Abstract
Dysregulated lipid metabolism induces an inflammatory and immune response leading to atherosclerosis. Conversely, inflammation may alter lipid metabolism. Recent treatment strategies in secondary prevention of atherosclerosis support beneficial effects of both anti-inflammatory and lipid-lowering therapies beyond current targets. There is a controversy about the possibility that anti-inflammatory effects of lipid-lowering therapy may be either independent or not of a decrease in low-density lipoprotein cholesterol. In this Position Paper, we critically interpret and integrate the results obtained in both experimental and clinical studies on anti-inflammatory actions of lipid-lowering therapy and the mechanisms involved. We highlight that: (i) besides decreasing cholesterol through different mechanisms, most lipid-lowering therapies share anti-inflammatory and immunomodulatory properties, and the anti-inflammatory response to lipid-lowering may be relevant to predict the effect of treatment, (ii) using surrogates for both lipid metabolism and inflammation as biomarkers or vascular inflammation imaging in future studies may contribute to a better understanding of the relative importance of different mechanisms of action, and (iii) comparative studies of further lipid lowering, anti-inflammation and a combination of both are crucial to identify effects that are specific or shared for each treatment strategy.
Keywords: Lipids, Inflammation, Immune response, Atherosclerosis, PCSK9, Statins
1. Introduction
Dyslipidaemia and inflammation are closely interconnected key drivers of atherosclerosis.1 Dysregulated lipid metabolism induces an inflammatory and immune response in atherosclerosis, whereas the beneficial effects of low-density lipoproteins (LDLs) lowering on cardiovascular outcomes are associated with decreased inflammation. However, the controversy on anti-inflammatory effects of lipid-lowering therapy has created a large confusion around the mechanism by which these drugs exert beneficial actions. In particular, whether the improved cardiovascular outcome of statins solely reflects a decrease in LDL cholesterol, or whether lipid-independent anti-inflammatory actions prevail has been a matter of debate for a long time. In this regard, there is a discrepancy between the results obtained in experimental and clinical studies. Since novel treatment strategies in secondary prevention of atherosclerosis argue for beneficial effects of both further lipid-lowering2 and anti-inflammation,3 it is crucial to clarify whether further lipid-lowering therapy leads to a sufficient anti-inflammatory response, and whether adding specific anti-inflammatory therapy can achieve additional risk reduction on top of the most efficient lipid lowering.
The recent introduction of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors represents an opportunity to deepen our understanding on the role of lipids on the immune and inflammatory responses in atherosclerosis. Likewise, the beneficial effects of IL-1β blockade3 provided the proof of concept for anti-inflammation as a therapeutic strategy in cardiovascular disease. In addition, recent post hoc analyses point to inflammatory biomarkers for the prediction of cardiovascular outcomes in patients treated with PCSK9 inhibitors.4,5 These findings have initiated a debate on whether personalized medicine can be anticipated based on either basal levels or changes in lipid levels and inflammatory biomarkers in response to lipid-lowering and/or anti-inflammatory treatments.
Thus, it is important to critically interpret and integrate the results obtained in both clinical and experimental studies to conclude on possible mechanisms behind these observations and to provide arguments for the design of future studies. This Position Paper will provide an overview of anti-inflammatory effects of lipid-lowering drugs, aiming to clarify the relation and the relevance of lipid-dependent and lipid-independent anti-inflammatory effects observed. In addition, this Position Paper underlines the mechanistic insights that translate into the observed outcomes and provides a consensus statement on how to identify the anti-inflammatory response to lipid lowering therapy.
2. Dyslipidaemia and immunity—cause or consequence?
Increased LDL levels are a well-recognized cause of inflammation in atherosclerosis. However, the fact that immune cells may also affect lipid metabolism in atherogenesis is less well known and may have been overlooked.
2.1 Lipid-induced immune responses
Modified LDL (mLDL) increases the endothelial expression of adhesion molecules, chemokines, as well as costimulatory and pro-inflammatory molecules, such as CD40 and nuclear factor-κB (NF-κB),6,7 which will promote the recruitment of inflammatory cells into the vascular wall. LDL accelerates monocyte to macrophage differentiation8 and this macrophage activation involves innate immune receptors, which are strong inducers of inflammation and have an established impact on atherosclerosis.9 For example, certain components of mLDL activate pattern recognition receptors, such as toll-like receptors (TLRs), triggering proinflammatory signals.10 These TLRs in addition prime the NLRP3 inflammasome through its activation by cholesterol crystals.11 Also, cholesterol-loaded smooth muscle cells acquire pro-inflammatory properties.12 Importantly, LDL also accumulates in circulating monocytes eliciting a pro-migratory phenotype, supporting that the pro-inflammatory paradigm induced by LDL appears both locally in the vascular wall and systemically in the circulation.13 B cells are involved in atherogenesis although their role is still not clear, with some data suggesting a protective action and others supporting a pro-atherogenic function, probably depending on the specific B-cell subset.14
In addition to innate immune cells, there is also evidence for an effect of lipids on adaptive immune responses. For example, stimulation of human CD4+ T cells with lysophosphatidylcholine enhances the expression of interferon-γ and CD40L, a molecule that binds to its receptor CD40 triggering the release of multiple inflammatory mediators.15 Lipoprotein-derived lysophosphatidic acid enhances atherosclerosis by releasing chemokine CXCL1 from endothelium to recruit monocytes, and oxidized LDL increases metalloproteinase-9 (MMP-9) expression and NF-κB activity in human macrophages.7 These data provide an essential mechanism by which lipids activate genes involved in immune responses in atherosclerosis.
High-density lipoprotein (HDL) has been said traditionally to induce atheroprotection. Bone marrow and splenic reservoirs of leucocytes accelerate atherosclerosis after myocardial infarction by liberating haematopoietic stem cells and progenitor cells.16 Conversely, HDL suppresses proliferation of these haematopoietic stem cells and myelopoiesis with anti-atherogenic effects mediated by ABCA1 (ATP-binding cassette transporter-1) and ABCG1 (ATP-binding cassette sub-family G member-1).17 Importantly, the benefits of HDL can be lost in hypercholesterolaemia18 and also in other conditions, such as chronic kidney disease.19 In addition, despite these reported benefits, recent data suggest that extreme high HDL may be associated with high mortality in the general population.20 Thus, more studies are needed to ascertain the mechanisms underlying these findings.
Dietary lipid modifications can also influence the immune response. In humans, NF-κB activity in circulating leucocytes is enhanced after a fat-enriched meal.21 Accordingly, low-fat diet reduces high-sensitivity C-reactive protein (CRP) levels,22 and Mediterranean diet decreases the expression of pro-inflammatory and prothrombotic genes.23 Also, monocyte cholesterol content increases following oral fat ingestion, showing a pro-inflammatory phenotype, comprising increased CD11c/CD18 expression.24 Then, besides LDL, triglyceride-rich particles, including cholesterol remnant particles, may equally contribute to pro-inflammatory changes.
In summary, lipid metabolism has profound effects on both innate and adaptive immunity via multiple mechanisms.
2.2 Inflammation modifies lipid metabolism
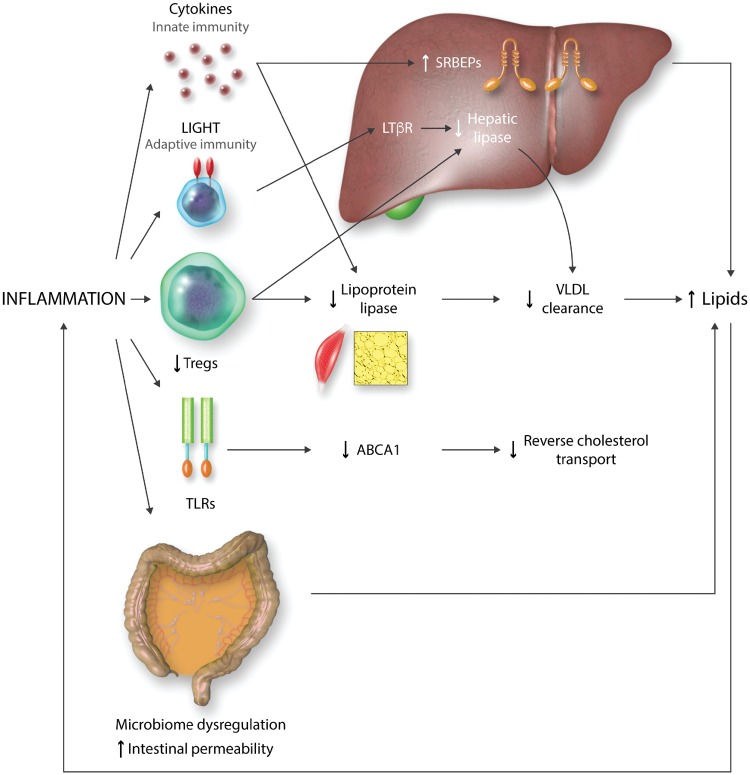
Both innate and adaptive immune processes regulate lipid metabolism (Figure 1). Several innate immune cytokines accelerate hepatic steatosis by influencing fatty acid biosynthesis and oxidation.25 Thus, subacute inflammation enhances the expression of sterol regulatory element-binding proteins (SREBPs),26 the master regulators of lipid biosynthesis. Innate immune cytokines can also influence lipoprotein lipase in adipose and muscle tissue27,28 leading to dysfunctional triglyceride clearance and increased plasma very-low density lipoprotein (VLDL) levels. Also, TLR activation inhibits cholesterol efflux by suppressing LXR (liver X receptor)-mediated induction of ABCA1 expression, thereby inhibiting reverse cholesterol transport.29
Figure 1.
