Abstract
The Epstein-Barr virus (EBV) is a B-lymphotropic gamma herpes virus associated with a number of malignancies. Most EBV-related cancers present complex medical management challenges; thus it has been essential to develop preclinical in vivo models allowing for the study of pathogenesis, prevention, and treatment of these diseases. Early in vivo models used nonhuman primates; however, such models were limited by the inability of EBV to achieve viral latency, availability, and cost. Immunodeficient mouse strains emerged as efficient models that allow for engraftment of human mononuclear cells and controlled evaluation of EBV-driven lymphoproliferative disease (EBV-LPD). By using highly immunodeficient strains of mice such as severe combined immune deficiency (SCID) and NOD/LtSz-scid ILrg−/− (NOG) mice, investigators have developed efficient platforms for evaluating pathogenesis of benign (HLH) and malignant (EBV-LPD) diseases associated with EBV. Humanized murine chimeric models have been essential tools for evaluating preventive strategies with vaccine and adoptive cellular approaches, as well as development of experimental therapeutic strategies. Manipulation of the human immune cells before engraftment or mutation of viral lytic and latent genes has enhanced our understanding of the oncogenic nature of EBV and the complexity of human immune responses to EBV. In this review, we discuss how the EBV murine models have evolved to become essential tools for studying the virology of EBV as it relates to human EBV-LPD pathogenesis, the immunobiology of innate and adaptive responses, and limitations of these models.
Keywords: Epstein-Barr virus, hu-PBL-SCID, humanized mice, mouse models, review
Introduction
The Epstein-Barr virus (EBV) is a human B-lymphotrophic gamma herpes virus that infects more than 90% of the world's population. Infection with EBV can lead to benign conditions such as infectious mononucleosis or malignant diseases such as Burkitt's lymphoma, nasopharyngeal carcinoma, Hodgkin's lymphoma, and post-transplant lymphoproliferative disorders (PTLD) (Cohen 2000). EBV-associated lymphoproliferative disorders (EBV-LPD) represent a heterogeneous spectrum of diseases that share a great degree of complexity involving viral (latent, lytic gene expression) and host (immunity, signal transduction, epigenetics) factors. Thus it has become essential to develop in vivo, preclinical models to allow for a comprehensive, integrated approach to evaluate pathogenesis and experimental therapeutic strategies of these diseases.
Several features of EBV-LPD have presented challenges to the development of useful animal models that provide an accurate representation of the human disease. These challenges include (1) narrow tropism for human B lymphocytes, (2) biphasic latent and lytic virus cycles, and (3) relevance of innate and adaptive immune networks to disease surveillance and pathogenesis. To meet such challenges, various strains of immunodeficient mice engrafted with human tissues and/or lymphocytes have emerged to provide scientists with tools for modeling EBV-LPD. The latent nature of EBV within the memory B cell compartment has led to development of humanized murine models that generate spontaneous EBV-LPD. Other models that focus on active infection of engrafted EBV-negative systems have also provided the scientific community with the opportunity to evaluate EBV-LPD pathogenesis and immune-based preventive and therapeutic approaches.
EBV and Associated Diseases
Denis Burkitt, a British surgeon working in Africa in the 1950s, observed the increased frequency of childhood lymphoma in equatorial Africa, where malaria was known to be endemic. This observation led him to suggest a relationship linking endemic lymphoma with vector-borne agents (Burkitt 1962). The quest for infectious agents led to the discovery of herpesvirus particles from several Burkitt's lymphoma biopsies by Epstein, Barr, and Achong (Epstein et al. 1965). This work led to EBV being the first human oncogenic herpesvirus to be described. Subsequent serologic studies identified EBV as the etiologic agent of infectious mononucleosis (Henle et al. 1968).
EBV belongs to the gamma subgroup of herpesviruses that includes two distinct genera: gamma 1 (Lymphocryptovirus) and gamma 2 (Rhadinovirus). EBV is a member of the gamma 1 herpesvirus genus that demonstrates a narrow tropism to naive human B lymphocytes. The EBV genome is approximately 172,000 base pairs in length and can assume both linear (in lytic activation) and episomal (latent) conformations. The virus genome contains terminal repeat sequences that mediate circularization during latent infection, a feature that can be used to examine the clonality of EBV-LPD (Raab-Traub and Flynn 1986).
Primary infection with EBV occurs in epithelial cells of the nasopharynx where the virus ultimately gains access to naive B cells in tonsillar lymphoid tissue. Infection of B cells occurs by binding of the viral glycoprotein (gp) 350 to CD21 and Human Leukocyte Antigen (HLA) class II molecules on the B cell surface. This typically results in latent infection, and the virus expresses genes of the viral latency III program where nine EBV latent proteins (EBV nuclear antigen [EBNA] 1, 2, 3A, 3B, 3C, and LP, and latent membrane protein [LMP] 1, 2A, and 2B) are expressed. Latency III infection drives B cell immortalization and proliferation of B lymphoblasts that eventually differentiate into memory B cells through the germinal center reaction. Primary infection leads to expression of oncogenic latent gene products that drive a self-limited lymphoproliferative disorder (infectious mononucleosis in some individuals); however, latency III gene products are also highly immunogenic and serve as primary targets for an efficient adaptive immune response (Paludan and Munz 2003). Although humans have evolved to control EBV infection, EBV ultimately establishes lifelong persistence within the host memory B cell compartment. Once memory B cells exit the germinal center, the viral genome becomes heavily methylated, which leads to transcriptional silencing of all latency proteins (latency 0) or limited expression of EBNA1 that effectively maintains the EBV episome (latency 1) and promotes its replication and passage to B proliferation of memory B cells. In the germinal centers transition, EBV expresses latency II, and only three latency proteins are expressed—EBNA1, LMP1, and LMP2A (Heslop 2009). The EBV noncoding RNAs (EBERs) are abundantly expressed in all EBV latency states and serve as specific biomarkers to identify EBV-LPDs.
