Abstract
Background
Polymer-free drug-eluting stents (PF-DES) were introduced with the aim of reducing the risk of stent thrombosis associated with durable polymer drug-eluting stents (DP-DES). The comparison of safety and efficacy profiles between these two stent platforms remains unclear.
Materials and methods
We conducted electronic database searches for randomized controlled trials (RCTs) comparing patients treated with either PF-DES or DP-DES. Outcomes included definite or probable stent thrombosis (ST), myocardial infarction (MI), cardiac death, all-cause death, target lesion revascularization (TLR), and target vessel revascularization (TVR). A random-effects model was used to derive risk ratios (RRs) with 95% confidence intervals (CIs). Subgroup analyses based on different variables were also performed. After screening a total of 1026 articles, the present meta-analysis included 13 RCTs comprising 8021 patients.
Results
No significant differences were found for the risks of definite or probable ST (RR, 0.94; 95% CI, 0.62–1.43; P = 0.77), MI (RR, 1.06; 95% CI, 0.85–1.33; P = 0.61), cardiac death (RR, 0.98; 95% CI, 0.80–1.21; P = 0.88), all-cause death (RR, 0.87; 95% CI, 0.76–1.00; P = 0.06), TLR (RR, 1.12; 95% CI, 0.94–1.33; P = 0.22), and TVR (RR, 1.18; 95% CI, 0.87–1.61; P = 0.29). Similarly, no significant differences were found for all outcomes regardless of anti-proliferative drug, except for an increased risk of TLR for polymer-free paclitaxel-eluting stents compared with DP-DES (RR, 2.32, 95% CI, 1.30–4.14; P = 0.005).
Conclusions
Our findings showed that PF-DES and DP-DES confer equivalent safety and efficacy profiles, with similar rates of stent thrombosis.
Keywords: Coronary artery disease, Drug-eluting stents, Durable polymer, Meta-analysis, Polymer-free
Highlights
-
•
Polymer-free and durable polymer drug-eluting stents confer similar safety and efficacy profiles.
-
•
There were comparable rates of stent thrombosis between the two stent platforms.
-
•
Further trials with greater length of follow-up are warranted to assess long-term safety and efficacy outcomes.
Abbreviations
- BMS
bare metal stents
- BP-DES
biodegradable polymer drug-eluting stents
- DAPT
dual antiplatelet therapy
- DP-DES
durable polymer drug-eluting stents
- M-H
Mantel-Haenszel
- MI
myocardial infarction
- OR
odds ratio
- PCI
percutaneous coronary intervention
- PF-DES
polymer-free drug-eluting stents
- PF-PES
polymer-free paclitaxel-eluting stents
- RCT
randomized controlled trial
- RR
risk ratio
- ST
stent thrombosis
- TLR
target lesion revascularization
- TVR
target vessel revascularization
1. Introduction
Drug-eluting stents have been a major advance in percutaneous coronary intervention (PCI). New developments in coronary stent technology have contributed to improved outcomes of patients with coronary artery disease. The sequential generations of devices have represented significant milestones in stent design, structure, and component materials [1]. These stent platforms have included bare metal stents (BMS), durable polymer drug-eluting stents (DP-DES) and polymer-free drug-eluting stents (PF-DES). First-generation DP-DES were developed to reduce the risk of in-stent restenosis and target lesion revascularization associated with BMS [2]. Despite these promising results, first-generation DP-DES were shown to have an increased risk of very late (>12 months) stent thrombosis compared with BMS [3]. The pathophysiology of stent thrombosis has been attributed to various factors, such as polymer-induced hypersensitivity reaction, stent malapposition, incomplete strut re-endothelialization, and accelerated neoatherosclerosis [4]. To address this issue, a new generation of DP-DES were developed, with improvements in anti-proliferative drugs, polymer coatings, and strut thickness [5]. The introduction of second-generation DP-DES reduced the risk of very late stent thrombosis associated with the preceding generation of devices [6]. Nevertheless, the potential thrombogenic nature of the polymer coating in second-generation DP-DES remains a concern, and suggestions have been made to extend the duration of dual antiplatelet therapy (DAPT) in patients receiving DP-DES [7].
PF-DES were designed to achieve similar advantages of BMS (reduced risk of stent thrombosis) and DP-DES (reduced risk of target lesion revascularization). These devices consist of a microporous metallic stent platform and an inorganic coating that can be loaded with an anti-proliferative drug [8]. The theoretical benefit of PF-DES is the elimination of the need for a polymer coating, which acts as a potential chronic inflammatory stimulus [9]. However, the main challenge for PF-DES has been the attainment of a sufficient level of anti-proliferative drug in the inorganic coating to ensure the inhibition of neointimal hyperplasia and in-stent restenosis [10]. Therefore, we performed a meta-analysis of randomized controlled trials (RCTs) to evaluate the safety and efficacy profiles of PF-DES compared with DP-DES.
2. Methods
2.1. Selection criteria
In the present meta-analysis, we included RCTs comparing patients with coronary artery disease who were randomized to receive PCI with either PF-DES or DP-DES. We included studies that assessed at least one of the following clinical outcomes: definite or probable stent thrombosis (ST), myocardial infarction (MI), cardiac death, all-cause death, target lesion revascularization (TLR), and target vessel revascularization (TVR). Only the most recent report with the greatest length of follow-up was included when institutions published progressive reports of an ongoing study. Studies that evaluated other types of stents, such as BMS, biodegradable polymer drug-eluting stents (BP-DES), or bioresorbable vascular scaffolds were excluded. Furthermore, studies that only assessed angiographic outcomes were excluded. Electronic database searches were limited to studies that involved human subjects. Conference abstracts, editorials, case reports, and review articles were excluded.
2.2. Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to perform the present meta-analysis [11]. Ovid Medline, PubMed, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), American College of Physicians (ACP) Journal Club, and Database of Abstracts of Reviews of Effectiveness (DARE) were searched from their dates of inception to October 2018. We identified potentially relevant studies in the electronic database searches using the following keywords or MeSH terms: “randomized controlled trial”, “drug-eluting stent”, “polymer-free”, “durable polymer”, “permanent polymer”, “sirolimus-eluting stent”, “paclitaxel-eluting stent”, “everolimus-eluting stent”, “zotarolimus-eluting stent”, and “stent thrombosis”. Reference lists of retrieved articles were screened and assessed according to the inclusion and exclusion criteria.
