Abstract
Background
The number of people with Internet gaming addiction (IGA) is increasing around the world. IGA is known to be associated with personal characteristics, psychosocial factors, and physiological factors. However, few studies have examined the genetic factors related to IGA. This study aimed to investigate the association between IGA and stress-related genetic variants.
Methods
This cross-sectional study was conducted with 230 male high school students in a South Korean city. We selected five stress-related candidate genes: DAT1, DRD4, NET8, CHRNA4, and CRHR1. The DAT1 and DRD4 genes were genotyped by polymerase chain reaction, and the NET8, CHRNA4, and CRHR1 genes were genotyped by pyrosequencing analysis. We performed a Chi-square test to examine the relationship of these five candidate genes to IGA.
Results
Having the AA genotype and the A allele of the CRHR1 gene (rs28364027) was associated with higher odds of belonging to the IGA participant group (p = .016 and p = .021, respectively) than to the non-IGA group. By contrast, the DAT1, DRD4, NET8, and CHRNA4 gene polymorphisms showed no significant difference between the IGA group and control group.
Conclusions
These results indicate that polymorphism of the CRHR1 gene may play an important role in IGA susceptibility in the Korean adolescent male population. These findings provide a justification and foundation for further investigation of genetic factors related to IGA.
Keywords: Internet gaming addiction, Gene polymorphism, CRHR1
Background
As the Internet grows, it provides faster and easier ways for people to access information and all types of media. Also expanding is the potential for overuse of the Internet such as online games that may lead to serious addiction. In particular, Internet gaming addiction (IGA) is one of the most serious public health issues among adolescents worldwide. IGA is a subtype of Internet addiction (IA) [1] that has become a socio-pathological phenomenon due to its negative personal and societal consequences. Additionally, IGA shares certain similarities in neurobiological features, personality traits, and behavioral characteristics with substance and behavioral addictions [2, 3].
For these reasons, Internet gaming disorder (IGD), also referred to as IGA, was formally recognized as a potential mental health disorder and was included in section III of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) as a condition warranting further study [4]. In the DSM-V, the classification of IGD is similar to that of pathological gambling and contains nine descriptive criteria: preoccupation; withdrawal; tolerance; loss of control; giving up other activities; continuation; deception; escape; and loss of significant relationships, job, or educational or career opportunities. However, lack of standardized definitions and diagnostic criteria for IGD has hampered progress on research of this condition [1, 2]. Thus, additional research is necessary to establish clear criteria for IGD diagnosis. In this paper, we focus on IGA rather than IGD because it is somewhat narrower in scope and is commonly referred to in the research literature.
IGA is known to be associated with several aspects of individuals’ personal characteristics, psychosocial factors, and neurobiological factors. Personal and psychosocial factors include, for example, age, gender, personality traits, self-esteem, stress, and depression [5–9]. As to neurobiological factors, studies have shown that the dopamine system, which is frequently assessed for addictive disorders, is involved in learning and reinforcement of behaviors. For example, dopamine levels have been associated with pleasure, novelty-seeking behavior, and reward dependency [10]. According to Weinstein and Lejoyeux [11], the level of dopamine release in the ventral striatum during online gaming is comparable to that induced by a substance such as amphetamine. Thus, IGA may be associated with the dopaminergic system in a fashion similar to substance-related addiction. In addition, stress has been reported to be one of the most important factors in addiction [12, 13]. Hypothalamic-pituitary-adrenal (HPA) axis is an intertwining of the central nervous system and endocrine system. Stress stimulates the release of corticotropin-releasing hormone (CRH) from the hypothalamus, and the HPA axis is driven by CRH signaling via Corticotropin releasing hormone receptor type 1 (CRHR1) gene. CRH stimulates secretion of adrenocorticotropic hormone (ACTH), and ACTH stimulation produces cortisol. Cortisol triggers negative feedback in the HPA axis [14]. In a recent study, serum cortisol levels were shown to be higher in excessive Internet game users than in non-excessive users [15]. In another study, serum cortisol levels were negatively correlated with severity of pathological gambling [16]. Although these findings are not entirely consistent, addiction is commonly related to HPA axis activity in study results.
