Abstract
Aims
Coronary high-intensity plaques (HIPs) with a high plaque-to-myocardial signal intensity ratio (PMR) on non-contrast T1-weighted imaging in patients with stable coronary artery disease (CAD) are associated with future coronary events. To characterize the morphological substrate of HIP, we performed a correlative optical coherence tomography (OCT) study.
Methods and results
We examined 137 lesions in 105 patients with stable angina pectoris or silent myocardial ischaemia scheduled for percutaneous coronary intervention (PCI) using a 3 T magnetic resonance scanner. Pre-interventional OCT was performed for PCI target lesions. HIP was defined as PMR ≥ 1.4. Of the 137 lesions, 34% were HIP and 66% were non-HIP. The prevalence of lipid-rich plaque (96% vs. 70%, P < 0.001), macrophage accumulation (65% vs. 46%, P = 0.046), cholesterol crystals (46% vs. 22%, P = 0.006), and healed plaque rupture (multiple layers of different optical densities overlaying a large lipid accumulation, 72% vs. 18%, P < 0.001) was significantly higher in the HIP group than the non-HIP group; no significant differences were observed for the presence of thin cap fibroatheroma, intracoronary thrombus, and plaque rupture between the two groups. Multivariable stepwise logistic regression analysis showed that HIP was significantly associated with the presence of healed plaque rupture [odds ratio (OR) 9.32; 95% confidence interval (95% CI) 4.05–22.71; P < 0.001] and lipid-rich plaque (OR 4.38; 95% CI 1.08–29.77; P = 0.038).
Conclusions
The significant association between HIP- and OCT-derived healed plaque rupture and large lipid core provides new insights into the characteristics of high-risk plaques, even in clinically stable CAD.
Keywords: coronary artery disease, magnetic resonance imaging, optical coherence tomography, myocardial infarction
Introduction
Magnetic resonance imaging (MRI) of the coronary vessels allows for the characterization of the extent of luminal stenosis, plaque burden, and plaque instability.1 Recently, the presence of a high-intensity plaque (HIP) with a plaque-to-myocardial signal intensity ratio (PMR) of ≥1.4 was shown to be a predictor of future coronary events in patients with stable coronary artery disease (CAD).2 Coronary HIPs with high PMR are found in up to one-third (28–36%) of patients with stable CAD.2,3 However, the mechanisms underlying the association between these lesions and adverse outcomes in patients with stable CAD are not fully understood.
To define the morphological substrate of atherosclerotic coronary plaques producing HIPs with high PMR, we used intracoronary optical coherence tomography (OCT) to precisely identify underlying plaque characteristics.4–6 An ex vivo post-mortem histopathological study confirmed the accuracy of OCT for identifying various features of high-risk plaques including thin cap fibroatheroma (TCFA), lipid-rich core, inflammation, neovascularization, healed plaque rupture, and luminal narrowing.4–10
Methods
Study population and percutaneous coronary intervention procedure
Between October 2012 and November 2016, 140 consecutive patients with stable angina pectoris (SAP) or silent myocardial ischaemia with >70% computed tomography angiography (CTA)-verified significant coronary artery stenosis were scheduled for elective percutaneous coronary intervention (PCI). They were prospectively enrolled in this study and underwent non-contrast T1-weighted imaging (T1WI) on cardiac magnetic resonance (CMR) within 24 h of the scheduled PCI and OCT procedures. All patients had demonstrated inducible myocardial ischaemia on stress myocardial scintigraphy and met the appropriate criteria for coronary intervention. We excluded 35 patients because OCT could not be performed before PCI (n = 27), CMR or OCT image quality was poor (n = 4; chronic atrial fibrillation in 1 patient and heart rate > 75 b.p.m. despite oral β-blocker therapy in 3 patients), or chronic kidney disease stage ≥3 was present (n = 4). Thus, 105 patients, consisting of 61 with stable effort angina pectoris and 44 with silent myocardial ischaemia, were evaluated in this correlative study. PCI was performed after intravenous administration of 10 000 IU heparin using a 6 Fr sheath and catheters. The target lesion was selected based on the presence of myocardial ischaemia diagnosed with stress myocardial scintigraphy. Antiplatelet therapy before PCI consisted of aspirin (100 mg) and clopidogrel (75 mg) for at least 7 days. This study was approved by the institutional review board of the National Cerebral and Cardiovascular Center. Written informed consent was obtained from all patients.
CMR protocol
Non-contrast T1WI was performed on a 3 T MR system with a 32-channel cardiac coil (MAGNETOM Verio; Siemens AG Healthcare Sector, Erlangen, Germany). The procedures used to acquire MR images in this study have been previously described.11,12 Briefly, coronary plaque imaging was performed using an inversion recovery-prepared 3D T1W turbo fast low-angle shot sequence with an electrocardiographic trigger, navigator-gated free-breathing, and fat suppression. Transaxial sections covered the entire heart (inversion time 650 ms; field of view 280 × 228 mm; acquisition matrix 256 × 187; reconstruction matrix 512 × 374; acquisition slice thickness 1.0 mm; reconstruction spatial resolution 0.6 × 0.5 × 0.6 mm; repetition time/echo 4.7 ms/2.13 ms; flip angle 12°; GRAPPA factor 2; navigator gating window ±1.5–2.5 mm; and data acquisition window 84–120 ms). Trigger delay and acquisition window were based on the duration of minimal right coronary artery motion as determined on cine-MR imaging. The mean acquisition time for plaque imaging was 23 ± 3 min.