2.3. Data extraction
We extracted data from texts, tables, figures, and supplementary materials. There were two investigators (JJW and JAW), who independently screened each retrieved article to determine the suitability for inclusion at the title or abstract level. Studies that met the inclusion and exclusion criteria were included for quantitative assessment. Discussion and consensus with the senior author (DB) occurred when there were discrepancies between the two reviewers. We extracted data for the following baseline characteristics: age, sex, diabetes mellitus, hypertension, hyperlipidemia, current smoking, previous MI, previous procedure (PCI and coronary artery bypass grafting), clinical presentation (stable and unstable angina), and target vessel location (left anterior descending, left circumflex, and right coronary artery). The main outcome of interest was definite or probable ST, as defined by the Academic Research Consortium (ARC) [12]. We also extracted data for other clinical outcomes, including MI, cardiac death, all-cause death, TLR, and TVR. All outcomes were assessed at the longest follow-up available.
2.4. Critical appraisal and statistical analysis
The included studies were qualitatively assessed using the risk of bias tool, which was proposed by the Cochrane Collaboration [13]. In addition, the risk of publication bias was evaluated by visually estimating funnel plots [14]. Summary statistics and risk estimates were expressed as risk ratios (RRs) with 95% confidence intervals (CIs) for the comparison of patients receiving PF-DES versus DP-DES. Heterogeneity between studies was evaluated using the χ2 test. The percentage of total variation across studies was estimated using the I2 statistic, with values greater than 50% indicating significant heterogeneity [15]. We used the Mantel-Haenszel (M-H) random-effects model because there were assumed variations in treatment effect between studies. Late safety and efficacy outcomes were assessed using a landmark analysis beyond 1 year of follow-up. We also performed subgroup analyses based on the following variables: PF-DES anti-proliferative drug (amphilimus, biolimus, paclitaxel, sirolimus, or sirolimus/probucol); DP-DES anti-proliferative drug (everolimus, paclitaxel, sirolimus, or zotarolimus); generation of DP-DES (first-generation or second-generation); and duration of DAPT (6 months or 12 months). Statistical analysis was conducted using RevMan Version 5.3 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen).
3. Results
3.1. Study selection
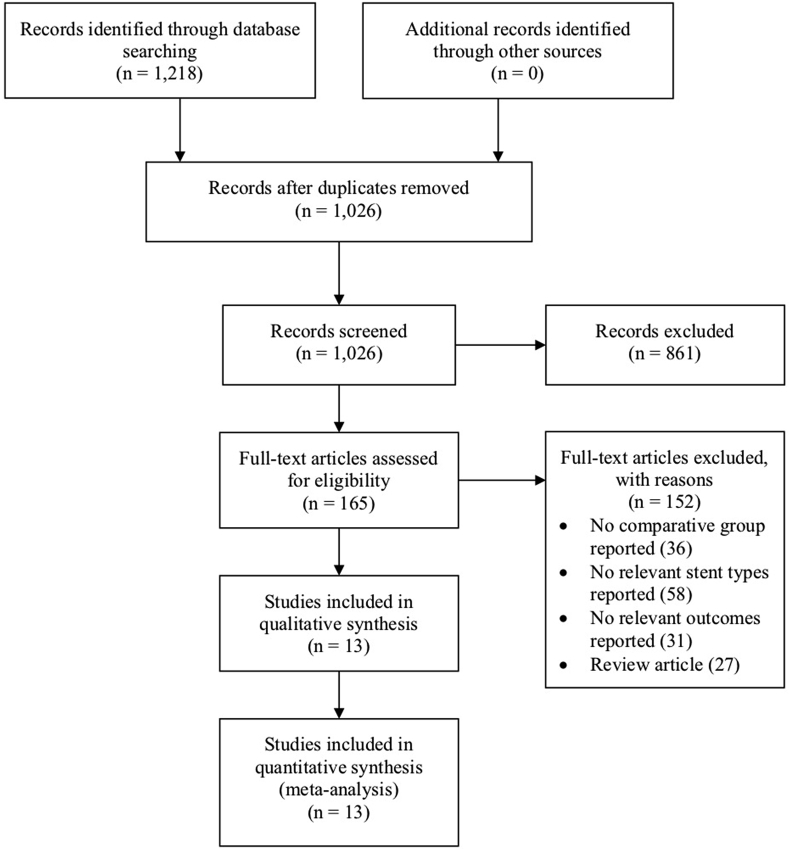
Fig. 1 shows the study selection process. A total of 1218 references were identified through electronic database searches. After duplicate references were removed, we retrieved 1026 potentially relevant articles. In the present meta-analysis, we included 13 RCTs [[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]] consisting of 8021 patients with coronary artery disease who were randomized to receive PCI with either PF-DES (n = 4545) or DP-DES (n = 3476). Table 1 outlines the study characteristics of the included trials. Patients receiving PF-DES were treated with either amphilimus-, biolimus-, paclitaxel-, sirolimus-, or sirolimus/probucol-eluting stent. Patients receiving DP-DES were treated with either everolimus-, paclitaxel-, sirolimus-, or zotarolimus-eluting stent.
Fig. 1.
Study selection process.
Table 1.
Study characteristics.