Regarding addiction and genetic factors, persistent behavioral changes induced by addiction may alter long-lasting gene expression, significantly contributing to the addiction phenotype [17]. Additionally, pharmacotherapy, a crucial strategy for the treatment of addiction, relies on genetic variation [18, 19]. According to previous studies, IGA seemingly shares some of the features of substance and behavioral addictions not only at the neurocircuitry, psychological, and behavioral levels but also at the genetic level [3, 20].
The majority of studies conducted to evaluate the association between genetic factors and IA or IGA have focused on genes of the dopamine system. A 2007 study found that Taq1A1 allele of the dopamine D2 receptor (DRD2) gene was more prevalent in an excessive online gaming group than in a control group [21]. The dopamine transporter 1 (DAT1) gene has been shown to be associated with pathological gambling [22]; however, Kim et al. [23] found that the DAT1 gene was not associated with IA. Given these inconsistent findings, further evaluation is needed to clarify the association between the DAT1 gene and IGA.
In addition to dopaminergic genes, stress-related hormone genes, including Catecholamine-O-Methyltransferase (COMT), serotonin transporter (5HTTLPR), and nicotinic acetylcholine receptor subunit alpha 4 (CHRNA4) genes have also been examined as potential associated factors with addiction. For example, the low-activity Val158Met variant of the COMT gene was more prevalent in an excessive online gaming group than in a control group [21]. Another study reported that the homozygous short allelic variant of the 5HTTLPR gene was more prevalent in an excessive Internet use group than in a control group [24]. In addition, in Montag’s study [25], an IA group demonstrated significantly more T - (CC) genotype of rs1044396 polymorphism in the CHRNA4 gene than a control group, and Montag suggested that IA might be partially explained by genetic differences. However, these results were not consistent with those of previous studies [23].
In recent years, the relevance of CRH systems to addictive disorders has received increased attention from researchers. The CRH pathways contribute to the withdrawal/negative affect and preoccupation/anticipation stages of the addiction cycle, and pharmacotherapeutic agents for the CRH systems may be effective in treating some key aspects of addiction [26]. The CRHR1 gene is involved in the anxiogenic action of CRH [27], and studies have shown that the CRHR1 gene was associated with addiction vulnerability and modulated the effects of exposure to childhood abuse on addictive disorders such as alcohol use disorder [14, 28, 29]. Although research has been conducted on changes in CRH pathways in relation to IGA [15, 16], few studies have analyzed genetic factors related to CRH systems.
In an effort to address some of the gaps in the current literature we investigated genetic variations associated with IGA. Specifically, we adopted an approach involving gene selection from stress-related genetic variants whose association with IGA has not been previously examined, including Dopamine Receptor D4 (DRD4), DAT1, Norepinephrine Transporter 8 (NET8), CHRNA4, and CRHR1 genes. These five genes are believed to exert effects on gaming behaviors through regulation of dopamine, norepinephrine, and cortisol levels. We then examined genetic variations of the selected five genes in the IGA and non-IGA groups, hypothesizing that these variations would differ between the two groups.
Methods
Participants
This study was a part of larger project that examined the role of the autonomic nervous system in development of IGA among adolescent males [15, 30, 31]. The current study focuses on identifying genomic differences between adolescent males with and without IGA. The 242 participants were 15- to 18-year-old boys recruited from nine high schools in a South Korean city using convenience and snowball sampling methods. We visited each high school to explain the study’s purpose and procedures and invited interested students to participate. We also asked interested students to visit the city’s public sports center—a hub for data collection—on a specific date for collection of study data. To maximize the sample size, we asked the students recruited to invite Internet game-using peers to come with them to the sport center, where we screened them for study eligibility. A minimum sample size of 110 for each of the two groups was estimated for the Chi-square test in order to analyze the genotype frequency between the two groups based on an effect size of 0.3, an alpha level of 0.05, and a power of 0.80 using the G-power program [32]. The study sample was limited to males belonging to only one ethnic group--Korean--because IGA is more common among male than female adolescents [9] and because there may be gender and ethnicity differences in genetic polymorphism [33, 34]. We excluded students with a diagnosed medical condition, including any kind of physical or psychiatric distress, and those taking medications that might affect HPA axis physiology and/or genetic stability (e.g., β-blockers or sedatives). Participants with missing data for any of the study variables were also excluded, and the final study sample consisted of 230 male students (118 with IGA and 112 without). The selection procedure for the study participants is depicted in Fig. 1. Study data were collected in a public sports center. Participants first completed a questionnaire in a private room at the sports center and then provided a blood sample. After completing data collection, we provided participants with health counseling and physical fitness measurements as rewards for participating in our study.
