Abstract
Background:
The consequences of excess copper in human tissue are the alterations in the oxidative stress markers and peroxidative damage of membrane lipids. Unselective copper binding may be the clue to damaging impact to protein construction and hence modifying their biological functions. The aim of this study is to match the hepatoprotective efficacy of curcumin (CM) or nanocurcumin (NCM) with that of desferrioxamine (DSF; standard heavy metal chelator) against toxic doses of copper sulphate (CuSO4).
Method:
All treatments were given simultaneously with CuSO4 for 7 days.
Result:
CuSO4 administration elevated serum alanine transaminase, and hepatic nitric oxide (NO), lipid peroxide, and caspase-3 as well as protein expression of cytochrome P4502E1, and nuclear factor-κB (NF-κB) and Bax gene expressions. On the other hand, hepatic levels of reduced glutathione, superoxide dismutase, and interleukin-10 were decreased, whereas DNA degradation was increased as well compared with the control group. The administration of the aforementioned antioxidants ameliorated all the previous altered measured parameters. Interestingly, NCM achieved the most pronounced hepatoprotective effect nearly equivalent to that of DSF.
Conclusion:
It was concluded that NCM is considered a promising candidate against CuSO4 toxicity, and cytochrome P450, NF-κB, and Bax are involved in its toxicity and treatment.
Keywords: nanocurcumin, CYP450, NF-κB, Bax, IL-10, DNA degradation, gene expressions
Introduction
Copper (Cu) is an essential transition metal whose deficiency or excess in the individual is associated with pathologic conditions.1 Copper sulphate (CuSO4) is used mainly in leather manufacturing as well as pesticide and fungicide in agricultural fields.2 In medicine, it is used as germicide and as antiseptic and in the treatment of schistosomiasis and gastric as well as topical exposure to phosphorous.3 CuSO4 produces toxic effects in the liver and brain due to its production of the highly reactive HO• species.4 Cu stimulates oxidative stress by triggering of reactive oxygen species (ROS) and declines of the activities of some free-radical scavenging enzymes including catalase (CAT) and superoxide dismutase (SOD), and the level of reduced glutathione (GSH).5 The inhibitory effect of copper on CAT, SOD, and GSH may be due to oxidative alteration of the enzymatic proteins and biomembrane lipids by ROS.6 It also initiates lipid peroxidation.7 It was indicated that copper–histidine complex induced nitric oxide.8 Oxidative stress mediated the upregulation of hepatic nuclear factor-κ B (NF-κB) protein expression in rats. It was reported that the induction of tumor necrosis factor-α (TNF-α), COX-2, and NF-κB is involved in the inflammatory response induced by Cu.8
Desferrioxamine (DSF) is a naturally occurring compound produced by Streptomyces pilosus.9 The DSF complexes with heavy metals to form a stable chelate, which prevents the metals from entering into further chemical reactions10; however, it has a limited use due to its various side effects.
The capability of curcumin (CM) to avoid liver dysfunction and the modifications of the antioxidant enzymes is attributable to that CM has a power to capture free radicals and possesses antioxidant activity and also may be to its Cu-chelation activity.6 Although there are abundant pharmacological functions of CM, its pharmacokinetic properties are less encouraging. Hence, there is an urgent need for novel effective strategies to overcome heavy metals toxicities. Nanocurcumin (NCM, particle size 200 nm) enhanced dissolution, stability, and bioavailability of the compounds. It is the first time NCM is used in managing CuSO4 toxicity. Nanoparticles can readily interact with biomolecules on both the surface and inside cells due to their small size.11
The current study is designed to compare the potential protective role of CM with that of NCM against CuSO4-induced hepatotoxicity. New mechanisms were also performed to assess the role of NCM in ameliorating CuSO4-induced hepatotoxicity.
Materials and Methods
Experimental Animals
Fifty male albino rats weighing 120 to 150 g were used. Rats were housed and maintained on a standard condition of temperature and humidity and were given standard diet and water ad libitum throughout the study. Ethical considerations for the use of animals were accomplished following the guidelines of the Experimental Animal Laboratory and approved by the Animal Care and Use Committee of the College of Pharmacy, King Saud University.