CMR image analysis
PMR was defined as the signal intensity of the coronary plaque divided by the signal intensity of nearby left ventricular myocardium. The methods used to evaluate plaque images in this study have been described previously.2,13 Two experienced investigators unaware of patient data used the T1 weighted images to calculate PMR independently. The highest signal intensity detected in each plaque was considered the PMR value for that plaque in segment-based analysis. In patient-based analysis, the highest PMR among the coronary plaques was defined as the PMR for that subject. A coronary plaque with PMR ≥ 1.4 was defined as a HIP. A coronary plaque with PMR < 1.4 was defined as a non-HIP since coronary plaques with PMR ≥ 1.4 have been shown to be associated with poor clinical prognosis in a previous study.2
To confirm that the location of an observed HIP corresponds to the presence of a coronary plaque, we used cross-sectional and reconstructed curved multiplanar CTA images. In addition, for plaque detection, we used co-registration images to facilitate confirmation of the anatomical position of high-intensity lesions on T1-weighted images and the coronary vessel on a standard console of a clinical MR system.2,13 The location of a non-HIP lesion was determined by carefully comparing the CTA and MRA images or invasive coronary arteriography (CAG) images during PCI using fiduciary points (e.g. side branches). Once a non-HIP lesion had been confirmed with both CTA and coronary MR angiography, the corresponding areas on coronary T1-weighted images were carefully matched using the surrounding cardiac and chest wall structures.14
To exclude breathing artefacts, the signal-to-noise ratio was defined as signal intensity (myocardium)/signal intensity (background), where background indicates extracorporeal background without artefacts. Plaque lesions with signal-to-noise ratio < 3 were excluded from the analysis.
Intraclass correlation coefficients with 95% confidence intervals (95% CIs) were calculated to assess intrareader and inter-reader agreement for PMR. The interval between initial analysis of PMR of HIPs and secondary analysis was 3 months. The intrareader intraclass coefficient was 0.947 (95% CI 0.911–0.969). The inter-reader intraclass correlation coefficient was 0.916 (95% CI 0.866–0.957). All correlation coefficients for PMR were >0.8 and had narrow CIs, indicating good intraobserver and interobserver agreement.
OCT image acquisition
Intravascular OCT of the entire target vessel was performed before PCI as follows.15 After intracoronary administration of nitroglycerin (100–300 μg), a commercially available frequency-domain OCT system (ILUMIEN/ILUMIEN OPTIS OCT Intravascular Imaging System, St. Jude Medical, St. Paul, MN, USA) was advanced to the distal site of a target lesion. OCT pullback was performed at 20 mm/s with continuous injection of contrast media through the guiding catheter. The raw OCT data were anonymized and digitally stored in offline review systems (St. Jude Medical) for subsequent analysis.
OCT data analysis
OCT image analysis was performed by two experienced observers who were blinded to the clinical data using previously established criteria for OCT plaque characterization.5,6,16,17 Non-target coronary plaques with 20–70% diameter stenosis in the same vessel as target lesions on quantitative CAG and target lesions undergoing PCI were analysed. Because OCT examination is an invasive technique performed during PCI, coronary vessels without target lesions for PCI were therefore excluded. The presence of lipid, TCFA, plaque rupture, calcification, thrombus, macrophage accumulation, cholesterol crystals, and microchannels on OCT images were evaluated within a 10 mm segment (5 mm proximal to 5 mm distal to the target lesion at the smallest lumen cross-sectional area), in accordance with previous studies.5,14,18 If the two observers had discordant diagnoses, a consensus diagnosis was obtained using repeated offline readings. When lipid, characterized by signal-poor regions with diffuse borders, was present for >90° in any cross-sectional images, the plaque was considered lipid-rich. For each plaque, fibrous cap thickness was measured three times at its thinnest part and the average value was calculated. TCFA was defined as lipid-rich plaque with cap thickness ≤65 µm. Lipid core length was defined as the longitudinal length of contiguous cross-sections that fulfilled the definition of lipid-rich plaque. Plaque rupture was defined as intimal interruption and cavity formation in a plaque. Calcification was defined as a well-delineated signal-poor region with sharp borders. Thrombus was defined as an irregular mass with high or low backscattering protruding into the lumen. Thrombus type was further classified as either red or white. Red thrombus was defined as a high backscattering protrusion with signal-free shadowing on OCT. White thrombus was defined as a signal-rich, low backscattering projection. Macrophage accumulation was defined as bright spots with high OCT backscattering signal variances. A microchannel was defined as a black hole or tubular structure within a plaque observed on ≥3 consecutive cross-sectional images. Healed plaque rupture was detected as a landmark with multiple tissue layers of different optical densities overlying a large lipid core in the presence or absence of calcification.9 A cholesterol crystal was defined as a thin linear region of high signal intensity within a lipid plaque.17 Interobserver and intraobserver variability were assessed based on the evaluation of all images by two independent readers and by the same reader at two separate time points, respectively.
CTA scanning and analysis
Coronary CTA was performed using a dual-source CT scanner (SOMATOM Definition Flash, Siemens Healthcare, Erlangen, Germany). Coronary images were acquired with 128 × 0.6-mm slice collimation, 280-ms gantry rotation time, 120-kV tube voltage, and 280-mAs quality reference current–time product. The images were reconstructed with 0.6-mm slice thickness in 0.3-mm increments with a medium smooth convolution kernel (B26f). Quantitative lesion analysis was performed using software that facilitates plaque volume measurement (Ziostation2, Ziosoft, Tokyo, Japan). Parameters assessed included (i) plaque volume; (ii) remodelling index (RI) with plaques that have RI ≥ 1.10 considered to be within an artery with positive remodelling19; (iii) spotty calcification; and (iv) Hounsfield units (HU), which was coded by the software as low-attenuation plaques (LAPs) (<30 HU), intermediate attenuation plaques (30–150 HU), calcified plaques (351–1000 HU), and lumen (151–350 HU) based on colour.20 The CT density of the plaques of at least three points was assessed and averaged.21 Spotty calcification was defined as <3 mm on focal multiplanar reconstruction images and cross-sectional images.13,19
Statistical analyses
Continuous variables are presented as means ± SD for normally distributed variables, which were compared using Student’s t-test. Non-normally distributed variables are presented as medians (interquartile range). They were compared using the Mann–Whitney U test. Baseline categorical variables were compared using the Fisher’s exact test or the χ2 test, as appropriate. Intraclass correlation coefficients with 95% CIs were calculated to assess intraobserver and interobserver agreement for lipid arc, and kappa statistics were used to assess for agreement of plaque characterization.