Fig. 1.
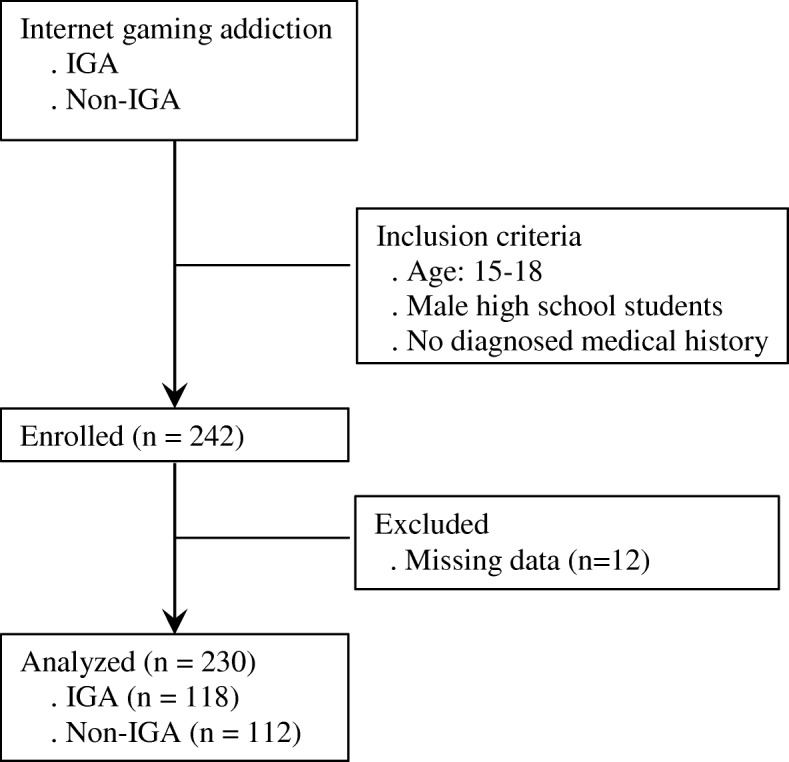
Diagram depicting the selection and flow of participants in the study. IGA, Internet gaming addiction; Non-IGA, non-Internet gaming addiction
Measures
Internet gaming addiction
Participants were screened using the online game addiction scale for adolescents, which was developed by the Korea Agency for Digital Opportunity and Promotion (KADO) [35]. The scale has established reliability and validity and has been used to screen for IGA among Korean adolescents in national surveys. The scale consists of 20 questions with responses on a 4-point Likert scale ranging from 1 (not at all) to 4 (always). Total scores range from 20 to 80, with higher scores reflecting a greater tendency toward addiction. A score of 49 or above indicates high IGA risk, and a score of 38 or above indicates overuse of Internet games and potential IGA risk [34]. Korean adolescents with high IGA risk and with potential IGA risk share most core characteristics of Internet-related addiction, such as daily life disturbance, withdrawal, tolerance, and preference for the virtual world [34, 36]. Based on KADO scale cut-off scores, our study participants were assigned to either the non-IGA group (scores < 38) or the IGA group (scores > 38). The scale’s Cronbach’s alpha in the current study was 0.93.