Chemicals
CuSO4 and CM were purchased from Sigma Chemicals Company for Pharmaceutical Industries, St Louis, Missouri. The NCM was obtained from Lipolife, Drakes Lane Industrial Estate, Drakes Lane (UK). The DSF was purchased from Novartis Pharma AG, Basle, Switzerland.
Experimental Design
Rats were randomly divided into 5 groups, 6 rats each, and left for acclimatization for 1 week before the experiment. All chemical compounds were dissolved in 1% carboxyl methyl cellulose (CMC). Group I: negative control, rats were injected with CMC. Group II: positive control, rats were administered CuSO4 orally (300 mg/kg)12 daily for 7 days. Group III (CuSO4): rats were administered CuSO4 and DSF (100 mg/kg).13 Groups IV (CM): rats were administered CuSO4 and CM (80 mg/kg).14 Group V (NCM): CuSO4 and NCM: rats were administered CuSO4 and NCM (80 mg/kg). All treatments were given orally simultaneously with CuSO4 for 7 days.
Blood and Liver Sampling
Rats were scarified and samples of blood and liver tissues were collected for analysis.
Blood was collected from the retro-orbital venous plexus of rats under sterile septic condition. Blood samples were centrifuged at 3000 rpm for serum separation and kept at −20°C. Livers were immediately excised, some livers were homogenized in phosphate-buffered saline and the others were fixed in 4% formalin. Other parts of livers were kept under liquid nitrogen for Western blot analysis (Table 1).
Table 1.
Primer Sequences Used for RT-PCR.
| Gene Name | Primer Sequence | Primer Size (bp) |
|---|---|---|
| Refer- actin | Forward: 5′ GAGACCTTCAACACCCCAGC 3′ | 263 |
| Reverse: 5′ ATGTCACGCACGATTTCCC 3′ | ||
| NF-κβ | Forward: 5′ CATGAAGAGAAGACACTGACCATGGAAA 3′ | 329 |
| Reverse: 5′ TGGATAGAGGCTAAGTGT AGACACG 3′ | ||
| Bax | Forward: 5′ GTTGCCCTCTTCTACTTTG 3′ | 194 |
| Reverse: 5′ AGCCACCCTGGTCTTG 3′ |
Abbreviations: RT-PCR, real-time polymerase chain reaction; NF-κB, nuclear factor-κB.
Biochemical Serum and Tissue Analysis
Serum alanine transaminase (ALT) level was evaluated using the kits (Randox Laboratories, Ltd, London, UK). Superoxide dismutase (SOD) was measured using ethylenediaminetetraacetic acid and Pyrogallol according to Svensson et al.15 Hepatic lipid peroxidation (MDA) and GSH levels were assessed using Uchiyama and Mihara16 and Ellman17 methods, respectively. Total nitrite (NO) was determined according to the method described by Moshage et al.18 Interleukin-10 (IL-10) level was measured using a high-sensitive rat ELISA kit (Immuno-Biological Laboratories Co, Ltd, Japan). Caspase-3 was evaluated calorimetrically using assay kit.
Western Blot
Hepatic cytochrome P450 (CYP450) expression of control and all treated group rats was determined using Western blotting according to the method of Mahmood et al.19 Briefly, the frozen liver samples were homogenized in RIPA buffer supplemented with proteinase inhibitors and the concentration of proteins was determined using Bradford reagent (Bio-Red, California, US). 60 μg of proteins were separated on sodium dodecyl sulphate-polyacrylamide gel electrophoresis and electrotransferred onto nitrocellulose membranes. The membranes were probed with anti-CYP450 and housekeeping anti-β-actin (Santa Cruz Biotechnology, Inc, Texas, US) overnight at 4°C. The membranes were incubated with the secondary antibody and developed using enhanced chemiluminescence kit. Band intensity was quantified using ImageJ (NIH, Maryland).
Gene Expression of NF-κB and Bax
NF-κB and Bax gene expression were estimated (Table 1) using Livak and Schmittgen method.20
DNA Fragmentation
DNA fragmentation quantitated by measuring oligonucleosome-bound DNA using an ELlSA kit (Boehringer, Mannheim, Germany).21
Statistical Analysis
Analysis was performed using GraphPad Prism (GraphPad Software, San Diego, California), and all statistical comparisons were made by means of the 1-way analysis of variance test followed by Tukey test post hoc analysis. Results were expressed as mean ± standard error of the mean and a P ≤ .05 was considered significant.