Since several lipid-rich plaque-derived indices in the univariable analysis were highly correlated, we used variance inflation factor (VIF) analysis to measure the impact of collinearity among significant variables in the multivariable linear regression model as a continuous variable reflecting the precision of the estimate. Variables with VIF > 10 were omitted from the multivariable analyses due to high collinearity. Next, multivariable stepwise logistic regression analysis was performed using covariates that significantly predicted HIP with PMR > 1.4 in linear regression models plus established OCT indices for plaque vulnerability such as presence of TCFA, plaque rupture, macrophage accumulation, microchannel, and intracoronary thrombus and fibrous cap thickness. Stepwise selection with a P-value of 0.1 for backward elimination was used. All statistical tests were two-sided and P-values <0.05 were considered statistically significant. Statistical analysis was performed with JMP, version 11 (SAS Institute, Cary, NC, USA) and Stata, version 13 (StataCorp LP, College Station, TX, USA).
Results
Clinical characteristics, angiographic interpretation, OCT findings, and CTA measures
Of the 105 study patients, 44 (42%) patients had HIP lesions. Of these, two patients had more than one HIP lesion; these lesions were in non-culprit lesions in the same vessel as target lesions. Therefore, we analysed these lesions during OCT pullback. Ultimately, of 105 study patients, 44 patients (46 HIP lesions) were categorized into the HIP group and 61 patients (91 lesions) were categorized into the non-HIP group (Supplementary data online, Table S1).
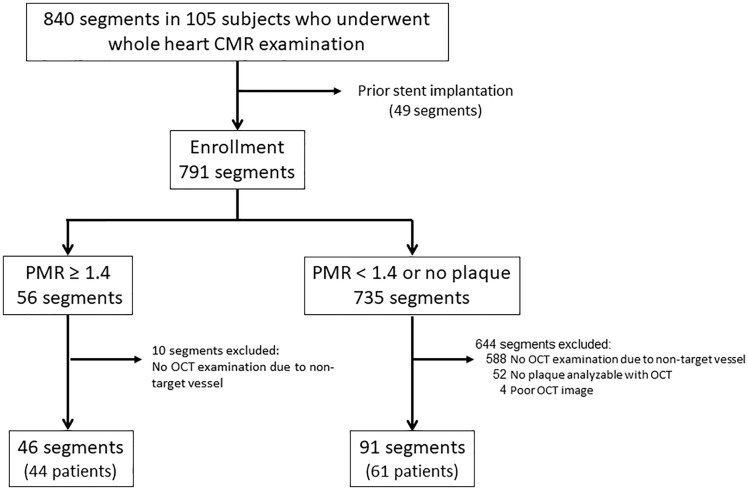
For segment-based analysis, 703 of 840 segments (105 patients) on CMR images were excluded because they either contained non-target lesions in a coronary vessel that did not undergo PCI or were previously treated with PCI and stenting, leaving 137 segments in the analysis (Figure 1). Ultimately, plaques with PMR ≥1.4 were identified in 46 segments and plaques with PMR < 1.4 were observed in 91 segments.
Figure 1.
Segment-based analysis of CMR and OCT. A total of 791 segments were analysed. They were divided into 56 segments with a plaque-to-myocardial signal intensity ratio (PMR) ≥1.4 and 735 segments that have plaques with PMR <1.4. Of the 56 segments that have plaques with PMR ≥1.4, 10 segments were excluded due to being in non-culprit vessels that underwent PCI. Ultimately, 46 segments that have plaques with PMR ≥1.4 and 91 segments that have plaques with PMR <1.4 were included in the final analysis.
Table 1 displays the clinical profiles of patients with and without HIP. There were no significant differences in age; gender; prevalence of hypertension, dyslipidaemia, diabetes mellitus; lipid profile; glycosylated haemoglobin levels; target vessel distribution; and medication use between the two groups. In particular, the proportion of patients taking statins was high and similar between the two groups.
Table 1.