Persistence and reactivation are hallmarks of EBV survival within its human host; a coexistence has evolved in immunocompetent humans. However, in select populations (Asian, immunodeficient), EBV can transform B lymphocytes and epithelial cells. Each EBV-associated disease demonstrates distinct patterns of latent gene expression (Table 1). Up to 200,000 new cases of EBV-related malignances are reported each year, and the virus has been estimated to cause 2% of all human cancers (Cohen et al. 2011). The prevalence of EBV-associated malignancies varies with geographical region, individual age, and immunocompetent status. (Andreone et al. 2003; Deeken et al. 2012; Grufferman and Delzell 1984; Kotton and Fishman 2005; Parkin 2006).
Table 1.
Epstein-Barr virus (EBV) latency programs, patterns of EBV latency genes expressed, and EBV-associated diseases
| Latency program | Gene expressed | EBV-associated diseases |
|---|---|---|
| Latency 0 | EBERs | None |
| Latency I | EBERs and EBNA1 | Burkitt's lymphoma Gastric carcinoma |
| Latency II | EBERs, EBNA1, and LMP2 | Hodgkin's lymphoma Nasopharyngeal carcinoma |
| Latency III | EBERs, EBNA-1, -2, -3A, -3B, -3C, and -LP, LMP1, LMP2 | Infectious mononucleosis Post-transplantation lymphoproliferative disorders |
EBER, EBV noncoding RNAs; EBNA, EBV nuclear antigen; LMP, latent membrane protein.
Early Preclinical Models of EBV-Associated Diseases
Although it is clear that EBV demonstrates a narrow tropism for humans, antibodies specific for various EBV-encoded proteins have been detected in nonhuman primate species. This led to the discovery that several nonhuman primates harbor viruses belonging to the gamma 1 herpesvirus genus and are homologues to EBV (Mosier 1996). Certain New World monkeys, such as the common marmoset (Callithrix jacchus) and cotton-top tamarin (Saguinus oedipus) are susceptible to experimental infection with EBV. Experimental infection of the common marmoset with EBV led to virus persistence in the peripheral blood leukocytes (PBLs) of infected animals. These animals developed an infectious mononucleosis–like syndrome with B cell lymphocytosis but did not go on to develop EBV-LPD (Farrell et al. 1997). Cotton-top tamarins infected with EBV developed EBV-LPD with unrestricted viral latency (Miller et al. 1977). These early findings led to attempts to develop vaccination strategies using the cotton-top tamarin as a host to deliver the lytic glycoprotein gp350. Although this approach led to protection against developing EBV-driven lymphomas, it had no prophylactic effect in preventing EBV infection (Sokal et al. 2007). The endangered status of such nonhuman primate species, high cost, limited availability, and inability of EBV to establish latency in the B cell compartment led investigators to explore other model systems.
Rodents are the preferred models in biomedical research because they are small in size, inexpensive, and easier to manage. However, rodents are not susceptible to EBV infection, and there are unknown lymphcryptoviruses in rodents (Davison 2010). The closest rodent virus to EBV is the murine ɣ-herpes virus 68 (MHV-68) (Ehlers et al. 2007; Flano et al. 2002). Yet, MHV-68 is molecularly distinct from EBV, and the host immune responses toward the two viruses are also different (Ehlers et al. 2010). In the early 1980s, Bosma and colleagues led the field in the discovery and experimental use of immunodeficient mice for studying cancer and infectious diseases (Bosma et al. 1983; Bosma and Carroll 1991). These early models of immunodeficient mice, which lacked sufficient adaptive cellular immune subsets (B and T lymphocytes), were found to be incapable of rejecting xenogeneic cellular and tissue grafts, including human mature PBLs, human hematopoietic precursor cells (HPCs), and fetal tissues.
Emergence of Murine Models to Study EBV-LPD
Severe Combined Immunodeficient Mouse Xenograft Model
Development of the hu-PBL-SCID Model
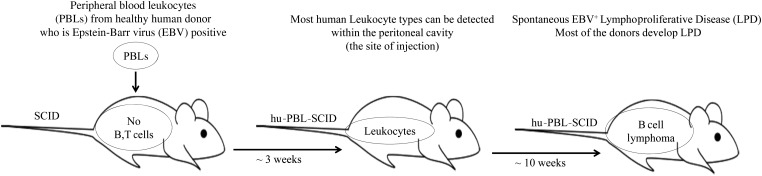
The severe combined immunodeficiency (SCID) mutation is an autosomal recessive mutation that impairs the rearrangement or repair of B and T cell receptors; therefore mice with the scid/scid phenotype lack mature B and T cells (Bosma et al. 1983). Thus these mice were found to readily accept cross-species tissue grafts (xenografts). Intraperitoneal injection of human PBL in the CB17 in-bred strain with the SCID mutation allowed for engraftment of human immune cells as measured by the detection of human immunoglobulin (Mosier et al. 1988). The hu-PBL-SCID model was originally developed out of the need to create a small animal model to study human immunodeficiency virus (HIV) biology and potential therapeutic approaches. Early studies, however, revealed human-chimeric mice to show evidence of EBV reactivation, presumably from co-engrafted memory B lymphocytes. Several groups followed up on these findings and found that some hu-PBL-SCID mice eventually developed spontaneous human, EBV+ B cell LPDs (Cannon et al. 1990; Mosier et al. 1992; Okano et al. 1990) (Figure 1), indicating that perhaps this model would be attractive for studying the pathogenesis of EBV-LPD.
Figure 1.
The hu-PBL-SCID model.
Most mononuclear cell subsets can be detected in the intraperitoneal space up to three weeks after engraftment but not in murine thymus or secondary lymphoid organs. Four weeks after injection of PBLsC, human T and B lymphocytes are the only cell types that can be recovered from murine spleen, liver, lung, and mesenteric lymph nodes (Tary-Lehmann et al. 1995). More than 90% of engrafted cells were T lymphocytes (including both CD4+ and CD8+ subsets) that appeared to express a mature activated phenotype (HLA-DR+ and CD45RO+) and show specificity for mouse major histocompatibility complex (MHC) antigens (Tary-Lehmann et al. 1994). Engrafted T cells recovered from SCID mice were found to be anergic; however, after prolonged culture in low-dose interleukin 2 (IL-2), these cells showed capacity to regain antigen responsiveness (Tary-Lehmann and Saxon 1992; Tary-Lehmann et al. 1995).