Results
Treatment with CuSO4 caused an elevation in serum ALT and hepatic NO, MDA, and caspase-3 levels compared to the negative control group, and all treatment compounds either DSF, CM, or NCM were effectively declined the previous serum biochemical parameters. On the other hand, the administration of CuSO4 caused a significant decrease in hepatic levels of GSH, SOD, and IL-10 in serum compared to the control group, whereas DSF, CM, and NCM attenuated these effects (Table 2 and Figure 1).
Table 2.
ALT, NO, MDA, GSH, and SOD Levels in Serum of Rats in Control, CuSO4-Intoxicated, and All Treated Groups.a
| Groups | CON | CuSO4 | DSF | CM | NCM |
|---|---|---|---|---|---|
| ALT (μ/L) | 1347 ± 66.84 | 5381 ± 81.0b | 1483 ± 71.92c | 1501 ± 33.20c | 1404 ± 42.3c |
| NO (mg/dL) | 2.03 ± 0.03 | 3.41 ± 0.1b | 2.07 ± 0.01c | 2.49 ± 0.1d | 2.12 ± 0.04c |
| MDA (mg/dL) | 139.8 ± 17.2 | 201.3 ± 5.5b | 145.8 ± 10.4c | 182.5 ± 14.02e | 166.2 ± 14.9d |
| GSH (mg/dL) | 0.62 ± 0.001 | 0.28 ± 0.01b | 0.51 ± 0.02c | 0.40 ± 0.01e | 0.61 ± 0.01c |
| SOD (μ/g) | 7.17 ± 0.2 | 4.3 ± 0.2b | 5.9 ± 0.1d | 5.3 ± 0.2e | 5.82 ± 0.2d |
Abbreviations: ALT, alanine transaminase; CM, curcumin; CON, control; DSF, desferrioxamine; GSH, reduced glutathione; NCM, nanocurcumin; NO, nitric oxide; SOD, superoxide dismutase.
a Data are presented as mean ± SEM (N = 6).
b P ≤ .001 versus control group.
c P ≤ .001.
d P ≤ .01.
e P ≤ .05 versus CuSO4-intoxicated group.
Figure 1.
IL-10 and caspase-3 levels in hepatic tissues in control, CuSO4-intoxicated group, as well as in all treated groups. Data are presented as mean ± SEM (N = 6). *** P ≤ .001 versus control group, and +++ P≤ .001, +++ P ≤ .01, and + P ≤ .05 versus CuSO4-intoxicated group. IL-10 indicates interleukin-10; SEM, standard error of the mean.
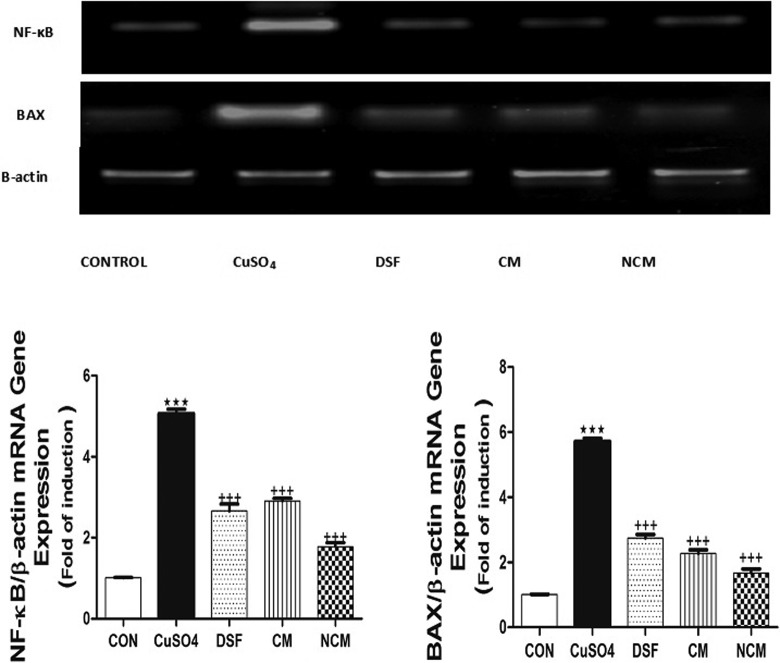
DNA fragmentation and protein expression of NF-κB and Bax messenger RNA (mRNA) were significantly elevated after consumption of CuSO4 (P ≤ .001). On the contrary, CYP450 protein expression was significantly decreased in CuSO4-treated group compared with control one (Figures 2 –4).The ingestion of the aforementioned antioxidants ameliorated all the previous altered measured parameters. Interestingly, NCM achieved the most pronounced hepatoprotective effect nearly equivalent to that of DSF.
