Abstract
We used 3 biological metrics highly relevant to health risks, that is, cell death, inflammation, and global DNA methylation, to determine the late effects of low doses (0.05 or 0.1 Gy) of 137Cs γ rays on the bone marrow, lung, and testis collected at 6 months post-irradiation from the same exposed BALB/cJ mouse. This integrative approach has not been used for such a purpose. Mice exposed to 0 or 1 Gy of radiation served as a sham or positive control group, respectively. The results could deliver information for better health risk assessment across tissues, including better scientific basis for radiation protection and clinical application. We found no changes in the levels of all studied biological metrics (except a significant increase in the levels of an anti-inflammatory cytokine, ie, interleukin 10) in tissues of 0.05-Gy exposed mice, when compared to those in sham controls. In contrast, significantly increased levels of cell death and inflammation, including a significant loss of global 5-hydroxymethylcytosine, were found in all tissues of the same mice exposed to 0.1 or 1.0 Gy. Our data demonstrated not only no harm but also hormesis in the 0.05-Gy exposed mice. However, the hormetic effect appears to be dependent on biological metrics and tissue.
Keywords: low-dose radiation, late effects, bone marrow, lung and testis, inflammation, DNA methylation
Introduction
There is increasing evidence showing differences between biological responses to low (as low as 0.05 Gy, the existing limit for exposure in the workplace, up to 0.1 Gy) and higher doses (more than 0.1 Gy) of low linear energy transfer radiation (ie, γ- and X-rays). However, the assessment of health risks from such low doses of radiation remains challenging and has been intensely debated. In spite of a substantial volume of publications opposing the conventional linear no-threshold (LNT) model for radiation-induced cancers or other health issues,1-9 the LNT is still applied.10,11 It should be noted that the majority of previous scientific reports (both in vitro and in vivo systems) focused only on the early effects of low-dose radiation on somatic cells (eg, bone marrow [BM] cells or and lymphocytes) using various biological metrics, that is, metaphase chromosome aberration (CA), micronucleus, and DNA damage.3,12-22 There is scant information on the effects of low-dose radiation on germinal cells (testis).23,24 This makes it necessary to further investigate the effects of low-dose radiation on germinal cells, since radiation-induced damage in germinal cells is likely to be transmitted to the offspring, and hence, such damage is highly relevant to genetic risks.
Results from previous studies on the effects of low-dose radiation on germinal cells showed that low doses of X-rays (200 kV, ranging from 0.025 to 0.1 Gy, delivered at a dose rate of 0.0125 Gy/min) induced significant increases in apoptosis in spermatogonia and spermatocytes of male Kunming mice collected at various times post-irradiation (ie, 6, 12, 18, and 24=hours).23,24 It should be noted that a dose of 0.075 Gy of 200 kV X-rays was the most effective dose in inducing apoptosis in germinal cells of exposed male Kunming mice. Nonetheless, in these studies, only initial or early responses (ie, 6 to 24 hours post-irradiation) were evaluated in previous studies. Recently, it was found that there were no detrimental effects on spermatogenesis, assessed by the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling assay, and sperm morphology in wild large Japanese field mice (Apodemus speciosus) which received about 4000 Bq/kg of γ-rays from 134Cs and 137Cs in different areas near the Fukushima Daiichi accident.25 However, a report from another group of investigators demonstrated an enhanced spermatogenesis in wild large Japanese field mice (A speciosus) captured from different locations near the Fukushima Daiichi accident where the external dose rates of a combined 134Cs and 137Cs γ rays were varied from 21, 304 to 306, or 407 to 447 μGy/d at sampling sites. Of note, the authors did not report the total amount of radiation that the mice received.26
Late effects of radiation exposure are highly relevant to health effects appearing later in life, such as cancer or other chronic diseases. In previous animal studies using the mouse as an animal model, there is no information on the effects of low-dose radiation across tissues (both somatic and germinal tissues) of the same exposed mouse. To improve the assessment of health risks of exposure to low-dose radiation, it is important to evaluate the biological responses in different tissues collected from the same exposed individual by means of various biological metrics relevant to health risks. Yet, such an approach has not been used to evaluate the effects of low-dose radiation (at the doses ≤0.1 Gy).
For these reasons, in this study, we evaluated the late effects of low-dose radiation (≤0.1 Gy of radiation) on the BM, lung, and testis collected from the same exposed individual BALBc/J mouse at 6 months after exposure to 0, 0.05, 0.1, or 1.0 Gy of 137Cs γ-rays (delivered at 0.75 Gy/min). These tissues were collected from mice included in our previous study investigating the effects of low-dose radiation on acute and late-occurring chromosome aberrations (Cas) in BM cells.22 Of note, in our previous low-dose study,22 there were 4 harvest times, that is, 1 hour, 4 hours, 1 month, and 6 months, post-irradiation. However, in this study, we selected only tissues from the 6 months post-irradiation, since the late-occurring damage was the focus of this study. This harvest time point is particularly important for our focus on the effects of low-dose radiation to the primitive-type Asingle spermatogonial stem cell (SSCs). It is known that the duration of mouse spermatogenesis from the primitive type Asingle SSCs to mature sperms (spermatozoa) is about 52 days, but 35 days from differentiated spermatogonia to mature sperms.27 Hence, the testis collected at 6 months post-irradiation are the most appropriate samples for studying the effects of radiation on the primitive type Asingle SSCs. It is known that high doses of X- or γ-rays induce DNA double-strand breaks, CAs, and mutagenesis in spermatogonia.28-36 However, very little is known about the effects of low-dose radiation on the primitive type Asingle SSCs.23,24
We focused on BM cells because these cells are highly sensitive to radiation-induced acute and long-term injuries (normally known as genomic instability)37-42 and are at risk of radiation-induced myeloid leukemia.43-45 Further, we previously demonstrated that low doses of 137Cs γ-rays (as low as 0.05 Gy) are incapable of inducing genomic instability, known to be one of the important steps in carcinogenesis,46 assessed by late-occurring CAs, while a single dose of 1.0 Gy of 137Cs γ-rays (a high dose) is capable of inducing genomic instability in BM cells collected from BALB/cJ mice used in this report.22 Likewise, our focus was also on the lung because it is one of the tissues known to be highly sensitive to radiation-induced acute and chronic inflammation and cell death.47-50 Further, an elevated incidence of lung cancer has been documented in atomic bomb survivors who were exposed to γ-rays and neutrons51 as well as in the Mayak workers of a nuclear facility in the Russian Federation who were exposed to both γ-rays and α particles from inhaling plutonium (239Pu).52