EBV-LPD in the hu-PBL-SCID Model
Injection of human PBLs from EBV-seropositive donors into the SCID mice produces spontaneous EBV-associated LPD of human B cell origin (Mosier et al. 1990). EBV-LPD in SCID mice is reproducible, and it is highly analogous to B cell lymphomas that arise in organ transplant recipients and acquired immune deficiency syndrome patients (Rowe et al. 1991). B cell tumors in chimeric mice are either monoclonal, oligoclonal, or polyclonal in nature. Interestingly, the clonality and efficiency of lymphomagenesis is highly variable among donors. Mononuclear cells from donors that are EBV seropositive do not give rise to EBV-LPD with equal efficiency. Donors can be categorized into three types: high incidence (75–100% engrafted mice develop EBV-LPD), intermediate incidence (10–74% EBV-LPD), and no incidence (0% EBV-LPD) (Picchio et al. 1992). The tumors are characterized by up-regulation of B cell activation surface antigens such as CD23 and CD39 and expression of adhesion molecules ICAM-1 and LFA-3. The disease is often highly disseminated in nature, involving the spleen, liver, bone marrow, and occasionally the central nervous system. Karyotypic analysis of the tumor cells reveals no chromosomal abnormalities. The Latency III program, where nine latent genes are expressed, is observed universally (Rowe et al. 1991).
EBV Pathogenesis in the hu-PBL-SCID Model
The lymphomagenesis in hu-PBL-SCID mice is a virus-driven process, presumably involves activation of B cells with differentiation into plasma cells and release of infectious virions that can infect bystander B cells (Mosier 1996). Outgrowth of latently infected, transformed B cells leads to lymphomagenesis that can be polyclonal, oligoclonal, or monoclonal in nature. In high-incidence donors, efficient EBV-LPD outgrowth is almost certainly due to deficits in human adaptive cellular surveillance. CD4+ Th cells in particular have been found to provide vital support of efficient lymphomagenesis by driving B cell activation, immunoglobulin production, and, ultimately, transformation (Baiocchi and Caligiuri 1994; Veronese et al. 1992). Cell depletion experimentations showed that injection of purified B cells only were poorly engrafted and failed to produce tumors in SCID mice (Veronese et al. 1992). Efficiency of lymphomagenesis was also impaired in presence of immunosuppressive agents such as cyclosporine A (CsA), which prevented T cell activation in the xenogeneic environment (Coles et al. 1994). In vitro and/or in vivo treatments with anti-CD3 immunotoxin also significantly reduced EBV-LPD development (Clinchy and Vitetta 1998). Moreover, tumor incidence is significantly reduced when CD4+ T helper cells are removed from the PBL inoculum (Baiocchi and Caligiuri 1994; Johannessen et al. 2000; Veronese et al. 1992).
After tumor formation, malignant B cells become independent of T cell help and produce their own growth factors that drive proliferation through an autocrine fashion. In situ studies confirmed that cytokines such as IL-2, IL-4, IL-6, IL-10, and interferon ɣ (IFNɣ) are expressed by the tumor cells themselves (Johannessen et al. 2000). The hu-PBL-SCID mice that developed EBV-LPD showed significant elevation of serum huIL-10 levels compared with mice that did not develop EBV-LPD (P = .005). The EBV+ tumor samples expressed transcript for huIL-10 and huIL-10 receptor, expressed huIL-10 protein by immunohistochemical staining, and showed specific binding of recombinant (r) huIL-10. IL-10 prevented programmed cell death and promoted proliferation of EBV-transformed ex vivo tumor cells at concentrations found in vivo. Thus, huIL-10 [and perhaps IL-6; unpublished data (R. Baiocchi)] production by EBV+ tumor cells may contribute directly to malignant outgrowth of EBV-LPD in this model (Baiocchi et al. 1995).
The hu-PBL-SCID Model as a Platform for Targeted and Immunomodulatory Therapy
The hu-PBL-SCID model has been particularly useful to assess novel preventive and treatment approaches for EBV-LPD, some of which have been translated to the clinical setting. The antiviral agent ganciclovir (GCV) has been tested in SCID mice and showed an inhibitory effect on tumor formation in treated mice. The drug inhibited B cell lymphoma development after active EBV infection (Boyle et al. 1992), a strategy that is commonly used in patients with PTLD. This preclinical work has led to translation of a targeted antiviral approach in patients with primary central nervous system PTLD (Roychowdhury et al. 2003) and systemic PTLD using the histone deacetylase (HDAC) inhibitor sodium butyrate to induce lytic gene activation, which allows for targeting of the EBV protein kinase BGLF4 by GCV (Perrine et al. 2007).
Utility of adoptive cellular therapy with EBV-specific cytotoxic T lymphocytes (CTLs) has been evaluated in the hu-PBL-SCID model. Intravenous or intraperitoneal injections of EBV-CTLs prevent or delay EBV-LPD in the hu-PBL-SCID model (Boyle et al. 1993). Autologous CTLs activity against EBV-related lymphoma is HLA-restricted and EBV-specific as shown by CTL chemotaxis to autologous tumors in SCID mice (Lacerda et al. 1996). Although the majority of EBV-specific CTLs were characterized as CD3+/CD8+/CD4− T cells (Lacerda and O'Reilly 1997), the protective effect of these effector cells was reliant on the presence of helper CD4+ T cells. Enhanced protection has also been reported when mice were treated with autologous EBV-specific CTLs and polyethylene glycol-modified recombinant human IL-2 (PEG-IL-2) (Buchsbaum et al. 1996).
The hu-PBL-SCID model has served as a useful platform to experimentally test cytokine immunotherapy. Daily injection of low-dose PEG-IL-2 (500 IU, subcutaneous, twice a day) into SCID mice engrafted with human PBLs from EBV+ high-incidence donors prevented EBV-LPD in more than 75% of engrafted animals. Murine natural killer (NK) cells were necessary for this PEG-IL-2–mediated protection outcome because protection was lost when murine NK cells were depleted. Moreover, human CD8+ T cells offered the cellular immunity required for PEG-IL-2–mediated protective effect against EBV-LPD in the model (Baiocchi and Caligiuri 1994). Another cytokine immunotherapy experimentally tested in the hu-PBL-SCID model was combination granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-2. Treatment with GM-CSF and IL-2 promoted the expansion of EBV-specific CD8+ T cells in vivo (Baiocchi et al. 2001), and the combination cytokine therapy was capable of prolonging survival even in the absence of murine NK cells. Experimental depletion of human leukocyte subsets from the PBLs demonstrated that human NK cells, CD8+ T cells, and monocytes were essential for the protective effects of the combination cytokine therapy (Baiocchi et al. 2001).