Clinical characteristics and angiographic findings
| All patients | HIP | Non-HIP | P-value | |
|---|---|---|---|---|
| (n = 105) | (n = 44) | (n = 61) | ||
| Age (years) | 67 ± 11 | 68 ± 10 | 65 ± 11 | 0.176 |
| Male | 88 (84) | 37 (84) | 51 (84) | 1.000 |
| Hypertension | 78 (74) | 37 (84) | 41 (67) | 0.070 |
| Dyslipidaemia | 91 (87) | 39 (89) | 52 (85) | 0.773 |
| Diabetes mellitus | 40 (38) | 16 (36) | 24 (39) | 0.840 |
| Current smoker | 15 (14) | 5 (11) | 10 (16) | 0.577 |
| BMI (kg/m2) | 24.4 ± 3.2 | 24.7 ± 3.4 | 24.2 ± 3.0 | 0.475 |
| TC (mg/dL) | 163 ± 34 | 163 ± 32 | 163 ± 36 | 0.899 |
| LDL (mg/dL) | 91 ± 31 | 89 ± 27 | 92 ± 33 | 0.961 |
| HDL (mg/dL) | 48 ± 12 | 48 ± 13 | 47 ± 12 | 0.773 |
| TG (mg/dL) | 138 ± 62 | 139 ± 60 | 137 ± 64 | 0.798 |
| HbA1c (%) | 6.2 ± 0.8 | 6.1 ± 0.8 | 6.2 ± 0.8 | 0.598 |
| PMR | NA | 1.77 (1.50, 2.20) | 0.91 (0.83, 0.98) | <0.001 |
| Medications | ||||
| Aspirin | 103 (98) | 43 (98) | 60 (98) | 1.000 |
| Beta-blocker | 61 (58) | 28 (64) | 33 (54) | 0.423 |
| ACEI/ARB | 63 (60) | 28 (64) | 35 (57) | 0.551 |
| CCB | 41 (39) | 20 (45) | 21 (34) | 0.312 |
| Statins | 101 (96) | 43 (98) | 58 (95) | 0.638 |
| Target vessel (n = 137 segments) | 0.885 | |||
| LAD | 84 (61) | 27 (59) | 57 (63) | |
| LCX | 12 (9) | 4 (9) | 8 (9) | |
| RCA | 41 (30) | 15 (32) | 26 (28) |
BMI, body mass index; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; HbA1c, glycosylated haemoglobin; HDL, high-density lipoprotein; HIP, high-intensity plaque; LAD, left anterior descending artery; LCX, left circumflex artery; LDL, low-density lipoprotein; NA, not applicable; PMR, plaque-to-myocardial signal intensity ratio; RCA, right coronary artery; TC, total cholesterol; TG, triglycerides.
Characteristics of OCT-verified plaques in the HIP and non-HIP lesions are presented in Table 2. There were no significant differences in the prevalence of TCFA, plaque rupture, calcification, microchannels, and intracoronary thrombus between the two groups. The prevalence of intracoronary thrombus was also similar in the two groups (11% in the HIP group and 8% in the non-HIP group, P = 0.537). Both groups demonstrated similar minimal fibrous cap thickness [180 μm (150–210) vs. 190 μm (150–220), P = 0.180]. On the other hand, HIP lesions had a higher frequency of lipid-rich plaque (96% vs. 70%, P < 0.001), macrophage accumulation (65% vs. 46%, P = 0.046), cholesterol crystals (46% vs. 22%, P = 0.006), and healed plaque rupture (72% vs. 18%, P < 0.001) compared with non-HIP lesions. Compared with non-HIP lesions, the HIP lesions had a wider lipid arc [188° (169–209) vs. 139° (90–173), P < 0.001] and a longer lipid length [5.9 mm (4.7–7.8) vs. 3.6 mm (2.6–4.6), P < 0.001]. Representative cases of HIP and non-HIP lesions and corresponding OCT images are shown in Figures 2 and 3.
Table 2.
Optical coherence tomography analysis
| HIP | Non-HIP | P-value | |
|---|---|---|---|
| (46 segments) | (91 segments) | ||
| Lipid-rich plaque | 44 (96%) | 64 (70%) | <0.001 |
| Lipid length, mm | 5.9 (4.7, 7.8) | 3.6 (2.6, 4.6) | <0.001 |
| Maximum lipid arc (°) | 188 (169, 209) | 139 (90, 173) | <0.001 |
| Average lipid arc (°) | 159 (133, 174) | 99 (80, 147) | <0.001 |
| Lipid index | 895 (716, 1212) | 336 (220, 635) | <0.001 |
| Minimum fibrous cap thickness (μm) | 180 (150, 210) | 190 (150, 220) | 0.180 |
| TCFA | 4 (9%) | 4 (4%) | 0.442 |
| Healed plaque rupture | 33 (72%) | 16 (18%) | <0.001 |
| Plaque rupture | 6 (13%) | 14 (15%) | 0.802 |
| Calcification | 14 (30%) | 36 (40%) | 0.350 |
| Thrombus | 5 (11%) | 7 (8%) | 0.537 |
| Red thrombus | 1 (20%) | 1 (14%) | |
| White thrombus | 4 (80%) | 6 (86%) | |
| Macrophage accumulation | 30 (65%) | 42 (46%) | 0.046 |
| Cholesterol crystal | 21 (46%) | 20 (22%) | 0.006 |
| Microchannel | 20 (43%) | 31 (34%) | 0.349 |
HIP, high-intensity plaque; TCFA, thin cap fibroatheroma.
Figure 2.
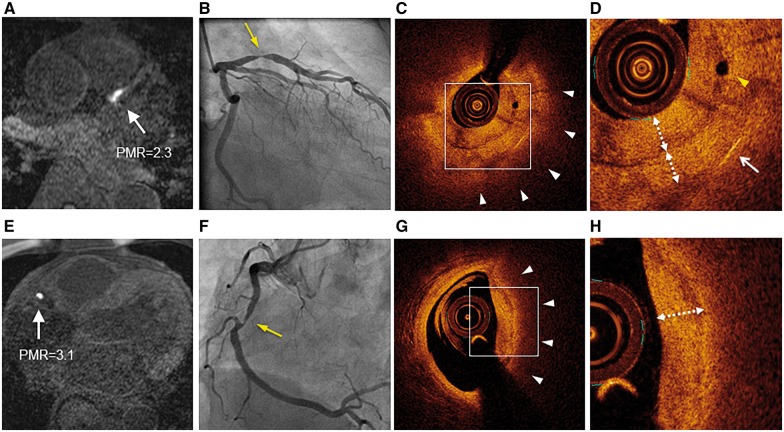
Representative HIP lesions on T1-weighted imaging and optical coherence tomography. Non-contrast T1-weighted imaging (T1WI) demonstrated HIPs (white arrows) in the proximal left anterior descending artery (A) and in the middle of the right coronary artery (E). These lesions had PMR of 2.3 and 3.1, respectively. Coronary artery angiography revealed significant coronary stenosis in lesions corresponding to HIPs (B and F) (yellow arrows). Optical coherence tomography (OCT) showed a layered signal pattern underlying a signal-poor region with diffuse borders (white arrowheads) in the areas corresponding to each HIP (C and G). The white dotted line with double arrows indicates the layered pattern of OCT signals in high-power images (D and H). The yellow arrowhead indicates a microchannel (D). The white arrow indicates a cholesterol crystal (D). HIPs, high-intensity plaques; PMR, plaque-to-myocardium signal intensity ratio.