Mechanisms through which inflammation may promote dyslipidaemia. Inflammation enhances SRBEPs (sterol regulatory element binding proteins). The interaction of co-stimulatory molecule LIGHT (lymphotoxin-related inducible ligand) with LTßR (lymphotoxin ß receptor), and the impairment of Tregs (T regulatory cells) responses decrease the expression of several lipases. TLR (Toll-like receptor) activation reduces reverse cholesterol transport through a suppression of ABCA1 expression. Finally, dysregulation of intestinal microbiome increases lipid levels via an enhanced permeability of the intestinal barrier.
The adaptive immune system can also influence lipoprotein metabolism. The interaction between the costimulatory molecule LIGHT (lymphotoxin-related inducible ligand) on T cells, and lymphotoxin β receptor (LTβR) on hepatocytes decreases hepatic lipase activity, impairs VLDL and LDL turnover, increasing plasma lipids.30 Similarly, enhanced hepatic inflammation due to impaired T regulatory cell (Treg) responses alters the expression of several lipid metabolism-related genes including sortilin-1 (Sort-1), lipoprotein lipase, hepatic lipase, and phospholipid transfer protein resulting in hypercholesterolaemia.31 In this regard, low Treg numbers or decreased Treg/effector T cell ratios are associated with cardiovascular disease.32 In adddition to these effects, during acute infections and sepsis, HDL gets dysfunctional and promotes inflammation.33,34
Interestingly, other chronic inflammatory diseases show increased triglycerides, small dense LDL, and lipoprotein (a) (Lp(a)), and decreased HDL levels.27 In addition, a dysregulated intestinal microbiome with enhanced permeability of the intestinal barrier affects metabolic diseases35 and atherosclerosis,36 being associated with increased LDL, VLDL, and total cholesterol, and with more extensive atherosclerosis in the apoE-/- mouse model.37 Also, intestinal inflammation may be associated to a decrease in trans-intestinal cholesterol efflux, increasing blood lipid levels.38
Altogether, these data indicate that the inflammatory and immune response affects lipid metabolism, establishing a vicious circle that promotes atherogenesis.
2.3 Effects of anti-inflammatory therapies on lipids and cardiovascular risk
Anti-inflammatory therapies show a varied range of effects on lipids and cardiovascular risk. First, non-steroidal anti-inflammatory drugs are associated with an increased risk of cardiovascular events.39,40 Glucocorticoids, the other widely used anti-inflammatory drugs, have been associated with increases in HDL/total cholesterol ratio,41 but also with enhanced VLDL and triglyceride production.42 Nevertheless, they show no effect on cardiovascular risk in secondary prevention.43
Anti-TNF therapy may lead to an increase in HDL without LDL changes,44 or to a modest rise in total cholesterol,45 although these effects may depend on the drug used. The impact of these changes on cardiovascular risk may be more complex, as anti-TNF therapies have been linked with a decreased number of cardiovascular events in rheumatoid arthritis patients. However, these results come mainly from observational studies,46 and large randomized clinical trials are needed to confirm this effect.
Another biologic therapy, tocilizumab, an IL-6 receptor blocker, increases total cholesterol, LDL, and triglyceride levels, but shifts HDL particles towards an anti-inflammatory composition.47 In contrast, the Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS) demonstrated that IL-1β blockade with canakinumab 150 mg every three months reduces the incidence of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death [hazard ratio (HR) 0.85, 95% confidence interval (CI) 0.74–0.98; P = 0.021] without affecting lipid levels, except for a mild increase in triglycerides.3
Methotrexate has been associated with both, unfavourable and beneficial lipid changes and an improved macrophage cholesterol handing.48,49 Regarding its effect on cardiovascular risk, the Cardiovascular Inflammation Reduction trial (CIRT) testing low dose methotrexate against placebo in secondary prevention has been discontinued earlier than scheduled (https://www.forbes.com/sites/larryhusten/2018/05/21/nih-halts-large-cardiovascular-inflammation-reduction-cirt-trial/#4cc3a12 c5b5f) but the results are still pending.
Finally, in a study including 532 patients with stable coronary artery disease, colchicine reduced the incidence of acute coronary syndrome, out-of-hospital cardiac arrest, or noncardioembolic ischaemic stroke (HR 0.33, 95% CI 0.18–0.59; P < 0.001).50 This effect is now being tested in two large clinical trials: the LoDoCo2 (http://www.anzctr.org.au/TrialSearch.aspx?searchTxt=LoDoCo2&isBasic=True) and the COLCOT studies (https://clinicaltrials.gov/ct2/show/NCT02551094).
Thus, studies of anti-inflammatory treatments have generated contradictory results in terms of their effects on lipids. Although the heterogeneous patient populations and different inflammatory targets studied preclude a definite conclusion, monitoring of lipid levels appears to be crucial in studies of anti-inflammation in cardiovascular prevention.
3. Statins and immunity: current status
3.1 Experimental data
In in vitro studies statins inhibit the expression of adhesion molecules in both endothelial cells and monocytes,51 as well as LDL-induced endothelial nitric oxide synthase down-regulation.52 In addition, statins reduce NF-κB activation, and chemokine and MMP expression53,54 and promote the expression of anti-inflammatory and cytoprotective molecules in endothelium.55
Statins also modulate the adaptive immune response by inhibiting the expression of major histocompatibility class II required for antigen presentation to effector T cells56 and divert T cell differentiation to Tregs, that supress pro-inflammatory responses of other immune cells and counteract pro-inflammatory IL-17-producing T cell differentiation (Th17).57 Statins also up-regulate the expression of Kruppel-like factor 2 (KLF2) in mouse and human T cells, diminishing interferon-γ expression,58 as KLF2 controls the expression of molecules essential for naive T cell recirculation and maintenance of T cell quiescence.
3.2 Evidence in humans
Further proof of concept for anti-inflammatory effects of statins was provided by studies of patients scheduled to elective carotid endarterectomy randomized to either statins or no lipid-lowering therapy.59 Plaques from patients under statins exhibited reduced NF-κB activity, decreased macrophage and T cell infiltrates, and less expression of proinflammatory mediators.59 Moreover, statins diminish plasma levels of CRP, cytokines, and adhesion molecules60,61 and decrease microparticle shedding from inflammatory cells.62
The anti-inflammatory effects of statins have been postulated to contribute to their clinical benefits. Accordingly, statins are especially effective when associated to a diminution in CRP levels.63 Furthermore, it has been suggested that statins may be more effective in patients with high CRP levels.61,64,65 However, this could be due to the higher cardiovascular risk of these patients and discordant data also exist.66 Also, mendelian randomization analyses suggest that CRP concentration itself is unlikely to be a causal factor of coronary artery disease.67 Thus, at present, CRP determination is not advised as its contribution to the existing methods of cardiovascular risk assessment seems to be small.68
The immunomodulatory effects of statins are also supported by data on clinical outcomes. Statins decrease the cytotoxicity of natural killer cells, and the incidence of coronary vasculopathy and rejection with dynamic impairment after a cardiac transplant.69 Other studies have also found a lower incidence of coronary artery disease and intimal thickening without reducing the incidence of cardiac rejection.70 Conversely, in patients with kidney transplantation, there are no consistent data confirming this immunomodulatory effect.71,72
In addition, imaging vascular inflammation by techniques such as PET (positron emission tomography) using FDG (18F-fluorodeoxyglucose) has evidenced that statins achieve a decrease of vascular inflammation consistent with the reduction of the cardiovascular risk observed in clinical trials.73 This is relevant, as a lack of reduction in arterial FDG uptake by anti-inflammatory therapies has been associated with an absence of clinical benefit.74
In conclusion, both experimental and clinical data support that statins have anti-inflammatory and immunomodulatory properties. Nevertheless, a legitimate question is whether these actions could be, at least in part, independent of their lipid-lowering effects.