| Study | Year | Patient (n) |
DAPT (months) | Follow-Up (months) | Anti-Proliferative Drug |
Outcomes | ||
|---|---|---|---|---|---|---|---|---|
| PF-DES | DP-DES | PF-DES | DP-DES | |||||
| BioFreedom FIM [19] | 2016 | 122 | 60 | 6 | 60 | Biolimus | Paclitaxel | Definite or probable ST, MI, cardiac death, all-cause death, TLR, TVR |
| Dang [20] | 2012 | 50 | 55 | 6 | 12 | Paclitaxel | Sirolimus | Definite or probable ST, MI, cardiac death, all-cause death, TLR |
| ISAR-TEST [21] | 2013 | 225 | 225 | 6 | 60 | Sirolimus | Paclitaxel | Definite or probable ST, MI, cardiac death, all-cause death, TLR |
| ISAR-TEST-2 [16] | 2010 | 333 | 335 | 12 | 24 | Sirolimus/Probucol | Sirolimus | Definite or probable ST, MI, all-cause death |
| ISAR-TEST-3 [17] | 2009 | 201 | 202 | 12 | 24 | Sirolimus | Sirolimus | Definite or probable ST, MI, all-cause death, TLR |
| ISAR-TEST-5 [22] | 2016 | 2002 | 1000 | 6 | 60 | Sirolimus/Probucol | Zotarolimus | Definite or probable ST, MI, cardiac death, all-cause death, TLR, TVR |
| LIPSIA Yukon [26] | 2014 | 118 | 114 | 12 | 60 | Sirolimus | Paclitaxel | Definite or probable ST, MI, cardiac death, all-cause death, TLR, TVR |
| Nano [27] | 2014 | 132 | 136 | 12 | 24 | Sirolimus | Sirolimus | Definite or probable ST, MI, cardiac death, all-cause death, TVR |
| NEXT [18] | 2012 | 148 | 148 | 6 | 12 | Amphilimus | Paclitaxel | Definite or probable ST, MI, cardiac death, all-cause death, TLR, TVR |
| ReCre8 [24] | 2018 | 747 | 744 | 12 | 12 | Amphilimus | Zotarolimus | Definite or probable ST, MI, cardiac death, all-cause death, TLR |
| RESERVOIR [23] | 2016 | 56 | 56 | 12 | 12 | Amphilimus | Everolimus | MI, cardiac death |
| Shiratori [25] | 2014 | 84 | 80 | 6 | 24 | Paclitaxel | Paclitaxel | Definite or probable ST, MI, cardiac death, all-cause death, TLR, TVR |
| Zhang [28] | 2013 | 327 | 321 | 12 | 24 | Paclitaxel | Sirolimus | Definite or probable ST, MI, cardiac death, all-cause death, TVR |
DAPT = dual antiplatelet therapy; DP-DES = durable polymer drug-eluting stents; MI = myocardial infarction; PF-DES = polymer-free drug-eluting stents; ST = stent thrombosis; TLR = target lesion revascularization; TVR = target vessel revascularization.
The Cochrane Collaboration risk of bias tool assessed the RCTs to be of high quality and appropriate for inclusion in the present meta-analysis, with minimal risk of bias (Supplementary Table 1) [13]. All trials had a randomized and multicenter design that defined the patient populations and clinical outcomes, with a median follow-up of 24 months. One trial [19] included three comparison arms of patients randomized to receive either standard-dose polymer-free biolimus-eluting stents (PF-BES), low-dose PF-BES, or DP-DES. Data for standard- and low-dose PF-BES were pooled together. Two trials [17,28] included a third comparison arm of patients randomized to receive BP-DES. Data from these third comparison arms were excluded, since BP-DES were irrelevant to our research question. Earlier reports of two trials [22,26] were excluded because the institutions published a more recent report with greater length of follow-up.
3.2. Patient and procedural characteristics
Table 2 outlines the baseline characteristics of the included trials. The weighted mean age of enrolled patients receiving PF-DES was 66.3 ± 10.9 years and those receiving DP-DES was 66.3 ± 10.6 years. Overall, the two comparison arms had similar proportions of male patients and with comorbidities, previous procedures, clinical presentations, and target vessel locations (all P > 0.05).
Table 2.
Baseline characteristics.
| Baseline Characteristic | PF-DES | DP-DES | RR or WMD (95% CI) | P Value |
|---|---|---|---|---|
| Age (years) | 66.3 ± 10.9 | 66.3 ± 10.6 | −0.39 (−1.03 to 0.25) | 0.23 |
| Male | 3403/4572 (74.4) | 2617/3503 (74.7) | 0.99 (0.97–1.02) | 0.49 |
| Diabetes mellitus | 1361/4572 (29.8) | 1025/3503 (29.3) | 1.00 (0.97–1.03) | 0.80 |
| Hypertension | 3002/4572 (65.7) | 2258/3503 (64.5) | 1.01 (0.98–1.04) | 0.49 |
| Hyperlipidemia | 2545/4452 (57.2) | 1939/3387 (57.2) | 0.97 (0.93–1.00) | 0.08 |
| Current smoking | 1028/4432 (23.2) | 794/3367 (23.6) | 1.06 (0.98–1.15) | 0.15 |
| Previous MI | 1120/4572 (24.5) | 846/3503 (24.2) | 0.97 (0.89–1.04) | 0.38 |
| Previous PCI | 338/1807 (18.7) | 353/1745 (20.2) | 0.90 (0.79–1.02) | 0.10 |
| Previous CABG | 352/4095 (8.6) | 258/3079 (8.4) | 1.00 (0.85–1.17) | 0.98 |
| Clinical presentation | ||||
| Stable angina | 1691/3254 (52.0) | 1001/2181 (45.9) | 1.04 (0.97–1.11) | 0.26 |
| Unstable angina | 1125/3767 (29.9) | 861/2700 (31.9) | 0.95 (0.89–1.01) | 0.09 |
| Target vessel location | ||||
| Left anterior descending | 2444/5177 (47.2) | 1809/3672 (31.9) | 0.96 (0.91–1.02) | 0.24 |
| Left circumflex | 1373/5177 (26.5) | 983/3672 (26.8) | 1.04 (0.94–1.14) | 0.43 |
| Right coronary | 1691/5177 (32.7) | 1195/3672 (32.5) | 1.03 (0.97–1.09) | 0.35 |
Values are n/N (%) or mean ± SD; DP-DES = durable polymer drug-eluting stents; PF-DES = polymer-free drug-eluting stents; RR, risk ratio; WMD = weighted mean difference.
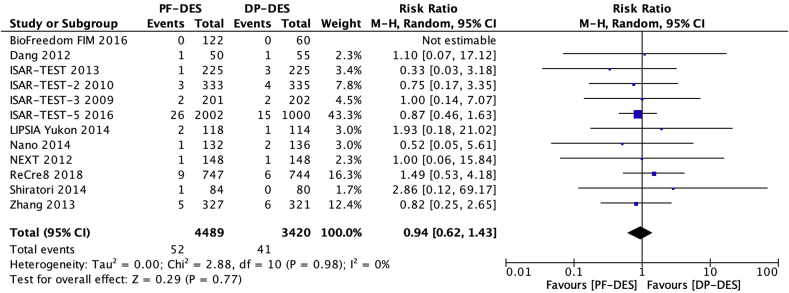
3.3. Definite or probable stent thrombosis
Twelve trials [[16], [17], [18], [19], [20], [21], [22],[24], [25], [26], [27], [28]] reported definite or probable stent thrombosis (ST) in 7909 patients. There was no significant difference between patients receiving PF-DES and those receiving DP-DES for the risk of definite or probable ST (1.2% vs 1.2%; RR, 0.94; 95% CI, 0.62–1.43; P = 0.77; I2 = 0%; Fig. 2).