In this study, we used 3 biological metrics closely linked to cell/tissue damage and cancer induction53-60 to determine the effects of low-dose radiation in the BM, lung, and testis collected from the same individual mouse: (1) cell death by measuring the levels of cleaved poly (ADP-ribose) polymerase (cleaved PARP), a marker of cell death associated with caspase activation; (2) inflammatory responses, that is, nuclear factor-κB (NF-κB, a redox-sensitive transcription factor), including pro- and anti-inflammatory cytokines; and (3) DNA methylation, that is, global 5-methylcytosine (5mC) and 5-hydroxymethylcytosine (5hmC), that is, epigenetic alterations. We routinely use these biological metrics to investigate the biological responses across tissues after exposure of mice to radiation with different qualities.49,50,61,62
Materials and Methods
Animals and Radiation Exposure
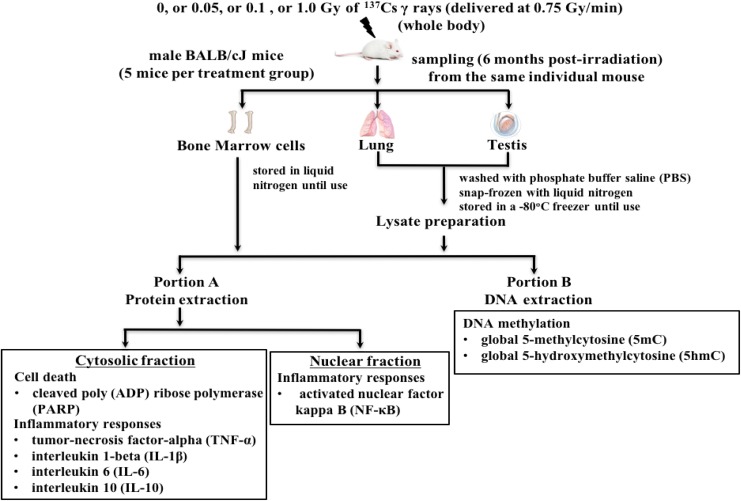
Details of mice and γ-irradiation have been presented elsewhere.22 In brief, all male BALB/cJ mice (8-10 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, Maine) and acclimatized for 2 weeks prior to γ-irradiation. It should be noted that the BALB/cJ mouse strain is known to be a radiosensitive strain.63 Mice were housed and cared for in a facility accredited by the American Association for Accreditation of Laboratory Animal Care. All animal handling procedures were performed under the approved guidelines by the Institutional Animal Care and Use Committee of Stony Brook University (SBU). Four groups of mice (5 in each) were exposed whole body to 0, 0.05, 0.1, or 1.0 Gy of 137Cs γ rays (at the dose rate of 0.75 Gy/min) using the GammaCell 40 (Atomic Energy of Canada, Ltd, Ontario, Canada) located in the Division of the Laboratory Animal Resources of SBU. Mice exposed to 0 or 1.0 Gy of 137Cs γ rays served as a sham or positive control, respectively. Figure 1 shows the experimental approach of this study.
Figure 1.
Diagram of the experimental approach.
Collection of BM Cells, Lung, and Testis
In each mouse, the methods for the collection and storage of BM cells from femurs and tibia,22 the lung,49,6 2 and the testis49 routinely used in our laboratory were followed. Prior to protein or DNA isolation, the total lung or testicular lysate was obtained by homogenization of the lung or the testis using a Bullet Blender Homogenizer (Next Advance Inc, Averill Park, New York). The protocols for homogenization of the lung or testis suggested by the manufacturer was followed, that is, the stainless steel beads (0.9-2.0 mm, part #SSB14B) for the lung and the zirconium oxide bead (multiple sizes of beads, part #ZSB05) for the testis. Then, the lung and testis lysates, including BM cells, of each individual mouse were divided into 2 portions, that is, 0.9-A and B. The portion A of each lysate was used to extract proteins from nuclear and cytosolic fractions using commercially available protein extraction kits (Active Motif Inc, Carlsbad, California).49,61,62 Protein contents in the cytosolic and the nuclear fractions of each lysate were measured by the Bradford assay using a BioPhotometer (Eppendorf, Inc, Westbury, New York). Subsequently, the total protein obtained from the nuclear fraction of each lysate was used for the quantitation of activated NF-κB, while the total protein obtained from the cytosolic fraction was used for the measurements of NF-κB-regulated pro- and anti-inflammatory cytokines, that is, tumor necrosis factor α (TNF-α), interleukin (IL)-1β, IL-6, and IL-10, including the levels of cleaved PARP. The portion B of each lysate of the same individual mouse was used to isolate DNA for the measurements of global 5mC and global 5hmC levels.
Measurements of Cleaved PARP
Similar to our previous studies,50,62 a commercially available enzyme-linked immunosorbent assay (ELISA) kit specific for cleaved PARP (Asp214) purchased from Cell Signaling Technology Inc (Beverly, Massachusetts) was used to measure the level of cleaved PARP. The data readouts were the levels of absorbance at 450 nm (using 50 μg protein per well as suggested by the manufacturer). The measurement of cleaved PARP was performed in duplicate wells for each lysate sample from each mouse. Then, the median, mean, and standard error (SE) values for each mouse in each treatment group were obtained. Subsequently, to simplify the comparison of the levels of cleaved PARP with other biological metrics used in this study, we presented the final data as the levels of absorbance at 450 nm in 100 µg protein.
Measurement of Activated NF-κB (NF-κB/p65)
We routinely used the specific NF-κB/p65 (referred to as NF-κB throughout the article) ELISA kits available from Active Motif North America, Inc (Carlsbad, California) to measure the levels of activated NF-κB in the nuclear fractions obtained from the BM, lung, and testis lysates collected from the same individual mouse.49,61,62 The procedure suggested by the manufacturer was followed. The level of absorbance at 450 nm was measured in 1 μg of protein per well. The assay was performed in duplicate wells for each tissue of each mouse. The data obtained from microplate spectrophotometer readings at 450 nm are the corrected values for the rate of tetra-methyl benzidine conversion, (expressed as optical density or OD/min) in actual samples after subtraction of the substrate conversion rates in reagent blank samples. The median, mean, and SE values of activated NF-κB levels of each tissue of each mouse were obtained. As with cleaved PARP, the final data are presented as the levels of absorbance at 450 nm in 100 µg protein.
Measurement of the Selected NF-κB-Regulated Pro- and Anti-Inflammatory Cytokines
We measured the expression, at the protein level, of selected cytokines known to be regulated by NF-κB activation: (1) proinflammatory cytokines, that is, TNF-α, IL-1β, and IL-6, and (2) an anti-inflammatory cytokine, that is, IL-10. We applied the methods suggested by the manufacturer (Biosource; Invitrogen, Carlsbad, California) that are routinely used in our laboratory for measuring the expression levels of these selected cytokines, at the protein level, in the cytosolic fraction of the BM, lung, and testis using the specific ELISA kits for TNF-α, IL-1β, IL-6, and IL10 from Biosource (Invitrogen).49,62 Briefly, serial dilutions of known concentrations of each cytokine were used to create a standard curve for each of the cytokines included in the study. The levels of each cytokine in the lysate of each tissue from the same mouse were measured using a microplate spectrophotometer (Molecular Devices, Sunnyvale, California) at 450 nm. The concentrations of the cytokines in each lysate sample (100 μL per well) were calculated from their respective standard curves. Ultimately, for each cytokine, the data are reported as picogram in 100 µg of protein. Similar to the measurements of activated NF-κB and cleaved PARP, the assay for each cytokine was performed in duplicate. The median, mean, and SE of each cytokine for each treatment group were obtained.