Figure 3.
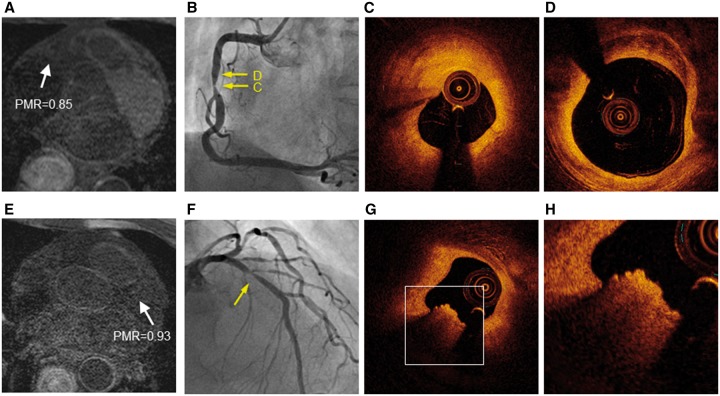
Representative non-HIP lesions on T1-weighted imaging with corresponding optical coherence tomography. Representative non-HIPs (white arrows) in the middle of the right coronary artery (PMR, 0.85) (A) and proximal left anterior descending artery (PMR, 0.93) (E). Coronary artery angiography revealed significant coronary stenosis (B and F) (yellow arrows). Optical coherence tomography showed that the target lesion was predominately comprised of fibrous plaque without attenuation (C and D). A typical red thrombus was observed in a non-HIP area (G). A higher power image of the white square in (G) is shown in (H). HIPs, high-intensity plaques; PMR, plaque-to-myocardium signal intensity ratio.
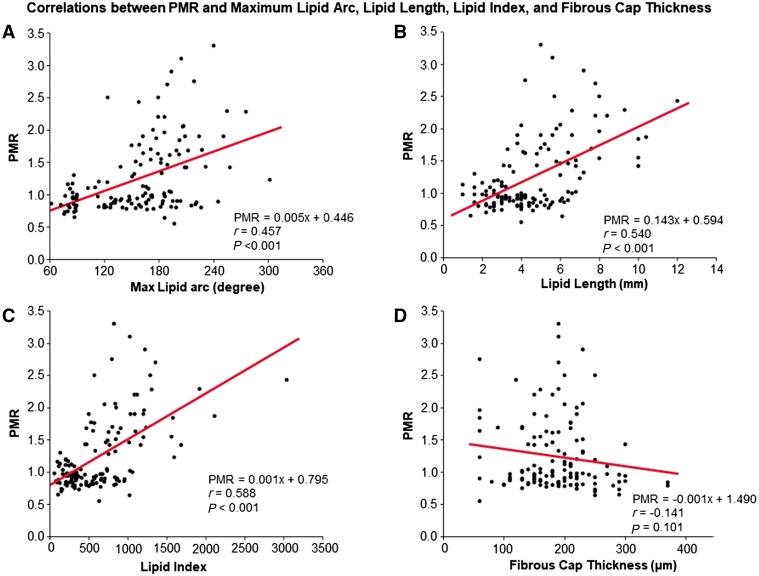
PMR was positively correlated with maximum lipid arc (r = 0.457, P < 0.001) (Figure 4A), lipid length (r = 0.540, P < 0.001) (Figure 4B), and lipid index (r = 0.588, P < 0.001) (Figure 4C), but not fibrous cap thickness (Figure 4D). As reported previously,2 we divided target lesions into high (PMR ≥ 1.4), medium (PMR 1.0–1.4), and low (PMR < 1.0) PMR groups. In the high PMR group 72% of lesions had healed plaque rupture compared with 27% in the medium group and 13% in the low PMR group (Figure 5).
Figure 4.
Correlations between PMR and maximum lipid arc, lipid length, and fibrous cap thickness. PMR was positively correlated with maximum lipid arc (A), lipid length (B) and lipid index (C), but not fibrous cap thickness (D). HIPs, high-intensity plaques; PMR, plaque-to-myocardium signal intensity ratio.
Figure 5.
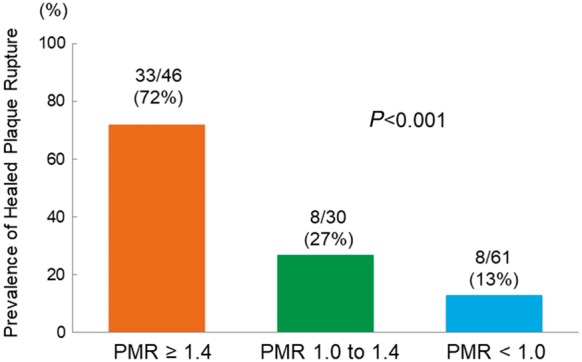
Prevalence of healed plaque rupture categorized by PMR. The prevalence of healed plaque rupture was significantly higher in the PMR > 1.4 group than in other groups.
