuraminidases (Fig. S9). Although the sialylated structures remained largely the same in MALDI-TOF/MS spectra after neuraminidase S treatment, they shifted after treatment with neuraminidase A, indicative of α2–6–linked Neu5Ac on these structures, as polysialic acid structures were not detected. The MS spectra suggested the existence of several monosialylated and disialylated N-glycans in ferret IgG, with no polysialylated structures. Sialylation could be either α2–6 and/or α2–3Neu5Ac. The MALDI-TOF spectrum showed that the peak of N-glycan with disialylation (m/z 2808) disappeared after treatment of α2–3/6/8/9neuraminidase A only but not with α2–3 neuraminidase S (Fig. S9), suggesting the existence of two terminal α2–6–linked Neu5Ac. Similarly, the monosialylated glycan (m/z 2406) lost one sialic acid upon treatment with neuraminidase A, indicating that this N-glycan contained one terminal α2–6–linked Neu5Ac.
Neuraminidase treatment of the RapiFluor-MS–tagged N-glycans on the UPLC chromatograms further confirmed that all sialylated N-glycans of ferret IgGs were α2–6Neu5Ac. Although there was little change in the chromatogram after neuramidase S treatment, the chromatogram of RapiFluor-MS–tagged N-glycans (Fig. 4) showed drastic changes after neuraminidase A treatment, with disappearance of those sialylated N-glycan peaks and an intensity increase of corresponding neutral N-glycan peaks. The conclusion of α2–6sialylation comes from the following findings: α2–3 neuraminidase S did not whereas α2–3/6/8/9 neuraminidase A did change the MALDI-TOF/MS profiles and UPLC fluorescence chromatograms of sialylated N-glycans; as positive controls, both neuraminidases did desialylate N-glycans of fetuin, which are mixtures of both α2–3 and α2–6 sialylation (Fig. S10); and the sialylated N-glycans of ferret IgGs did not contain polysialic acids such as α2–8Neu5Ac.
The αGal epitope is on the α1–6Man arm of ferret IgG N-glycans, and α2–6Neu5Ac predominantly presents on the α1–3Man arm of monosialylated N-glycans
Exoglycosidase treatment combined with isomeric separation by HILIC UPLC made additional isomeric assignments of the glycans possible. After neuraminidase A treatment, along with the loss of one Neu5Ac, peaks P14, 15, and 16 decreased and, correspondingly, peaks P6a, 8, and 10 increased, whereas the peaks of their related isomers P6, 7, and 9, respectively, remained the same (Fig. 4). Peaks 6a, 8, and 10 contain isomers with Galβ1–4GlcNAc on the α1–3Man arm, indicating that the α2–6Neu5Ac predominantly presents on the antenna of α1–3–linked core trimannosyl mannose α1–3 Man branch; therefore, peaks P14, 15, and 16 were also assigned to be α1–3 Man–branched isomers. Similarly, after β1–4 galactosidase treatment, with the loss of one β1–4–linked galactose, peaks 17, 18, and 20 were found to be related to peaks 14, 15, and 16, respectively. Therefore, with one additional β1–4–linked terminal galactose on the α1–6Man arm, peaks 17, 18, and 20 were assigned to be the extension of isomers 14, 15, and 16, respectively. With a similar approach, α1–3galactosidase treatment proved that αGal in peaks 14, 21, 22, and 24 was linked to β1–4Gal on the α1–6Man arm.
Unusual hybrid N-glycans are found in ferret IgGs
In the MALDI-TOF/MS and LC-MS analyses, α1–2Man residues were found in two hybrid N-glycans ((M + Na)+ at m/z 2406 and 2580). Hybrid structures commonly contain complex-type structures on the α1–3Man arm, whereas on the α1–6 arm, high-mannose type structures are seen that can be linked by α1–3mannose and α1–6mannose. To confirm that the terminal mannose residue was an α1–2 Man in the N-glycans of ferret IgG, the N-glycans were released by PNGase F, treated with mannosidases, permethylated, and analyzed on MALDI-TOF. Treatment with α1–2mannosidase resulted in collapses of peaks of high-mannose N-glycans, including Man9, Man8, Man7, and Man6 to Man5, which was the final product with the loss of all α1–2 Man residues, indicating that α1–2mannosidase was highly specific and efficient (Fig. S11 and Table S3). Furthermore, in a parallel experiment with α1–2/3/6mannosidase, the cleavage process continued past the Man5 glycoform. This is indicated by the abundance of 1187 and 1391 m/z forms. After removal of two more Man, either α1–3– or α1–6–linked Man residues were removed, the structure Man2-Manβ1–4GlcNAcβ became dominant, and hydrolysis seemed to be declining drastically toward the end. It is likely that the kinetics of enzymatic cleavage decreased while approaching the inner core. These results confirmed the expected specificity and efficiency of mannosidases. The molecular ion (M + Na)+ of a hybrid structure at m/z 2580 (Fig. S12) was clearly reduced after being treated with α1–2mannosidase, which confirmed the α1–2mannose in this hybrid N-glycan. The abundance of this hybrid N-glycan was also reduced upon α1–2/3/6 mannosidase treatment, which is additional proof of the α-mannose–linked hybrid N-glycan structure. Similarly, a related nonfucosylated hybrid glycan (m/z 2406) showed a similar pattern of shift, but with less change, after being treated with α1–2 mannosidase and α1–2/3/6 mannosidase. This glycan seemed to be less susceptible to these two mannosidases. These results support the conclusion of the presence of single branches as in the CID fragmentation experiments (Fig. S4) but with additional linkage confirmation.
RapiFluor-labeled N-glycans of ferret IgG were also treated with α mannosidases and analyzed using HILIC UPLC. Treatment of α1–2 mannosidase reduced the peak intensity of two hybrid glycans and two Man6 isomers correspondingly, resulting in the presence of two new glycans (retention times: 17.6 and 18.6 min) and enhanced intensity of Man5, respectively (Fig. S13). With α1–2/3/6 mannosidase treatment, the shift was more obvious for the two hybrid glycans. Interestingly, after treatment with α1–2/3/6 mannosidase, Man6 and Man5 peaks disappeared, accompanied by the appearance of three new peaks that eluted before 10 min. This finding agrees with previous MALDI-TOF/MS results of α1–2/3/6 mannosidase-treated and permethylated glycans, in which three new lighter glycans with sequential loss of three mannoses were detected.
Collectively, the MS data from CID fragmentation and the results of nanoLC-MS/MS spectra and UPLC chromatograms with and without α-mannosidase treatments confirmed the assignment of single-branched α1–2 mannose to the two hybrid N-glycan structures with (M + Na)+ at m/z of 2580 and 2406.