Fig. 2.
Risk of definite or probable stent thrombosis. DP-DES = durable polymer drug-eluting stents. M-H = Mantel-Haenszel. PF-DES = polymer-free drug-eluting stents.
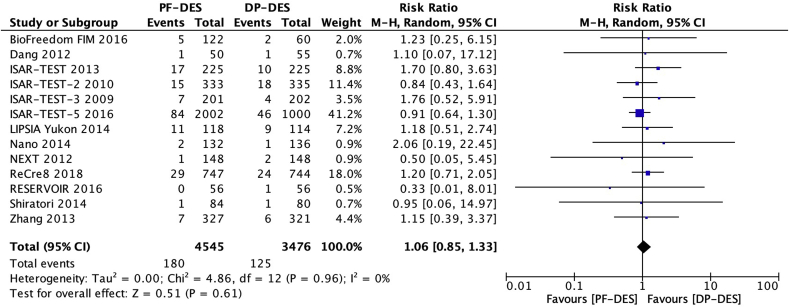
3.4. Myocardial infarction
Thirteen trials [[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]] reported myocardial infarction (MI) in 8021 patients. There was no significant difference between patients receiving PF-DES and those receiving DP-DES for the risk of MI (4.0% vs 3.6%; RR, 1.06; 95% CI, 0.85–1.33; P = 0.61; I2 = 0%; Fig. 3).
Fig. 3.
Risk of myocardial infarction. DP-DES = durable polymer drug-eluting stents. M-H = Mantel-Haenszel. PF-DES = polymer-free drug-eluting stents.
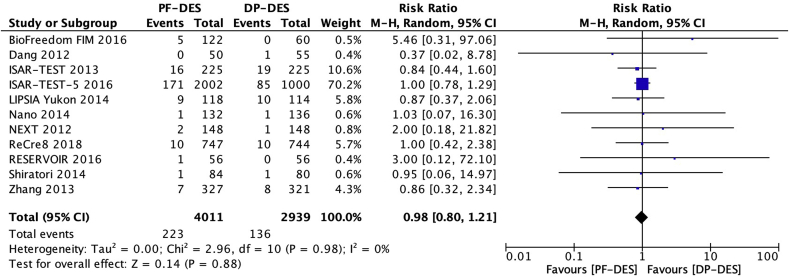
3.5. Cardiac death
Eleven trials [[18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]] reported cardiac death in 6950 patients. There was no significant difference between patients receiving PF-DES and those receiving DP-DES for the risk of cardiac death (5.6% vs 4.6%; RR, 0.98; 95% CI, 0.80–1.21; P = 0.88; I2 = 0%; Fig. 4).
Fig. 4.
Risk of cardiac death. DP-DES = durable polymer drug-eluting stents. M-H = Mantel-Haenszel. PF-DES = polymer-free drug-eluting stents.
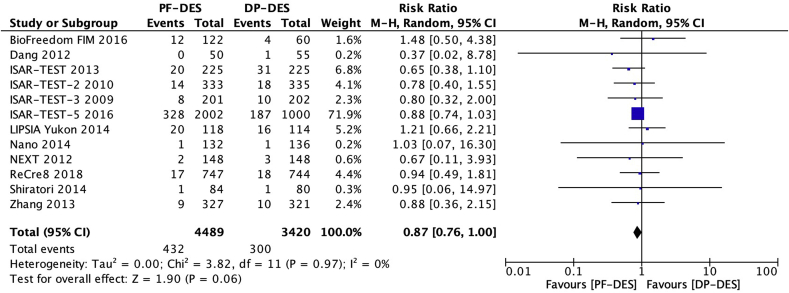
3.6. All-cause death
Twelve trials [[16], [17], [18], [19], [20], [21], [22],[24], [25], [26], [27], [28]] reported all-cause death in 7909 patients. There was no significant difference between patients receiving PF-DES and those receiving DP-DES for the risk of all-cause death (9.6% vs 8.8%; RR, 0.87; 95% CI, 0.76–1.00; P = 0.06; I2 = 0%; Fig. 5).
Fig. 5.
Risk of all-cause death. DP-DES = durable polymer drug-eluting stents. M-H = Mantel-Haenszel. PF-DES = polymer-free drug-eluting stents.
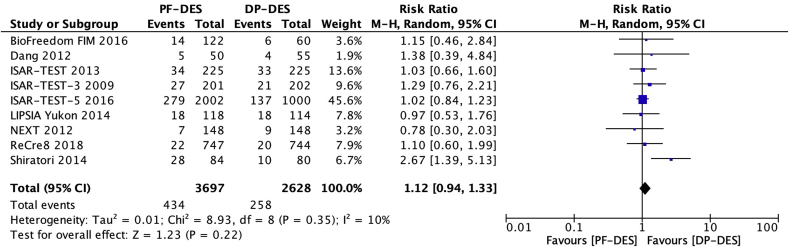
3.7. Target lesion revascularization
Nine trials [[17], [18], [19], [20], [21], [22],[24], [25], [26]] reported target lesion revascularization (TLR) in 6325 patients. There was no significant difference between patients receiving PF-DES and those receiving DP-DES for the risk of TLR (11.7% vs 9.8%; RR, 1.12; 95% CI, 0.94–1.33; P = 0.22; I2 = 10%; Fig. 6).
Fig. 6.
Risk of target lesion revascularization. DP-DES = durable polymer drug-eluting stents. M-H = Mantel-Haenszel. PF-DES = polymer-free drug-eluting stents.