Figure 2.
A, Western blot analysis of the expression of cytochrome-P450 in control, CuSO4-intoxicated, and all treated groups. B, The densitometry analysis of the expression of cytochrome-P450 proteins in control, CuSO4-intoxicated, and all treated groups. (Data corrected by β-actin and expressed as protein/β-actin). Data are presented as mean ± SEM (N = 6). *** P ≤ .001 versus control group, and +++ P ≤ .001 versus CuSO4-intoxicated group. SEM indicates standard error of the mean.
Figure 3.
Hepatic DNA fragments in control group, CuSO4-intoxicated group, as well as in all treated groups. Data are expressed as means ± SEM of 6 rats. *** P ≤ .001 versus control group, and +++ P ≤ .001, +++ P ≤ .01, and + P ≤ .05 versus CuSO4-intoxicated group. SEM indicates standard error of the mean.
Figure 4.
mRNA expression of hepatic NF-κB and Bax in control, CuSO4-intoxicated group, and different treated groups. Data are presented as mean ± SEM (N = 6). *** P ≤ .001 versus control group, and +++ P ≤ .001 versus CuSO4-intoxicated group. mRNA indicates messenger RNA; NF-κB, nuclear factor-κB; SEM, standard error of the mean.
Discussion
Copper is an imperative element, extensively dispersed in both animal and human tissues. It is a component of a number of metalloenzymes, including catalase, peroxides, SOD, and cytochrome oxidase.1 Elevated level of Cu in drinking water exhibited a prospective ecological hazard.7 Cu leads to oxidative stress by the induction of ROS and initiation of lipid peroxidation.22 The gastrointestinal tract, lungs, and skin are the main routes of Cu absorption into circulation. It is translocated to the liver where it is incorporated into ceruloplasmin and released into the plasma. Liver and kidney are the chief target organs of CuSO4 leading to hepatic injury and renal damage.23,24 Therefore, studies on the copper toxicity are of especial importance.
In the present study, CuSO4 induced elevation in the serum level of ALT, MDA, and NO representing liver damage. Herein, treatments with CM, NCM, and DSF along with CuSO4 decreased serum levels of ALT, MDA, and NO. It is well known that Cu2+ depletes intracellular GSH, which is considered as the first antioxidant in the body.25 Letelier et al26 confirmed that lipid peroxidation is elevated as a result of an overdose of CuSO4.
In the current work, CuSO4 induced a reduction in hepatic levels of GSH, IL-10, and SOD whereas treatment with the aforementioned antioxidants restored their levels. The CM is a bioactive phenolic component in turmeric spice (Curcuma longa). The CM has various pharmacological activities, including anti-inflammatory, anti-mutagenic, and antioxidant potential, and despite its multiple activities, it has poor bioavailability.25 Nanoparticles can also offer several other advantages, such as prolonged half-life of injected drugs, drug delivery targeted to specific tissues, and increased metabolic stability. Furthermore, it can increase membrane permeability through its specific physiochemical properties, such as size, surface, charge, and hydrophobicity. These advantages have enabled improvement of clinical outcomes and reduced the side effects of CUR treatment.27 The effective role of NCM against liver injury induced by CuSO4 was discussed for the first time.