Preferences of β-galactosylation and sialylation are observed in N-glycans of ferret IgG
In neutral core-fucosylated glycans, addition of the first β-galactose showed a slight preference for the α1–3Man arm for nonbisected glycans (P8 > P7). This trend was reversed for bisected glycans, in which the first galactose was dominantly located on the α1–6Man arm (P9 > P10). For sialylated N-glycans, more sialylation of the β-galactose on the α1–3Man arm was observed, indicating that ST6Gal-I prefers the galactose on the α1–3Man arm for both bisected and nonbisected structures, as evidenced by the migration of P14, P15, and 16 toward corresponding α1–3Man–branched glycans P6a, P8, and P10, respectively, after neuraminidase A treatment. The trend of preferential sialylation on the α1–3 Man arm did not change in the presence of the second galactose, as evidenced by the disappearance of the P17, P18, and P20 intensity increase of P14, P15, and P16, respectively. Interestingly, the α1–3galactosidase treatment revealed that the addition of α1–3Gal seemed to prefer the α1–6Man branch (P14, 21, 22, and 24).
Bisecting GlcNAc-containing N-glycans of ferret IgGs increase after influenza A infection
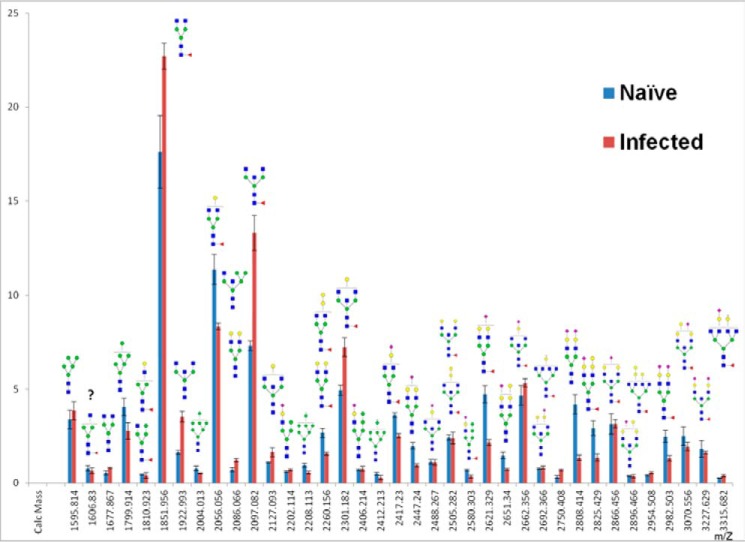
A fundamental question related to IgG N-glycosylation is whether the N-glycosylation profile of ferret IgG changes upon infection with influenza viruses. Thus, IgGs purified from ferrets before and after infection with seasonal H3N2 HK/4801 virus were analyzed. There were 42 N-glycans in IgG isolated from infected ferrets according to MALDI-TOF/MS analysis (Fig. S14). The abundances of those permethylated N-glycans of IgGs before and after HK/4801 infection are shown in Fig. 5. The relative abundance of F1A2G0 ((M + Na)+ ion at m/z 1851) and F1A2G1 ((M + Na)+ ion at m/z 2056) (with a difference of one Gal), the two most abundant and related glycans in ferret IgG, showed opposite changes after infection; the abundance of F1A2G0 increased, whereas the abundance of F1A2G1 decreased, suggesting a decrease in the galactosylation of N-glycans. In contrast to galactosylation, the relative abundance of bisecting GlcNAc-containing neutral glycans increased significantly, whereas two of the largest bisecting GlcNAc-containing sialylated N-glycans (m/z 3070 and 3227) appeared to be slightly reduced in their relative abundance. For the high-mannose type N-glycans, the abundance of Man5 increased, whereas the abundance of Man6∼9 N-glycans decreased after infection. Interestingly, the abundance of sialylated complex-type biantennary glycans decreased significantly after infection (∼50%). On the contrary, the abundance of core-fucosylated glycans showed no substantial change before and after infection (75% versus 78%). Quantitative measurement of RapiFluor-MS–labeled glycans on IgG of infected ferrets by UPLC fluorescence also revealed 26 peaks (Fig. 6A). The two UPLC chromatograms were very similar in the retention time of each peak, but the relative intensity or abundance of some peaks changed after infection. The peak areas of the corresponding peaks were compared after integration (Fig. 6B). Overall, the changes in N-glycans of IgG after infection resembled that seen by MALDI-TOF/MS measurement, e.g. increased abundance of bisecting GlcNAc-containing N-glycans and decreased abundance for F1A2G1 as well as a decrease of sialylated glycans, P21, 22, 23, and 25. It is worth noting that there were some differences in results between these two measurements because of the different methods of calculation. MALDI-TOF/MS measurement calculates relative abundance and showed an abundance of lighter glycans in the sample with infection. In addition, because the most intensified monoisotopic peaks were used to calculated relative abundance in MALDI-TOF/MS measurements, it became more diluted for heavier glycans with wider isotopic distribution compared with lighter glycans. UPLC fluorescence measurement is quantitative and gives the absolute quantity of each peak, although many peaks contain more than one major glycan and cannot be used for direct quantification of individual glycans. UPLC fluorescence measurement also did not show a differential change between isomeric pairs after infection, except for isomers of Man6, in which P11, terminal α1–2 Man linked to inner α1–6 Man, decreased, whereas P12, terminal α1–2 Man linked to inner α1–3 Man, remained the same. Currently, it is not known whether these changes in N-glycans of IgG are on the Fc or/and on its Fab in a subpopulation of total IgG or antigen-specific IgG from infected animals.
Figure 5.
Comparison of relative abundance of N-glycans of IgGs from naïve with that from infected ferrets determined by MALDI-TOF/MS. N-glycans released from IgGs purified from pooled sera from four ferrets before (naïve) and after influenza A/H3 infection (infected) were reduced and permethylated and measured by MALDI-TOF/MS. The relative abundance of each glycan was calculated based on the most abundant isotopic peaks. The error bars represent the variation of data of three experiments.
Figure 6.
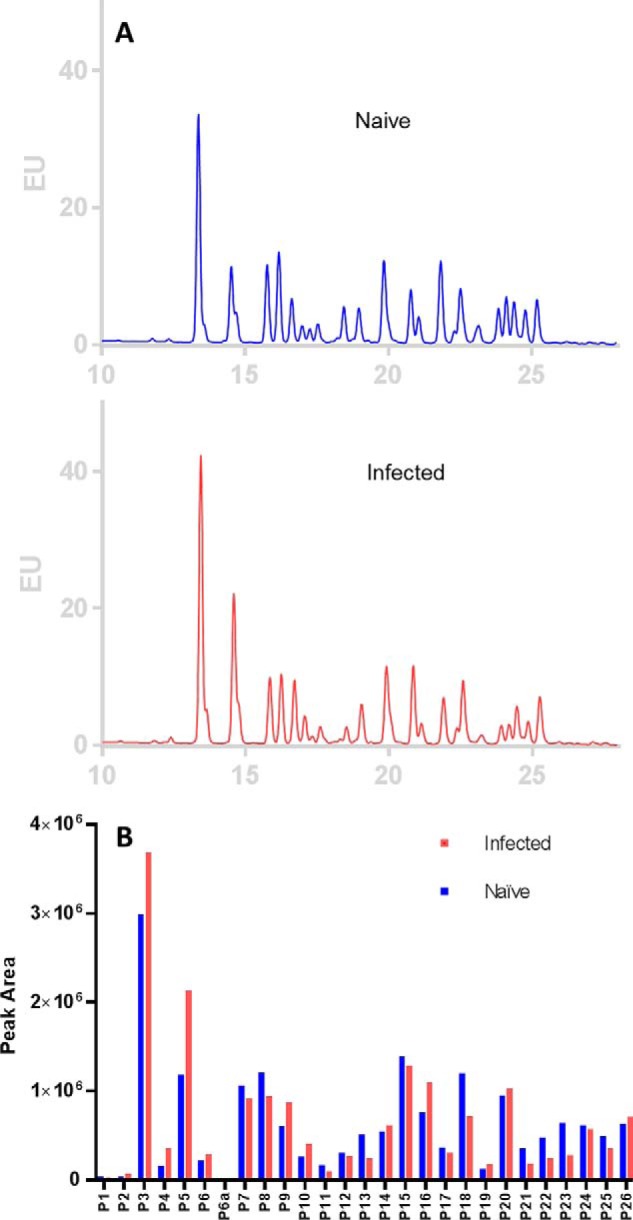
Quantification and comparison of N-glycans of IgGs from naïve to infected ferrets determined by HILIC UPLC fluorescence. A, N-glycans released from IgGs purified from pooled sera of four ferrets before (naïve) and after influenza A/H3 infection (infected). N-glycans were labeled by RapiFluor-MS and separated on HILIC UPLC monitored by fluorescence, and their chromatograms were overlaid. B, the relative abundance of corresponding peaks of N-glycans of IgGs from naïve and infected samples was compared.