DNA extraction
Whole blood was collected from participants into tubes containing Ethylenediaminetetraacetic acid (EDTA), and blood samples were preserved in a deep freezer. Genomic Deoxyribonucleic acid (DNA) was extracted directly from the blood samples using standard procedures. After thawing the frozen blood at room temperature, the DNA was extracted from whole blood using a QIAamp Blood Kit (Qiagen, Hilden, Germany) in accordance with manufacturer instructions. The DNA was eluted in 50 μl (μL) of Tris- Ethylenediaminetetraacetic acid (TE) buffer (10 mM Tris-HCl, 1 mM EDTA, PH 8.0) and amplified using a C1000 thermal cycler (Bio-Rad, Hercules, USA).
Selection of polymorphism of candidate genes
We selected five genes that were likely to exert an effect on Internet gaming through regulation of dopamine, norepinephrine and cortisol: the DRD4 variable number of tandem repeats (VNTR) polymorphism, DAT1 VNTR polymorphism, NET 8 (rs5569), CHRNA4 (rs1044396) and CRHR1 (rs28364027) genes. The selected genes have previously been reported to contain polymorphic variants associated with addiction. The genotype distribution of the five Single nucleotide polymorphisms (SNP) did not deviate from Hardy–Weinberg equilibrium (HWE).
Genotyping
The VNTR polymorphisms in the DRD4 and DAT1 genes were amplified using polymerase chain reaction (PCR) primers [37, 38]. To determine the repeat numbers of the different alleles of both genes, PCR products were evaluated by means of electrophoresis on ethidium bromide-stained agarose gels.
The NET8, CHRNA4, and CRHR1 genes were first amplified using PCR primers. Primers were designed with Pyrosequencing Assay Design Software V1.0.6 (Biotage AB, Uppsala, Sweden); a full list of primer sequences and annealing temperatures for each PCR reaction is provided in Table 1. All PCR reactions were checked on a 2.0% agarose gel to ensure successful amplification and specificity before pyrosequencing. Thereafter, genotype was determined by means of pyrosequencing on a PyroMark Q24 (Biotage AB, Uppsala, Sweden). For the pyrosequencing analysis, PCR products were processed in accordance with the manufacturer’s standard protocol (Biotage). A 20 μL PCR template was pipetted into a PSQ 24 Plate Low (Biotage AB, Uppsala, Sweden) containing 2 μL streptavidin-coated Sepharose beads (Streptavidin Sepharose High Performance, GE Healthcare Bio-Science AB, Uppsala, Sweden), 40 μL 2X binding buffer [10 nmol/l Tris (hydroxymethyl)-aminomethan, 2 mol/l NaCl, 1 mmol/l EDTA and 0.1% polyoxyethylenesorbitan monolaureate (Tween 20), pH 7.6], and 18 μL high-performance liquid chromatography-purified water. This mixture was incubated for 10 min at room temperature (shaker speed 1400 rpm). The complexes were purified and separated from the non-biotinylated strands using a PyroMark Vacuum Prep Worktable (Biotage). The beads were then suspended with 20 μL of annealing buffer (20 mM Tris-acetate and 2 mM Mg-acetate at PH 7.6) containing 0.8 μL of sequencing primer. The template-sequencing primer mixture was transferred onto a PSQ 24 Plate (Biotage), heated to 85 °C for 2 min, and cooled to room temperature. Sequencing reactions were performed with a PyroMark Gold Q24 Reagent Kit (Qiagen, Germantown, MD, USA) in accordance with the manufacturer’s instructions.
Table 1.
PCR primers, PCR conditions and sequencing primers for genotyping five gene using pyrosequencing
| Primer for PCR | Sequencing primer for Pyrosequencing | Annealing temperature |
||
|---|---|---|---|---|
|
DRD4
VNTR |
Forward | 5’-GCGACTACGTGGTCTACTCG-3′ | – | 60 °C |
| Reverse | 5’-AGGACCCTCATGGCCTTG-3′ | |||
|
DAT1
VNTR |
Forward | 5’-TGTGGTGTAGGGAACGGCCTGAG-3′ | – | 65 °C |
| Reverse | 5’-CTTCCTGGAGGTCACGGCTCAAGG-3’ | |||
|
NET8
(rs5569) |
Forward | 5’-B-GTGAAGAGTTTCCGGTGTCGC-3’ | 5’-GCATGGAGGCTGTCATC-3’ | 57 °C |
| Reverse | 5’-GAGGCATGGAGGCTGTCA-3’ | |||
|
CHRNA4
(rs1044396) |
Forward | 5’-CAGCCCTCTCCGTGCAAAT-3’ | 5’-CTCTTCGGTGTCCCC-3’ | 57 °C |
| Reverse | 5’-B-GGTGCTGCGGGTCTTGAC-3’ | |||
|
CRHR1
(rs28364027) |
Forward | 5’-B-AGGAACCCTGGAGACAGAAGT-3’ | 5′-GGTTAAGTGAGGGGAA-3’ | 55 °C |
| Reverse | 5′-TGGTATGGGGTGGTTAAGTGA-3’ | |||
PCR polymerase chain reaction, B biotin
Ethical considerations
This study was approved by the Institutional Review Board of a university in South Korea. Informed and written consent was obtained from all participants as well as their legal guardians prior to data collection.