3.3 Lipid-independent anti-inflammatory and immunomodulatory effects of statins
Some intermediate compounds of the mevalonate pathway that is blocked by statins to decrease cholesterol synthesis, are implicated in post-translational modifications of key proteins75 involved in important cell functions. Among them, small G proteins75 play a role in cell signal transduction, and their blockade in atherosclerotic cells might interfere with atherogenesis irrespective of the inhibition of cholesterol synthesis.75 In this regard, statins have anti-inflammatory effects in vitro in the absence of changes in lipid concentrations.53 In animal models, they have stronger anti-inflammatory effects than diet modification in spite of less decrease in cholesterol levels.76 Moreover, they reduce inflammation in models of inflammatory disorders likely unrelated to lipids.77–79
In humans, however, data are less clear. In the Atorvastatin in Rheumatoid Arthritis (TARA) trial, atorvastatin modestly decreased inflammation, with a diminution of −0.5 (95% CI −0.75 to −0.25) in the disease activity score, when compared with 0.03 (95% CI −0.23 to 0.28) in the placebo group (P = 0.004),80 an effect that could be lipid-independent. However, in another study ezetimibe, that decrease LDL through a different mechanism, achieved also a mild but significant reduction in the activity score of −0.55 ± 1.01 (P = 0.002) that was similar to that obtained by simvastatin (−0.67 ± 0.91; P = 0.002).81 Accordingly, PCSK9-antibodies reduce the pro-inflammatory changes in circulating monocytes, coinciding with a marked decrease in monocyte-cholesterol content.13 Then, LDL lowering may be the predominant factor explaining anti-inflammatory effects in humans. In fact, risk reduction of major vascular events is similar in statin and non-statin therapies [0.77 (95% CI 0.71–0.84) and 0.77 (95% CI 0.75–0.79) per 38.7 mg/dL LDL decrease, respectively; P < 0.001 for both].82
Thus, although basic research suggests that statins have lipid-independent anti-inflammatory effects, this seems difficult to confirm in humans. This discrepancy may reflect the biodistribution of statins. In this regard, a nano-particulate formulation statin packaged in HDL particles exerts potent anti-inflammatory effects in plaques, as it delivers the statin into the plaque macrophages directly.83,84 In contrast, oral statins in equal doses hardly affect plaque inflammation, illustrating that the first-pass clearance of statins by the liver precludes a strong anti-inflammatory effect in vivo.
4. Non-statin LDL-lowering drugs and inflammation
The relationship between lipids and inflammation is supported by the observation that non-statin lipid-lowering drugs also have anti-inflammatory effects. In this regard, ezetimibe also diminishes plaque inflammation in models of atherosclerosis.85 Accordingly, in patients at high cardiovascular risk, ezetimibe reduces plasma levels of inflammatory markers.86 Similarly to data reported with statins, achievement of both CRP and LDL prespecified targets in patients receiving ezetimibe and statin combination therapy is associated with better outcomes than reaching only LDL target levels.87 Then, non-statin LDL-lowering drugs also show anti-inflammatory properties despite working through different mechanisms, suggesting that lipid reduction plays a key role in these effects. In this regard, recent data show that lipid reduction is associated to a modulation of the inflammatory response irrespective of the lipid-lowering therapy used.88 Specifically, the reduction obtained in CRP levels with statins and with their combination with ezetimibe is proportional to the reduction observed in LDL levels.88
5. PCSK9 inhibition
5.1 PCSK9: an endogenous inhibitor of the LDL receptor
PCSK9 mediates intracellular degradation of the hepatic LDL receptor (LDLR).89 Once secreted by the hepatocyte, PCSK9 binds to the extra-cellular EGF-A domain of the LDLR, leading to the internalization of the LDLR-PCSK9 complex through clathrin-coated pits.90 In addition, PCSK9 could also enhance LDLR degradation by an intra-cellular pathway not requiring PCSK9 secretion.91 In this regard, the S127R PCSK9 gain-of-function variant leads to autosomal dominant hypercholesterolaemia without PCSK9 secretion.92
PCSK9 monoclonal antibodies block the extracellular PCSK9 pathway reducing LDL, triglyceride, cholesterol and Lp(a) plasma levels, and increasing HDL and ApoA1 levels.2 These data translate into a decrease in the incidence of cardiovascular events in secondary prevention2 (http://www.acc.org/latest-in-cardiology/clinical-trials/2018/03/09/08/02/odyssey-outcomes).
5.2 PCSK9 and inflammation
The fact that the recently developed PCSK9 inhibitors do not reduce plasma CRP13 has lead to the idea that they may not have anti-inflammatory effects (Figure 2). However, many data indicate that these drugs share anti-inflammatory actions with other lipid-lowering drugs. Even more, PCSK9 itself could have pro-inflammatory effects.
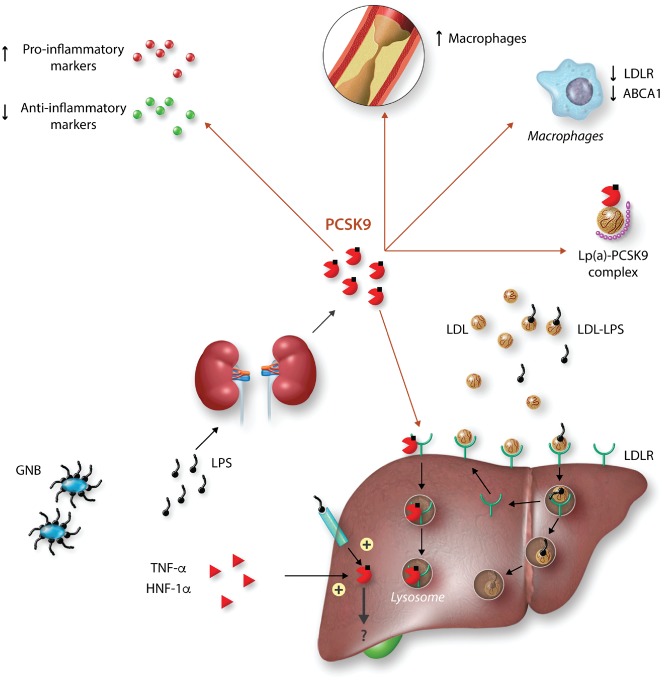
Figure 2.
Role of PCSK9 in sepsis and inflammation. Hepatic PCSK9 expression is induced by TNFα hepatocyte nuclear factor-1α (HNF-1α) and lipopolysaccharide (LPS). Once in the plasma, PCSK9 binds to the LDL receptor facilitating LDL degradation in lysosomes, thus decreasing clearance of LDL-bound LPS. Extracellular PCSK9 also enhances the expression of pro-inflammatory markers, decreases macrophages in atheroma, and the expression of LDLR and ABCA1 in these cells. It also forms a complex with Lp(a). The intracellular function of PCSK9 in inflammation remains unknown. GNB, gram-negative bacteria; mab, monoclonal antibody.
The transcriptional regulation of PCSK9 suggests that it may have a role in inflammation. PCSK9 expression is induced by hepatocyte nuclear factor-1α (HNF-1α), which regulates the expression of acute phase pro-inflammatory proteins.93 Also, lipopolysaccharide induces early hepatic and renal PCSK9 mRNA expression in mice,94 as well as in endothelial and vascular smooth muscle cells.95 Finally, TNFα increases PCSK9 expression in vitro in cultured macrophages.96 In humans, plasma PCSK9 concentrations increase in sepsis,97 trauma,98 and in acute coronary syndromes. Furthermore, they are positively associated with white blood cell count, fibrinogen,99 and CRP100 in coronary patients. To date, there are no data reporting the effects of anti-inflammatory molecules on PCSK9 levels.