Discussion
In this study, we have shown for the first time that ferret serum IgG is an N-glycosylated protein and that the N-glycan structures are highly diverse like those of other mammalian species. Our results from this comprehensive N-glycan characterization show that there are some similarities in N-glycans between human IgGs and ferret IgGs. Like humans, ferrets only have Neu5Ac but no detectable Neu5Gc on their N-glycans, which is a feature distinct from most other mammals (32, 33). This is consistent with a recent report that ferrets have a mutation in CMAH, as do humans, that inactivates the hydrolase that converts Neu5Ac to Neu5Gc (29, 34). In addition, all major N-glycans observed in human IgG are also found on ferret IgGs, including core-fucosylated or noncore fucosylated N-glycans with or without bisecting GlcNAc, and a minor fraction of them is also mono- or disialylated. Similar to human IgG, sialylated N-glycans containing α2–6Neu5Ac represent a minor fraction of the total N-glycans in ferret IgG. One major difference between human and ferret IgG is that ferret IgGs possess αGal epitopes, whereas human IgG does not have this epitope. Interestingly, the αGal epitope is predominantly on the α1–6Man arm of complex N-glycans in ferret IgG, whereas mono-sialylated N-glycans predominantly have α2–6Neu5Ac on their α1–3Man arm. This suggests that the α1–3galacosyltransferase prefers the β-galactose on the α1–6Man arm as its acceptor, and ST6Gal-I prefers the β-galactose on the other arm i.e. α1–3Man of N-glycans with or without the presence of core fucose. In humans, terminal Galα1–3Gal does not exist, and instead, natural antibodies against the Galα1–3Gal epitope occur in the blood (35, 36). It will be interesting to determine whether the Galα1–3Gal motif affects the function of IgGs in ferrets. Furthermore, our results also suggest that addition of the first galactose was preferentially on the GlcNAc on the α1–3 Man arm of nonbisecting glycans (P8 > P7) in ferret IgG, whereas it was reversed for bisected glycans in N-glycan processing (P9 > P10). This preference switch could be explained by the substrate specificity of β4galactosyltransferases and GnT-III and accessibility of the substrate in the context of protein structure (30). This resembles human IgG2 and bovine IgG as well but is different from human IgG1 (24, 30). In humans, IgG1 is the dominant subclass that has high binding affinity for Fc receptors, whereas IgG2 binds the Fc receptor weakly. Exposure to T-independent antigens such as carbohydrates carried by pathogens can alter IgG class switch recombination, resulting in selective suppression of highly functional IgG1 while enhancing less functional IgG2 (20, 37).
To obtain detailed structural information on the N-glycans of ferret IgGs, two labeling strategies in combination with a set of comprehensive tools were utilized. These strategies included permethylation after reduction and RapiFluor-MS labeling. Permethylation after reduction provides very informative clues when examining the structure through MS/MS fragmentation because reducing end and nonreducing end GlcNAc can be easily differentiated; branching, including core fucosylation and the linear structure at the core α1–6 Man in hybrid structures, was identified; and, importantly, bisecting GlcNAc structures were directly identified through HCD fragmentation. Identification of a bisecting GlcNAc is tedious. It can be done either through GC-MS linkage analysis of chemically hydrolyzed permethylated glycans, which can only identify the existence of bisected mannose residues (5), or by stepwise exoglycosidase trimming to identify bisecting glycans. Diagnostic D ions with the loss of one GlcNAc residue were also reported in addition to the identification of bisecting GlcNAc in positive or negative mode (38, 39). Our approach with HCD fragmentation is direct and reliable and can be utilized to identify bisecting GlcNAc structures in permethylated glycans, in which a fragment ion with m/z of 681.3440 unique to bisecting GlcNAc was identified. In addition, permethylation is widely considered to prevent the loss of sialic acids from glycans, neutralize the charge of sialic acids, increase the sensitivity of MS detection, and facilitate quantification of the relative abundance of each glycan in the sample. Furthermore, LC separation of the permethylated glycans allowed us to separate glycans with terminal α1–3Gal (αGal) from its isomers, nonreducing end βGal-linked N-glycans. It is worth noting that two unique hybrid glycans with α1–2–linked Man to the inner Man on the α1–6Man arm were identified. This supports the possibility of a pathway other than the GnT1/Man5 pathway to form this hybrid structure. This is unusual for a pathway for the biosynthesis of N-glycans. It is a general perception that GnT-I utilizes Man5 as its acceptor substrate and transfers the first GlcNAc to α1–3 Man of the trimannosyl core via β1–2 linkage, forming one single branch. This is further extended by β-4Gal and then α2–3 or 2–6Sia, and the α1–6Man of the trimannosyl core has two branches, terminal α1–3 and α1–6Man. This unconventional structure was confirmed by α-mannosidase treatment (40, 41). The strategy by RapiFluor-MS–labeled glycans on HILIC UPLC fluorescence gives us a much-needed quantitative method for the measurement of glycans. Also, to complement the permethylation/LC-MS-MS strategy, some of the RapiFluor-MS–labeled glycan isomers were better resolved on HILIC UPLC, providing more tools to identify additional glycan structures. With these practical and comprehensive strategies, analysis of permethylated reducing end–reduced glycans on MALDI-TOF/MS and (C18) LC-MS/MS and analysis of RapiFluor-MS N-glycans on HILIC UPLC fluorescence and MS, we were successfully able to assign 42 different N-glycan structures for serum IgG from naïve ferrets.