The IL-10 has been considered as a potential anti-inflammatory cytokine, and there are several studies supporting the view that IL-10 shows a strong suppressive effect on Th1 lymphocytes, antigen-presenting cells, and the production of inflammatory mediators.28 Our results are in accordance with that of Bruzell et al29 and Joe et al,30 which stated that CM has strong antioxidant potential and acts as oxygen free-radicals scavenger. It markedly suppressed ROS generation like superoxide anions and hydrogen peroxide by macrophages activation, which plays an important role in the inflammation.30 The ability of CM to prevent liver dysfunction and the alterations of the antioxidant parameters may be due to the fact that CM possesses scavenging free-radicals properties and antioxidant activity.6 Therefore, the protective effects of CM may be related to their ability to chelate/sequester Cu via formation of complexes.4,31
Persichini et al8 reported that hyper-cupremic situations may lead to the onset of inflammation through production of ROS and activation of NF-κB-dependent gene which involved in the inflammatory response and hence production of TNF-α and induction of NOS-II and COX.2 Herein, hepatic NO and gene expression of NF-κB were upregulated by toxic dose of CuSO4, while treatments with the antioxidants in question declined the previous parameters. It was reported that Cu exposure induces metallothionein and catalase and increases the concentrations of NAD (+) and lactate, which are consistent with a shift toward anaerobic metabolism and changes in gene expression.32
CYP450 is a key marker of corruption with many toxins.33 The existing data indicated that CuSO4 declined hepatic CYP450. The reduction of the activity of this enzyme might be associated with the overproduction of cytokines. Abdel-Razzak et al34 revealed the direct inhibitory effects of hepatic CYP-dependent drug metabolism on inflammatory cytokines. The inhibitory effect of Cu2+ on the CYP450-catalyzed reactions may come from the inability of an efficient electron transfer from NADPH−P450 reductase to P450 and also the dysfunctional conformation of NADPH−CYP450 reductase and CYP450.35 The inflammatory cytokines inhibition of CYP enzymes may also be linked to the stimulation of heme oxygenase activity, which initiates oxygen radicals and hence lead to the damage of CYP apoproteins.36 The marked elevation of the CYP450 enzyme activity by the co-ingestion of the agents in question may be attributed to their ability to suppress free radical production and inflammatory cytokine creation as previously reported.37-40
Copper may promote apoptosis along with DNA base modifications, strand breaks, and rearrangements. Moreover, Cu may induce apoptosis by p53-dependent and p53-independent pathways and suggests that disorders of apoptosis may play a critical role in Cu-induced hepatotoxicity and neurotoxicity.41 In the current study, caspase-3 as well as mRNA expression of Bax in CuSO4-treated group were elevated, whereas the consumption of the antioxidants in question downregulated their levels.
In conclusion, the present study showed that the exposure to CuSO4 induced adverse effects and profound alterations on the hepatic function associated with oxidative stress and diminished activities of antioxidant enzymes. The CM causes indirect reduction of ROS through a decline of oxygen radicals and the downregulation of the expression of hepatic NO, lipid peroxide, and caspase-3 as well as protein expression of CYP450 and mRNA expression of NF-κB and Bax, which is responsible for inflammation, a common process during liver injury. The hepatoprotective effects of NCM have shown promising prospects for remission of hepatotoxicity due to its superior bioavailability behavior over native CM.
Footnotes
Authors’ Note: All authors contributed to writing this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the Undergraduate Student’s Research Support Program, Project no. URSP - 3 - 18 - 116.