Data analysis
Statistical analysis was performed to examine the potential relationships between IGA and SNPs. Data were analyzed using IBM SPSS statistics ver. 20.0 (IBM Co., Armonk, NY, USA). During the analysis, χ2-tests were performed to check for significant differences between the IGA and non-IGA groups for HWE. Also, the χ2-tests were carried out to identify differences in genetic polymorphism, including genotype and allele frequency, between the IGA and non-IGA groups. An uncorrected p value of < .05 was adopted as the threshold for statistical significance.
Results
Participants’ demographic characteristics, with the exception of daily sleep time, did not significantly differ between the IGA and non-IGA groups. The mean age of the participants was 16.63 years, with ages ranging from 15 to 18 years, and their mean body mass index (BMI) was 21.91 kg per square meter (kg/m2), with values ranging from 15.4 to 36.7 kg/m2. About one-quarter of the participants reported that they currently smoked cigarettes and drank alcoholic beverages; the percentage was similar in the IGA and non-IGA groups. Perceived academic performance was also similar in the two groups. However, daily sleep time was significantly shorter in the IGA group, with about one-third of the participants indicating that they slept less than 6 h per day. Regarding Internet gaming-related characteristics, participants’ daily Internet gaming time averaged 171.96 min (227.29 min in the IGA group and 113.66 min in the non-IGA group), their average duration of Internet gaming was 7.21 years (7.64 years in the IGA group and 6.82 years in the non-IGA group), and their average IGA score was 36.50 (46.05 in the IGA group and 26.43 in the non-IGA group). All these Internet gaming-related characteristics significantly differed between the IGA and non-IGA groups. Additional details of the participants’ demographic and Internet gaming-related characteristics are provided in our previous study [31].
As shown in Table 2, the 48 base pair (bp) VNTR polymorphism in the DRD4 gene was classified as long alleles (repeats ≥5) and short alleles (repeats < 5) based on a previous study [23]. The homozygous short allelic variant of the DRD4 gene VNTR polymorphism was the most common in the IGA and non-IGA groups. The DAT1 gene VNTR polymorphism showed 7- to 11-repeat alleles of 40 bp, and the 10-repeat allele was approximately 94% in both groups. There was no significant difference in the DRD4 and DAT1 VNTR polymorphisms between the IGA and non-IGA groups.
Table 2.