Exoglycosidase treatments are essential to confirm unique epitopes and terminal structures of glycans as well as to derive isomeric structural information of glycans. The loss of one Gal and the shift of certain peaks on the HILIC UPLC profile after α1–3 galactosidase treatment and MALDI-TOF/MS, respectively, confirmed that that there are four major N-glycans carrying the αGal epitope. Similarly, treatment with neuraminidases confirmed that all sialylation seen on N-glycans of ferret IgG is α2–6Neu5Ac–linked. In addition, more isomers were identified by observing the integration of glycan peaks of ambiguous isomeric identification into known isomeric glycan peaks after exoglycosidase treatments, for example. αGal is present on the α1–6Man arm, whereas α2–6Neru5Ac is on the α1–3Man arm. Furthermore, α1–2mannosidase treatment confirms the unusual hybrid structure of N-glycans with α1–2Manα1–3Man on the α1–6 Man arm (no branching) of the trimannosyl core.
The N-glycan profiles of bulk serum IgGs of the ferret shifted after infection with a seasonal H3N2 virus. The major changes include more bisecting GlcNAc, less galactosylation of neutral N-glycans, and less sialylated N-glycans. Although there may be only a fraction of IgGs that are antigen-specific, as reported for other infectious diseases (42), these changes in N-glycan profiles of ferret IgGs are still intriguing. It suggests that virus-specific ferret IgG may carry more N-glycans with bisecting GlcNAc. Sialylated glycoforms of human IgGs are reported to have an anti-inflammatory effect (43), whereas bisecting glycans can enhance antibody-dependent cellular cytotoxic activity (ADCC) in humans (44, 45). It has been reported that an abundance of sialylated Fc glycans predicts influenza vaccine efficiency in humans (21). In ferrets, influenza exposure elicits a light chain–biased antibody response against the surface glycoprotein hemagglutinin (HA) (18). The shift of ferret IgG glycan profiles observed in this study might be a programmed inflammatory reaction after exposure to influenza virus or may just reflect an augmented antibody-mediated immune response in infected ferrets or decreased/increased clearance of certain glycoforms. The exact mode of action requires profiling the N-glycans of HA-specific ferret IgG with regard to effector functions, which is still an ongoing investigation.
In this study, we observed no dramatic change in core fucosylation of N-glycans in ferret IgGs before and after infection. A similar phenomenon was also observed during HIV infection (42). HIV patients showing persistent viral suppression were found to have antibodies with lower fucosylation (42). These HIV patients were also found to have more functionally coordinated immune responses between IgG3/IgG1 and effector functions, including ADCC and monocyte/neutrophil phagocytosis (46). It has been reported that core fucosylation on N-glycans of Fc in IgG can drastically reduce antibody effector functions by attenuating the interactions between IgG and Fc receptors (47). To fine-tune this interaction, it might require a secondary level of adjustment. Recent studies show that differences in sialylation-mediated modulation only occurred in the context of core fucosylation. Without core fucosylation, sialylated and nonsialylated mAbs had similar ADCC activity (48). In our study, we observed a notable decrease in sialylation of core-fucosylated complex-type biantennary glycans. However, the decrease in these biantennary core-fucosylated sialylated glycans might be a minor factor because the decrease in biantennary afucosylated sialylated glycans was more significant. Again, it would be much more meaningful to assess and interpret the changes in N-glycans of antigen-specific IgGs from infected ferrets.
To defend against specific infectious agents, antibodies perform two roles: directly neutralizing the pathogen and mounting an innate immunity response by recruiting innate immune machinery (42). Glyocoform diversity can play a role in the relative strength of these two pathways. Adjustment of the glycosylation profile of antibodies triggered by infection strengthens the innate immune recruiting capacity of antibodies. Although HA-specific neutralizing antibodies are the primary defense mechanism, antibody Fc-mediated effector functions are also critical for broad cross-protection against different subtypes of influenza A viruses (49, 50). The efficacy of a broadly neutralizing antibody depends on its FcR binding capacity, which is modulated by glycosylation (21, 51). Introducing sialylated glycoforms of broadly neutralizing IgG into immune complexes has been shown to elicit high-affinity IgGs against conserved HA stem regions for broad protection against influenza viruses (21, 52).
In summary, N-glycans in ferrets are highly diverse, as they are in other mammalian species. Our comprehensive profiling of the N-glycans in ferret IgGs before and after H3N2 infection filled the knowledge gap regarding the ferret immune process and influenza viruses, opening the door to in-depth investigation of the structure–function relationships of ferret IgG for improvement of vaccine strain selection and universal vaccine development.
Experimental procedures
Reagents and chemicals
Endoglycosidase H (Endo H), α1–2/3/6 mannosidase, β1–3 galactosidase, β1–4 galactosidase, α1–3/4/6 galactosidase, α2–3 neuraminidase S, and α2–3/6/8/9 neuraminidase A were obtained from New England Biolabs (Ipswich, MA). Enzyme α1–2 mannosidase was obtained from ProZyme (Hayward, CA). Peptide-N-glycosidase F (PNGase F) was purchased from either Prozyme or New England Biolabs. Ammonia borane complex, β-mercaptoethanol, sodium hydroxide beads, and methyl iodide were obtained from Sigma-Aldrich (St. Louis, MO). Micro SpinColumn active charcoal, Micro SpinColumn C18, and Micro SpinColumn empty were purchased from Harvard Apparatus (Hayward, CA). Ultrapure MALDI matrix 2,5-dihydroxybenzoic acid was purchased from Alfa Aesar (Ward Hill, MA). The GlycoWorks RapiFluor-MS N-glycan kit was purchased from Waters (Milford, MA).
Ferret infection
Seronegative male ferrets (15–16 weeks old) were purchased from Triple F Farms (Gillett, PA) and were housed in a biosafety level 2 facility at the White Oak campus of the Food and Drug Administration. Under anesthesia with a ketamine/xylazine mixture, ferrets were inoculated with H3N2 A/Hong Kong/4801/2014 (HK/4801) via both nostrils. Blood was collected via the anterior vena cava before and 1 month after infection. All procedures were carried out in accordance with the protocol approved by the Institutional Animal Care and Use Committee of the Food and Drug Administration.
Ferret IgG purification and gel electrophoresis
Ferret IgGs were purified from pooled sera collected from four animals before and after infection using the NAb Protein A Plus Spin kit (Thermo Fisher). Total ferret IgG was quantitated using the reducing agent-compatible Pierce microplate BCA protein assay kit (Thermo Fisher). Purified ferret IgGs were separated on 4–12% NuPAGE BisTris gels (Invitrogen) under both nonreducing and reducing conditions. Nonspecific mouse monoclonal IgG and rabbit polyclonal IgG were used as controls.
Influenza-specific antibody determination
H3N2-specific hemagglutinin inhibition titers in ferret sera were determined using 8 HA units/50 μl of HK/4801 virus and 0.75% guinea pig erythrocytes in the presence of 20 nm oseltamivir as described before (7, 8, 53). Influenza-specific IgG was assessed by ELISA using recombinant HA (Immune Technology) as the coating antigen and peroxidase-conjugated goat anti-ferret IgG (Abcam) as the detection antibody (8).
Endoglycosidases, PNGase F, and Endo H treatment
To an aliquot of 20 μg of purified ferret IgGs, 25 μl of 10 mm phosphate buffer (pH 7.4) containing 0.1% SDS and 0.1% β-mercaptoethanol was added. The reaction mixture was incubated at 60 °C in a water bath for 1 h to denature the ferret IgGs. At room temperature, 2.5 μl of 10% Nonidet P-40 was added and incubated for 10 min. Of the 40-μl solution, 6 μl was aliquoted and 0.8 μl of 10× Glycobuffer 2 or 3 and 1 μl of 10-fold diluted PNGase F or Endo H (New England Biolabs) were added and incubated at 37 °C for 21 h. 5 μl of the enzymatic reaction mixture containing 2 μg of protein was separated on SDS-PAGE under reducing conditions.