Limitations of the hu-PBL-SCID Model
The hu-PBL-SCID model has several limitations. There is variation in the number of human cells that can repopulate the SCID mice engrafted with human PBLs (Mosier et al. 1988). Moreover, the SCID mice in the model have intact innate immunity, which can compromise engraftment of human cells. The level of human cell engraftment is very low, with only 0.01–0.1% of human cells being detected in peripheral blood and tissues (Tary-Lehmann et al. 1995). Additionally, transfer of mature human cells is not always well tolerated and can lead to graft-versus-host disease (Murphy et al. 1992). Furthermore, T cells engrafted in this model fail to function without exogenous immune-based support (i.e., cytokine/antibodies). Finally, the variation in EBV-LPD among donors makes it difficult to establish a consistent pool of donors for reliable, reproducible results.
New Generation Humanized Mouse Models of EBV-LPD
Background Strains and Model Types
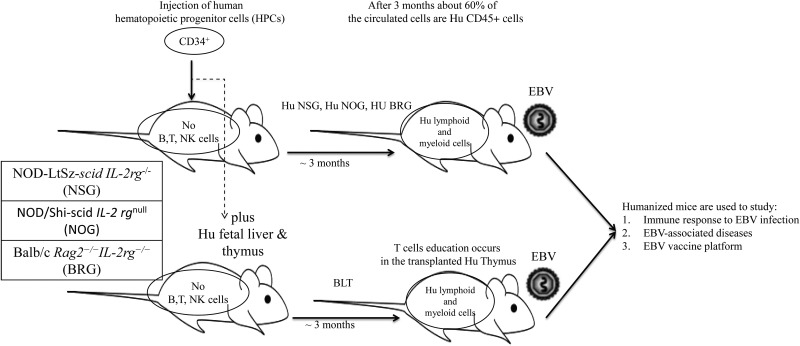
The two main background strains of mice used in the humanized mouse model for EBV infection are the Bagg albino (BALB/c) and nonobese diabetic (NOD) mice. Genetic manipulations such as scid gene (Bosma et al. 1983) and the recombination-activating gene (rag) mutations were introduced to the recipient strains to facilitate engraftment of human cells and to enhance the function of engrafted cells. The Rag proteins are essential for V(D)J recombination in early stages of lymphoid development. Mutation in the rag genes prevents mouse B and T cells somatic recombination (Mombaerts et al. 1992; Shinkai et al. 1992). Additional genetic alterations added to these background strains included the common gamma chain mutation (Shultz et al. 2007). The common gamma chain is found in many cytokine and cell signaling receptors, and knockout mutations of this gene eliminate signaling from IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21, which severely impairs the development of innate and adaptive immunity as well as neutrophils and dendritic cells (DCs). The most frequent strains with multiple mutations used for EBV infection modeling are NOD-LtSz-scid IL-2rg−/− (NSG), NOD/Shi-scid IL-2rgnull (NOG), and Balb/c Rag2−/−IL-2rg−/− (BRG) (Munz 2015) (Figure 2).
Figure 2.
Humanized mice model of Epstein-Barr virus (EBV) infection.
Mice in the EBV humanized mouse model are injected with human CD34+ hematopoietic progenitor cells (HPCs) and subsequently infected with EBV. Unlike mature blood cells engraftment, the naive human cells are well tolerated in the mice and almost universally EBV negative. A robust immunocompetent model of humanized mice was generated by transplantation of fetal liver and thymus organoids under the kidney capsule along with the HPCs from the autologous fetal liver (BLT model) (Melkus et al. 2006). An earlier model using fetal tissue transplantation is the SCID-Hu mice (McCune et al. 1988) led to sluggish peripheral blood engraftment. Injection of human HPCs in the BLT model can overcome the difficulty of robust peripheral engraftment encountered with the SCID-Hu mice model. Development of human T cells in the BLT model shows efficient and intact HLA restriction, which enhances the adaptive immune responses (Figure 2). However, the BLT mouse strain is technically very challenging to develop and maintain, is expensive, and is available in a limited number of states given legislation precluding use of such models.
Human Immune System Components Reconstituted
Transplantation of human CD34+ HPCs into SCID mice leads to reconstitution of human myeloid and lymphoid cells approximately three months after engraftment. Approximately 60% of the cells in the peripheral blood and spleen of engrafted mice are CD45+ human cells (Pearson et al. 2008). The B and T cells comprise the majority of human mononuclear cells found reconstituted in engrafted mice (up to 90%); however the B cell to T cell ratio is aberrantly skewed. The B cell to T cell ratio has been reported to be up to 1:2 (Strowig et al. 2009). The majority of T cells are CD4+ cells, and all subsets of the T lymphocytes were detected, including αβ and γδ T cells, as well as earlier thymic T cell stages (Traggiai et al. 2004). Other human cells reconstituted in the model are NK cells (approximately 5%), monocytes (approximately 3%), and DCs (approximately 2%).
Variation in engraftment rates of human cells have been reported and are likely reflective of differences in the percentage of CD34+ HPCs across the donor pool. Additionally there are variations in the source of human CD34+ HPCs, including cord blood, adult peripheral blood, bone marrow, or human fetal liver. An additional variable in these models includes mouse age, with higher level of T cells engraftment reported in newborn mice compared with adult animals (Pearson et al. 2008). Moreover, different murine strains demonstrated variation in percentages of human cell reconstitution. For example, myeloid cells in NOD mice (in contrast with BALB/c mice) express the myeloid cell inhibitory receptor, signal regulatory protein α (SIRP-α). The SIRP-α receptor promotes the development of a human bone marrow–like microenvironment, which allows for recognition of the human leukocyte surface antigen CD47 on engrafted human cells. This association between engrafted cell populations and microenvironment prevents phagocytosis by murine myeloid cells and ultimately enhances the efficiency of reconstitution in vivo (Takenaka et al. 2007). Human cells reconstitution in BRG mice is only 20% of the total cells in peripheral blood and spleen (Takenaka et al. 2007). However, more recent work evaluating genetic manipulations of the SIRP-α gene in BALB/c background mice overcome this issue (Strowig et al. 2011).