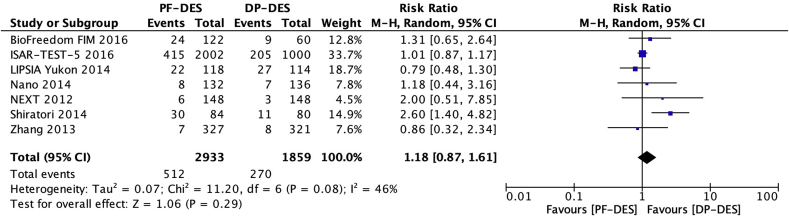
3.8. Target vessel revascularization
Seven trials [18,19,21,22,25,26,28] reported target vessel revascularization (TVR) in 4792 patients. There was no significant difference between patients receiving PF-DES and those receiving DP-DES for the risk of TVR (17.5% vs 14.5%; RR, 1.18; 95% CI, 0.87–1.61; P = 0.29; I2 = 46%; Fig. 7).
Fig. 7.
Risk of target vessel revascularization. DP-DES = durable polymer drug-eluting stents. M-H = Mantel-Haenszel. PF-DES = polymer-free drug-eluting stents.
3.9. Subgroup analyses
Table 3 outlines the safety and efficacy outcomes in different subgroups. At 1 year, 2 years, and 5 years of follow-up, there were no significant differences between patients with PF-DES and those with DP-DES for the risks of definite or probable ST, MI, cardiac death, all-cause death, TLR, and TVR (all P > 0.05). In addition, our landmark analysis beyond 1 year of follow-up showed no significant differences between patients with PF-DES and those with DP-DES for all outcomes (all P > 0.05). Our subgroup analysis based on PF-DES anti-proliferative drug (amphilimus, biolimus, paclitaxel, sirolimus, or sirolimus/probucol) showed no significant differences between patients with PF-DES and those with DP-DES for the risks of definite or probable ST, MI, cardiac death, all-cause death, and TVR (all P > 0.05). A similar result was observed in the risk of TLR for all PF-DES anti-proliferative drugs except for paclitaxel. Two trials [20,25] reported TLR in 134 patients receiving polymer-free paclitaxel-eluting stents (PF-PES) and 135 patients receiving DP-DES. Patients with PF-PES had a significantly increased risk of TLR than those with DP-DES (24.6% vs 10.4%; RR, 2.32; 95% CI, 1.30–4.14; P = 0.005; I2 = 0%). Our subgroup analyses based on DP-DES anti-proliferative drug (everolimus, paclitaxel, sirolimus, or zotarolimus), generation of DP-DES (first-generation or second-generation) and duration of DAPT (6 months or 12 months) showed no significant differences between patients with PF-DES and those with DP-DES for all outcomes (all P > 0.05).
Table 3.
Safety and efficacy outcomes.
| Analysis | Definite or Probable ST | MI | Cardiac Death | All-Cause Death | TLR | TVR |
|---|---|---|---|---|---|---|
| Outcomes at longest follow-up | 0.94 (0.62–1.43) | 1.06 (0.85–1.33) | 0.98 (0.80–1.21) | 0.87 (0.76–1.00) | 1.12 (0.94–1.33) | 1.18 (0.87–1.61) |
| Outcomes at 1 year | 1.38 (0.56–3.43) | 1.12 (0.68–1.85) | 1.08 (0.50–2.32) | 0.87 (0.48–1.60) | 1.04 (0.65–1.67) | 2.00 (0.51–7.85) |
| Outcomes at 2 years | 0.85 (0.39–1.82) | 1.06 (0.64–1.73) | 0.88 (0.36–2.16) | 0.82 (0.52–1.29) | 1.81 (0.89–3.68) | 1.52 (0.75–3.08) |
| Outcomes at 5 years | 0.91 (0.50–1.69) | 0.96 (0.70–1.32) | 1.01 (0.79–1.28) | 0.90 (0.77–1.06) | 1.02 (0.85–1.21) | 1.00 (0.87–1.15) |
| Landmark analysis beyond 1 year | 0.34 (0.01–8.20) | 0.44 (0.13–3.50) | 0.46 (0.24–81.57) | 0.85 (0.45–1.62) | 1.09 (0.51–2.33) | 1.07 (0.43–2.66) |
| Subgroup analysis | ||||||
| PF-DES anti-proliferative drug | ||||||
| Amphilimus | 1.42 (0.54–3.73) | 1.12 (0.67–1.87) | 1.15 (0.52–2.54) | 0.90 (0.49–1.67) | 1.00 (0.60–1.65) | 2.00 (0.51–7.85) |
| Biolimus | NR | 1.23 (0.25–6.15) | 5.46 (0.31–97.06) | 1.48 (0.50–4.38) | 1.15 (0.46–2.84) | 1.31 (0.65–2.64) |
| Paclitaxel | 0.97 (0.35–2.70) | 1.12 (0.43–2.87) | 0.81 (0.33–2.00) | 0.84 (0.37–1.90) | *2.32 (1.30–4.14) | 1.61 (0.55–4.71) |
| Sirolimus | 0.67 (0.17–2.60) | 1.52 (0.92–2.50) | 0.86 (0.52–1.42) | 0.84 (0.59–1.21) | 1.09 (0.81–1.46) | 0.85 (0.55–1.34) |
| Sirolimus/Probucol | 0.85 (0.47–1.52) | 0.90 (0.66–1.22) | 1.00 (0.78–1.29) | 0.87 (0.74–1.02) | 1.02 (0.84–1.23) | 1.01 (0.87–1.17) |
| DP-DES anti-proliferative drug | ||||||
| Everolimus | NR | 0.33 (0.01–8.01) | 3.00 (0.12–72.10) | NR | NR | NR |
| Paclitaxel | 0.77 (0.17–3.55) | 1.43 (0.75–2.72) | 0.97 (0.54–1.74) | 0.75 (0.48–1.19) | 1.29 (0.76–2.20) | 1.93 (0.23–3.02) |
| Sirolimus | 0.81 (0.38–1.72) | 1.03 (0.62–1.71) | 0.80 (0.31–2.07) | 0.80 (0.51–1.27) | 1.30 (0.80–2.14) | 0.86 (0.32–2.34) |
| Zotarolimus | 1.01 (0.59–1.72) | 0.99 (0.74–1.33) | 1.00 (0.79–1.28) | 0.88 (0.75–1.03) | 1.02 (0.85–1.23) | 1.01 (0.87–1.17) |
| Generation of DP-DES | ||||||
| First-generation | 0.85 (0.44–1.64) | 1.19 (0.83–1.69) | 0.91 (0.59–1.39) | 0.86 (0.65–1.14) | 1.23 (0.93–1.64) | 1.29 (0.83–2.02) |
| Second-generation | 1.01 (0.59–1.72) | 0.98 (0.73–1.32) | 1.01 (0.80–1.28) | 0.88 (0.75–1.03) | 1.02 (0.85–1.23) | 1.01 (0.87–1.17) |
| Duration of DAPT | ||||||
| 6 months | 0.85 (0.48–1.53) | 1.01 (0.75–1.38) | 0.99 (0.79–1.25) | 0.86 (0.74–1.00) | 1.18 (0.87–1.60) | 1.48 (0.87–2.51) |
| 12 months | 1.04 (0.57–1.91) | 1.12 (0.80–1.56) | 0.94 (0.57–1.56) | 0.94 (0.69–1.30) | 1.12 (0.81–1.57) | 0.86 (0.57–1.29) |
Values are risk ratio (95% confidence interval); MI = myocardial infarction; NR = not reported; PF-DES = polymer-free drug-eluting stents; TLR = target lesion revascularization; TVR = target vessel revascularization; ST = stent thrombosis; * = statistical significance (P < 0.05).