References
- 1. Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101(3):294–291. [DOI] [PubMed] [Google Scholar]
- 2. Barceloux DG. Copper. J Toxicol Clin Toxicol. 1999;37(2):217–230. [DOI] [PubMed] [Google Scholar]
- 3. King CH, Bertsch D. Historical perspective: snail control to prevent schistosomiasis. PLoS Negl Trop Dis. 2015;9(4):e0003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Musacco-Sebio R, Ferrarotti N, Saporito-Magriñá C, et al. Oxidative damage to rat brain in iron and copper overloads. Metallomics. 2014;6(8):1410–1416. [DOI] [PubMed] [Google Scholar]
- 5. Zhang SS, Noordin MM, Rahman SO, Haron J. Effects of copper overload on hepatic lipid peroxidation and antioxidant defense in rats. Vet Hum Toxicol. 2000;42(5):261–264. [PubMed] [Google Scholar]
- 6. Trujillo J, Chirino YI, Molina-Jijon E, Anderica-Romero AC, Tapia E, Pedraza-Chaverri J. Renoprotective effect of the antioxidant curcumin: recent findings. Redox Biol. 2013;1(1):448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hashish EA, Elgaml SA. Hepatoprotective and nephroprotective effect of curcumin against copper toxicity in rats. Indian J Clin Biochem. 2016;31(3):270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Persichini T, Percario Z, Mazzon E, Colasanti M, Cuzzocrea S, Musci G. Copper activates the NF-kappaB pathway in vivo. Antioxid Redox Signal. 2006;8(9-10):1897–1904. [DOI] [PubMed] [Google Scholar]
- 9. Aronson J, Meyler L. Meyler’s side effects of drugs. In: The International Encyclopedia of Adverse Drug Reactions and Interactions. 16th ed Oxford: Elsevier, 2016. pp. 846–860. [Google Scholar]
- 10. Flora S, Pachauri V. Chelation in metal intoxication. Int J Environ Res Public Health. 2010;7(12):2745–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang A, Kopečková P, Kopeček J. Binding and cytotoxicity of HPMA copolymer conjugates to lymphocytes mediated by receptor-binding epitopes. Pharm Res. 2003;20(3):360–367. [DOI] [PubMed] [Google Scholar]
- 12. Luo XG, Ji F, Lin YX, et al. Effects of dietary supplementation with copper sulfate or tribasic copper chloride on broiler performance, relative copper bioavailability, and oxidation stability of vitamin E in feed. Poult Sci. 2005;84(6):888–893. [DOI] [PubMed] [Google Scholar]
- 13. Tokyol C, Yilmaz S, Kahraman A, Cakar H, Polat C. The effects of desferrioxamine and quercetin on liver injury induced by hepatic ischaemia-reperfusion in rats. Acta Chir Belg. 2006;106(1):68–72. [DOI] [PubMed] [Google Scholar]
- 14. Chuang SE, Cheng AL, Lin JK, Kuo ML. Inhibition by curcumin of diethylnitrosamine induced hepatic hyperplasia, inflammation, cellular gene products and cell-cycle-related proteins in rats. Food Chem Toxicol. 2000;38(11):991–995. [DOI] [PubMed] [Google Scholar]
- 15. Svensson AK, Bilsel O, Kayatekin C, Adefusika JA, Zitzewitz JA, Matthews CR. Metal-free ALS variants of dimeric human Cu, Zn-superoxide dismutase have enhanced populations of monomeric species. PLoS One. 2010;5(4):e10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271–278. [DOI] [PubMed] [Google Scholar]
- 17. Ellman GL. Tissue sulfhydral group. Arch Biochem Biophys. 1959;82(1):70–77. [DOI] [PubMed] [Google Scholar]
- 18. Moshage H, Kok B, Huizenga JR, Jansen PL. Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem. 1995;41(6 pt 1):892–896. [PubMed] [Google Scholar]
- 19. Mahmood T, Yang PC. Western blot: technique, theory, and troubleshooting. N Am J Med Sci. 2012;4(9):429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−Delta Delta C (T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 21. Leist M, Gantner F, Bohlinger I, German PG, Tiegs G, Wendel A. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-alpha requires transcriptional arrest. I Immunol. 1994;153(4):1778–1787. [PubMed] [Google Scholar]
- 22. Abuja PM, Albertini R. Methods for monitoring oxidative stress, lipid peroxidation and oxidation resistance of lipoproteins. Clin Chim Acta. 2001;306(1-2):1–17. [DOI] [PubMed] [Google Scholar]
- 23. Hassan S, Shaikh MU, Ali N, Riaz M. Copper sulphate toxicity in a young male complicated by methemoglobinemia, rhabdomyolysis and renal failure. J Coll Phys Surg-Pak. 2010;20(7):490–491. [PubMed] [Google Scholar]