Measurement of 5mC and 5hmC
The methods for DNA isolation from the mouse BM cells and tissue lysates, using the DNeasy kit (Qiagen, ValenciaCalifornia), have been previously presented.40,49 Prior to using for the measurements of levels of global 5mC and 5hmC, DNA concentrations and purities were measured using the BioPhotometer (Eppendorf Co). Subsequently, we used commercially available ELISA kits for the detection of global 5mC and 5hmC (Zymo Research, Inc, Irvine, California) to measure the percentage of global 5mC and 5hmC in the DNA samples isolated from the BM cells and the lung/testis lysates of these mice. The methods suggested by the manufacturer were followed. In brief, the levels of global 5mC and 5hmC were measured using a microplate spectrophotometer (Molecular Devices) at 405 nm. Thereafter, the % 5mC and % 5hmC were calculated from a standard curve obtained using the control DNA set provided by the manufacturer. The measurements of 5mC and 5hmC in the DNA sample from each tissue of each mouse were also done in duplicate (using 200 ng of DNA per well). Finally, the median, mean, and SE values of global 5mC and global 5hmC were obtained for each treatment group.
Statistical Analyses
We presented the levels of each biological metric as mean ± standard error of the mean. For each tissue, the mean value for each assay of each mouse was used as a single datum point for statistical analyses. The Student t test was used to evaluate statistical differences in the mean values of each exposed group and the corresponding sham control group. A P value of ≤.05 was considered as statistically significant.
Results
Supplemental Table 1 presents the raw data and descriptive statistics (median, mean, and SE) of each biological metric used to investigate the effects of low doses of radiation (0.05 or 0.1Gy), including a high dose (1.0 Gy) of radiation, on the BM, lung, and testis collected from the same individual mouse. There were 5 mice in each group. Our data indicated that the majority of the median values of each group are very similar to the mean value. More importantly, the values of these 2 parameters are the same in many cases. These findings suggest the normal distribution of the data. Further, the data obtained in this study are similar to those in our previously published work regarding the levels of cleaved PARP,50,62 activated NF-κB, TNF-α, IL-1β, IL-6, 5mC, and 5hmC.40,49,50,61,62 Hence, we applied a similar statistical approach, that is, Student t test, to determine the differences in each biological metric between exposed and corresponding sham control groups. Figures 2 to 7 show the levels of cleaved PARP, activated NF-κB, TNF-α, IL-1β, IL-6, Il-10, 5mC, and 5hmC, respectively, in the BM, lung, and testis collected from the same individual mouse at 6 months after irradiation. P values (Student t test) shown in each figure indicate statistical differences between exposed and the corresponding sham control groups. Details of our results for each biological metric are shown as follows.
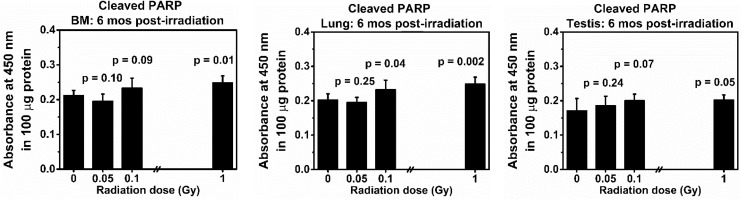
Figure 2.
Levels of cleaved poly (ADP-ribose) polymerase (PARP) in the bone marrow (BM), lung, and testis collected at 6 months from the same BALB/cJ mouse after exposure to low doses (0.05 or 0.1 Gy) of 137Cs γ rays. Mice exposed to 0 or 1.0 Gy of 137Cs γ rays were used as a sham or a positive control group, respectively. The data reflect the absorbance at 450 nm of 100 μg protein. There were 5 mice in each group. P values (Student t test) indicate statistical differences (at the significant level of P value <.05) in the levels of cleaved PARP between exposed and corresponding tissues from the sham control group.
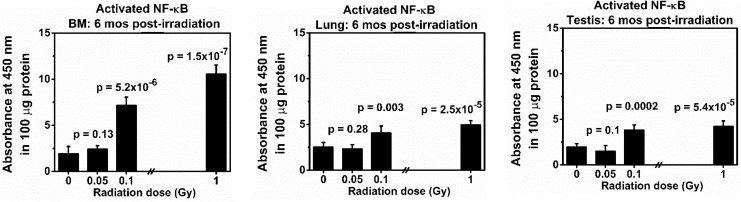
Figure 3.
Levels of activated nuclear factor κB (NF-κB) in the bone marrow (BM), lung, and testis collected at 6 months from the same BALB/cJ mouse after exposure to low doses (0.05 or 0.1 Gy) of 137Cs γ rays. Mice exposed to 0 or 1.0 Gy of 137Cs γ rays were used as a sham or a positive control group, respectively. The data reflect the absorbance at 450 nm of 100 μg protein. There were 5 mice in each group. P values (Student t test) indicate statistical differences (at the significant level of P value <.05) in the levels of activated NF-κB between exposed and corresponding tissues from the sham control group.
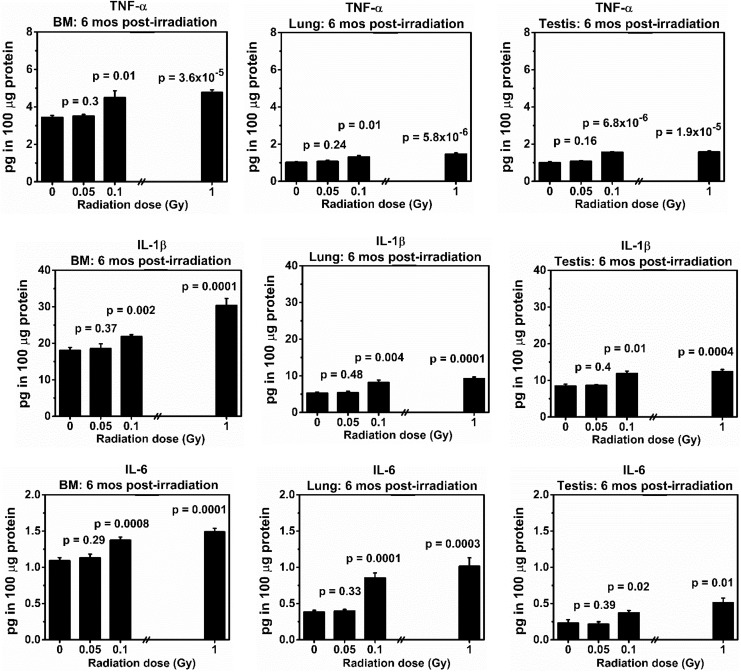
Figure 4.
Levels (picograms in 100 μg of protein) of tumor necrosis factor α (TNF-α), interleukin (IL) 1β, and IL-6 in the bone marrow (BM), lung, and testis collected at 6 months from the same BALB/cJ mouse after exposure to low doses (0.05 or 0.1 Gy) of 137Cs γ rays. Mice exposed to 0 or 1.0 Gy of 137Cs γ rays were used as a sham or positive control, respectively. There were 5 mice in each group. P values (Student t test) indicate statistical differences (at the significant level of P value <.05) in the levels of TNF-α, IL-1β, and IL-6 between exposed and corresponding tissues from the sham control group.
Figure 5.