While PCSK9 expression in macrophages is debatable, data suggest that it modulates LDLR expression in these cells, either through a paracrine101 and/or autocrine mechanism102 and also stimulates the expression of scavenger receptors (principally LOX-1) and ox-LDL uptake in these cells.96 Furthermore, PCSK9 silencing reduces oxLDL-induced cytokine expression in THP-1 derived macrophages through NF-κB inhibition103 and decreases TLR4 expression and NF-κB activation in oxLDL-treated macrophages.104 Conversely, overexpression of human PCSK9 in lipopolysaccharide-stimulated macrophages promotes the expression of pro-inflammatory markers, while inhibiting anti-inflammatory molecules.102 Similarly, PCSK9 overexpression in TNFα-primed macrophages enhances the expression of scavenger receptors.96 Bone marrow transplantation from mice expressing human PCSK9 (hPCSK9tg) in apoE knockout mice generated a chimeric model expressing hPCSK9 only in macrophages.102 Interestingly, despite not modifying lipid levels, transplanted animals showed a LDLR-dependent increased number of Ly6Chi inflammatory monocytes within atherosclerotic lesions and spleens, and a reduction of LDLR expression in macrophages.102 Furthermore, PCSK9 decreases ABCA1 expression, thereby reducing cholesterol efflux in macrophages at least partly in a LDLR-dependent manner.105 In addition, human recombinant PCSK9 induces the expression of monocyte chemoattractant protein-1, IL6 and other pro-inflammatory cytokines in both THP-1 derived and human primary macrophages.106 Altogether, these data suggest that PCSK9 may locally regulate atherosclerotic plaque inflammation. Accordingly, the ATHEROREMO-IVUS study found that circulating PCSK9 levels were positively associated with the extent of necrotic core in atheroma, independently of LDL levels in patients with acute coronary syndrome.107 Moreover, PCSK9 inhibition with alirocumab in APOE*3 Leiden.CETP mice, decreases macrophage and necrotic core content and increases vascular smooth muscle cells and collagen content.108
Regarding the effects of PCSK9 inhibition on inflammation in humans, it reduces Lp(a) levels,109 a molecule that circulates bound to PCSK9 in plasma,110 and promotes inflammation and oxidative stress and coagulation. Also, PCSK9-antibody therapy markedly reduces monocyte inflammatory phenotype in patients with familial hypercholesterolaemia, without any change in plasma hsCRP concentration.13 A similar lack of effect on hsCRP levels has been described in large cardiovascular outcomes trials.4 This emphasizes the potential for anti-inflammatory effects without reduction in the liver-derived acute phase reactant hsCRP.111
5.3 PCSK9 and septic shock
Beyond its lipid-lowering action, as lipopolysaccharide circulates bound to LDL, up-regulation of hepatic LDLR by PCSK9 inhibition has been suggested to result in increased lipopolysaccharide clearance (Figure 2), a decreased inflammatory response, and improved survival following sepsis in mice112,113 although, in a recent study, PCSK9 inhibition failed to reduce LPS-induced mortality in mice.114 Importantly, humans with PCSK9 loss-of-function variants also exhibit improved clinical outcomes during septic shock.115 Finally, enhanced plasma PCSK9 levels during sepsis are associated with multiple organ failure.97 Based on these results, clinical trials are planned to assess the effect of PCSK9 inhibition outcomes during sepsis.
5.4 Vascular actions of PCSK9
PCSK9 is expressed in many other tissues than the liver, including atherosclerotic plaques,101,116 and vascular areas of low shear stress, that are prone to develop atherosclerosis.95 PCSK9 expression is found mainly in vascular smooth muscle cells,101 and to a lesser extent in endothelial cells.95 PCSK9 deficiency reduces neointimal formation following injury of the carotid artery in mice beyond cholesterol lowering by decreasing vascular smooth muscle cell migration and proliferation rate.117
Recent epidemiological studies have demonstrated that plasma PCSK9 levels are associated with carotid atherosclerosis118 independently of LDL. However, other data challenge the idea of a lipid-independent effects of PCSK9 inhibitors. For instance, plasma PCSK9 levels have not proven consistently to predict cardiovascular events.100,119,120 Moreover, in the GLAGOV trial a linear relationship was found between the regression of atheroma and the decrease of LDL achieved with evolocumab.121 Finally, the risk reduction observed with PCSK9 inhibitors in clinical trials, seems to be fully explained by the decrease of LDL achieved, suggesting that the anti-atherosclerotic benefit of this therapy is directly related to LDL reduction.122
However, inflammation may affect the response to PCSK9 inhibition as suggested by very recent post hoc analyses of PCSK9 trials.4 First, patients with high CRP at baseline obtain greater benefit with PCSK9 inhibitors.4 Second, patients with persistent high CRP levels after initiating treatment with statins and PSCK9 inhibitors have a worse prognosis.5 Although the risk reductions obtained in these trials seem to be fully explained by the decrease in LDL cholesterol, a more marked beneficial response to PCSK9 inhibition may hence be predicted in patients with high inflammatory levels.
Consensus statements
Lipid reduction is associated with modulation of the inflammatory and immune responses irrespective of the lipid-lowering therapy used. Despite decreasing cholesterol through different mechanisms, most lipid-lowering therapies, including dietary interventions, share anti-inflammatory, and immunomodulatory properties. This observation provides strong evidence that lipid lowering per se causes alterations in inflammation and immunity. Some lipid-lowering drugs also directly target lipid-independent pathways to reduce inflammation in experimental and exploratory studies. However, a contribution of these effects to cardiovascular outcomes is unclear, and further studies are required to address this question. However, regardless of the mechanism involved, an anti-inflammatory response to lipid-lowering may be of clinical importance to predict the effect of treatment.
Using surrogates for both lipid metabolism and inflammation as biomarkers in future studies may contribute to a better understanding of the relative importance of different mechanisms of action. Given the strong association between inflammation, lipids and atherosclerosis, assessment of the inflammatory response to lipid-lowering interventions could be helpful to establish optimal dose and type of lipid-lowering therapy in cardiovascular prevention. There is still an unmet need for new biomarkers and further validation of existing biomarkers that more closely reflect the inflammatory activity in atherosclerosis before such approach can be implemented.123 In this regard, at present, CRP determination is not adviced as it adds small value to the existing methods of cardiovascular risk assessment.68 Also, imaging vascular inflammation by techniques such as PET show promise to assess the anti-inflammatory effect of lipid-lowering therapies. Furthermore, we raise the notion of lipid monitoring in studies of anti-inflammatory therapies.
Comparative studies of further lipid lowering, anti-inflammation and a combination of both will be crucial to identify effects that are specific or shared for each treatment strategy. Current experimental and clinical research evidence discussed in the present Position Paper can be used to design a head-to-head comparison of the potential beneficial effects of additional anti-inflammation or lipid lowering therapies or the combination of both regimens to current medical standards in secondary prevention. In this context, personalized medicine could be anticipated based on predictive factors for a beneficial response to lipid lowering and/or inflammatory levels in secondary prevention.
Conflict of interest: J.T. reports personal fees from Sanofi-Renegeron and Pfizer. L.B. reports grants and personal fees from ASTRA ZENECA, and personal fees from Sanofi. B.C. reports grants and personal fees from Amgen, Sanofi and Regeneron; personal fees from Merck, and grants from Pfizer. J.E. reports personal fees from Sanofi and Pfizer. E.S. reports speaker fees/reimbursement from Amgen and Sanofi. C.M.M. reports grants from MSD, Bayer, AstraZeneca, and EliLilly; and personal fees from MSD, AstraZeneca, Roche, Sanofi, and Amgen. All other authors declared no conflict of interest.
Funding
J.T. is supported by FIS (PI17/01615), and Spanish Societies of Cardiology and Arteriosclerosis. L.B. is supported by PNS-2016, Tercel 2016 and CiberCV from Institute Carlos III and Spanish Society of Cardiology. B.C. is supported by Fondation Leducq (13CVD03) and by the French national project CHOPIN (ANR-16-RHUS-0007). J.E. is supported by FIS-FEDER (PI14/00386). P.C.E. is supported by the British Heart Foundation. D.F.J.K. is supported by the Swedish Heart-Lung Foundation and Novo Nordisk Foundation (NNF15CC0018346). E.L. is funded by the NWO (VICI), the European Research Council (ERC-con) and the DFG (SFB1123). S.S. is supported by the DFG (SFB1123). E.S.S. is supported by the HORIZON2020 program (REPROGRAM) and the Dutch Heart Foundation (CVON-GENIUS-II). C.W. is funded by the European Research Council (ERC-Adv) and the DFG (SFB1123). M.B. is funded by Swedish Research Council (2014-2312); and the Swedish Heart and Lung Foundation (20150600 and 20150683).