- 24. Galhardi CM, Diniz YS, Faine LA, Rodrigues HG, Burneiko RC, Ribas BO, et al. Toxicity of copper intake: lipid profile, oxidative stress and susceptibility to renal dysfunction. Food Chem Toxicol. 2004;42(12):2053–2060. [DOI] [PubMed] [Google Scholar]
- 25. Eigner D, Scholz D. Ferula asafoetida and Curcuma longa in traditional medical treatment and diet in Nepal. J Ethnopharmacol. 1999;67(1):1–6. [DOI] [PubMed] [Google Scholar]
- 26. Letelier ME, Lepe AM, Faúndez M, Salazar J, Marín R, Aracena P, Speisky H. Possible mechanisms underlying copper-induced damage in biological membranes leading to cellular toxicity. Chem Biol Interact. 2005;151(2):71–82. [DOI] [PubMed] [Google Scholar]
- 27. Onoue S, Yamada S, Chan K. Nanodrugs: pharmacokinetics and safety. Int J Nanomed. 2014;9:1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou W, Merrick BA, Khaledi MG, Tomer KB. Detection and sequencing of phosphopeptides affinity bound to immobilized metal ion beads by matrix-assisted laser desorption/ionization mass spectrometry. J Am Soc Mass Spectrom. 2000;11(4):273–282. [DOI] [PubMed] [Google Scholar]
- 29. Bruzell EM, Morisbak E, Tonnesen HH. Studies on curcumin and curcuminoids. XXIX. Photoinduced cytotoxicity of curcumin in selected aqueous preparations. Photochem Photobiol Sci. 2005;4(7):523–530. [DOI] [PubMed] [Google Scholar]
- 30. Joe B, Lokesh BR. Role of capsaicin, curcumin and dietary n-3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochim Biophys Acta. 1994;1224(2):255–263. [DOI] [PubMed] [Google Scholar]
- 31. Pinlaor S, Yongvanit P, Prakobwong S, et al. Curcumin reduces oxidative and nitrative DNA damage through balancing of oxidant-antioxidant status in hamsters infected with Opisthorchis viverrini. Mol Nutr Food Res. 2009;53(10):1316–1328. [DOI] [PubMed] [Google Scholar]
- 32. Gaetke LM, Chow-Johnson HS, Chow CK. Copper: toxicological relevance and mechanisms. Arch Toxicol. 2014;88(11):1929–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cao X, Bi R, Song Y. Toxic responses of cytochrome P450 sub-enzyme activities to heavy metals exposure in soil and correlation with their bioaccumulation in Eisenia fetida. Ecotoxicol Environ Saf. 2017;144:158–165. [DOI] [PubMed] [Google Scholar]
- 34. Park HJ, Choi YJ, Kim JW, et al. Differences in the epigenetic regulation of cytochrome P450 genes between human embryonic stem cell-derived hepatocytes and primary hepatocytes. PLoS One. 2015;10(7):e0132992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim JS, Ahn T, Yim SK, Yun CH. Differential effect of copper (II) on the cytochrome P450 enzymes and NADPH-cytochrome P450 reductase: inhibition of cytochrome P450-catalyzed reactions by copper (II) ion. Biochemistry. 2002;41(30):9438–9447. [DOI] [PubMed] [Google Scholar]
- 36. Mannering G J, Deloria L B, Abbot V. Role of xanthine oxidase in the interferon-mediated depression of the hepatic cytochrome P-450 system in mice. Cancer Res. 1988;48(8):2107–2112. [PubMed] [Google Scholar]
- 37. Alonso M, Collado PS, González-Gallego J. Melatonin inhibits the expression of the inducible isoform of nitric oxide synthase and nuclear factor kappa B activation in rat skeletal muscle. J Pineal Res. 2006;41(1):8–14. [DOI] [PubMed] [Google Scholar]
- 38. El-Sokkary GH, Khidr BM, Younes HA. Role of melatonin in reducing hypoxia induced oxidative stress and morphological changes in the liver of male mice. Eur J Pharmacol 2006;540(1-3):107–114. [DOI] [PubMed] [Google Scholar]
- 39. Velez S, Nair NG, Reddy VP. Transition metal ion binding studies of carnosine and histidine: biologically relevant antioxidants. Colloids Surf B Biointerf. 2008;66(2):291–294. [DOI] [PubMed] [Google Scholar]
- 40. Tsai SJ, Kuo WW, Liu WH, Yin MC. Antioxidative and anti-inflammatory protection from carnosine in the striatum of MPTP-treated mice. J Agric Food Chem. 2010;58(21):11510–11516. [DOI] [PubMed] [Google Scholar]
- 41. Rana SV. Metals and apoptosis: recent developments. J Trace Elem Med Biol. 2008;22(4):262–284. [DOI] [PubMed] [Google Scholar]