DRD4 and DAT1 VNTR genotypes and allele frequencies between IGA and non-IGA groups
| Gene | Genotype | IGA (n = 116) |
Non-IGA (n = 114) |
χ21 | p1 | Allele type | IGA (n = 116) |
Non-IGA (n = 114) |
χ22 | p2 |
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |||||||
| DRD4 | l/l | 0 (0.0) | 0 (0.0) | 0.32 | .571 | l | 2 (0.9) | 1 (0.4) | 0.31 | .573 |
| l/s | 2 (1.7) | 1 (0.9) | s | 230 (99.1) | 227 (99.6) | |||||
| s/s | 114 (98.3) | 113 (99.1) | ||||||||
| DAT1 | 7/10 | 4 (3.4) | 5 (4.4) | 2.11 | .806 | 7-repeats | 4 (7.1) | 5 (2.2) | 1.05 | .787 |
| 9/10 | 7 (6.0) | 4 (3.5) | 9-repeats | 7 (3.0) | 4 (1.8) | |||||
| 10/10 | 102 (87.9) | 102 (89.4) | 10-repeats | 218 (94.0) | 215 (94.2) | |||||
| 10/11 | 3 (2.7) | 2 (1.8) | 11-repeats | 3 (1.3) | 4 (1.8) | |||||
| 11/11 | 0 (0.0) | 1 (0.9) |
VNTR variable number of tandem repeats, IGA Internet game addiction, non-IGA non-Internet game addiction; χ21, χ2 in comparison of genotype frequency between IGA and non-IGA; p1, p-value in comparison of genotype frequency between IGA and non-IGA; χ22, χ2 in comparison of allele frequency between IGA and non-IGA; p2, p-value in comparison of allele frequency between IGA and non-IGA; l, 48-base pair repeats ≥5; s, 48-base pair repeats <
As Table 3 shows, the genotype and allele frequencies of the CRHR1 (rs28364027) gene differed significantly between the IGA and non-IGA groups (χ2=5.76, p = .016 and χ2=5.36, p = .021, respectively). The presence of the AA genotype increased the risk of IGA (odds ratio: 2.68, 95% CI: 1.172–6.149), whereas the presence of G-allele carriers did not. No significant differences in the NET8 (rs5569) or CHRNA4 (rs1044396) genes were observed between the IGA and non-IGA groups.
Table 3.
NET8 (rs5569), CHRNA4 (rs1044396) and CRHR1 (rs28364027) genotypes and allele frequencies between IGA and non-IGA groups
| Gene (SNP) | MAF | Genotype | IGA (n = 116) | Non-IGA (n = 114) | χ21 | p1 | Allele type | IGA (n = 116) | Non-IGA (n = 114) | χ22 | p2 | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |||||||||
| NET8 (rs5569) | 0.25 | AA | 65 (58.6) | 70 (50.2) | 0.89 | .640 | A | 177 (76.0) | 167 (73.2) | 0.50 | .450 | 0.78 (0.47–1.32) |
| AG | 41 (35.3) | 47 (41.3) | G | 56 (24.0) | 61 (26.8) | |||||||
| GG | 7 (6.1) | 7 (6.1) | ||||||||||
| CHRNA4 (rs1044396) | 0.21 | CC | 68 (58.6) | 71 (62.3) | 0.59 | .730 | C | 180 (77.6) | 180 (78.9) | 0.10 | .730 | 1.17 (0.63–1.95) |
| CT | 44 (37.9) | 38 (33.3) | T | 52 (22.4) | 48 (21.1) | |||||||
| TT | 4 (3.5) | 5 (4.4) | ||||||||||
| CRHR1 (rs28364027) | 0.07 | AA | 107 (92.2) | 93 (81.6) | 5.76 | .016 | A | 223 (97.8) | 207 (94.7) | 5.36 | .021 | 2.63 (1.17–6.15) |
| AG | 9 (7.8) | 21 (18.4) | G | 9 (2.2) | 21 (5.3) | |||||||
| GG | 0 (0.0) | 0 (0.0) |
IGA Internet gaming addiction, non-IGA non-Internet gaming addiction, SNP single nucleotide polymorphism, MAF minor allele frequency; χ21, χ2 in comparison of genotype frequency between IGA and non-IGA; p1, p-value in comparison of genotype frequency between IGA and non-IGA; χ22, χ2 in comparison of allele frequency between IGA and non-IGA; p2, p-value in comparison of allele frequency between IGA and non-IGA; OR, odds ratio; CI, confidence interval
Discussion
In our study, we investigated the influence of gene polymorphism on IGA risk for five stress-related candidate genes: the DRD4 VNTR, DAT1 VNTR, NET8 (rs5569), CHRNA4 (rs1044396), and CRHR1 (rs28364027). We selected a gender- and age-controlled sample to minimize the impact of confounding variables on our genetic findings; in addition, our study was conducted with ethnically homogeneous participants. Notably, we found significant differences in the genotypes and allele frequency of rs28364027 between the IGA and non-IGA groups, but not in those of the other candidate genes.