Preparation of reducing end–reduced and permethylated N-glycans for MALDI-TOF/MS analysis
N-Glycans were released from the purified ferret IgG, reduced, and permethylated using a well-established protocol (54–56). An aliquot of 20 μg of purified ferret IgGs was taken and denatured as described above. After adding 2.5 μl of 10% Nonidet P-40 and incubating at room temperature for 10 min, an aliquot of 0.25 milliunits of peptide N-glycosidase F from ProZyme was added. The reaction mixture was incubated at 37 °C for 21 h for a complete release of the N-linked glycans. The released N-glycans were purified by Micro SpinColumn active charcoal. The samples were dried and reduced by adding 10 μl of 10 mg/ml ammonia borane complex in H2O at 60 °C for 1 h. Excess reactants were removed by adding methanol and evaporated under a vacuum three times. Subsequently the reduced glycans were permethylated by methyl iodide in sodium hydroxide beads. The permethylated N-glycans with the reducing ends reduced were mixed with matrix dihydroxybenzoic acid, spotted on a MALDI target plate, and analyzed by a Bruker UltrafleXtreme MALDI-TOF/TOF mass spectrometer in positive ion reflector mode.
Nano-LC-MS/MS analysis of the permethylated glycans
The permethylated glycans were analyzed by nanoLC/MS/MS using a Thermo Fisher Ultimate LC and Fusion Orbitrap MS (San Jose, CA). Briefly, the permethylated glycans were first loaded onto a trap cartridge (Thermo Fisher PepMap, C18, 5 μm, 0.3 × 5 mm) and then eluted onto a reverse-phase Easy-Spray column (Thermo Fisher PepMap, C18, 3 μm, 100 Å) using a linear 120-min gradient of acetonitrile (2–50%) containing 0.1% formic acid at a 300 nl/min flow rate. The eluted glycans were sprayed into the Fusion Orbitrap. Data-dependent acquisition mode was enabled, and each Fourier transform mass spectrometry MS1 scan (120,000 resolution, scanned from 300–2000 m/z) was followed by Orbitrap MS2 scans (15,000 resolution) using top speed (acquiring as many MS2 scans as possible within a 3-s cycle time). Precursor ions were fragmented using HCD and CID, respectively, with a normalized collision energy of 27% and 35%, respectively. Automated gain control targets were 2.0 × 105 and 1.0 × 104, respectively, for MS1 and MS2. The spray voltage and ion transfer tube temperature were set at 1.8 kV and 250 °C, respectively.
Rapid release and labeling of N-glycans with the RapiFluor-MS tag
N-glycans of ferret IgG were released and labeled by RapiFluor-MS tag according to the manufacturer's protocol. Before the enzymatic release, buffer exchange was performed, and 1.2 mg/ml ferret IgG in 50 mm HEPES (pH 7) was obtained. 12 μl of the IgG solution was aliquoted, and 10.8 μl of water was added to a total volume of 22.8 μl. Next, 5 μl of 5% RapiGest SF was added, and the reaction mixture was heated at 90 °C to denature the protein. N-glycans were released as glycosylamines by adding 1.2 μl of Rapid PNGase F and incubating at 50 °C for 5 min. After the reaction mixture was cooled at room temperature for 3 min, 12 μl of RapiFluor-MS labeling reagent solution was added, and the reaction proceeded for 5 min at room temperature. 358 μl of acetonitrile was added and purified on HILIC solid phase extraction provided by the manufacturer. The final eluate from HILIC solid phase extraction, containing RapiFluor-MS–labeled glycans in 90 μl of 200 mm ammonium acetate in 5% acetonitrile, was mixed with 310 μl of GlycoWorks sample diluent–N,N-dimethylformamide/acetonitrile.
UPLC fluorescence analysis and ultra-high-performance liquid chromatography–MS analysis of the RapidFluor-MS–labeled N-glycans
To quantify the released N-glycans from ferret IgG, the above solution was aliquoted and analyzed by UPLC with a fluorescence detector. 5 μl was injected and separated on an Acquity UPLC BEH (Ethylene Bridged Hybrid) amide column (130 Å, 1.7 μm, 2.1 × 150 mm). A 55-min gradient was used as recommended by the manufacturer. At 60 °C, with 50 mm ammonium formate (pH 4.4) as mobile phase A and acetonitrile as mobile phase B, a 35-min linear gradient changing from 25% to 46% A at a flow rate of 0.4 ml/min was used for separation. From 35 to 36.5 min, the gradient was ramped up to 100% A, and the flow rate was reduced to 0.2 ml/min, and this was maintained for 3 min to clean the column. From 39.5 to 43.1 min, the gradient was gradually changed back to 25% A, and then, from 43.1 to 47.6 min, the flow rate was increased to 0.4 ml/min and maintained until 55 min to equilibrate the column. The fluorescence detector was set with excitation at 265 nm, emission at 425 nm, and sampling rate at 2 Hz.
Another aliquot of the samples was analyzed by LC/MS using a Thermo Fisher Ultimate LC connected to Q Exactive Orbitrap MS (San Jose, CA). Briefly, the same LC conditions as on the Aquity UPLC were used, and the eluted glycans were sprayed into the Q Exactive Orbitrap under the following source conditions: spray voltage, 3.5 kV; ion transfer tube temperature, 300 °C, S-lens radiofrequency level, 50; sheath gas, 40; and auxiliary gas, 10. The Fourier transform MS1 was acquired with the resolution set at 70,000 with a scan range of 200–2000 m/z and an automated gain control target of 3.0 × 106.
Exoglycosidase experiments
α1–2 mannosidase and α1–2/3/6 mannosidase were chosen to determine the linkage of nonreducing end-terminal Man; β1–3 galactosidase, β1–4 galactosidase, and α1–3/4/6 galactosidase were used to clarify the linkage of nonreducing end-terminal Gal. α2–3 neuraminidase S and α2–3/6/8/9 neuraminidase A were selected to characterize the linkage of terminal sialic acids. Briefly, N-glycans were released from purified ferret IgGs by PNGase F and purified and dried as described earlier. The dried N-glycans were redissolved in the corresponding buffers and subjected to different exoglycosidase treatments for 2 h at 37 °C as suggested by the manufacturers, except for α1–2 mannosidase, which was treated for 22.5 h. The exoglycosidase-treated glycans were purified and dried again, followed by reduction and permethylation, and then measured by MALDI-TOF-MS. The shift of glycan peaks and change of intensities were used as clues to determine the linkages.