Immune Responses to EBV in Humanized Mice
Humanized mice are capable of mounting both innate and adaptive immune responses in the context of EBV infection (Yajima et al. 2008). Innate immunity, driven in large part by NK cells, is a critical factor in controlling EBV lytic replication and limiting the virus's ability to infect neighboring bystander B cells. Antibody depletion of NK cells from humanized mice infected with EBV increased the level of EBV lytic replication and led to a higher rate of EBV-LPD compared with controls. Lymphomas developing in NK-depleted mice are often monoclonal and widely disseminated throughout the animals (Chijioke et al. 2013). Interestingly, NK depletion showed no effect on latent EBV infection. No significant difference was observed between NK depletion and nondepletion groups in tumor formation when mice were infected with an EBV mutant strain lacking the key lytic switch gene, BZLF1 (Chijioke et al. 2013).
Humoral immune responses in humanized mice are very limited because most B lymphocytes are naive or in transitional stage upon engraftment. There is no development of human secondary lymphoid organs in humanized mice, which diminishes an efficient germinal center, somatic hypermutation, and class switching. Upon EBV infection, humanized mice can only generate EBV-specific immunoglobulin M (IgM) antibody and mainly against the EBV BFRF3 protein (Yajima et al. 2008). However, the amount of antibody produced is very low, approximately 1000-fold less than the average levels in an adult human (Traggiai et al. 2004).
Humanized mouse adaptive cellular immune responses toward EBV have been well established and characterized. Humanized mice can generate both CD4+ and CD8+ T cell responses against EBV lytic and latent antigens (Traggiai et al. 2004) and diminish EBV-driven cellular transformation. Depletion of CD4+ and CD8+ T cells impaired the immune control against EBV and led to high levels of EBV viremia. However, deletion of CD4+ Th cells alone led to high viral load but had no effect in tumor development. Infection of BLT mice with EBV led to the expansion of human memory T cells (CD45RA−CD27+) in peripheral blood that mimics spontaneous human immune responses that occur in acute EBV infection (Melkus et al. 2006).
EBV-Associated Diseases in Humanized Mice
Both lytic and latent EBV infection can be established in humanized murine models, yet lytic replication is not required to establish latency in humanized mice. This is nicely illustrated by using a mutant strain of EBV that lacks the lytic antigen BZLF1 that still shows capacity to establish latency in B cells (Ma et al. 2011).
Humanized mice infected with EBV have been shown to induce EBV-LPD with consistent latency III gene expression profile where all nine latency proteins are expressed (Yajima et al. 2008). Another EBV-associated malignancy that is recapitulated in the humanized mice is the diffuse large B cell lymphomas (DLBCLs). The EBV EBNA3B mutant strain developed DLBCLs upon infection in humanized mice (White et al. 2012). Lack of EBNA3B protein compromised the production of the chemokine CXCL10, and therefore tumors induced by EBANA3B mutant strain showed reduced T cell infiltration within the tumor microenvironment (White et al. 2012). The disease was also reported in humanized NSG mice infected with an EBV strain that lacks the BZLF1 lytic protein (Ma et al. 2011). Interestingly, the numbers of mice that develop the disease are less than the wild-type strain. Additionally, infection with EBV mutant strain with enhanced expression of BZLF1 lytic antigen (superlytic strain) also developed DLBCLs, but the ability of the superlytic strain to induce DLBCLs was similar to the wild type (Ma et al. 2012). These intriguing findings point toward lytic infection as a potential driving factor for EBV-induced lymphomagenesis.
Sato and colleagues (2011) reported the induction of EBV-associated hemophagocytic lymphohistiocytosis (HLH) in NOG mice intrahepatically infected with the EBV Akata strain (Sato et al. 2011). In this study, humanized mice infected with the Akata strain of EBV experienced thrombocytopenia, hepercytokinemia, and erythrocytopenia, which mimics HLH pathology in humans. Infiltration of activated CD8+ T cells was reported in most organs, especially the spleen and liver. The level of viral load was highly correlated with the frequency of CD8+ T cells and with the level of IFNγ cytokine in the blood (Sato et al. 2011). HLH is a disease associated with high mortality that frequently is treated with intensive chemotherapy (HLH94 protocol). This model therefore provides investigators with a unique opportunity to explore novel, less toxic, and more effective approaches to this challenging condition.
Humanized Mouse Models as a Platform to Assess Prevention of EBV-LPD
Humanized mice are susceptible to EBV, and they can generate both innate and adaptive immune response toward the virus. Therefore, they provide a potentially useful platform to experimentally test EBV vaccine candidates and to boost the immune system with a variety of immune modulatory agents. The utility of antigen-presenting cells such as DCs has been tested to enhance immune cell specificity to EBV-LPD. Gurer and colleagues (2008) targeted the DC receptor DEC-205 with anti-DEC-205 antibody fused to recombinant EBNA1 antigen to boost the humanized immune responses. Intraperitoneal injection of humanized mice with anti-DEC-205-EBNA1 and Polyinosinic:polycytidylic acid (polyI:C) adjuvant generated EBNA1-specific CD4+ and CD8+ T cells. Moreover, vaccination with αDEC-205-EBNA1 elicited EBNA1-specific IFNγ secreted T cells. Immunized mice also generated humoral immune responses, and IgM antibody specific for EBNA1 antigen has been detected. More important, the vaccine efficiently controlled the outgrowth of transformed B cells. Another study targeted specific subset of DCs, CD141+ cDCs, by using double-stranded RNA (ds-RNA) as adjuvant. Endocytic receptor DEC-205 is highly expressed by cDCs, and vaccination with αDEC-205-EBNA1 and ds-RNA adjuvant generated EBNA-specific CD4+ T cells (Meixlsperger et al. 2013).