Specifically, we found no association between 48 bp VNTR polymorphism in the DRD4 gene and IGA. Our findings are seemingly quite different from study results for Western populations. In Western studies, the 48 bp VNTR polymorphism in the DRD4 gene has been associated with a number of addictive disorders, including substance abuse [39–41], alcohol dependence [42, 43], and smoking [44]. In particular, the 7-repeat allele of the DRD4 48 bp VNTR polymorphism has been reported as posing a risk for addictive disorders in Western populations [39, 40, 42, 44]. However, in Asians, including the Korean participants in our study, the DRD4 gene has not been associated with addictive disorders [45–47]. The rarity of the 7-repeat allele in Asian populations, including the Japanese, Chinese, and Taiwanese, may have contributed to the inconsistency between our findings and Western study results [34, 48]. On the whole, the genotype distribution of the DRD4 gene has shown considerable variation by ethnicity. Further research is needed to clarify whether the genotype and allele frequency of the DRD4 gene polymorphism influences IGA in particular races and ethnicities.
In addition, we found no differences in the genotypes and allele frequencies of the DAT1, NET8, and CHRNA4 gene polymorphisms between the IGA and non-IGA groups. The VNTR polymorphism in the DAT1 gene has been shown to alter DAT1 gene expression [49], and prior studies have demonstrated that the presence of the 9-repeat allele increased susceptibility to addiction more than alleles having more than 9-repeats [50, 51]. In Western populations, the DAT1 gene was associated with alcohol dependency [51], substance abuse [52], and pathological gambling [22]. In contrast, we found no association between the 40 bp VNTR polymorphism in the DAT1 gene and IGA. On the other hand, our results are consistent with the findings of previous studies of Korean populations with addictive disorders--like, alcohol and nicotine use disorders [53, 54].
The NET8 gene polymorphism was shown to be correlated with reward dependency in Cloninger’s temperament dimensions [55]. Based on this and findings from other previous studies, we hypothesized that the NET8 gene polymorphism was associated with IGA, but we found no differences in the genotype and allele frequency of the NET8 gene polymorphism between the IGA and non-IGA groups. Nonetheless, our non-significant findings in this regard provide information about stress-related gene traits that has not previously been reported in IGA studies.
Regarding the CHRNA4 gene, we found no significant differences in polymorphism between the IGA and non-IGA groups. The CHRNA4 gene has been reported to impact dopaminergic neurotransmission in animal models [56]; additionally, this gene has been found to be a factor in addictive disorder in several animal and human studies. For example, the CC genotype of rs1044396 in the CHRNA4 gene was found at a much higher frequency in individuals exhibiting addictive behavior and showed relatively consistent results across ethnicities [25, 57–59]. Similarly, our study indicated that the CC genotype of rs1044396 was present at a higher frequency in the IGA group than in the non-IGA group, but there was not a significant difference. The IGA group’s having a more frequent CC genotype of rs1044396 than the non-IGA group but not significantly so can be explained in several ways. First, prolonged and excessive Internet gaming might activate the HPA axis [60, 61], resulting in CC genotype of rs1044396; however, this pathway did not seem to cause a significant change in our Korean population. Second, our participants’ demographic characteristics might have contributed to the statistical non-significance. Our participants were restricted to male adolescents in high school, whereas Montag et al. [25] employed male and female university students with and without IA in identifying the candidate gene of IA—the CHRNA4 gene. In Montag’s study, the CC genotype of rs1044396 in the CHRNA4 gene occurred significantly more frequently in the IA group—specifically, this effect was driven by female gender [25]. Third, our study had a relatively small sample, which may have increased the possibility of false-negative results. Because ours is the first study to reveal an association between variants in the CHRNA4 gene and IGA, we cannot be certain that statistically significant differences in polymorphism would have been found in a larger sample. Therefore, replication studies with larger samples are needed to determine whether CHRNA4 gene polymorphism contributes to IGA.