Exoglycosidase treatments of RapiFluor-MS–labeled N-glycans
RapiFluor-MS–labeled N-glycans were subjected to treatments with exoglycosidases to determine the glycosidic linkages. An aliquot of 20 μl of RapiFluor-MS–labeled N-glycans was dried on SpeedVac and redissolved in 8 μl of manufacturer-recommended exoglycosidase reaction buffer. Then 1 μl of exoglycosidase was added. The reaction mixtures were incubated at 37 °C for 2 h, except for α1–2 mannosidase, which was incubated for 24 h. The reactions were mixed with10 μl of N,N-dimethylformamide and 21 μl of acetonitrile, and then analyzed by UPLC fluorescence using the same condition as described above. The UPLC chromatogram profiles of exoglycosidase-treated samples were compared with their controls to identify the shifted glycans with corresponding glycosidic linkages and monosaccharide residues.
Author contributions
G. Z. and H. X. conceptualization; G. Z., M. K., S.-R. K., S. K., and D. N. P. data curation; G. Z., M. K., S.-R. K., D. N. P., H. X., and T. J. formal analysis; G. Z., M. K., S.-R. K., S. K., D. N. P., H. X., and T. J. validation; G. Z., M. K., S.-R. K., S. K., H. X., and T. J. investigation; G. Z., M. K., S.-R. K., S. K., D.N. P., H. X., and T. J. visualization; G. Z., M. K., S.-R. K., S. K., W. W. W., R. S., H. X., and T. J. methodology; G. Z., H. X., and T. J. writing-original draft; G. Z., M. K., S.-R. K., S. K., W. W. W., R. S., D. N. P., C. A., H. X., and T. J. writing-review and editing; R. S., C. A., H. X., and T. J. resources; R. S. and C. A. software; R. S., C. A., H. X., and T. J. supervision; R. S. and H. X. project administration; H. X. and T. J. funding acquisition.
Supplementary Material
Acknowledgments
We thank Drs. Ashutosh Rao and Amy Rosenberg for scientific critique and suggestions regarding the manuscript.
This work is supported by Food and Drug Administration intramural funds. This project was supported in part by an appointment to the Internship/Research Participation Program at the Office of Biotechnology Products, Food and Drug Administration, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the Department of Energy and Food and Drug Administration. The authors declare that they have no conflicts of interest with the contents of this article. The views expressed in this article are those of the authors, and do not necessarily reflect the official policy or position of the U.S. Food and Drug Administration and the Dept. of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
This article contains Figs. S1–S14 and Tables S1–S3.
- HILIC
- hydrophilic interaction liquid chromatography
- UPLC
- ultra-performance liquid chromatography
- CID
- collision-induced dissociation
- HCD
- high-energy collisional dissociation
- Neu5Ac
- N-acetylneuraminic acid
- Neu5Gc
- N-glycolylneuraminic acid
- ADCC
- antibody-dependent cellular cytotoxic activity
- HA
- hemagglutinin
- Endo H
- endoglycosidase H
- PNGase F
- peptide-N-glycosidase F.
References
- 1. Flannery B., Clippard J., Zimmerman R. K., Nowalk M. P., Jackson M. L., Jackson L. A., Monto A. S., Petrie J. G., McLean H. Q., Belongia E. A., Gaglani M., Berman L., Foust A., Sessions W., Thaker S. N., et al. (2015) Early estimates of seasonal influenza vaccine effectiveness: United States, January 2015. MMWR Morb. Mortal. Wkly. Rep. 64, 10–15 [PMC free article] [PubMed] [Google Scholar]
- 2. Skowronski D. M., Janjua N. Z., De Serres G., Sabaiduc S., Eshaghi A., Dickinson J. A., Fonseca K., Winter A. L., Gubbay J. B., Krajden M., Petric M., Charest H., Bastien N., Kwindt T. L., Mahmud S. M., et al. (2014) Low 2012–13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS ONE 9, e92153 10.1371/journal.pone.0092153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skowronski D. M., Chambers C., Sabaiduc S., De Serres G., Winter A. L., Dickinson J. A., Krajden M., Gubbay J. B., Drews S. J., Martineau C., Eshaghi A., Kwindt T. L., Bastien N., and Li Y. (2016) A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–2015 Season. Clin. Infect. Dis. 63, 21–32 10.1093/cid/ciw176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sullivan S. G., Chilver M. B., Carville K. S., Deng Y. M., Grant K. A., Higgins G., Komadina N., Leung V. K., Minney-Smith C. A., Teng D., Tran T., Stocks N., and Fielding J. E. (2017) Low interim influenza vaccine effectiveness, Australia, 1 May to 24 September 2017. Euro. Surveill. 22, 10.2807/1560-7917.ES.2017.22.43.17-00707 10.2807/1560-7917.ES.2017.22.43.17-00707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jia N., Barclay W. S., Roberts K., Yen H. L., Chan R. W., Lam A. K., Air G., Peiris J. S., Dell A., Nicholls J. M., and Haslam S. M. (2014) Glycomic characterization of respiratory tract tissues of ferrets: implications for its use in influenza virus infection studies. J. Biol. Chem. 289, 28489–28504 10.1074/jbc.M114.588541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tripp R. A., and Tompkins S. M. (2009) Animal models for evaluation of influenza vaccines. Curr. Top. Microbiol. Immunol. 333, 397–412 [DOI] [PubMed] [Google Scholar]
- 7. Xie H., Wan X. F., Ye Z., Plant E. P., Zhao Y., Xu Y., Li X., Finch C., Zhao N., Kawano T., Zoueva O., Chiang M. J., Jing X., Lin Z., Zhang A., and Zhu Y. (2015) H3N2 mismatch of 2014–15 northern hemisphere influenza vaccines and head-to-head comparison between human and ferret antisera derived antigenic maps. Sci. Rep. 5, 15279 10.1038/srep15279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kosikova M., Li L., Radvak P., Ye Z., Wan X. F., and Xie H. (2018) Imprinting of repeated influenza A/H3 exposures on antibody quantity and antibody quality: implications on seasonal vaccine strain selection and vaccine performance. Clin. Infect. Dis. 67, 1523–1532 10.1093/cid/ciy327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barr I. G., McCauley J., Cox N., Daniels R., Engelhardt O. G., Fukuda K., Grohmann G., Hay A., Kelso A., Klimov A., Odagiri T., Smith D., Russell C., Tashiro M., Webby R., et al. (2010) Epidemiological, antigenic and genetic characteristics of seasonal influenza A(H1N1), A(H3N2) and B influenza viruses: basis for the WHO recommendation on the composition of influenza vaccines for use in the 2009–2010 northern hemisphere season. Vaccine 28, 1156–1167 10.1016/j.vaccine.2009.11.043 [DOI] [PubMed] [Google Scholar]
- 10. Russell C. A., Jones T. C., Barr I. G., Cox N. J., Garten R. J., Gregory V., Gust I. D., Hampson A. W., Hay A. J., Hurt A. C., de Jong J. C., Kelso A., Klimov A. I., Kageyama T., Komadina N., et al. (2008) Influenza vaccine strain selection and recent studies on the global migration of seasonal influenza viruses. Vaccine 26, D31–D34 10.1016/j.vaccine.2008.07.078 [DOI] [PubMed] [Google Scholar]