References
- 1. Tuñón J, Bäck M, Badimón L, Bochaton-Piallat M-L, Cariou B, Daemen MJ, Egido J, Evans PC, Francis SE, Ketelhuth DF, Lutgens E, Matter CM, Monaco C, Steffens S, Stroes E, Vindis C, Weber C, Hoefer IE.. Interplay between hypercholesterolaemia and inflammation in atherosclerosis: translating experimental targets into clinical practice. Eur J Prev Cardiol 2018;25:948–955. [DOI] [PubMed] [Google Scholar]
- 2. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR.. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 3. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ.. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 4. Bohula EA, Giugliano RP, Leiter LA, Verma S, Park J-G, Sever PS, Lira Pineda A, Honarpour N, Wang H, Murphy SA, Keech A, Pedersen TR, Sabatine MS.. Inflammatory and cholesterol risk in the FOURIER trial. Circulation 2018;138:131–140. [DOI] [PubMed] [Google Scholar]
- 5. Pradhan AD, Aday AW, Rose LM, Ridker PM.. Residual inflammatory risk on treatment with PCSK9 inhibition and statin therapy. Circulation 2018;138:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shapiro MD, Fazio S.. From lipids to inflammation. Circ Res 2016;118:732–749. [DOI] [PubMed] [Google Scholar]
- 7. Xu X-P, Meisel SR, Ong JM, Kaul S, Cercek B, Rajavashisth TB, Sharifi B, Shah PK.. Oxidized low-density lipoprotein regulates matrix metalloproteinase-9 and its tissue inhibitor in human monocyte-derived macrophages. Circulation 1999;99:993–998. [DOI] [PubMed] [Google Scholar]
- 8. Escate R, Padro T, Badimon L.. LDL accelerates monocyte to macrophage differentiation: effects on adhesion and anoikis. Atherosclerosis 2016;246:177–186. [DOI] [PubMed] [Google Scholar]
- 9. Cole JE, Kassiteridi C, Monaco C.. Toll-like receptors in atherosclerosis: a ‘Pandora’s box' of advances and controversies. Trends Pharmacol Sci 2013;34:629–636. [DOI] [PubMed] [Google Scholar]
- 10. Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL.. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem 2003;278:1561–1568. [DOI] [PubMed] [Google Scholar]
- 11. Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E.. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010;464:1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AAC, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK.. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 2015;21:628.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bernelot Moens SJ, Neele AE, Kroon J, van der Valk FM, Van den Bossche J, Hoeksema MA, Hoogeveen RM, Schnitzler JG, Baccara-Dinet MT, Manvelian G, de Winther MPJ, Stroes ESG.. PCSK9 monoclonal antibodies reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia. Eur Heart J 2017;38:1584–1593. [DOI] [PubMed] [Google Scholar]
- 14. Tsiantoulas D, Diehl CJ, Witztum JL, Binder CJ.. B cells and humoral immunity in atherosclerosis. Circ Res 2014;114:1743–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schönbeck U, Libby P.. CD40 signaling and plaque instability. Circ Res 2001;89:1092–1103. [DOI] [PubMed] [Google Scholar]
- 16. Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M.. Myocardial infarction accelerates atherosclerosis. Nature 2012;487:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR.. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 2010;328:1689–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Padró T, Cubedo J, Camino S, Béjar MT, Ben-Aicha S, Mendieta G, Escolà-Gil JC, Escate R, Gutiérrez M, Casani L, Badimon L, Vilahur G.. Detrimental effect of hypercholesterolemia on high-density lipoprotein particle remodeling in pigs. J Am Coll Cardiol 2017;70:165–178. [DOI] [PubMed] [Google Scholar]
- 19. Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Krankel N, Kania G, Zewinger S, Akhmedov A, Shi Y, Martin T, Perisa D, Winnik S, Muller MF, Sester U, Wernicke G, Jung A, Gutteck U, Eriksson U, Geisel J, Deanfield J, von Eckardstein A, Luscher TF, Fliser D, Bahlmann FH, Landmesser U.. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 2013;38:754–768. [DOI] [PubMed] [Google Scholar]
- 20. Madsen CM, Varbo A, Nordestgaard BG.. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J 2017;38:2478–2486. [DOI] [PubMed] [Google Scholar]
- 21. Blanco-Colio LM, Valderrama M, Alvarez-Sala LA, Bustos C, Ortego M, Hernández-Presa MA, Cancelas P, Gómez-Gerique J, Millán J, Egido J.. Red wine intake prevents nuclear factor-κB activation in peripheral blood mononuclear cells of healthy volunteers during postprandial lipemia. Circulation 2000;102:1020–1026. [DOI] [PubMed] [Google Scholar]
- 22. Camhi SM, Stefanick ML, Ridker PM, Young DR.. Changes in C-reactive protein from low-fat diet and/or physical activity in men and women with and without metabolic syndrome. Metabolism 2010;59:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Llorente-Cortés V, Estruch R, Mena MP, Ros E, González MAM, Fitó M, Lamuela-Raventós RM, Badimon L.. Effect of Mediterranean diet on the expression of pro-atherogenic genes in a population at high cardiovascular risk. Atherosclerosis 2010;208:442–450. [DOI] [PubMed] [Google Scholar]
- 24. Gower RM, Wu H, Foster GA, Devaraj S, Jialal I, Ballantyne CM, Knowlton AA, Simon SI.. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol 2011;31:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaneda M, Kashiwamura S, Ueda H, Sawada K, Sugihara A, Terada N, Kimura-Shimmyo A, Fukuda Y, Shimoyama T, Okamura H.. Inflammatory liver steatosis caused by IL-12 and IL-18. J Interferon Cytokine Res 2003;23:155–162. [DOI] [PubMed] [Google Scholar]
- 26. Endo M, Masaki T, Seike M, Yoshimatsu H.. TNF-alpha induces hepatic steatosis in mice by enhancing gene expression of sterol regulatory element binding protein-1c (SREBP-1c). Exp Biol Med (Maywood) 2007;232:614–621. [PubMed] [Google Scholar]
- 27. Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C.. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res 2004;45:1169–1196. [DOI] [PubMed] [Google Scholar]
- 28. Guzik TJ, Skiba DS, Touyz RM, Harrison DG.. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res 2017;113:1009–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ito A, Hong C, Rong X, Zhu X, Tarling EJ, Hedde PN, Gratton E, Parks J, Tontonoz P.. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. eLife 2015;4:e08009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lo JC, Wang Y, Tumanov AV, Bamji M, Yao Z, Reardon CA, Getz GS, Fu Y-X.. Lymphotoxin ß receptor-dependent control of lipid homeostasis. Science 2007;316:285–288. [DOI] [PubMed] [Google Scholar]
- 31. Klingenberg R, Gerdes N, Badeau RM, Gister A, Ketelhuth DF, Lundberg AM, Rudling M, Nilsson SK, Olivecrona G, Zoller S, Lohmann C, Scher TF, Jauhiainen M, Sparwasser T, Hansson GK.. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest 2013;123:1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Emoto T, Sasaki N, Yamashita T, Kasahara K, Yodoi K, Sasaki Y, Matsumoto T, Mizoguchi T, Hirata KI.. Regulatory/effector T-cell ratio is reduced in coronary artery disease. Circ J 2014;78:2935–2941. [DOI] [PubMed] [Google Scholar]
- 33. Catapano AL, Pirillo A, Bonacina F, Norata GD.. HDL in innate and adaptive immunity. Cardiovasc Res 2014;103:372–383. [DOI] [PubMed] [Google Scholar]
- 34. Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, La Du BN, Fogelman AM, Navab M.. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest 1995;96:2758–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G, Le Chatelier E, Levenez F, Dore J, Mattila I, Plichta DR, Poho P, Hellgren LI, Arumugam M, Sunagawa S, Vieira-Silva S, Jorgensen T, Holm JB, Trost K, Meta HITC, Kristiansen K, Brix S, Raes J, Wang J, Hansen T, Bork P, Brunak S, Oresic M, Ehrlich SD, Pedersen O.. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016;535:376–381. [DOI] [PubMed] [Google Scholar]