Limitations of Humanized Murine Models of EBV-LPD
An obvious limitation of the humanized mice is the lack of human epithelial cells, the entry site of EBV in human primary infection. This limits modeling the EBV normal route of infection through the oropharyngeal site. Another limitation to the model is the limited development of the germinal center and secondary lymphoid organs because this leads to impaired humoral responses. Humanized mice can only develop EBV-specific IgM antibodies specific for a limited number of described antigens and incompletely recapitulate the complex latency programs by demonstrating restriction to latency III. With the exception of the BLT model, T cells in the humanized model are educated within the mouse thymus, which impairs cellular immune responses. The BLT model allows for the development of robust T cell responses. However, the major drawbacks of this model include the high costs, technically intensive nature, requirement of human fetal tissues, and legislative challenges present in many states.
Conclusions
Suitable animal models that recapitulate human EBV-associated malignancies are vital tools to gain better understanding of disease pathogenesis and development of novel preventive and therapeutic strategies. There is now abundant evidence that human-murine chimeric model systems can serve as useful models to study EBV-LPD. In contrast to other higher-order vertebrate models, murine models are cost effective, have relatively short latency periods for development of EBV-LPD, and have the capacity to simultaneously examine viral and human immune factors. Immunodeficient mouse strains constituted with human mononuclear cells and or tissues provide a well-controlled and reproducible approach to model EBV-LPD and have been vital to the discovery and translation of targeted therapeutics (monoclonal antibodies, epigenetic modifiers, antivirals) and immune-based preventive and therapeutic strategies.
References
- Andreone P, Gramenzi A, Lorenzini S, Biselli M, Cursaro C, Pileri S, Bernardi M. 2003. Posttransplantation lymphoproliferative disorders. Arch Intern Med 163:1997–2004. [DOI] [PubMed] [Google Scholar]
- Baiocchi RA, Caligiuri MA. 1994. Low-dose interleukin 2 prevents the development of Epstein-Barr virus (EBV)–associated lymphoproliferative disease in scid/scid mice reconstituted i.p. with EBV-seropositive human peripheral blood lymphocytes. Proc Natl Acad Sci U S A 91:5577–5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiocchi RA, Ross ME, Tan JC, Chou CC, Sullivan L, Haldar S, Monne M, Seiden MV, Narula SK, Sklar J. 1995. Lymphomagenesis in the SCID-hu mouse involves abundant production of human interleukin-10. Blood 85:1063–1074. [PubMed] [Google Scholar]
- Baiocchi RA, Ward JS, Carrodeguas L, Eisenbeis CF, Peng R, Roychowdhury S, Vourganti S, Sekula T, O'Brien M, Moeschberger M, Caligiuri MA. 2001. GM-CSF and IL-2 induce specific cellular immunity and provide protection against Epstein-Barr virus lymphoproliferative disorder. J Clin Invest 108:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma GC, Custer RP, Bosma MJ. 1983. A severe combined immunodeficiency mutation in the mouse. Nature 301:527–530. [DOI] [PubMed] [Google Scholar]
- Bosma MJ, Carroll AM. 1991. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol 9:323–350. [DOI] [PubMed] [Google Scholar]
- Boyle TJ, Berend KR, DiMaio JM, Coles RE, Via DF, Lyerly HK. 1993. Adoptive transfer of cytotoxic T lymphocytes for the treatment of transplant-associated lymphoma. Surgery 114:218–225; discussion 226. [PubMed] [Google Scholar]
- Boyle TJ, Tamburini M, Berend KR, Kizilbash AM, Borowitz MJ, Lyerly HK. 1992. Human B-cell lymphoma in severe combined immunodeficient mice after active infection with Epstein-Barr virus. Surgery 112:378–386. [PubMed] [Google Scholar]
- Buchsbaum RJ, Fabry JA, Lieberman J. 1996. EBV-specific cytotoxic T lymphocytes protect against human EBV-associated lymphoma in scid mice. Immunol Lett 52:145–152. [DOI] [PubMed] [Google Scholar]
- Burkitt D. 1962. Determining the climatic limitations of a children's cancer common in Africa. Br Med J 2:1019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon MJ, Pisa P, Fox RI, Cooper NR. 1990. Epstein-Barr virus induces aggressive lymphoproliferative disorders of human B cell origin in SCID/hu chimeric mice. J Clin Invest 85:1333–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijioke O, Muller A, Feederle R, Barros MH, Krieg C, Emmel V, Marcenaro E, Leung CS, Antsiferova O, Landtwing V, Bossart W, Moretta A, Hassan R, Boyman O, Niedobitek G, Delecluse HJ, Capaul R, Munz C. 2013. Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein-Barr virus infection. Cell Rep 5:1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinchy B, Vitetta ES. 1998. The use of an anti-CD3 immunotoxin to prevent the development of lymphoproliferative disease in SCID/PBL mice. J Immunol Methods 218:141–153. [DOI] [PubMed] [Google Scholar]
- Cohen JI. 2000. Epstein-Barr virus infection. N Engl J Med 343:481–492. [DOI] [PubMed] [Google Scholar]
- Cohen JI, Fauci AS, Varmus H, Nabel GJ. 2011. Epstein-Barr virus: An important vaccine target for cancer prevention. Sci Transl Med 3:107fs7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles RE, Boyle TJ, DiMaio JM, Berend KR, Via DF, Lyerly HK. 1994. T cells or active Epstein-Barr virus infection in the development of lymphoproliferative disease in human B cell-injected severe combined immunodeficient mice. Ann Surg Oncol 1:405–410. [DOI] [PubMed] [Google Scholar]