- 11. Li Y., Myers J. L., Bostick D. L., Sullivan C. B., Madara J., Linderman S. L., Liu Q., Carter D. M., Wrammert J., Esposito S., Principi N., Plotkin J. B., Ross T. M., Ahmed R., Wilson P. C., and Hensley S. E. (2013) Immune history shapes specificity of pandemic H1N1 influenza antibody responses. J. Exp. Med. 210, 1493–1500 10.1084/jem.20130212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen Z., Wang W., Zhou H., Suguitan A. L. Jr., Shambaugh C., Kim L., Zhao J., Kemble G., and Jin H. (2010) Generation of live attenuated novel influenza virus A/California/7/09 (H1N1) vaccines with high yield in embryonated chicken eggs. J. Virol. 84, 44–51 10.1128/JVI.02106-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Popova L., Smith K., West A. H., Wilson P. C., James J. A., Thompson L. F., and Air G. M. (2012) Immunodominance of antigenic site B over site A of hemagglutinin of recent H3N2 influenza viruses. PLoS ONE 7, e41895 10.1371/journal.pone.0041895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koel B. F., Burke D. F., Bestebroer T. M., van der Vliet S., Zondag G. C., Vervaet G., Skepner E., Lewis N. S., Spronken M. I., Russell C. A., Eropkin M. Y., Hurt A. C., Barr I. G., de Jong J. C., Rimmelzwaan G. F., et al. (2013) Substitutions near the receptor binding site determine major antigenic change during influenza virus evolution. Science 342, 976–979 10.1126/science.1244730 [DOI] [PubMed] [Google Scholar]
- 15. Li G. M., Chiu C., Wrammert J., McCausland M., Andrews S. F., Zheng N. Y., Lee J. H., Huang M., Qu X., Edupuganti S., Mulligan M., Das S. R., Yewdell J. W., Mehta A. K., Wilson P. C., and Ahmed R. (2012) Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc. Natl. Acad. Sci. U.S.A. 109, 9047–9052 10.1073/pnas.1118979109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wrammert J., Koutsonanos D., Li G. M., Edupuganti S., Sui J., Morrissey M., McCausland M., Skountzou I., Hornig M., Lipkin W. I., Mehta A., Razavi B., Del Rio C., Zheng N. Y., Lee J. H., et al. (2011) Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 208, 181–193 10.1084/jem.20101352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abe Y., Takashita E., Sugawara K., Matsuzaki Y., Muraki Y., and Hongo S. (2004) Effect of the addition of oligosaccharides on the biological activities and antigenicity of influenza A/H3N2 virus hemagglutinin. J. Virol. 78, 9605–9611 10.1128/JVI.78.18.9605-9611.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirchenbaum G. A., Allen J. D., Layman T. S., Sautto G. A., and Ross T. M. (2017) infection of ferrets with influenza virus elicits a light chain-biased antibody response against hemagglutinin. J. Immunol. 199, 3798–3807 10.4049/jimmunol.1701174 [DOI] [PubMed] [Google Scholar]
- 19. Hensley S. E. (2014) Challenges of selecting seasonal influenza vaccine strains for humans with diverse pre-exposure histories. Curr. Opin. Virol. 8, 85–89 10.1016/j.coviro.2014.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jennewein M. F., and Alter G. (2017) The immunoregulatory roles of antibody glycosylation. Trends Immunol. 38, 358–372 10.1016/j.it.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 21. Wang T. T., Maamary J., Tan G. S., Bournazos S., Davis C. W., Krammer F., Schlesinger S. J., Palese P., Ahmed R., and Ravetch J. V. (2015) Anti-HA glycoforms drive B cell affinity selection and determine influenza vaccine efficacy. Cell 162, 160–169 10.1016/j.cell.2015.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Selman M. H., de Jong S. E., Soonawala D., Kroon F. P., Adegnika A. A., Deelder A. M., Hokke C. H., Yazdanbakhsh M., and Wuhrer M. (2012) Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination. Mol. Cell. Proteomics 11, M111.014563 10.1074/mcp.M111.014563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nesspor T. C., and Scallon B. (2014) Chimeric antibodies with extended half-life in ferrets. Influenza Other Respir. Viruses 8, 596–604 10.1111/irv.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pucic M., Knezevic A., Vidic J., Adamczyk B., Novokmet M., Polasek O., Gornik O., Supraha-Goreta S., Wormald M. R., Redzic I., Campbell H., Wright A., Hastie N. D., Wilson J. F., Rudan I., et al. (2011) High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol. Cell. Proteomics 10, M111.010090 10.1074/mcp.M111.010090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Halim A., Westerlind U., Pett C., Schorlemer M., Rüetschi U., Brinkmalm G., Sihlbom C., Lengqvist J., Larson G., and Nilsson J. (2014) Assignment of saccharide identities through analysis of oxonium ion fragmentation profiles in LC-MS/MS of glycopeptides. J. Proteome Res. 13, 6024–6032 10.1021/pr500898r [DOI] [PubMed] [Google Scholar]
- 26. Shields R. L., Lai J., Keck R., O'Connell L. Y., Hong K., Meng Y. G., Weikert S. H., and Presta L. G. (2002) Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcγ RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277, 26733–26740 10.1074/jbc.M202069200 [DOI] [PubMed] [Google Scholar]
- 27. Zhou S., Dong X., Veillon L., Huang Y., and Mechref Y. (2017) LC-MS/MS analysis of permethylated N-glycans facilitating isomeric characterization. Anal. Bioanal. Chem. 409, 453–466 10.1007/s00216-016-9996-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mucha E., Lettow M., Marianski M., Thomas D. A., Struwe W. B., Harvey D. J., Meijer G., Seeberger P. H., von Helden G., and Pagel K. (2018) Fucose migration in intact protonated glycan ions: a universal phenomenon in mass spectrometry. Angew. Chem. Int. Ed. Engl. 57, 7440–7443 10.1002/anie.201801418 [DOI] [PubMed] [Google Scholar]
- 29. Ng P. S., Böhm R., Hartley-Tassell L. E., Steen J. A., Wang H., Lukowski S. W., Hawthorne P. L., Trezise A. E., Coloe P. J., Grimmond S. M., Haselhorst T., von Itzstein M., Paton A. W., Paton J. C., and Jennings M. P. (2014) Ferrets exclusively synthesize Neu5Ac and express naturally humanized influenza A virus receptors. Nat. Commun. 5, 5750 10.1038/ncomms6750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fujii S., Nishiura T., Nishikawa A., Miura R., and Taniguchi N. (1990) Structural heterogeneity of sugar chains in immunoglobulin G: conformation of immunoglobulin G molecule and substrate specificities of glycosyltransferases. J. Biol. Chem. 265, 6009–6018 [PubMed] [Google Scholar]
- 31. Gornik O., Wagner J., Pucić M., Knezević A., Redzic I., and Lauc G. (2009) Stability of N-glycan profiles in human plasma. Glycobiology 19, 1547–1553 10.1093/glycob/cwp134 [DOI] [PubMed] [Google Scholar]
- 32. Adamczyk B., Tharmalingam-Jaikaran T., Schomberg M., Szekrényes A., Kelly R. M., Karlsson N. G., Guttman A., and Rudd P. M. (2014) Comparison of separation techniques for the elucidation of IgG N-glycans pooled from healthy mammalian species. Carbohydr. Res. 389, 174–185 10.1016/j.carres.2014.01.018 [DOI] [PubMed] [Google Scholar]