The comparison of CTA measures between HIPs and non-HIPs is summarized in Supplementary data online, Table S4. The median RI in the HIP group was significantly higher than in the non-HIP group [1.24 (1.08–1.40) vs. 0.99 (0.91–1.12), P < 0.001]. Positive remodelling was observed in 42 (91.3%) of 46 segments with HIPs, as opposed to 13 (14.3%) of 91 segments with non-HIPs. In addition, total atheroma volume and LAP volume were significantly larger in HIPs [199.8 (148.5–265.2) mm3 vs. 100.8 (68.2–130.3) mm3, P < 0.001; 27.3 (15.4–49.6) mm3 vs. 6.7 (3.4–12.1) mm3, P < 0.001, respectively]. The frequency of spotty calcification was not statistically significant (41.3% vs. 30.8%, P = 0.061).
Factors related to the presence of HIP
Univariable analysis of OCT characteristics showed that lipid-rich plaque, lipid length, maximum lipid arc, average lipid arc, healed plaque rupture, and cholesterol crystal were all significant predictors of HIP (P < 0.01). These variables were further analysed in a multivariable linear regression model with VIF analysis. Lipid length, maximum lipid arc, and average lipid arc were omitted from the VIF analysis because of collinearity. After stepwise selection to adjust for factors that were significant in the univariable analysis and known OCT-derived high-risk morphological features (TCFA, plaque rupture, calcification, thrombus, macrophage accumulation, microchannel), presence of healed plaque rupture remained a significant independent predictor of HIP (Table 3, Model 1). To remove insignificant variables from Model 1 for parsimony, multivariable stepwise selection analysis was performed. As a result, both the presence of lipid-rich plaque [odds ratio (OR) 4.38; 95% CI 1.08–29.77; P = 0.038] and healed plaque rupture (OR 9.32; 95% CI 4.05–22.71; P < 0.001) were identified as significant determinants of HIP (Table 3, Model 2). In contrast, the presence of intracoronary thrombus, TCFA, and fibrous cap thickness were not associated with HIP. Thus, multivariable stepwise logistic regression analysis showed that the presence of HIP was most significantly associated with lipid-rich plaque and healed plaque rupture.
Table 3.
Association between HIP and OCT-derived parameters based on univariable and multivariable logistic regression analysis
| Univariable analysis |
Multivariable analysis: Model 1 |
Multivariable analysis: Model 2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Lipid-rich plaque | 9.28 | 2.60–59.37 | <0.001 | 3.34 | 0.73–24.16 | 0.125 | 4.38 | 1.08–29.77 | 0.038 |
| Lipid length (mm) | 2.24 | 1.72–3.08 | <0.001 | ||||||
| Maximum lipid arc (°) | 1.03 | 1.02–1.04 | <0.001 | ||||||
| Average lipid arc (°) | 1.03 | 1.02–1.05 | <0.001 | ||||||
| Lipid index | 1.42 | 1.21–1.80 | <0.001 | ||||||
| Minimum fibrous cap thickness (μm) | 1.00 | 0.99–1.00 | 0.091 | 1.00 | 0.99–1.01 | 0.486 | |||
| TCFA | 2.07 | 0.47–9.15 | 0.324 | 5.69 | 0.71–47.33 | 0.101 | |||
| Healed plaque rupture | 11.74 | 5.21–28.11 | <0.001 | 11.30 | 4.55–33.15 | <0.001 | 9.32 | 4.05 –22.71 | <0.001 |
| Plaque rupture | 0.83 | 0.28–2.23 | 0.712 | 0.75 | 0.21–2.63 | 0.652 | |||
| Calcification | 0.67 | 0.31–1.41 | 0.291 | 1.13 | 0.41–3.05 | 0.808 | |||
| Thrombus | 0.88 | 0.23–3.34 | 0.855 | 1.96 | 0.40–9.69 | 0.404 | |||
| Macrophage accumulation | 2.18 | 1.06–4.63 | 0.034 | 1.62 | 0.64–4.21 | 0.309 | |||
| Cholesterol crystal | 2.98 | 1.40–6.46 | 0.005 | 1.67 | 0.63–4.43 | 0.304 | |||
| Microchannel | 1.48 | 0.72–3.08 | 0.283 | 1.29 | 0.52–3.21 | 0.576 | |||
CI, confidence interval; HIP, high-intensity plaque; OCT, optical coherence tomography; OR, odds ratio; TCFA, thin cap fibroatheroma.
Interobserver and intraobserver variability for plaque characterization
The interval between initial analysis of OCT-derived features and secondary analysis was 1 month. Intraclass correlation coefficients with 95% CIs for interobserver and intraobserver reliability for lipid arc were 0.87 (95% CI 0.73–0.93) and 0.91 (0.79–0.95), respectively. There was acceptable interobserver concordance for intracoronary thrombus (κ = 0.89), healed plaque rupture (κ = 0.73), lipid-rich plaque (κ = 0.69), and TCFA (κ = 0.73). There also was acceptable intraobserver concordance for intracoronary thrombus (κ = 0.92), healed plaque rupture (κ = 0.73), lipid-rich plaque (κ = 0.82), and TCFA (κ = 0.87).
Discussion
HIP and healed plaque rupture
In the present study of patients with stable CAD, OCT-verified presence of healed plaque rupture and lipid-rich plaque were associated with HIP regardless of intracoronary thrombus status. Healed plaque rupture is pathologically characterized as a disrupted fibrous cap consisting of collagen type I with an underlying necrotic core and an overlying repair reaction, typically consisting of smooth muscle cells, proteoglycan, and collagen type III.8 Healed plaque rupture can be detected by OCT as a layered pattern of signals with an underlying signal-poor region9 (Figure 2). Since episodic rupture and healing potentially contribute to increased lipid burden and luminal narrowing, it is conceivable that the presence of healed plaque rupture itself may reflect an increased risk of future events. A previous autopsy study had reported that in patients with sudden coronary death, the prevalence of healed plaque rupture was 80% in hearts with stable plaque and healed myocardial infarction.7 Higher numbers of healed plaque rupture sites might be associated with larger necrotic cores and higher plaque volume. During healing of disrupted plaques, thrombus is incorporated into the repaired territory. Dying red blood cells produce substantial amounts of free cholesterol and cholesterol crystals, which contribute to higher lipid content in the plaque.22 However, further clinical studies are warranted to investigate whether healed plaque rupture contributes to plaque progression.