- Davison AJ. 2010. Herpesvirus systematics. Veterinary Microbiology 143:52–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeken JF, Tjen ALA, Rudek MA, Okuliar C, Young M, Little RF, Dezube BJ. 2012. The rising challenge of non-AIDS-defining cancers in HIV-infected patients. Clin Infect Dis 55:1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers B, Kuchler J, Yasmum N, Dural G, Voigt S, Schmidt-Chanasit J, Jakel T, Matuschka FR, Richter D, Essbauer S, Hughes DJ, Summers C, Bennett M, Stewart JP, Ulrich RG. 2007. Identification of novel rodent herpesviruses, including the first gammaherpesvirus of Mus musculus. J Virol 81:8091–8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers B, Spiess K, Leendertz F, Peeters M, Boesch C, Gatherer D, McGeoch DJ. 2010. Lymphocryptovirus phylogeny and the origins of Epstein-Barr virus. J Gen Virol 91(Pt 3):630–642. [DOI] [PubMed] [Google Scholar]
- Epstein MA, Barr YM, Achong BG. 1965. The behaviour and morphology of a second tissue culture strain (EB2) of lymphoblasts from Burkitt's lymphoma. Br J Cancer 19:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell PJ, Hollyoake M, Niedobitek G, Agathanggelou A, Morgan A, Wedderburn N. 1997. Direct demonstration of persistent Epstein-Barr virus gene expression in peripheral blood of infected common marmosets and analysis of virus-infected tissues in vivo. J Gen Virol 78(Pt 6):1417–1424. [DOI] [PubMed] [Google Scholar]
- Flano E, Woodland DL, Blackman MA. 2002. A mouse model for infectious mononucleosis. Immunol Res 25:201–217. [DOI] [PubMed] [Google Scholar]
- Grufferman S, Delzell E. 1984. Epidemiology of Hodgkin's disease. Epidemiol Rev 6:76–106. [DOI] [PubMed] [Google Scholar]
- Gurer C, Strowig T, Brilot F, Pack M, Trumpfheller C, Arrey F, Park CG, Steinman RM, Munz C. 2008. Targeting the nuclear antigen 1 of Epstein-Barr virus to the human endocytic receptor DEC-205 stimulates protective T-cell responses. Blood 112:1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G, Henle W, Diehl V. 1968. Relation of Burkitt's tumor-associated herpes-type virus to infectious mononucleosis. Proc Natl Acad Sci U S A 59:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop HE. 2009. How I treat EBV lymphoproliferation. Blood 114:4002–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen I, Asghar M, Crawford DH. 2000. Essential role for T cells in human B-cell lymphoproliferative disease development in severe combined immunodeficient mice. Br J Haematol 109:600–610. [DOI] [PubMed] [Google Scholar]
- Kotton CN, Fishman JA. 2005. Viral infection in the renal transplant recipient. J Am Soc Nephrol 16:1758–1774. [DOI] [PubMed] [Google Scholar]
- Lacerda JF, Ladanyi M, Louie DC, Fernandez JM, Papadopoulos EB, O'Reilly RJ. 1996. Human Epstein-Barr virus (EBV)–specific cytotoxic T lymphocytes home preferentially to and induce selective regressions of autologous EBV-induced B cell lymphoproliferations in xenografted C.B-17 scid/scid mice. J Exp Med 183:1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda JF, O'Reilly RJ. 1997. Characteristics of human EBV-specific cytotoxic T lymphocytes utilized for adoptive immunotherapy of EBV-induced lymphoproliferations in xenografted SCID mice. Ann Oncol 8Suppl 2:137–140. [PubMed] [Google Scholar]
- Ma SD, Hegde S, Young KH, Sullivan R, Rajesh D, Zhou Y, Jankowska-Gan E, Burlingham WJ, Sun X, Gulley ML, Tang W, Gumperz JE, Kenney SC. 2011. A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas. J Virol 85:165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SD, Yu X, Mertz JE, Gumperz JE, Reinheim E, Zhou Y, Tang W, Burlingham WJ, Gulley ML, Kenney SC. 2012. An Epstein-Barr Virus (EBV) mutant with enhanced BZLF1 expression causes lymphomas with abortive lytic EBV infection in a humanized mouse model. J Virol 86:7976–7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. 1988. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science 241:1632–1639. [DOI] [PubMed] [Google Scholar]
- Meixlsperger S, Leung CS, Ramer PC, Pack M, Vanoaica LD, Breton G, Pascolo S, Salazar AM, Dzionek A, Schmitz J, Steinman RM, Munz C. 2013. CD141+ dendritic cells produce prominent amounts of IFN-alpha after dsRNA recognition and can be targeted via DEC-205 in humanized mice. Blood 121:5034–5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT, Garcia JV. 2006. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med 12:1316–1322. [DOI] [PubMed] [Google Scholar]
- Miller G, Shope T, Coope D, Waters L, Pagano J, Bornkamn G, Henle W. 1977. Lymphoma in cotton-top marmosets after inoculation with Epstein-Barr virus: tumor incidence, histologic spectrum antibody responses, demonstration of viral DNA, and characterization of viruses. J Exp Med 145:948–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68:869–877. [DOI] [PubMed] [Google Scholar]
- Mosier DE. 1996. Viral pathogenesis in hu-PBL-SCID mice. Semin Immunol 8:255–262. [DOI] [PubMed] [Google Scholar]
- Mosier DE, Baird SM, Kirven MB, Gulizia RJ, Wilson DB, Kubayashi R, Picchio G, Garnier JL, Sullivan JL, Kipps TJ. 1990. EBV-associated B-cell lymphomas following transfer of human peripheral blood lymphocytes to mice with severe combined immune deficiency. Curr Top Microbiol Immunol 166:317–323. [DOI] [PubMed] [Google Scholar]
- Mosier DE, Gulizia RJ, Baird SM, Wilson DB. 1988. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature 335:256–259. [DOI] [PubMed] [Google Scholar]
- Mosier DE, Picchio GR, Kirven MB, Garnier JL, Torbett BE, Baird SM, Kobayashi R, Kipps TJ. 1992. EBV-induced human B cell lymphomas in hu-PBL-SCID mice. AIDS Res Hum Retroviruses 8:735–740. [PubMed] [Google Scholar]
- Munz C. 2015. EBV infection of mice with reconstituted human immune system components. Curr Top Microbiol Immunol 391:407–423. [DOI] [PubMed] [Google Scholar]