- 33. Raju T. S., Briggs J. B., Borge S. M., and Jones A. J. (2000) Species-specific variation in glycosylation of IgG: evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology 10, 477–486 10.1093/glycob/10.5.477 [DOI] [PubMed] [Google Scholar]
- 34. Irie A., Koyama S., Kozutsumi Y., Kawasaki T., and Suzuki A. (1998) The molecular basis for the absence of N-glycolylneuraminic acid in humans. J. Biol. Chem. 273, 15866–15871 10.1074/jbc.273.25.15866 [DOI] [PubMed] [Google Scholar]
- 35. Galili U., Rachmilewitz E. A., Peleg A., and Flechner I. (1984) A unique natural human IgG antibody with anti-α-galactosyl specificity. J. Exp. Med. 160, 1519–1531 10.1084/jem.160.5.1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sandrin M. S., Vaughan H. A., Dabkowski P. L., and McKenzie I. F. (1993) Anti-pig IgM antibodies in human serum react predominantly with Gal(α1–3)Gal epitopes. Proc. Natl. Acad. Sci. U.S.A. 90, 11391–11395 10.1073/pnas.90.23.11391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vidarsson G., Dekkers G., and Rispens T. (2014) IgG subclasses and allotypes: from structure to effector functions. Front. Immunol. 5, 520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harvey D. J., Martin R. L., Jackson K. A., and Sutton C. W. (2004) Fragmentation of N-linked glycans with a matrix-assisted laser desorption/ionization ion trap time-of-flight mass spectrometer. Rapid Commun. Mass Spectrom. 18, 2997–3007 10.1002/rcm.1709 [DOI] [PubMed] [Google Scholar]
- 39. Harvey D. J. (2005) Fragmentation of negative ions from carbohydrates: part 3: fragmentation of hybrid and complex N-linked glycans. J. Am. Soc. Mass Spectrom. 16, 647–659 10.1016/j.jasms.2005.01.006 [DOI] [PubMed] [Google Scholar]
- 40. Shivatare V. S., Shivatare S. S., Lee C. D., Liang C. H., Liao K. S., Cheng Y. Y., Saidachary G., Wu C. Y., Lin N. H., Kwong P. D., Burton D. R., Wu C. Y., and Wong C. H. (2018) Unprecedented role of hybrid N-glycans as ligands for HIV-1 broadly neutralizing antibodies. J. Am. Chem. Soc. 140, 5202–5210 10.1021/jacs.8b00896 [DOI] [PubMed] [Google Scholar]
- 41. Zhou S., Huang Y., Dong X., Peng W., Veillon L., Kitagawa D. A. S., Aquino A. J. A., and Mechref Y. (2017) Isomeric separation of permethylated glycans by porous graphitic carbon (PGC)-LC-MS/MS at high temperatures. Anal. Chem. 89, 6590–6597 10.1021/acs.analchem.7b00747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ackerman M. E., Crispin M., Yu X., Baruah K., Boesch A. W., Harvey D. J., Dugast A. S., Heizen E. L., Ercan A., Choi I., Streeck H., Nigrovic P. A., Bailey-Kellogg C., Scanlan C., and Alter G. (2013) Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Invest. 123, 2183–2192 10.1172/JCI65708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaneko Y., Nimmerjahn F., and Ravetch J. V. (2006) Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313, 670–673 10.1126/science.1129594 [DOI] [PubMed] [Google Scholar]
- 44. Umaña P., Jean-Mairet J., Moudry R., Amstutz H., and Bailey J. E. (1999) Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat. Biotechnol. 17, 176–180 10.1038/6179 [DOI] [PubMed] [Google Scholar]
- 45. Zou G., Ochiai H., Huang W., Yang Q., Li C., and Wang L. X. (2011) Chemoenzymatic synthesis and Fcγ receptor binding of homogeneous glycoforms of antibody Fc domain: presence of a bisecting sugar moiety enhances the affinity of Fc to FcγIIIa receptor. J. Am. Chem. Soc. 133, 18975–18991 10.1021/ja208390n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ackerman M. E., Mikhailova A., Brown E. P., Dowell K. G., Walker B. D., Bailey-Kellogg C., Suscovich T. J., and Alter G. (2016) Polyfunctional HIV-specific antibody responses are associated with spontaneous HIV control. PLoS Pathog. 12, e1005315 10.1371/journal.ppat.1005315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ferrara C., Grau S., Jäger C., Sondermann P., Brünker P., Waldhauer I., Hennig M., Ruf A., Rufer A. C., Stihle M., Umaña P., and Benz J. (2011) Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. U.S.A. 108, 12669–12674 10.1073/pnas.1108455108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li T., DiLillo D. J., Bournazos S., Giddens J. P., Ravetch J. V., and Wang L. X. (2017) Modulating IgG effector function by Fc glycan engineering. Proc. Natl. Acad. Sci. U.S.A. 114, 3485–3490 10.1073/pnas.1702173114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. DiLillo D. J., Tan G. S., Palese P., and Ravetch J. V. (2014) Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat. Med. 20, 143–151 10.1038/nm.3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. DiLillo D. J., Palese P., Wilson P. C., and Ravetch J. V. (2016) Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J. Clin. Invest. 126, 605–610 10.1172/JCI84428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Corti D., Voss J., Gamblin S. J., Codoni G., Macagno A., Jarrossay D., Vachieri S. G., Pinna D., Minola A., Vanzetta F., Silacci C., Fernandez-Rodriguez B. M., Agatic G., Bianchi S., Giacchetto-Sasselli I., et al. (2011) A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333, 850–856 10.1126/science.1205669 [DOI] [PubMed] [Google Scholar]
- 52. Maamary J., Wang T. T., Tan G. S., Palese P., and Ravetch J. V. (2017) Increasing the breadth and potency of response to the seasonal influenza virus vaccine by immune complex immunization. Proc. Natl. Acad. Sci. U.S.A. 114, 10172–10177 10.1073/pnas.1707950114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xie H., Li L., Ye Z., Li X., Plant E. P., Zoueva O., Zhao Y., Jing X., Lin Z., Kawano T., Chiang M. J., Finch C. L., Kosikova M., Zhang A., Zhu Y., and Wan X. F. (2017) Differential effects of prior influenza exposures on H3N2 cross-reactivity of human postvaccination sera. Clin. Infect. Dis. 65, 259–267 10.1093/cid/cix269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alley W. R. Jr, Madera M., Mechref Y., and Novotny M. V. (2010) Chip-based reversed-phase liquid chromatography-mass spectrometry of permethylated N-linked glycans: a potential methodology for cancer-biomarker discovery. Anal. Chem. 82, 5095–5106 10.1021/ac100131e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kang P., Mechref Y., Klouckova I., and Novotny M. V. (2005) Solid-phase permethylation of glycans for mass spectrometric analysis. Rapid Commun. Mass Spectrom. 19, 3421–3428 10.1002/rcm.2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zou G., Benktander J. D., Gizaw S. T., Gaunitz S., and Novotny M. V. (2017) Comprehensive analytical approach toward glycomic characterization and profiling in urinary exosomes. Anal. Chem. 89, 5364–5372 10.1021/acs.analchem.7b00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.