4. Discussion
The present meta-analysis of 13 RCTs compared the safety and efficacy profiles between PF-DES and DP-DES in a total of 8021 patients with coronary artery disease. Our findings demonstrated that the two stent platforms had similar rates of definite or probable ST, MI, cardiac death, all-cause death, TLR, and TVR. Overall, there were no identifiable safety and efficacy advantages of PF-DES over DP-DES. A meta-analysis [29] of 6178 patients found no significant differences between PF-DES and DP-DES for the risk of definite or probable ST at both short- (≤1 year) (odds ratio [OR], 0.95; 95% CI 0.54–1.67; P = 0.43) and long-term (>1 year) follow-up (OR, 0.75; 95% CI, 0.36–1.55; P = 0.53). Similar outcomes were observed for the risks of mortality, TLR, and TVR at both short- and long-term follow-up (all P > 0.05).
PF-DES were introduced with the aim to overcome the risks of late safety and efficacy outcomes associated with the preceding generations of devices. The polymer coating used in DP-DES has been shown to cause chronic inflammation and delayed vascular healing [30]. A meta-analysis [31] of 6575 patients reported that PF-DES had a significantly reduced risk of all-cause death compared with DP-DES (OR, 0.77; 95% CI, 0.61–0.98; P = 0.03). The present meta-analysis showed that the reduced risk of all-cause death associated with PF-DES was attenuated, and no longer reached statistical significance, with minimal heterogeneity between studies. This finding may be attributed to the inclusion of recent RCTs [23,24] comparing PF-DES with second-generation DP-DES. The new generation of DP-DES were found to have a significantly reduced risk of all-cause death compared with first-generation DP-DES (RR, 0.58; 95% CI, 0.37–0.90; P = 0.01) [32].
The polymer coating in DP-DES has multiple functions, such as stabilizing and binding the anti-proliferative drug to the stent platform and slowing down the rate of drug elution [33]. The ongoing challenge in the development of PF-DES has been maintaining these functions, while improving biocompatibility without the polymer coating. In general, PF-DES have a microporous metallic stent platform and an inorganic coating that can be loaded with an anti-proliferative drug [8]. These modifications in stent design and structure compared to preceding generations of devices will affect the elution profile of different anti-proliferative drugs [34]. Hence, we performed subgroup analyses based on PF-DES anti-proliferative drug (amphilimus, biolimus, paclitaxel, sirolimus, or sirolimus/probucol) and DP-DES anti-proliferative drug (everolimus, paclitaxel, sirolimus, or zotarolimus), which showed no significant differences between PF-DES and DP-DES for all outcomes except for TLR in PF-PES. Our subgroup analyses found that PF-PES had a significantly increased risk of TLR compared with DP-DES. This finding might not reflect a true difference due to the small sample size from two trials [20,25]. Nevertheless, a trial [35] of 1043 patients with PF-PES showed that approximately 40% of the anti-proliferative drug was lost from the stent surface during delivery of the device. This issue in drug release kinetics causes non-uniform local drug distribution, abnormal neointimal hyperplasia, and in-stent restenosis, which may contribute to the increased risk of TLR associated with PF-PES, as observed in our sensitivity analyses.
Second-generation DP-DES were shown to have improved clinical outcomes compared with first-generation DP-DES [6]. Our subgroup analysis based on generation of DP-DES found no significant differences for all outcomes. However, this finding should be interpreted with caution due to the small sample size. The comparison of safety and efficacy outcomes between PF-DES and second-generation DP-DES was derived from three trials [[22], [23], [24]].
The proposed rationale for developing PF-DES was to improve vascular healing in the stented segment, resulting in a shorter duration of DAPT following stent implantation [36]. However, our subgroup analysis based on duration of DAPT (6 months or 12 months) found no significant differences between PF-DES and DP-DES for all outcomes. The latest American College of Cardiology and American Heart Association (ACC/AHA) guidelines [7] have reduced the recommended duration of DAPT from 12 months to 6 months in patients receiving DES. There have been suggestions that short-term (1 month) DAPT may be safe following PF-DES implantation [37]. The LEADERS FREE trial [38] of 2466 patients found that PF-DES were superior to BMS with respect to primary safety endpoint (composite of definite or probable ST, MI, and cardiac death; hazards ratio [HR], 0.80; 95% CI, 0.64–0.99; P < 0.039) and primary efficacy endpoint (TLR; HR, 0.54; 95% CI, 0.41–0.72; P < 0.0001) when used with 1-month DAPT. The elimination of the polymer coating in PF-DES is thought to reduce the risk of very late ST compared with DP-DES. However, this theoretical benefit was not demonstrated at 1 year, 2 years, and 5 years of follow-up, as well as in our landmark analysis beyond 1 year of follow-up, which showed no significant difference between PF-DES and DP-DES for the risk of definite or probable ST.
In the present meta-analysis, electronic database searches were limited to RCTs to reduce the risk of bias. However, there were several limitations that deserve consideration. Firstly, as with any meta-analysis, our study should be interpreted in light of the limitations in design and quality of the original studies, which often compared stents with various anti-proliferative drugs, durations of DAPT, and lengths of follow-up. To address this source of bias, we performed subgroup analyses based on these variables. A network meta-analysis comparing different types of DES may be considered for future research when further trials become available. Secondly, the original studies did not report outcomes according to baseline characteristics, so we were unable to perform subgroup analyses to determine if these variables might influence the results. Thirdly, the exclusion of data from unpublished RCTs may have reduced the potential number of patients in each comparison arm. Fourthly, some reports of the original studies did not describe the randomization techniques, which made it difficult to assess the risk of bias. Finally, trials with greater length of follow-up in larger number of patients are necessary to evaluate the long-term safety and efficacy profiles of PF-DES compared with DP-DES. Further trials with shortened duration of DAPT are warranted to realize the theoretical benefits of PF-DES.