Levels (picograms in 100 μg of protein) of interleukin 10 (IL-10) in the bone marrow (BM), lung, and testis collected at 6 months from the same BALB/cJ mouse after exposure to low doses (0.05 or 0.1 Gy) of 137Cs γ rays. Mice exposed to 0 or 1.0 Gy of 137Cs γ rays were used as a sham or positive control, respectively. There were 5 mice in each group. P values (Student t test) indicate statistical differences (at the significant level of P value <.05) in the levels of IL-10 between exposed and corresponding tissues from the sham control group.
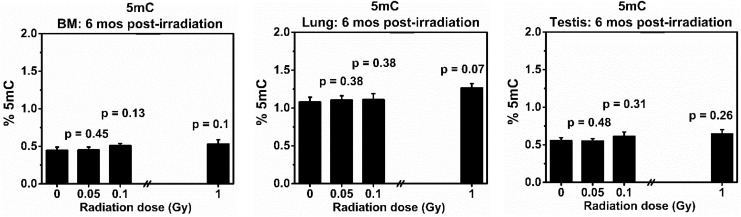
Figure 6.
Levels of global 5-methylcytosine (5mC) in the bone marrow (BM), lung, and testis collected at 6 months from the same BALB/cJ mouse after exposure to low doses (0.05 or 0.1 Gy) of 137Cs γ rays. Mice exposed to 0 or 1.0 Gy of 137Cs γ rays were used as a sham or positive control, respectively. There were 5 mice in each group. P values (Student t test) indicate statistical differences (at the significant level of P value <.05) in the levels of global 5mC between exposed and corresponding tissues from the sham control group.
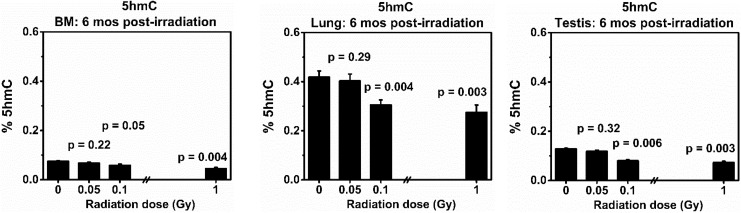
Figure 7.
Levels of global and 5-hydroxymethylcytosine (5hmC) in the BM, lung, and testis collected at 6 months from the same BALB/cJ mouse after exposure to low doses (0.05 or 0.1 Gy) of 137Cs γ rays. Mice exposed to 0 or 1.0 Gy of 137Cs γ rays were used as a sham or positive control, respectively. There were 5 mice in each group. P values (Student t test) indicate statistical differences (at the significant level of P value <.05) in the levels of global 5mhC between exposed and corresponding tissues from the sham control group.
Levels of Cleaved PARP
Figure 2 shows the levels of cleaved PARP in the BM, lung, and testis from the same mouse of each exposed group collected at 6 months post-exposure to various doses of 137Cs γ rays. Our results indicate that the levels of cleaved PARP in the BM, lung, and testis collected from mice exposed to 0.05 Gy of 137Cs γ-rays did not differ from those in the corresponding tissues collected from the sham control groups (P values = .1, .25, and .24 for BM, lung, and testis, respectively). Notably, the levels of cleaved PARP in the BM and the lung from the 0.05 Gy exposed groups were lower than those in the sham controls. Nonetheless, such decreases were not statistically different. For the 0.1 Gy exposed group, a significant level of cleaved PARP was detected in the lung tissue (P = .04) only. However, there were significant increases in the levels of cleaved PARP in all tissues of mice exposed to a high dose of 137Cs γ-rays used in this study (ie, 1.0 Gy), when compared to those in the corresponding sham controls (P values = .005, .002, and .01 for BM, lung, and testis, respectively).
In summary, our data clearly demonstrated there were no tissues injuries (determined by the levels of cleaved PARP in the BM, lung, and testis) in mice exposed to a single dose of 0.05 Gy of 137Cs γ-rays. In contrast, the effectiveness of a single dose of 0.1 or 1.0 Gy of 137Cs γ-rays in inducing tissue injuries was evident.
Levels of Activated NF-κB
Figure 3 presents our data on the levels of activated NF-κB in the BM, lung, and testis collected from the same exposed mouse (5 mice in each group) at 6 months post-irradiation. Similar to the levels of cleaved PARP, our data demonstrated that there was no statistical difference in the levels of activated NF-κB in the BM, lung, and testis from the same mouse, when compared to such levels in the corresponding tissue from the sham controls (P values = .12, .28, and .09 for the BM, lung, and testis, respectively). However, a reduction (but not statistically significant) was found in the levels of activated NF-κB in the lung and testis (not the BM) from mice exposed to 0.05 Gy of 137Cs γ-rays in relation to the sham control levels. Our data also revealed that a single dose of 0.1 or 1.0 Gy of 137Cs γ-rays induced highly significant increases in the levels of activated NF-κB in all tissues from the same individual mouse collected at 6 months post-irradiation, when compared to the levels in corresponding tissues (P values ranging from .003 to 1.5 × 10−7). It should be noted that a single dose of 0.1 or 1.0 Gy of 137Cs γ-rays induced a higher level of activated NF-κB in the BM than in the lung or testis.
Undoubtedly, our data demonstrated that a single dose of 0.05 Gy of 137Cs γ rays did not increase the levels of activated NF-κB. Further, our data showed a reduction (but not significant) in the levels of activated NF-κB in the lung and testis collected from the same exposed mice exposed to this low dose of radiation. Such findings suggest the trend of beneficial effects, normally known as “hormesis,” of this low dose level (0.05 Gy) of radiation in the lung and testis of the same exposed mouse (evaluated by the levels of activated NF-κB). This phenomenon was not detected in tissues of mice exposed to 0.1 or 1.0 Gy of 137Cs γ-rays.
Levels of Selected Pro-inflammatory Cytokines, That is, TNF-α, IL-1β, and IL-6
Figure 4 shows that there were no statistical differences in levels of all 3 selected NF-κB-regulated proinflammatory cytokines (ie, TNF-α, IL-1β, and IL-6) in the BM, lung, and testis from the same mice collected at 6 months after exposure to a single dose of 0.05 Gy of 137Cs γ-rays, in relation to the levels in the corresponding tissues collected from sham controls. However, a single dose of 0.1 or 1.0 Gy of 137Cs γ-rays induced highly significant increases in the levels of these 3 pro-inflammatory cytokines in all tissues from the same mouse collected at 6 months post-irradiation in relation to the levels in the corresponding tissues from the same mouse. Our data also showed that the control levels of TNF-α, IL-1β, and IL-6 differ among tissues of the BALB/cJ mouse, with the highest control level in the BM.
Our results unambiguously indicated no increases in the expression levels of all pro-inflammatory cytokines included in the studies in all types of tissues collected from the same mouse exposed to a single dose of 0.05 Gy of 137Cs γ rays. Overall, our results showed that there was no increased risk of chronic inflammation (harmful effects) from exposure to this low dose level, evaluated as a lack of increased expression of pro-inflammatory cytokines. In contrast, our data clearly showed significant increases in the expression levels of pro-inflammatory cytokines (indicative of chronic inflammation) in all tissues selected for analyses that were collected from the same individual mice exposed to a single dose of 0.1 or 1.0 Gy of 137Cs γ rays.