- Murphy WJ, Bennett M, Anver MR, Baseler M, Longo DL. 1992. Human-mouse lymphoid chimeras: Host-vs.-graft and graft-vs.-host reactions. Eur J Immunol 22:1421–1427. [DOI] [PubMed] [Google Scholar]
- Okano M, Taguchi Y, Nakamine H, Pirruccello SJ, Davis JR, Beisel KW, Kleveland KL, Sanger WG, Fordyce RR, Purtilo DT. 1990. Characterization of Epstein-Barr virus-induced lymphoproliferation derived from human peripheral blood mononuclear cells transferred to severe combined immunodeficient mice. Am J Pathol 137:517–522. [PMC free article] [PubMed] [Google Scholar]
- Paludan C, Munz C. 2003. CD4+ T cell responses in the immune control against latent infection by Epstein-Barr virus. Curr Mol Med 3:341–347. [DOI] [PubMed] [Google Scholar]
- Parkin DM. 2006. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 118:3030–3044. [DOI] [PubMed] [Google Scholar]
- Pearson T, Greiner DL, Shultz LD. Creation of “humanized” mice to study human immunity. Curr Protoc Immunol 15:15.21. 2008 doi: 10.1002/0471142735.im1521s81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SP, Hermine O, Small T, Suarez F, O'Reilly R, Boulad F, Fingeroth J, Askin M, Levy A, Mentzer SJ, Di Nicola M, Gianni AM, Klein C, Horwitz S, Faller DV. 2007. A phase 1/2 trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus-associated lymphoid malignancies. Blood 109:2571–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picchio GR, Kobayashi R, Kirven M, Baird SM, Kipps TJ, Mosier DE. 1992. Heterogeneity among Epstein-Barr virus-seropositive donors in the generation of immunoblastic B-cell lymphomas in SCID mice receiving human peripheral blood leukocyte grafts. Cancer Res 52:2468–2477. [PubMed] [Google Scholar]
- Raab-Traub N, Flynn K. 1986. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell 47:883–889. [DOI] [PubMed] [Google Scholar]
- Rowe M, Young LS, Crocker J, Stokes H, Henderson S, Rickinson AB. 1991. Epstein-Barr virus (EBV)–associated lymphoproliferative disease in the SCID mouse model: implications for the pathogenesis of EBV-positive lymphomas in man. J Exp Med 173:147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychowdhury S, Peng R, Baiocchi RA, Bhatt D, Vourganti S, Grecula J, Gupta N, Eisenbeis CF, Nuovo GJ, Yang W, Schmalbrock P, Ferketich A, Moeschberger M, Porcu P, Barth RF, Caligiuri MA. 2003. Experimental treatment of Epstein-Barr virus-associated primary central nervous system lymphoma. Cancer Res 63:965–971. [PubMed] [Google Scholar]
- Sato K, Misawa N, Nie C, Satou Y, Iwakiri D, Matsuoka M, Takahashi R, Kuzushima K, Ito M, Takada K, Koyanagi Y. 2011. A novel animal model of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in humanized mice. Blood 117:5663–5673. [DOI] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68:855–867. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Pearson T, King M, Giassi L, Carney L, Gott B, Lyons B, Rossini AA, Greiner DL. 2007. Humanized NOD/LtSz-scid IL2 receptor common gamma chain knockout mice in diabetes research. Ann N Y Acad Sci 1103:77–89. [DOI] [PubMed] [Google Scholar]
- Sokal EM, Hoppenbrouwers K, Vandermeulen C, Moutschen M, Leonard P, Moreels A, Haumont M, Bollen A, Smets F, Denis M. 2007. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J Infect Dis 196:1749–1753. [DOI] [PubMed] [Google Scholar]
- Strowig T, Gurer C, Ploss A, Liu YF, Arrey F, Sashihara J, Koo G, Rice CM, Young JW, Chadburn A, Cohen JI, Munz C. 2009. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J Exp Med 206:1423–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Rongvaux A, Rathinam C, Takizawa H, Borsotti C, Philbrick W, Eynon EE, Manz MG, Flavell RA. 2011. Transgenic expression of human signal regulatory protein alpha in Rag2-/-gamma(c)-/- mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci U S A 108:13218–13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, Dick JE, Danska JS. 2007. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol 8:1313–1323. [DOI] [PubMed] [Google Scholar]
- Tary-Lehmann M, Lehmann PV, Schols D, Roncarolo MG, Saxon A. 1994. Anti-SCID mouse reactivity shapes the human CD4+ T cell repertoire in hu-PBL-SCID chimeras. J Exp Med 180:1817–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tary-Lehmann M, Saxon A. 1992. Human mature T cells that are anergic in vivo prevail in SCID mice reconstituted with human peripheral blood. J Exp Med 175:503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tary-Lehmann M, Saxon A, Lehmann PV. 1995. The human immune system in hu-PBL-SCID mice. Immunol Today 16:529–533. [DOI] [PubMed] [Google Scholar]
- Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. 2004. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 304:104–107. [DOI] [PubMed] [Google Scholar]
- Veronese ML, Veronesi A, D'Andrea E, Del Mistro A, Indraccolo S, Mazza MR, Mion M, Zamarchi R, Menin C, Panozzo M. 1992. Lymphoproliferative disease in human peripheral blood mononuclear cell-injected SCID mice. I. T lymphocyte requirement for B cell tumor generation. J Exp Med 176:1763–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RE, Ramer PC, Naresh KN, Meixlsperger S, Pinaud L, Rooney C, Savoldo B, Coutinho R, Bodor C, Gribben J, Ibrahim HA, Bower M, Nourse JP, Gandhi MK, Middeldorp J, Cader FZ, Murray P, Munz C, Allday MJ. 2012. EBNA3B-deficient EBV promotes B cell lymphomagenesis in humanized mice and is found in human tumors. J Clin Invest 122:1487–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima M, Imadome K, Nakagawa A, Watanabe S, Terashima K, Nakamura H, Ito M, Shimizu N, Honda M, Yamamoto N, Fujiwara S. 2008. A new humanized mouse model of Epstein-Barr virus infection that reproduces persistent infection, lymphoproliferative disorder, and cell-mediated and humoral immune responses. J Infect Dis 198:673–682. [DOI] [PubMed] [Google Scholar]