- 36. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL.. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kappel B, Stohr R, De Angelis L, Menghini R, Marx N, Federici M.. Disruption of gut microbiota alters intestinal lipid metabolism and worsens atherosclerosis. Eur Heart J 2016;37(Suppl 1):790.26834189 [Google Scholar]
- 38. Herbert KE, Erridge C.. Regulation of low-density lipoprotein cholesterol by intestinal inflammation and the acute phase response. Cardiovasc Res 2018;114:226–232. [DOI] [PubMed] [Google Scholar]
- 39. Farkouh ME, Greenberg JD, Jeger RV, Ramanathan K, Verheugt FWA, Chesebro JH, Kirshner H, Hochman JS, Lay CL, Ruland S, Mellein B, Matchaba PT, Fuster V, Abramson SB.. Cardiovascular outcomes in high risk patients with osteoarthritis treated with ibuprofen, naproxen or lumiracoxib. Ann Rheum Dis 2007;66:764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nissen SE, Yeomans ND, Solomon DH, Lüscher TF, Libby P, Husni ME, Graham DY, Borer JS, Wisniewski LM, Wolski KE, Wang Q, Menon V, Ruschitzka F, Gaffney M, Beckerman B, Berger MF, Bao W, Lincoff AM.. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med 2016;375:2519–2529. [DOI] [PubMed] [Google Scholar]
- 41. Choi HK, Seeger JD.. Glucocorticoid use and serum lipid levels in US adults: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2005;53:528–535. [DOI] [PubMed] [Google Scholar]
- 42. Ettinger WH Jr, Hazzard WR.. Prednisone increases very low density lipoprotein and high density lipoprotein in healthy men. Metabolism 1988;37:1055–1058. [DOI] [PubMed] [Google Scholar]
- 43. Azar RR, Rinfret S, Théroux P, Stone PH, Dakshinamurthy R, Feng YJ, Wu AHB, Rangé G, Waters DD.. A randomized placebo-controlled trial to assess the efficacy of antiinflammatory therapy with methylprednisolone in unstable angina (MUNA trial). Eur Heart J 2000;21:2026–2032. [DOI] [PubMed] [Google Scholar]
- 44. Daien CI, Duny Y, Barnetche T, Daures JP, Combe B, Morel J.. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: a systematic review with meta-analysis. Ann Rheum Dis 2012;71:862–868. [DOI] [PubMed] [Google Scholar]
- 45. van Sijl AM, Peters MJ, Knol DL, de Vet RH, Sattar N, Dijkmans BA, Smulders YM, Nurmohamed MT.. The effect of TNF-alpha blocking therapy on lipid levels in rheumatoid arthritis: a meta-analysis. Semin Arthritis Rheum 2011;41:393–400. [DOI] [PubMed] [Google Scholar]
- 46. Barnabe C, Martin BJ, Ghali WA.. Systematic review and meta-analysis: anti-tumor necrosis factor alpha therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res 2011;63:522–529. [DOI] [PubMed] [Google Scholar]
- 47. McInnes IB, Thompson L, Giles JT, Bathon JM, Salmon JE, Beaulieu AD, Codding CE, Carlson TH, Delles C, Lee JS, Sattar N.. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis 2015;74:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ronda N, Greco D, Adorni MP, Zimetti F, Favari E, Hjeltnes G, Mikkelsen K, Borghi MO, Favalli EG, Gatti R, Hollan I, Meroni PL, Bernini F.. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol 2015;67:1155–1164. [DOI] [PubMed] [Google Scholar]
- 49. Navarro-Millan I, Charles-Schoeman C, Yang S, Bathon JM, Bridges SL Jr, Chen L, Cofield SS, Dell'Italia LJ, Moreland LW, O'Dell JR, Paulus HE, Curtis JR.. Changes in lipoproteins associated with methotrexate or combination therapy in early rheumatoid arthritis: results from the treatment of early rheumatoid arthritis trial. Arthritis Rheum 2013;65:1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL.. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013;61:404–410. [DOI] [PubMed] [Google Scholar]
- 51. Takeuchi S, Kawashima S, Rikitake Y, Ueyama T, Inoue N, Hirata K-I, Yokoyama M.. Cerivastatin suppresses lipopolysaccharide-induced ICAM-1 expression through inhibition of rho GTPase in BAEC. Biochem Biophys Res Commun 2000;269:97–102. [DOI] [PubMed] [Google Scholar]
- 52. Martinez-Gonzalez J, Raposo B, Rodríguez C, Badimón L.. 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition prevents endothelial NO synthase downregulation by atherogenic levels of native LDLs: balance between transcriptional and posttranscriptional regulation. Arterioscler Thromb Vasc Biol 2001;21:804–809. [DOI] [PubMed] [Google Scholar]
- 53. Bustos C, Hernández-Presa MA, Ortego M, Tuñón J, Ortega L, Pérez F, Díaz C, Hernández G, Egido J.. HMG-CoA reductase inhibition by atorvastatin reduces neointimal inflammation in a rabbit model of atherosclerosis. J Am Coll Cardiol 1998;32:2057–2064. [DOI] [PubMed] [Google Scholar]
- 54. Hernández-Presa MA, Martı´n-Ventura JL, Ortego M, Gómez-Hernández A, Tuñón J, Hernández-Vargas P, Blanco-Colio LM, Mas S, Aparicio C, Ortega L, Vivanco F, Gerique JG, Dı´az C, Hernández G, Egido J, Atorvastatin reduces the expression of cyclooxygenase-2 in a rabbit model of atherosclerosis and in cultured vascular smooth muscle cells. Atherosclerosis 2002;160:49–58. [DOI] [PubMed] [Google Scholar]
- 55. Ali F, Zakkar M, Karu K, Lidington EA, Hamdulay SS, Boyle JJ, Zloh M, Bauer A, Haskard DO, Evans PC, Mason JC.. Induction of the cytoprotective enzyme heme oxygenase-1 by statins is enhanced in vascular endothelium exposed to laminar shear stress and impaired by disturbed flow. J Biol Chem 2009;284:18882–18892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kwak B, Mulhaupt F, Myit S, Mach F.. Statins as a newly recognized type of immunomodulator. Nat Med 2000;6:1399–1402. [DOI] [PubMed] [Google Scholar]
- 57. Kagami S-I, Owada T, Kanari H, Saito Y, Suto A, Ikeda K, Hirose K, Watanabe N, Iwamoto I, Nakajima H.. Protein geranylgeranylation regulates the balance between Th17 cells and Foxp3+ regulatory T cells. Int Immunol 2009;21:679–689. [DOI] [PubMed] [Google Scholar]
- 58. Bu D, Tarrio M, Grabie N, Zhang Y, Yamazaki H, Stavrakis G, Maganto-Garcia E, Pepper-Cunningham Z, Jarolim P, Aikawa M, Garcia-Cardeña G, Lichtman AH.. Statin-induced Kruppel-like factor 2 expression in human and mouse T cells reduces inflammatory and pathogenic responses. J Clin Invest 2010;120:1961–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Martín-Ventura JL, Blanco-Colio LM, Gómez-Hernández A, Muñoz-García B, Vega M, Serrano J, Ortega L, Hernández G, Tuñón J, Egido J.. Intensive treatment with atorvastatin reduces inflammation in mononuclear cells and human atherosclerotic lesions in one month. Stroke 2005;36:1796–1800. [DOI] [PubMed] [Google Scholar]
- 60. Ferro D, Parrotto S, Basili S, Alessandri C, Violi F.. Simvastatin inhibits the monocyte expression of proinflammatory cytokines in patients with hypercholesterolemia. J Am Coll Cardiol 2000;36:427–431. [DOI] [PubMed] [Google Scholar]
- 61. Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, Gotto AMJ.. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med 2001;344:1959–1965. [DOI] [PubMed] [Google Scholar]
- 62. Suades R, Padro T, Alonso R, Mata P, Badimon L.. Lipid-lowering therapy with statins reduces microparticle shedding from endothelium, platelets and inflammatory cells. Thromb Haemost 2013;110:366–377. [DOI] [PubMed] [Google Scholar]
- 63. Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E.. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005;352:20–28. [DOI] [PubMed] [Google Scholar]
- 64. Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AMJ, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ.. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 65. Ridker PM. A test in context: high-sensitivity C-reactive protein. J Am Coll Cardiol 2016;67:712–723. [DOI] [PubMed] [Google Scholar]