Levels of a Selected Anti-Inflammatory Cytokine, That is, IL-10
Figure 5 demonstrates significant increases in the levels of IL-10 in the BM, lung, and testis collected at 6 months from the same mouse exposed to 0.05 Gy of 137Cs γ-rays, in relation to the levels in the corresponding tissues (P values = .01, .0001, and .009 for the BM, lung, and testis, respectively). However, the levels of IL-10 in the BM, lung, and testis collected from the same individual mouse after exposure of a single dose of 0.1 or 1.0 Gy of 137Cs γ-rays are similar to those detected in the corresponding tissues from the sham control group (P values are .18 and .49 for the BM; .39 and .26 for the lung; and .14 and .25 for the testis). Similar to the control levels of pro-inflammatory cytokines, our data showed that the control levels of the anti-inflammatory cytokine IL-10 differ among tissues of the BALB/cJ mouse. The highest control level of IL-10 was found in the testis.
Our results clearly showed that a single dose of 0.05 Gy of 137Cs γ-rays induced highly significant expression of an anti-inflammatory cytokine IL-10 in all tissues collected from the same exposed mouse. Hence, our results suggest that this low-dose radiation protects exposed individuals from cell/tissue injuries by stimulating the immunity, enabling cells/tissues to maintain homeostasis and integrity. Such a phenomenon was not observed in any tissue of mice exposed to 0.1 or 1.0 Gy of 137Cs γ-rays.
Levels of Global 5mC
Figure 6 shows the effects of 137Cs γ-rays (at the doses of 0, 0.05, 0.1 or 1.0 Gy) on the levels of global 5mC in the BM, lung, and testis from the same mouse collected at 6 months after irradiation. There was a trend of increased levels of global 5mC in all examined tissues of exposed mice, regardless of radiation dose, in relation to those of the corresponding sham controls. However, such increases were not statistically different (P values ranging from .13 to .48). The control level of global 5mC was highest in the lung of BALB/cJ mice, while those in the BM and testis were relatively similar.
At the global level, our data showed no differences in the levels of global 5mC in all tissues included in the analyses that were collected from the same exposed mice, regardless of radiation dose.
Levels of 5hmC
Figure 7 shows that there were no differences in the levels of global 5hmC in the BM, lung, and testis in mice exposed to 0.05 Gy of 137Cs γ-rays compared to the corresponding tissues collected from the sham control group (P values = .22, 0.29, and .32 for the BM, lung, and testis, respectively). In contrast, the levels of global 5hmC were significantly reduced in all tissues collected from mice exposed to either 0.1 or 1.0 Gy of 137Cs γ rays when compared to the levels in the corresponding tissues from the sham control group. Of note, the lung of sham control mice possesses the highest levels of global 5hmC in relation to those in the BM or testis from the same individual mouse. Further, the control level of global 5hmC is lowest in BM cells.
Our data unequivocally demonstrated that there were no epigenetic alterations (determined by the levels of global 5hmC) in all studied tissues collected from the same mice exposed to a single dose of 0.05 Gy of 137Cs γ-rays. However, our results clearly showed that a single dose of 0.1 or 1.0 Gy of 137Cs γ rays induced a significant loss of global 5hmC (a phenomenon normally observed in cancer cells).60
Discussion
Our study is the first to use 3 different biological metrics closely linked to cell/tissue damage and cancer induction to evaluate the late effects of low-dose radiation (0.05 or 0.1 Gy of 137Cs γ-rays) in both somatic (ie, the BM and lung) and germinal (ie, the testis) tissues collected from the same individual BALB/cJ mouse at 6 months after irradiation. The results from our integrative approach could deliver information for better assessment of health risks across tissues of the same exposed individual and could provide the better scientific basis for radiation protection and clinical application.
We observed a reduction in the levels of cleaved PARP in the BM collected from mice exposed to 0.05 Gy of 137Cs γ rays (Figure 2) and in the levels of activated NF-κB in the lung and testis (not in the BM) from the same individual mouse exposed to a single dose of 0.05 Gy of 137Cs γ-rays (Figure 3). Although such reduction is not statistically significant (in relation to those observed in the sham control group), our data showed not only no harmful late effects but also a trend of hormetic effects in tissues of exposed male BALB/cJ mice. Of note, our data demonstrated that such beneficial effects are dependent on the tissue and the biological metric used for analyses, that is, cleaved PARP in the BM and activated NF-κB in the lung and testis. Overall, the hormesis beneficial effect of low-dose radiation does not always occur. Similar findings have been reported in both in vitro and in vivo systems.21,64,65 At higher doses of radiation (ie, 0.1 or 1.0 Gy of 137Cs γ rays), we detected highly significant increases in the levels of cleaved PARP (Figure 2) and activated NF-κB in all tissues included in the analyses (Figure 3).
In this study, we detected no statistical differences in the levels of the NF-κB-regulated proinflammatory cytokines included in our study (ie,TNF-α, IL-1β, and IL-6) in all tissues collected from the same individual mouse exposed to 0.05 Gy of 137Cs γ-rays, in relation to those in the corresponding tissues from the sham control group (Figure 4). Importantly, our data show that a single dose of 0.05 Gy of 137Cs γ-rays induced significant increases in the levels of an anti-inflammatory cytokine IL-10 in all tissues collected from the same individual mouse (Figure 5). Hence, the findings of increased levels of an anti-inflammatory cytokine IL-10, combined with no induction of proinflammatory cytokines, suggests that there are no harmful effects to the immune system in the BM, lung, and testis after exposure of male BALB/cJ mice to a single dose of 0.05 Gy of 137Cs γ rays. Taken together, it is possible that this low-dose radiation protects exposed individuals from cell/tissue injuries by stimulating the immunity that, in turn, enables cells/tissues to maintain homeostasis and integrity.
It should be noted that although our data suggest beneficial effects of low-dose radiation, the investigation of only one signaling pathway (ie, NF-κB activation) and a small number of cytokines limits the clinical value of our data. Therefore, to properly assess the benefits/risks of exposure to low-dose radiation, other transduction signaling pathways66 should be included in future analyses. Examples of such pathways are the activation of nuclear factor erythroid 2-related factor 2 transcription factor, activator protein-1, signal transducer and activator of transcription 3, tumor suppressor p53, and mitogen-activated protein kinases. Many other cytokines/chemokines involved in various functional responses (eg, innate immunity, adaptive immunity, growth factor, and chemokines) should also be measured in different tissues, including plasma, collected from the same individual mouse after exposure. The available information on both plasma (circulating) and tissue cytokines of the same mouse would help to determine the strength or limitation of plasma cytokines in clinical applications for radiation exposure. Further, the levels of cytoprotective antioxidant enzymes, for example NAD(P)H quinone dehydrogenase 1, superoxide dismutase, and heme oxygenase-1, should be investigated in different tissues collected from the same mouse. Additionally, histopathological evaluations in these tissues of the same mouse are needed. Such an approach would deliver the most reliable information on the potential health benefits/risks of low-dose radiation (<0.1 Gy).
In contrast, we detected statistically significant increases in the levels of all of the pro-inflammatory cytokines selected for analyses (ie, TNF-α, IL-1β, and Il-6) in all tissues from the same mouse exposed to 0.1 or 1.0 Gy of 137Cs γ-rays (Figure 4). Taken together, our data also suggest that a single dose of 0.1 or 1.0 Gy of 137Cs γ rays induced impairment of the immune system (the manifestation of chronic inflammation) at 6 months post-irradiation that may trigger genomic instability and/or other downstream signaling pathways associated with health risks.