In addition to healed plaque rupture, HIP was also significantly associated with the presence of lipid-rich plaque, and PMR was positively correlated with maximum lipid arc and lipid length (Figure 4A–C). These findings were consistent with a previous intravascular ultrasound study of patients with stable CAD that showed a significantly higher prevalence of a lipid pool in patients with HIP. The presence of a lipid pool was strongly correlated with periprocedural myocardial infarction.3
Importantly, no significant differences were observed in the presence of TCFA, microchannels, and plaque rupture between the HIP and non-HIP groups in this study (Table 2). Our present findings are markedly different from those in previous OCT studies that involved mostly ACS patients.14,23 Kubo et al.24 reported that in infarct-related or PCI target lesions in patients with acute myocardial infarction (AMI) or SAP, OCT-derived plaque rupture (AMI 77% vs. SAP 7%, P < 0.001) and intracoronary thrombus (AMI 100% vs. SAP 0%, P < 0.001) were observed more frequently in AMI than in SAP. In addition, the frequency of OCT-derived TCFA was significantly lower in SAP than in AMI (SAP 6% vs. AMI 38%, P = 0.03). Consistent with these previous imaging studies, the composition of HIP is different in patients with stable CAD or silent myocardial infarction vs. patients with ACS.
Potential mechanisms for high signal intensity in coronary plaques
In previous carotid MR studies, the presence of lesions with high-intensity signal was considered to indicate intraplaque haemorrhage (IPH) that contributes to a significant shortening of T1 relaxation time. Because IPH often occurs within necrotic cores, lipid-rich core regions also contribute to high-intensity signals on T1WI.25,26 The histopathological basis of these associations has been demonstrated in carotid endarterectomy specimens.25,27 In coronary lesions, we have demonstrated that HIPs with high PMR are associated with adverse outcomes.2 Since the presence of HIP in a coronary artery is considered to indicate a necrotic core with IPH, the association between coronary HIP and increased risk of future cardiovascular events may be partly attributable to IPH involvement. Aspiration of coronary specimens corresponding to coronary HIP visualized on a 3 T MR system has confirmed that a large portion of the necrotic core has fibrin-rich thrombus and numerous immunohistologically confirmed CD68+ macrophages.12 Intraluminal thrombus has also been previously shown to be associated with coronary HIP in patients with ACS based on intravascular OCT or specimens obtained through an aspiration catheter during PCI.14,18,23 Along with the present OCT findings in patients with stable CAD, HIP seems to represent several important components of pathologically advanced plaques. However, the actual cause of high signal intensity in atherosclerotic coronary plaques on T1WI remains under discussion. Further studies are needed to clarify the coronary plaque characteristics representing HIP on non-contrast T1WI.
Study limitations
The number of subjects in this study was small. In addition, recruiting patients with significant coronary stenosis might have resulted in selection bias. Since OCT has limited axial penetration depth (≤2.0 mm), we could not evaluate the number of layers overlying a large necrotic core when evaluating healed plaque rupture, vascular remodelling index, and total lipid volume. However, CTA helped overcome some limitations of OCT in HIP characterization. Namely, the HIP group had a significantly higher prevalence of positive remodelling, larger total atheroma volume, and larger LAP volume (Supplementary data online, Table S4).
OCT findings in HIP and non-HIP lesions overlap, although OCT allows us to visualize the micromorphology of coronary arteries with near-histological resolution. Since OCT findings and histopathological data were not directly compared in this study, the precise characteristics of HIPs remain unknown. The development of atherosclerosis is a continuous process that involves numerous interconnected cellular and acellular processes that influence the behaviour of each other. The precise roles of many of these processes are unknown, and further studies are needed.
Given the observational nature of our present study, there might have been selection bias due to patient recruitment in a single centre. In addition, we did not explore the entire coronary tree using OCT imaging. Therefore, the exclusion of some non-target lesions might have introduced some bias.
Conclusions
HIP on non-contrast T1WI in patients with stable CAD is strongly associated with healed plaque rupture and a large lipid core as detected by OCT. These findings suggest the morphological basis of HIP and explain the high-risk nature of HIP with high PMR.
Funding
The present work was supported in part by grants from the Takeda Science Foundation and the Japan Cardiovascular Research Foundation.
Conflict of interest: None declared.