5. Conclusions
In summary, our findings demonstrated similar safety and efficacy profiles between PF-DES and DP-DES. The two stent platforms had equivalent safety and efficacy outcomes, including comparable rates of definite or probable ST.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Provenance and peer review
Not commissioned externally peer reviewed.
Ethical approval
This research does not contain any studies with human participants or animals.
Sources of funding
None declared.
Author contribution
James Wu: study design, data collection, data analysis, writing.
Joshua Way: study design, data collection, data analysis, writing.
Leonard Kritharides: study design, writing.
David Brieger: study design, writing.
Conflicts of interest
None declared.
Research registration number
reviewregistry619.
Guarantor
James Wu.
David Brieger.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2018.12.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Iqbal J., Gunn J., Serruys P.W. Coronary stents: historical development, current status, and future directions. Br. Med. Bull. 2013;106:193–211. doi: 10.1093/bmb/ldt009. [DOI] [PubMed] [Google Scholar]
- 2.Goto K., Zhao Z., Matsumura M., Dohi T., Kobayashi N., Kirtane A.J. Mechanisms and patterns of intravascular ultrasound in-stent restenosis among bare metal stents and first- and second-generation drug-eluting stents. Am. J. Cardiol. 2015;116(9):1351–1357. doi: 10.1016/j.amjcard.2015.07.058. [DOI] [PubMed] [Google Scholar]
- 3.Holmes D.R., Kereiakes D.J., Garg S., Serruys P.W., Dehmer G.J., Ellis S.G. Stent thrombosis. J. Am. Coll. Cardiol. 2010;56(17):1357–1365. doi: 10.1016/j.jacc.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Joner M., Finn A.V., Farb A., Mont E.K., Kolodgie F.D., Ladich E. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 2006;48(1):193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 5.Dores H., Raposo L., Teles R.C., Machado C., Leal S., Gonçalves P.A. Stent thrombosis with second- versus first-generation drug-eluting stents in real-world percutaneous coronary intervention: analysis of 3806 consecutive procedures from a large-volume single-center prospective registry. J. Invasive Cardiol. 2013;25(7):330–336. [PubMed] [Google Scholar]
- 6.Navarese E.P., Kowalewski M., Kandzari D., Lansky A., Górny B., Kołtowski L. First-generation versus second-generation drug-eluting stents in current clinical practice: updated evidence from a comprehensive meta-analysis of randomised clinical trials comprising 31,379 patients. Open Heart. 2014;1(1) doi: 10.1136/openhrt-2014-000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine G.N., Bates E.R., Bittl J.A., Brindis R.G., Fihn S.D., Fleisher L.A. ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelies. Circulation. 2016;134(10):e123–e155. doi: 10.1161/CIR.0000000000000404. 2016. [DOI] [PubMed] [Google Scholar]
- 8.Byrne R.A., Iijima R., Mehilli J., Pinieck S., Bruskina O., Schömig A. Durability of antirestenotic efficacy in drug-eluting stents with and without permanent polymer. JACC Cardiovasc. Interv. 2009;2(4):291–299. doi: 10.1016/j.jcin.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Chen W., Habraken T.C., Hennink W.E., Kok R.J. Polymer-free drug-eluting stents: an overview of coating strategies and comparison with polymer-coated drug-eluting stents. Bioconjug. Chem. 2015;26(7):1277–1288. doi: 10.1021/acs.bioconjchem.5b00192. [DOI] [PubMed] [Google Scholar]
- 10.Chen D., Jepson N. Coronary stent technology: a narrative review. Med. J. Aust. 2016;205(6):277–281. doi: 10.5694/mja16.00444. [DOI] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 12.Cutlip D.E., Windecker S., Mehran R., Boam A., Cohen D.J., van Es G.A. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 13.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne J.A., Egger M., Smith G.D. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323(7304):101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrne R.A., Kastrati A., Tiroch K., Schulz S., Pache J., Pinieck S. 2-year clinical and angiographic outcomes from a randomized trial of polymer-free dual drug-eluting stents versus polymer-based Cypher and Endeavor drug-eluting stents. J. Am. Coll. Cardiol. 2010;55(23):2536–2543. doi: 10.1016/j.jacc.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Byrne R.A., Kufner S., Tiroch K., Massberg S., Laugwitz K.L., Birkmeier A. Randomised trial of three rapamycin-eluting stents with different coating strategies for the reduction of coronary restenosis: 2-year follow-up results. Heart. 2009;95(18):1489–1494. doi: 10.1136/hrt.2009.172379. [DOI] [PubMed] [Google Scholar]
- 18.Carrié D., Berland J., Verheye S., Hauptmann K.E., Vrolix M., Violini R. A multicenter randomized trial comparing amphilimus- with paclitaxel-eluting stents in de novo native coronary artery lesions. J. Am. Coll. Cardiol. 2012;59(15):1371–1376. doi: 10.1016/j.jacc.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Costa R.A., Abizaid A., Mehran R., Schofer J., Schuler G.C., Hauptmann K.E. Polymer-free Biolimus A9-coated stents in the treatment of de novo coronary lesions: 4- and 12-month angiographic follow-up and final 5- year clinical outcomes of the prospective, multicenter BioFreedom FIM clinical trial. JACC Cardiovasc. Interv. 2016;9(1):51–64. doi: 10.1016/j.jcin.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Dang Q., Li Y.J., Gao L., Jin Z., Gou L.X. Six-month angiographic and one-year clinical outcomes of polymer free paclitaxel-eluting stent in patients with ST-segment elevation myocardial infarction: a comparison with permanent polymer sirolimus-eluting stent. Chin. Med. J. (Engl). 2012;125(19):3393–3397. [PubMed] [Google Scholar]