It is known that DNA methylation is one of the key epigenetic events associated with cancer induction. A high level of global 5mC (hypermethylation) in specific loci has been linked to suppression of gene activation,67,68 while a reduction in levels of global 5hmC has been linked to cancer induction.57,58,60 In this study, we found no changes in the levels of global 5hmC in the BM, lung, and testis from mice exposed to 0.05 Gy of 137Cs γ-rays only (Figure 6). In contrast, a significant reduction in the levels of global 5hmC was detected in all tissues from mice exposed to 0.1 or 1.0 Gy of 137Cs γ-rays. Such observations were similar to those detected in the lung and testis collected from CBA/Ca mice exposed to various doses (0.1, 0.25, or 0.5 Gy) of heavy titanium ions (48Ti ions, one type of radiation found in the space environment).49 Consequently, our data suggest that the loss of global 5hmC is an important biological response to radiation exposure (at the dose range of ≥0.1 Gy), regardless of radiation quality and tissue. Since a loss of global 5hmC and chronic inflammation (high levels of activated NF-κB and proinflammatory cytokines) were detected in the same tissue of the same mouse exposed to 0.1 or 1.0 Gy of 137Cs γ-rays, it is plausible to postulate that there is a link between chronic inflammation and a loss of global 5hmC. However, it remains unclear whether chronic inflammation enhances the loss of global 5hmC or the loss of global 5hmC heightens chronic inflammation.
With respect to the levels of global 5mC in the BM, lung, and testis from the same mouse, our data showed no significant change in the levels of global 5mC for all doses of 137Cs γ-rays radiation (Figure 6). Similar observations were reported in our previous studies with space radiation (heavy ions).40,49,61 Thus, based on our data from this study, in combination with our data previously published, a loss of global 5hmC may be a more appropriate biomarker of radiation exposure than the changes in the levels of global 5mC. In the future, the identification of genes affected by methylation/hydroxymethylation should be performed to enhance our understanding of epigenetic events induced by low or high doses of radiation. Such studies may help to discover genes/proteins that serve as the molecular shift of responses from low doses (less than 0.1 Gy) to high doses of radiation (equal to or more than 0.1 Gy).
Our investigation of the late effects of low-dose radiation on the primitive Asingle SSCs is important because the SSCs are capable of self-renewal and differentiation into spermatocytes and mature sperm. Thus, any damage to the SSC compartment, if no repair, will be transmitted to the next generation and will have adverse effects on self-renewal, proliferation, and differentiation, leading to genetic risk. The damage in the SSCs has also been linked to long-term effects of radiation on fertility.31,69 70, On the other hand, the induced damage occurring in other stages of male germ cell development (eg, spermatocytes) will have an impact on the progeny that are conceived shortly after irradiation.
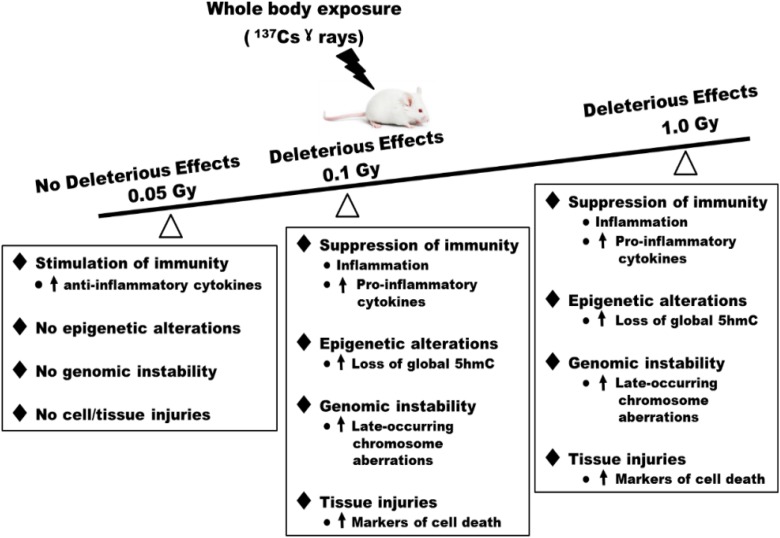
In summary, our data showed no alterations in the levels of cell death (tissue injuries), inflammation, and epigenetics at the global levels (global 5mC and 5hmC) in tissues of 0.05 Gy exposed mice, when compared to those in sham controls. Further, we found a significant increase in the levels of an anti-inflammatory cytokine, that is, IL10, suggesting the stimulatory effects on the immunity, in tissues of 0.05 Gy exposed mice. Additionally, our data suggested the hormetic effect of a single dose of 0.05 Gy of 137Cs γ-rays. However, such hormesis beneficial effect is not robust and appears to be dependent on the tissue and biological metric used for the analyses. At the dose level of 0.1 or 1.0 Gy of 137Cs γ-rays, however, significant levels of induced damage were detected. Figure 8 is a diagram summarizing the biological responses to low-dose (≤0.1 Gy) and high-dose (1.0 Gy) radiation. Together with our data on late-occurring CAs (genomic instability) previously published,22 we conclude that exposure to a single dose of 0.05 Gy of 137Cs γ-rays (the existing limit for exposure in the workplace) does not pose health risks in the BM, lung, and testis of exposed individuals. Our inference is based on the findings that this low-dose radiation fails to induce the biological events known to be linked to chronic diseases such as cancer (ie, genomic instability,58 chronic inflammation,57,58 and loss of global 5hmC60), as well as cell/tissue injuries (ie, cleaved PARP as the cellular marker for cell death) in all tissue types included in the analyses.
Figure 8.
Differences in response to low and high doses of radiation at 6 months after exposure of male BALB/cJ mice. Deleterious effects were not detected in the bone marrow (BM), lung, and testis collected from the same mouse exposed to a single dose of 0.05 Gy of 137Cs γ rays when compared to the corresponding tissues collected from the sham control group. In contrast, deleterious effects were detected in all tissues collected from mice exposed to either 0.1 or 1.0 Gy of 137Cs γ rays.