Supplementary Material
References
- 1. Noguchi T, Yamada N, Kawasaki T, Tanaka A, Yasuda S.. Detection of high-risk atherosclerotic plaques by magnetic resonance imaging. Circ J 2013;77:1975–83. [DOI] [PubMed] [Google Scholar]
- 2. Noguchi T, Kawasaki T, Tanaka A, Yasuda S, Goto Y, Ishihara M. et al. High-intensity signals in coronary plaques on noncontrast T1-weighted magnetic resonance imaging as a novel determinant of coronary events. J Am Coll Cardiol 2014;63:989–99. [DOI] [PubMed] [Google Scholar]
- 3. Hoshi T, Sato A, Akiyama D, Hiraya D, Sakai S, Shindo M. et al. Coronary high-intensity plaque on T1-weighted magnetic resonance imaging and its association with myocardial injury after percutaneous coronary intervention. Eur Heart J 2015;36:1913–22. [DOI] [PubMed] [Google Scholar]
- 4. Kume T, Akasaka T, Kawamoto T, Ogasawara Y, Watanabe N, Toyota E. et al. Assessment of coronary arterial thrombus by optical coherence tomography. Am J Cardiol 2006;97:1713–7. [DOI] [PubMed] [Google Scholar]
- 5. Tearney GJ, Yabushita H, Houser SL, Aretz HT, Jang IK, Schlendorf KH. et al. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation 2003;107:113–9. [DOI] [PubMed] [Google Scholar]
- 6. Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH. et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation 2002;106:1640–5. [DOI] [PubMed] [Google Scholar]
- 7. Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J. et al. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation 2001;103:934–40. [DOI] [PubMed] [Google Scholar]
- 8. Mann J, Davies MJ.. Mechanisms of progression in native coronary artery disease: role of healed plaque disruption. Heart 1999;82:265–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Otsuka F, Joner M, Prati F, Virmani R, Narula J.. Clinical classification of plaque morphology in coronary disease. Nat Rev Cardiol 2014;11:379–89. [DOI] [PubMed] [Google Scholar]
- 10. Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M. et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol 2016;13:79–98. [DOI] [PubMed] [Google Scholar]
- 11. Asaumi Y, Noguchi T, Morita Y, Fujiwara R, Kanaya T, Matsuyama TA. et al. High-intensity plaques on noncontrast T1-weighted imaging as a predictor of periprocedural myocardial injury. JACC Cardiovasc Imaging 2015;8:741–3. [DOI] [PubMed] [Google Scholar]
- 12. Asaumi Y, Noguchi T, Morita Y, Matsuyama TA, Otsuka F, Fujiwara R. et al. Non-contrast T1-weighted magnetic resonance imaging at 3.0 Tesla in a patient undergoing elective percutaneous coronary intervention—clinical and pathological significance of high-intensity plaque. Circ J 2014;79:218–20. [DOI] [PubMed] [Google Scholar]
- 13. Kawasaki T, Koga S, Koga N, Noguchi T, Tanaka H, Koga H. et al. Characterization of hyperintense plaque with noncontrast T(1)-weighted cardiac magnetic resonance coronary plaque imaging: comparison with multislice computed tomography and intravascular ultrasound. JACC Cardiovasc Imaging 2009;2:720–8. [DOI] [PubMed] [Google Scholar]
- 14. Matsumoto K, Ehara S, Hasegawa T, Sakaguchi M, Otsuka K, Yoshikawa J. et al. Localization of coronary high-intensity signals on T1-weighted MR imaging: relation to plaque morphology and clinical severity of angina pectoris. JACC Cardiovasc Imaging 2015;8:1143–52. [DOI] [PubMed] [Google Scholar]
- 15. Kataoka Y, Puri R, Hammadah M, Duggal B, Uno K, Kapadia SR. et al. Sex differences in nonculprit coronary plaque microstructures on frequency-domain optical coherence tomography in acute coronary syndromes and stable coronary artery disease. Circ Cardiovasc Imaging 2016;9:pii: e004506.. [DOI] [PubMed] [Google Scholar]
- 16. Kume T, Akasaka T, Kawamoto T, Watanabe N, Toyota E, Neishi Y. et al. Assessment of coronary arterial plaque by optical coherence tomography. Am J Cardiol 2006;97:1172–5. [DOI] [PubMed] [Google Scholar]
- 17. Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG. et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol 2012;59:1058–72. [DOI] [PubMed] [Google Scholar]
- 18. Ehara S, Hasegawa T, Nakata S, Matsumoto K, Nishimura S, Iguchi T. et al. Hyperintense plaque identified by magnetic resonance imaging relates to intracoronary thrombus as detected by optical coherence tomography in patients with angina pectoris. Eur Heart J Cardiovasc Imaging 2012;13:394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T. et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54:49–57. [DOI] [PubMed] [Google Scholar]
- 20. Noguchi T, Tanaka A, Kawasaki T, Goto Y, Morita Y, Asaumi Y. et al. Effect of intensive statin therapy on coronary high-intensity plaques detected by noncontrast T1-weighted imaging: the AQUAMARINE pilot study. J Am Coll Cardiol 2015;66:245–56. [DOI] [PubMed] [Google Scholar]
- 21. Watabe H, Sato A, Akiyama D, Kakefuda Y, Adachi T, Ojima E. et al. Impact of coronary plaque composition on cardiac troponin elevation after percutaneous coronary intervention in stable angina pectoris. A computed tomography analysis. J Am Coll Cardiol 2012;59:1881–8. [DOI] [PubMed] [Google Scholar]
- 22. Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK. et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 2003;349:2316–25. [DOI] [PubMed] [Google Scholar]
- 23. Jansen CH, Perera D, Makowski MR, Wiethoff AJ, Phinikaridou A, Razavi RM. et al. Detection of intracoronary thrombus by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 2011;124:416–24. [DOI] [PubMed] [Google Scholar]
- 24. Kubo T, Imanishi T, Kashiwagi M, Ikejima H, Tsujioka H, Kuroi A. et al. Multiple coronary lesion instability in patients with acute myocardial infarction as determined by optical coherence tomography. Am J Cardiol 2010;105:318–22. [DOI] [PubMed] [Google Scholar]
- 25. Moody AR, Murphy RE, Morgan PS, Martel AL, Delay GS, Allder S. et al. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation 2003;107:3047–52. [DOI] [PubMed] [Google Scholar]
- 26. Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G. et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 2001;104:2051–6. [DOI] [PubMed] [Google Scholar]
- 27. Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr. et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995;92:1355–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.