- 66. Heart Protection Study Collaborative Group. C-reactive protein concentration and the vascular benefits of statin therapy: an analysis of 20 536 patients in the Heart Protection Study. Lancet 2011;377:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. C Reactive Protein Coronary Heart Disease Genetics Collaboration (CCGC). Association between C reactive protein and coronary heart disease: Mendelian randomisation analysis based on individual participant data. BMJ 2011;342:d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FD, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WM; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kobashigawa JA, Katznelson S, Laks H, Johnson JA, Yeatman L, Wang XM, Chia D, Terasaki PI, Sabad A, Cogert GA, Trosian K, Hamilton MA, Moriguchi JD, Kawata N, Hage A, Drinkwater DC, Stevenson LW.. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med 1995;333:621–627. [DOI] [PubMed] [Google Scholar]
- 70. Wenke K, Meiser B, Thiery J, Nagel D, von Scheidt W, Steinbeck G, Seidel D, Reichart B.. Simvastatin reduces graft vessel disease and mortality after heart transplantation: a four-year randomized trial. Circulation 1997;96:1398–1402. [DOI] [PubMed] [Google Scholar]
- 71. Holdaas H, Fellström B, Jardine AG, Holme I, Nyberg G, Fauchald P, Grönhagen-Riska C, Madsen S, Neumayer H-H, Cole E, Maes B, Ambuhl P, Olsson AG, Hartmann A, Solbu DO, Pedersen TR.. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet 2003;361:2024–2031. [DOI] [PubMed] [Google Scholar]
- 72. Sahu K, Sharma R, Gupta A, Gulati S, Agarwal D, Kumar A, Bhandari M.. Effect of lovastatin, an HMG CoA reductase inhibitor, on acute renal allograft rejection. Clin Transplant 2001;15:173–175. [DOI] [PubMed] [Google Scholar]
- 73. Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, Subramanian SS, Abdelbaky A, Rudd JHF, Farkouh ME, Nunes IO, Beals CR, Shankar SS.. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol 2013;62:909–917. [DOI] [PubMed] [Google Scholar]
- 74. Tawakol A, Singh P, Rudd JHF, Soffer J, Cai G, Vucic E, Brannan SP, Tarka EA, Shaddinger BC, Sarov-Blat L, Matthews P, Subramanian S, Farkouh M, Fayad ZA.. Effect of treatment for 12 weeks with rilapladib, a lipoprotein-associated phospholipase A2 inhibitor, on arterial inflammation as assessed with 18F-fluorodeoxyglucose-positron emission tomography imaging. J Am Coll Cardiol 2014;63:86–88. [DOI] [PubMed] [Google Scholar]
- 75. Van Aelst L, D'Souza-Schorey C.. Rho GTPases and signaling networks. Genes Dev 1997;11:2295–2322. [DOI] [PubMed] [Google Scholar]
- 76. Hernández-Presa MA, Ortego M, Tuñón J, Martín-Ventura JL, Mas S, Blanco-Colio LM, Aparicio C, Ortega L, Gómez-Gerique J, Vivanco F, Egido J.. Simvastatin reduces NF-κB activity in peripheral mononuclear and in plaque cells of rabbit atheroma more markedly than lipid lowering diet. Cardiovasc Res 2003;57:168–177. [DOI] [PubMed] [Google Scholar]
- 77. Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, Bravo M, Mitchell DJ, Sobel RA, Steinman L, Zamvil SS.. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 2002;420:78–84. [DOI] [PubMed] [Google Scholar]
- 78. Leung BP, Sattar N, Crilly A, Prach M, McCarey DW, Payne H, Madhok R, Campbell C, Gracie JA, Liew FY, McInnes IB.. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol 2003;170:1524–1530. [DOI] [PubMed] [Google Scholar]
- 79. Sparrow CP, Burton CA, Hernandez M, Mundt S, Hassing H, Patel S, Rosa R, Hermanowski-Vosatka A, Wang P-R, Zhang D, Peterson L, Detmers PA, Chao Y-S, Wright SD.. Simvastatin has anti-inflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Arterioscler Thromb Vasc Biol 2001;21:115–121. [DOI] [PubMed] [Google Scholar]
- 80. McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakova O, Ford I, Capell HA, Sattar N.. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet 2004;363:2015–2021. [DOI] [PubMed] [Google Scholar]
- 81. Mäki-Petäjä KM, Booth AD, Hall FC, Wallace SML, Brown J, McEniery CM, Wilkinson IB.. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol 2007;50:852–858. [DOI] [PubMed] [Google Scholar]
- 82. Silverman MG, Ference BA, Im K, Wiviott S, Giugliano R, Grundy S, Braunwald E, Sabatine M.. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 2016;316:1289–1297. [DOI] [PubMed] [Google Scholar]
- 83. Duivenvoorden R, Tang J, Cormode DP, Mieszawska AJ, Izquierdo-Garcia D, Ozcan C, Otten MJ, Zaidi N, Lobatto ME, van Rijs SM, Priem B, Kuan EL, Martel C, Hewing B, Sager H, Nahrendorf M, Randolph GJ, Stroes ESG, Fuster V, Fisher EA, Fayad ZA, Mulder WJM.. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat Commun 2014;5:3065–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tang J, Lobatto ME, Hassing L, van der Staay S, van Rijs SM, Calcagno C, Braza MS, Baxter S, Fay F, Sanchez-Gaytan BL, Duivenvoorden R, Sager HB, Astudillo YM, Leong W, Ramachandran S, Storm G, Pérez-Medina C, Reiner T, Cormode DP, Strijkers GJ, Stroes ESG, Swirski FK, Nahrendorf M, Fisher EA, Fayad ZA, Mulder WJM.. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci Adv 2015;1:e1400223.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gómez-Garre D, Muñoz-Pacheco P, González-Rubio ML, Aragoncillo P, Granados R, Fernández-Cruz A.. Ezetimibe reduces plaque inflammation in a rabbit model of atherosclerosis and inhibits monocyte migration in addition to its lipid-lowering effect. Br J Pharmacol 2009;156:1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chan DC, Watts GF, Gan SK, Ooi EMM, Barrett PHR.. Effect of ezetimibe on hepatic fat, inflammatory markers, and apolipoprotein B-100 kinetics in insulin-resistant obese subjects on a weight loss diet. Diabetes Care 2010;33:1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bohula EA, Giugliano RP, Cannon CP, Zhou J, Murphy SA, White JA, Tershakovec AM, Blazing MA, Braunwald E.. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation 2015;132:1224–1233. [DOI] [PubMed] [Google Scholar]
- 88. Catapano AL, Pirillo A, Norata GD.. Vascular inflammation and low-density lipoproteins: is cholesterol the link? A lesson from the clinical trials. Br J Pharmacol 2017;174:3973–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Leren TP. Sorting an LDL receptor with bound PCSK9 to intracellular degradation. Atherosclerosis 2014;237:76–81. [DOI] [PubMed] [Google Scholar]
- 90. Zhang DW, Lagace TA, Garuti R, Zhao Z, McDonald M, Horton JD, Cohen JC, Hobbs HH.. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J Biol Chem 2007;282:18602–18612. [DOI] [PubMed] [Google Scholar]
- 91. Poirier S, Mayer G, Poupon V, McPherson PS, Desjardins R, Ly K, Asselin MC, Day R, Duclos FJ, Witmer M, Parker R, Prat A, Seidah NG.. Dissection of the endogenous cellular pathways of PCSK9-induced low density lipoprotein receptor degradation: evidence for an intracellular route. J Biol Chem 2009;284:28856–28864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Homer VM, Marais AD, Charlton F, Laurie AD, Hurndell N, Scott R, Mangili F, Sullivan DR, Barter PJ, Rye KA, George PM, Lambert G.. Identification and characterization of two non-secreted PCSK9 mutants associated with familial hypercholesterolemia in cohorts from New Zealand and South Africa. Atherosclerosis 2008;196:659–666. [DOI] [PubMed] [Google Scholar]
- 93. Dong B, Wu M, Li H, Kraemer FB, Adeli K, Seidah NG, Park SW, Liu J.. Strong induction of PCSK9 gene expression through HNF1α and SREBP2: mechanism for the resistance to LDL-cholesterol lowering effect of statins in dyslipidemic hamsters. J Lipid Res 2010;51:1486–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Feingold KR, Moser AH, Shigenaga JK, Patzek SM, Grunfeld C.. Inflammation stimulates the expression of PCSK9. Biochem Biophys Res Commun 2008;374:341–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ding Z, Liu S, Wang X, Deng X, Fan Y, Sun C, Wang Y, Mehta JL.. Hemodynamic shear stress via ROS modulates PCSK9 expression in human vascular endothelial and smooth muscle cells and along the mouse aorta. Antioxid Redox Signal 2015;22:760–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ding Z, Liu S, Wang X, Theus S, Deng X, Fan Y, Zhou S, Mehta JL.. PCSK9 regulates expression of scavenger receptors and ox-LDL uptake in macrophages. Cardiovasc Res 2018;114:1145–1153. [DOI] [PubMed] [Google Scholar]