- 21.King L., Byrne R.A., Mehilli J., Schömig A., Kastrati A., Pache J. Five-year clinical outcomes of a polymer-free sirolimus-eluting stent versus a permanent polymer paclitaxel-eluting stent: final results of the intracoronary stenting and angiographic restenosis - test equivalence between two drug-eluting stents (ISAR-TEST) trial. Cathet. Cardiovasc. Interv. 2013;81(1):E23–E28. doi: 10.1002/ccd.24375. [DOI] [PubMed] [Google Scholar]
- 22.Kufner S., Sorges J., Mehilli J., Cassese S., Repp J., Wiebe J. Randomized trial of polymer-free sirolimus- and probucol-eluting stents versus durable polymer zotarolimus-eluting stents: 5-year results of the ISAR-TEST-5 trial. JACC Cardiovasc. Interv. 2016;9(8):784–792. doi: 10.1016/j.jcin.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Romaguera R., Gómez-Hospital J.A., Gomez-Lara J., Brugaletta S., Pinar E., Jiménez-Quevedo P. A randomized comparison of reservoir-based polymer-free amphilimus-eluting stents versus everolimus-eluting stents with durable polymer in patients with diabetes mellitus: the RESERVOIR clinical trial. JACC Cardiovasc. Interv. 2016;9(1):42–50. doi: 10.1016/j.jcin.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Rozemeijer R., Stein M., Voskuil M., van der Bor R., Frambrach P., Pereira B. Randomized all-comers evaluation of a permanent polymer zotarolimus-eluting stent versus a polymer-free amphilimus-eluting stent: (ReCre8) a multicenter, non-inferiority trial. Circulation. 2018 doi: 10.1161/CIRCULATIONAHA.118.037707. [DOI] [PubMed] [Google Scholar]
- 25.Shiratori Y., Cola C., Brugaletta S., Alvarez-Contreras L., Martin-Yuste V., del Bianco B.G. Randomized comparison between polymer-free versus polymer-based paclitaxel-eluting stent: two-year final clinical results. Circ. Cardiovasc. Interv. 2014;7(3):312–321. doi: 10.1161/CIRCINTERVENTIONS.113.000800. [DOI] [PubMed] [Google Scholar]
- 26.Stiermaier T., Heinz A., Schloma D., Kleinertz K., Dänschel W., Erbs S. Five-year clinical follow-up of a randomized comparison of a polymer-free sirolimus-eluting stent versus a polymer-based paclitaxel-eluting stent in patients with diabetes mellitus (LIPSIA Yukon trial) Cathet. Cardiovasc. Interv. 2014;83(3):418–424. doi: 10.1002/ccd.25131. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y., Chen F., Muramatsu T., Xu B., Li Z., Ge J. Nine-month angiographic and two-year clinical follow-up of polymer-free sirolimus-eluting stent versus durable-polymer sirolimus-eluting stent for coronary artery disease: the Nano randomized trial. Chin. Med. J. (Engl). 2014;127(11):2153–2158. [PubMed] [Google Scholar]
- 28.Zhang Y., Shen J., Li Z., Zhu A., Yuan Y., Yue R. Two-year clinical outcomes of different drug-eluting stents with different polymer coating strategies in coronary artery heart disease: a multi-centre, randomised, controlled clinical trial. Int. J. Cardiol. 2013;168(3):2646–2652. doi: 10.1016/j.ijcard.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 29.Navarese E.P., Kowalewski M., Cortese B., Kandzari D., Dias S., Wojakowski W. Short and long-term safety and efficacy of polymer-free vs. durable polymer drug-eluting stents. A comprehensive meta-analysis of randomized trials including 6178 patients. Atherosclerosis. 2014;233(1) doi: 10.1016/j.atherosclerosis.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 30.Wilson G.J., McGregor J., Conditt G., Shibuya M., Sushkova N., Eppihimer M.J. Impact of bioresorbable versus permanent polymer on longterm vessel wall inflammation and healing: a comparative drug-eluting stent experimental study. EuroIntervention. 2018;13(14):1670–1679. doi: 10.4244/EIJ-D-17-00332. [DOI] [PubMed] [Google Scholar]
- 31.Wu D., Yu M., Gao H., Zhang L., Song F., Zhang X. Polymer-free versus permanent polymer drug-eluting stents in coronary artery disease: a meta-analysis of 10 RCTs with 6575 patients. Chronic. Dis. Transl. Med. 2015;1(4):221–231. doi: 10.1016/j.cdtm.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan P., Dong P., Li Z. Second- versus first-generation drug-eluting stents for diabetic patients: a meta-analysis. Arch. Med. Sci. 2014;10(2):213–221. doi: 10.5114/aoms.2014.42571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acharya G., Park K. Mechanisms of controlled drug release from drug-eluting stents. Adv. Drug Deliv. Rev. 2006;58(3):387–401. doi: 10.1016/j.addr.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Hsiao H.M., Chiu Y.H., Wu T.Y., Shen J.K., Lee T.Y. Effects of through-hole drug reservoirs on key clinical attributes for drug-eluting depot stent. Med. Eng. Phys. 2013;35(7):884–897. doi: 10.1016/j.medengphy.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 35.Lansky A.J., Costa R.A., Mintz G.S., Tsuchiya Y., Midei M., Cox D.A. Non-polymer-based paclitaxel-coated coronary stents for the treatment of patients with de novo coronary lesions: angiographic follow-up of the DELIVER clinical trial. Circulation. 2004;109(16):1948–1954. doi: 10.1161/01.CIR.0000127129.94129.6F. [DOI] [PubMed] [Google Scholar]
- 36.Gibson C.M. Going polymer free and dual antiplatelet free earlier: the coevolution of stent and pharmacotherapy. J. Am. Coll. Cardiol. 2017;69(2):172–175. doi: 10.1016/j.jacc.2016.10.056. [DOI] [PubMed] [Google Scholar]
- 37.Onuma Y., Serruys P.W. Freedom from long DAPT signals the demise of the bare-metal stent. Eur. Heart J. 2017;38(13):970–973. doi: 10.1093/eurheartj/ehw291. [DOI] [PubMed] [Google Scholar]
- 38.Garot P., Morice M.C., Tresukosol D., Pocock S.J., Meredith I.T., Abizaid A. 2-year outcomes of high bleeding risk patients after polymer-free drug-coated stents. J. Am. Coll. Cardiol. 2017;69(2):162–171. doi: 10.1016/j.jacc.2016.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.