Supplemental Material
Supplemental Material, Table_1_word_format for Late Effects of Low-Dose Radiation on the Bone Marrow, Lung, and Testis Collected From the Same Exposed BALB/cJ Mice by Witawat Jangiam, Chatchanok Udomtanakunchai, Paiboon Reungpatthanaphong, Montree Tungjai, Louise Honikel, Chris R. Gordon, and Kanokporn Noy Rithidech in Dose-Response
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Aeronautics and Space Administration (NASA) Grant # NNX11AK91G, the DOE Grant #DE-FG02-02ER6331, and Stony Brook University.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Brooks AL, Hui TE, Couch LA. Very large amounts of radiation are required to produce cancer. Dose-Response. 2007;5(4):263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scott BR. Low-dose radiation risk extrapolation fallacy associated with the linear-no-threshold model. Hum Exp Toxicol. 2008;27(2):163–168. [DOI] [PubMed] [Google Scholar]
- 3. Redpath JL, Liang D, Taylor TH, Christie C, Elmore E. The shape of the dose-response curve for radiation-induced neoplastic transformation in vitro: evidence for an adaptive response against neoplastic transformation at low doses of low-LET radiation. Radiat Res. 2001;156(6):700–707. [DOI] [PubMed] [Google Scholar]
- 4. Tubiana M, Feinendegen LE, Yang C, Kaminski JM. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology. 2009;251(1):13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calabrese EJ. An abuse of risk assessment: how regulatory agencies improperly adopted LNT for cancer risk assessment. Arch Toxicol. 2015;89:647–648. [DOI] [PubMed] [Google Scholar]
- 6. Calabrese EJ, O’Connor MK. Estimating risk of low radiation doses—a critical review of the BEIR VII report and its use of the linear no-threshold (LNT) hypothesis. Radiat Res. 2014;182(5):463–474. [DOI] [PubMed] [Google Scholar]
- 7. Hooker AM, Bhat M, Day TK, et al. The linear no-threshold model does not hold for low-dose ionizing radiation. Radiat Res. 2004;162(4):447–452. [DOI] [PubMed] [Google Scholar]
- 8. Doss M. Linear no-threshold model vs. radiation hormesis. Dose-Response. 2013;11(4):495–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cuttle JM. Commentary on using LNT for radiation protection and risk assessment. Dose Response. 2010;8(3):378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. BEIR. Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2 Vol BEIR VII. Washington, DC: The National Academies; 2005. [PubMed] [Google Scholar]
- 11. UNSCEAR. Biological mechanisms of radiation actions at low doses. http://wwwunscearorg/docs/reports/Biological_mechanisms_WP_12-57831pdf.%202012.
- 12. Azzam EI, de Toledo SM, Raaphorst GP, Mitchel REJ. Low-dose ionizing radiation decreases the frequency of neoplastic transformation to a level below the spontaneous rate in C3 H 10T1/2 Cells. Radiat Res. 1996;146(4):369–373. [PubMed] [Google Scholar]
- 13. Bond VP, Benary V, Sondhaus CA. A different perception of the linear, nonthreshold hypothesis for low-dose irradiation. Proc Natl Acad Sci U S A. 1991;88(19):8666–8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elmore E, Lao XY, Kapadia R, Giedzinski E, Limoli C, Redpath JL. Low doses of very low-dose-rate low-LET radiation suppress radiation-induced neoplastic transformation in vitro and induce an adaptive response. Radiat Res. 2008;169(3):311–318. [DOI] [PubMed] [Google Scholar]
- 15. Feinendegen LE. Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol. 2005;78(925):3–7. [DOI] [PubMed] [Google Scholar]
- 16. Mitchel REJ. The dose window for radiation-induced protective adaptive responses. Dose Response. 2010;8(2):192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Olivieri G, Bodycote J, Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984;223(4636):594–597. [DOI] [PubMed] [Google Scholar]
- 18. Scott BR, Di Palma J. Sparsely ionizing diagnostic and natural background radiation are likely preventing cancer and other genomic-instability associated diseases. Dose Response 2006;5(3):230–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolff S. The adaptive response in radiobiology: evolving insights and implications. Environ Health Perspect. 1998;106(suppl 1):277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rithidech K, Udomtanakunchai C, Honikel L, Whorton E. Lack of genomic instability in bone marrow cells of SCID mice exposed whole-body to low-dose radiation. Int J Environ Res Public Health. 2013;10(4):1356–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rithidech KN, Scott BR. Evidence for radiation hormesis after in vitro exposure of human lymphocytes to low doses of ionizing radiation. Dose-Response. 2008;6(3):252–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rithidech KN, Udomtanakunchai C, Honikel LM, Whorton EB. No evidence for the in vivo induction of genomic instability by low doses of 137Cs gamma rays in bone marrow cells of BALB/CJ and C57BL/6 J mice. Dose Response. 2012;10(1):11–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu G, Gong P, Zhao H, Wang Z, Gong S, Cai L. Effect of low-level radiation on the death of male germ cells. Radiat Res. 2006;165(4):379–389. [DOI] [PubMed] [Google Scholar]
- 24. Liu G, Gong P, Bernstein LR, Bi Y, Gong S, Cai L. Apoptotic cell death induced by low-dose radiation in male germ cells: hormesis and adaptation. Crit Rev Toxicol. 2007;37(7):587–605. [DOI] [PubMed] [Google Scholar]
- 25. Okano T, Ishiniwa H, Onuma M, Shindo J, Yokohata Y, Tamaoki M. Effects of environmental radiation on testes and spermatogenesis in wild large Japanese field mice (Apodemus speciosus) from Fukushima. Sci Rep. 2016;6:23601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takino S, Yamashiro H, Sugano Y, et al. Analysis of the effect of chronic and low-dose radiation exposure on spermatogenic cells of male large Japanese field mice (Apodemus speciosus) after the Fukushima Daiichi nuclear power plant accident. Radiat Res. 2017;187(2):161–168. [DOI] [PubMed] [Google Scholar]
- 27. Adler ID. Male germ cell cytogenetics In: Chu TC, ed. Cytogenetics Assay of Environmental Mutagen. Cambridge, UK: Allanheld, Osmun Publisher, 1982:249–276. [Google Scholar]
- 28. van Buul PP. Dose-response relationship for radiation-induced translocations in somatic and germ cells of mice. Mutat Res. 1977;45(1):61–68. [DOI] [PubMed] [Google Scholar]
- 29. Vaiserman AM, Mekhova LV, Koshel NM, Voitenko VP. Cancer incidence and mortality after low-dosage radiation exposure: epidemiological aspects. Biophys. 2011;56(2):371–380. [PubMed] [Google Scholar]
- 30. Vaiserman AM. Epigenetic programming by early-life stress: evidence from human populations. Dev Dynam. 2015;244(3):254–265. [DOI] [PubMed] [Google Scholar]
- 31. Hacker-Klom U. Long term effects of ionizing radiation on mouse spermatogenesis. Acta Radiol Oncol. 1985;24(4):363–367. [DOI] [PubMed] [Google Scholar]
- 32. Hamer G, Roepers-Gajadien HL, van Duyn-Goedhart A, et al. DNA double-strand breaks and γ-H2AX signaling in the testis. Biol Reprod. 2003;68(2):628–634. [DOI] [PubMed] [Google Scholar]
- 33. Cordelli E, Eleuteri P, Grollino MG, et al. Direct and delayed X-ray-induced DNA damage in male mouse germ cells. Environ Mol Mutagen. 2012;53(6):429–439. [DOI] [PubMed] [Google Scholar]