- 97. Boyd JH, Fjell CD, Russell JA, Sirounis D, Cirstea MS, Walley KR.. Increased plasma PCSK9 levels are associated with reduced endotoxin clearance and the development of acute organ failures during sepsis. J Innate Immun 2016;8:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bras ML, Roquilly A, Deckert V, Langhi C, Feuillet F, Sébille V, Mahé P-J, Bach K, Masson D, Lagrost L, Costet P, Asehnoune K, Cariou B.. Plasma PCSK9 Is a late biomarker of severity in patients with severe trauma injury. J Clin Endocrinol Metab 2013;98:E732–E736. [DOI] [PubMed] [Google Scholar]
- 99. Li S, Guo Y-L, Xu R-X, Zhang Y, Zhu C-G, Sun J, Qing P, Wu N-Q, Jiang L-X, Li J-J.. Association of plasma PCSK9 levels with white blood cell count and its subsets in patients with stable coronary artery disease. Atherosclerosis 2014;234:441–445. [DOI] [PubMed] [Google Scholar]
- 100. Gencer B, Montecucco F, Nanchen D, Carbone F, Klingenberg R, Vuilleumier N, Aghlmandi S, Heg D, Räber L, Auer R, Jüni P, Windecker S, Lüscher TF, Matter CM, Rodondi N, Mach F.. Prognostic value of PCSK9 levels in patients with acute coronary syndromes. Eur Heart J 2016;37:546–553. [DOI] [PubMed] [Google Scholar]
- 101. Ferri N, Tibolla G, Pirillo A, Cipollone F, Mezzetti A, Pacia S, Corsini A, Catapano AL.. Proprotein convertase subtilisin kexin type 9 (PCSK9) secreted by cultured smooth muscle cells reduces macrophages LDLR levels. Atherosclerosis 2012;220:381–386. [DOI] [PubMed] [Google Scholar]
- 102. Giunzioni I, Tavori H, Covarrubias R, Major AS, Ding L, Zhang Y, DeVay RM, Hong L, Fan D, Predazzi IM, Rashid S, Linton MF, Fazio S.. Local effects of human PCSK9 on the atherosclerotic lesion. J Pathol 2016;238:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhihan T, Lu J, Juan P, Zhong R, Dangheng W, Chunyang W, Lihong P, Zhisheng J, Lushan L.. PCSK9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF-κB activation in THP-1-derived macrophages. Int J Mol Med 2012;30:931–938. [DOI] [PubMed] [Google Scholar]
- 104. Tang Z-H, Peng J, Ren Z, Yang J, Li T-T, Li T-H, Wang Z, Wei D-H, Liu L-S, Zheng X-L, Jiang Z-S.. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-κB pathway. Atherosclerosis 2017;262:113–122. [DOI] [PubMed] [Google Scholar]
- 105. Adorni MP, Cipollari E, Favari E, Zanotti I, Zimetti F, Corsini A, Ricci C, Bernini F, Ferri N.. Inhibitory effect of PCSK9 on Abca1 protein expression and cholesterol efflux in macrophages. Atherosclerosis 2017;256:1–6. [DOI] [PubMed] [Google Scholar]
- 106. Ricci C, Ruscica M, Camera M, Rossetti L, Macchi C, Colciago A, Zanotti I, Lupo MG, Adorni MP, Cicero AFG, Fogacci F, Corsini A, Ferri N.. PCSK9 induces a pro-inflammatory response in macrophages. Sci Rep 2018;8:2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Cheng JM, Oemrawsingh RM, Garcia-Garcia HM, Boersma E, van Geuns R-J, Serruys PW, Kardys I, Akkerhuis KM.. PCSK9 in relation to coronary plaque inflammation: results of the ATHEROREMO-IVUS study. Atherosclerosis 2016;248:117–122. [DOI] [PubMed] [Google Scholar]
- 108. Kühnast S, van der Hoorn JWA, Pieterman EJ, van den Hoek AM, Sasiela WJ, Gusarova V, Peyman A, Schäfer H-L, Schwahn U, Jukema JW, Princen HMG.. Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin. J Lipid Res 2014;55:2103–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gaudet D, Kereiakes DJ, McKenney JM, Roth EM, Hanotin C, Gipe D, Du Y, Ferrand A-C, Ginsberg HN, Stein EA.. Effect of alirocumab, a monoclonal proprotein convertase subtilisin/kexin 9 antibody, on lipoprotein(a) concentrations (a pooled analysis of 150 mg every two weeks dosing from phase 2 trials). Am J Cardiol 2014;114:711–715. [DOI] [PubMed] [Google Scholar]
- 110. Tavori H, Christian D, Minnier J, Plubell D, Shapiro MD, Yeang C, Giunzioni I, Croyal M, Duell PB, Lambert G, Tsimikas S, Fazio S.. PCSK9 association with lipoprotein(a): novelty and significance. Circ Res 2016;119:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sahebkar A, Di Giosia P, Stamerra CA, Grassi D, Pedone C, Ferretti G, Bacchetti T, Ferri C, Giorgini P.. Effect of monoclonal antibodies to PCSK9 on high-sensitivity C-reactive protein levels: a meta-analysis of 16 randomized controlled treatment arms. Br J Clin Pharmacol 2016;81:1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Topchiy E, Cirstea M, Kong HJ, Boyd JH, Wang Y, Russell JA, Walley KR.. Lipopolysaccharide is cleared from the circulation by hepatocytes via the low density lipoprotein receptor. PLoS One 2016;11:e0155030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dwivedi DJ, Grin PM, Khan M, Prat A, Zhou J, Fox-Robichaud EA, Seidah NG, Liaw PC.. Differential expression of PCSK9 modulates infection, inflammation, and coagulation in a murine model of sepsis. Shock 2016;46:672–680. [DOI] [PubMed] [Google Scholar]
- 114. Berger J-M, Loza Valdes A, Gromada J, Anderson N, Horton JD.. Inhibition of PCSK9 does not improve lipopolysaccharide-induced mortality in mice. J Lipid Res 2017;58:1661–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Walley KR, Thain KR, Russell JA, Reilly MP, Meyer NJ, Ferguson JF, Christie JD, Nakada T-A, Fjell CD, Thair SA, Cirstea MS, Boyd JH.. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci Transl Med 2014;6:258ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cariou B, Si-Tayeb K, Le May C.. Role of PCSK9 beyond liver involvement. Curr Opin Lipidol 2015;26:155–161. [DOI] [PubMed] [Google Scholar]
- 117. Ferri N, Marchianò S, Tibolla G, Baetta R, Dhyani A, Ruscica M, Uboldi P, Catapano AL, Corsini A.. PCSK9 knock-out mice are protected from neointimal formation in response to perivascular carotid collar placement. Atherosclerosis 2016;253:214–224. [DOI] [PubMed] [Google Scholar]
- 118. Xie W, Liu J, Wang W, Wang M, Qi Y, Zhao F, Sun J, Liu J, Li Y, Zhao D.. Association between plasma PCSK9 levels and 10-year progression of carotid atherosclerosis beyond LDL-C: a cohort study. Int J Cardiol 2016;215:293–298. [DOI] [PubMed] [Google Scholar]
- 119. Leander K, Mälarstig A, van’t Hooft FM, Hyde C, Hellénius M-L, Troutt JS, Konrad RJ, Öhrvik J, Hamsten A, de Faire U.. Circulating proprotein convertase subtilisin/kexin type 9 (PCSK9) predicts future risk of cardiovascular events independently of established risk factors. Circulation 2016;133:1230–1239. [DOI] [PubMed] [Google Scholar]
- 120. Ridker PM, Rifai N, Bradwin G, Rose L.. Plasma proprotein convertase subtilisin/kexin type 9 levels and the risk of first cardiovascular events. Eur Heart J 2016;37:554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Nicholls S, Puri R, Anderson T, Ballantyne C, Cho L, Kastelein J, Koenig W, Somaratne R, Kassahun H, Yang J, Wasserman S, Scott R, Ungi I, Podolec J, Ophuis A, Cornel J, Borgman M, Brennan D, Nissen S.. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA 2016;316:2373–2384. [DOI] [PubMed] [Google Scholar]
- 122. Ference BA, Cannon CP, Landmesser U, Lüscher TF, Catapano AL, Ray KK.. Reduction of low density lipoprotein-cholesterol and cardiovascular events with proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors and statins: an analysis of FOURIER, SPIRE, and the Cholesterol Treatment Trialists Collaboration. Eur Heart J 2018;39:2540–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hoefer IE, Steffens S, Ala-Korpela M, Bäck M, Badimon L, Bochaton-Piallat M-L, Boulanger CM, Caligiuri G, Dimmeler S, Egido J, Evans PC, Guzik T, Kwak BR, Landmesser U, Mayr M, Monaco C, Pasterkamp G, Tuñón J, Weber C; ESC Working Group Atherosclerosis and Vascular Biology. Novel methodologies for biomarker discovery in atherosclerosis. Eur Heart J 2015;36:2635–2642. [DOI] [PubMed] [Google Scholar]