- 34. Spalding JF, Wellnitz JM, Schweitzer WH. Effects of rapid massive doses of gamma-rays on the testes and germ cells of the rat. Radiat Res. 1957;7(1):65–70. [PubMed] [Google Scholar]
- 35. Generoso WM, Cain KT, Cacheiro NLA, Cornett CV. Response of mouse spermatogonial stem cells to X-ray induction of heritable reciprocal translocations. Mutat Res. 1984;126(2):177–187. [DOI] [PubMed] [Google Scholar]
- 36. Adler ID. Stage-sensitivity and dose-response study after γ-irradiation of mouse primary spermatocytes. Int J Radiation Biology. 1977;31(1):79–85. [DOI] [PubMed] [Google Scholar]
- 37. Shao L, Wang Y, Chang J, Luo Y, Meng A, Zhou D. Hematopoietic stem cell senescence and cancer therapy-induced long-term bone marrow injury. Transl Cancer Res. 2013;2(5):397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Y, Liu L, Pazhanisamy SK, Li H, Meng A, Zhou D. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med. 2010;48(2):348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rithidech KN, Honikel L, Whorton EB, mFISH analysis of chromosomal damage in bone marrow cells collected from CBA/CaJ mice following whole body exposure to heavy ions (56Fe ions). Radiat Environ Biophys. 2007;46(2):137–145. [DOI] [PubMed] [Google Scholar]
- 40. Rithidech KN, Honikel LM, Reungpathanaphong P, Tungjai M, Jangiam W, Whorton EB. Late-occurring chromosome aberrations and global DNA methylation in hematopoietic stem/progenitor cells of CBA/CaJ mice exposed to silicon (28Si) ions. Mutat Res. 2015;781:22–31. [DOI] [PubMed] [Google Scholar]
- 41. Rithidech KN, Honikel LM, Reungpatthanaphong P, Tungjai M, Golightly M, Whorton EB. Effects of 100 MeV protons delivered at 0.5 or 1 cGy/min on the in vivo induction of early and delayed chromosomal damage. Mutat Res. 2013;756(1–2):127–140. [DOI] [PubMed] [Google Scholar]
- 42. Chang J, Feng W, Wang Y, et al. Whole-body proton irradiation causes long-term damage to hematopoietic stem cells in mice. Radiat Res. 2015;183(2):240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–737. [DOI] [PubMed] [Google Scholar]
- 44. Cronkite EP, Bond VP, Carsten AL, Inoue T, Miller ME, Bullis JE. Effects of low level radiation upon the hematopoietic stem cell: implications for leukemogenesis. Radiat Environ Biophys. 1987;26(2):103–114. [DOI] [PubMed] [Google Scholar]
- 45. Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30(6):513–528. [DOI] [PubMed] [Google Scholar]
- 46. Ullrich RL, Ponnaiya B. Radiation-induced instability and its relation to radiation carcinogenesis. Int J Radiat Biol. 1998;74(6):747–754. [DOI] [PubMed] [Google Scholar]
- 47. Finkelstein J, Johnston C, Baggs R, Rubin P. Early alterations in extracellular matrix and transforming growth factor beta gene expression in mouse lung indicative of late radiation fibrosis. Int J Radiat Oncol Biol Phys. 1994;28(3):621–631. [DOI] [PubMed] [Google Scholar]
- 48. Rübe CE, Palm J, Erren M, et al. Cytokine plasma levels: reliable predictors for radiation pneumonitis? PLoS One. 2008;3(8):e2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rithidech KN, Jangiam W, Tungjai M, Gordon C, Honikel L, Whorton EB. Induction of chronic inflammation and altered levels of DNA hydroxymethylation in somatic and germinal tissues of CBA/CaJ mice exposed to (48)Ti ions. Front Oncol. 2016;6:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rithidech KN, Reungpatthanaphong P, Tungjai M, Jangiam W, Honikel L, Whorton EB. Persistent depletion of plasma gelsolin (pGSN) after exposure of mice to heavy silicon ions. Life Sci Space Res. 2018;17:83–90. [DOI] [PubMed] [Google Scholar]
- 51. Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiat Res. 2007;168(1):1–64. [DOI] [PubMed] [Google Scholar]
- 52. Sokolnikov ME, Gilbert ES, Preston DL, et al. Lung, liver and bone cancer mortality in Mayak workers. Int J Cancer. 2008;123(4):905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boulares AH, Yakovlev AG, Ivanova V, et al. Role of Poly(ADP-ribose) Polymerase (PARP) cleavage in apoptosis: caspase 3-resistant parp mutant increases rates of apoptosis in transfected cells. J of Biol Chem. 1999;274(33):22932–22940. [DOI] [PubMed] [Google Scholar]
- 54. Cesselli D, Jakoniuk I, Barlucchi L, et al. Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ Res. 2001;89(3):279–286. [DOI] [PubMed] [Google Scholar]
- 55. Los M, Mozoluk M, Ferrari D, et al. Activation and caspase-mediated Inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol Biol Cell. 2002;13(3):978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wallach D, Kang TB, Kovalenko A. Concepts of tissue injury and cell death in inflammation: a historical perspective. Nat Rev Immunol. 2013;14(1):51–59. [DOI] [PubMed] [Google Scholar]
- 57. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. [DOI] [PubMed] [Google Scholar]
- 58. Hanahan D, Weinberg Robert A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 59. Puukila S, Lemon JA, Lees SJ, Tai TC, Boreham DR, Khaper N. Impact of ionizing radiation on the cardiovascular system: a review. Radiat Res. 2017;188(4.2):539–546. [DOI] [PubMed] [Google Scholar]
- 60. Pfeifer G, Kadam S, Jin SG. 5-hydroxymethylcytosine and its potential roles in development and cancer. Epigenet Chromatin. 2013;6(1):631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jangiam W, Tungjai M, Rithidech K. Induction of chronic oxidative stress, chronic inflammation and aberrant patterns of DNA methylation in the liver of titanium-exposed CBA/CaJ mice. Int J Radiat Biol. 2015;91(5):389–398. [DOI] [PubMed] [Google Scholar]
- 62. Tungjai M, Whorton E, Rithidech K. Persistence of apoptosis and inflammatory responses in the heart and bone marrow of mice following whole-body exposure to 28Silicon (28Si) ions. Radiat Environ Biophys. 2013;52(3):339–350. [DOI] [PubMed] [Google Scholar]
- 63. Ponnaiya B, Cornforth MN, Ullrich RL. Radiation-induced chromosomal instability in BALB/c and C57BL/6 mice: the difference is as clear as black and white. Radiat Res. 1997;147(2):121–125. [PubMed] [Google Scholar]
- 64. Bruce VR, Belinsky SA, Gott K, et al. Low-dose gamma-radiation inhibits benzo[A]pyrene-induced lung adenoma development in A/J mice. Dose-Response. 2012;10(4):516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ducoff SC. Radiation hormesis: incredible of inevitable? Korean J Biol Sci. 2002;6(3):187–193. [Google Scholar]
- 66. Schmidt-Ullrich RK, Dent P, Grant S, Mikkelsen RB, Valerie K. Signal transduction and cellular radiation responses. Radiat Res. 2000;153(3):245–257. [DOI] [PubMed] [Google Scholar]
- 67. Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16(1):6–12. [DOI] [PubMed] [Google Scholar]
- 68. Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7(1):21–33. [DOI] [PubMed] [Google Scholar]
- 69. Meistrich ML. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril. 2013;100(5):1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hedger MP. Toll-like receptors and signalling in spermatogenesis and testicular responses to inflammation—a perspective. J Reprod Immunol. 2011;88(2):130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Table_1_word_format for Late Effects of Low-Dose Radiation on the Bone Marrow, Lung, and Testis Collected From the Same Exposed BALB/cJ Mice by Witawat Jangiam, Chatchanok Udomtanakunchai, Paiboon Reungpatthanaphong, Montree Tungjai, Louise Honikel, Chris R. Gordon, and Kanokporn Noy Rithidech in Dose-Response