Interestingly, we found that the AA genotype of rs28364027 in the CRHR1 gene was associated with IGA, as it was much more frequent in the IGA group than in the non-IGA group. The CRHR1 gene polymorphism has been primarily associated with stress-related phenotypes, including altered cortisol response [62, 63] and alcohol dependency [64–66]. Kim and colleagues [67] reported that an allele of rs28364027 was associated with alcohol dependency in the Korean population.
Furthermore, we observed that the AA genotype of rs28364027 showed a 2.69 times greater increase in the odds for IGA (95% CI [1.17–6.15]) compared to the G-carrier genotype. According to the literature, the CRHR1 gene is well known to be related to alcohol dependency, but in our study, alcohol consumption and even smoking rate did not differ between the IGA and non-IGA groups. Among the other covariates, daily sleep time was significantly shorter with IGA than without IGA. However, previous research has provided little evidence that the CRHR1 gene is related to sleep behavior; rather, circadian clock and serotonin transporter genes have mainly been related to sleep phenotypes [68–70]. Thus, our findings suggest that the CRHR1 gene is a promising candidate for IGA risk research.
Regarding the genetic differences between individuals with and without a certain addiction, researchers have emphasized the framework of gene-environment (GxE) interaction to explain those variations. For example, an interaction between genetic polymorphism and stressful life events was tentatively identified as a predictor or moderator and triggered alcohol use disorders [14, 28, 29], nicotine dependence [71], and cannabis use [72]. Although a variety of stressors are known to be important risk factors for addiction [12, 13], some researchers have argued that addiction symptoms or addictive behaviors such as cravings [73, 74], immersion [75, 76], withdrawal [77], pathologic gambling [16], and game playing [60, 61, 78] could also produce biological stress responses by activating the HPA axis or sympathetic nervous system [15, 16, 73, 79, 80]. Specifically, studies of Internet/online gaming have reported that the activity of the HPA axis and sympathetic nervous system were increased during and after gaming [15, 60, 61, 78, 79] and even in the resting state in prolonged game users [30, 31].
Based on the results of this and previous studies, we offer two possible explanations for the genetic association of the CRHR1 gene with IGA in Korean male adolescents. First, the IGA group in the current study had been involved in Internet gaming for 7.21 years on average. Thus, it is possible that Internet gaming itself stimulated the addiction tendency through long-term exposure to gaming and to stress responses induced by gaming. Second, given our findings and those of previous studies, it seems reasonable that an interaction exists between the AA genotype of rs28364027 as a risk factor for IGA and excessive gaming immersion, which constitutes a stress event that reinforces the addictive behavior.
Gene polymorphism is an important concept that explains not only differences in appearance, but also susceptibility to diseases, expression patterns of diseases, and responses to medications [81]. However, environmental factors also play an important role in individual traits, although genetic predispositions affect much of a given trait [82]. In particular, investigation of the relationship between the CRHR1 gene polymorphism and GxE interaction in IGA is worthy of future study. Such research could inform the development more effective intervention programs by addressing environmental factors based on a greater understanding of genetic predisposition.
We conducted this investigation because no previous studies have examined the potential associations between stress-related genes and IGA, although some studies have reported relationships between these genes and other addictive disorders. Our results should be interpreted in light of the strengths and limitations of our study. The strengths include well-ascertained stress-related phenotypes and a relatively homogeneous sample in terms of age, gender, and Korean-only ethnicity. Moreover, our findings provide a justification and foundation for further investigation of genetic factors related to IGA.
Despite the strengths of our study, some limitations should be acknowledged. First, even though our sample was homogeneous on the whole, our sample size was relatively small, which may have limited the generalizability of our findings. Second, the study design was cross-sectional, and we did not directly examine the function of rs28364027 in the CRHR1 gene or whether CRHR1 gene polymorphism and stress events have a GxE interaction in IGA. Therefore, we could not identify a causal association between IGA and the CRHR1 gene polymorphism. Finally, we did not correct for multiple comparisons, and therefore the associations between variants in the CRHR1 gene and IGA reported here may be limited to nominal significant associations. Further studies are needed to investigate the potential interaction between the CRHR1 gene polymorphism and IGA in a larger sample and to identify the functionality of rs28364027. In addition, studies of varying racial and ethnic populations are warranted to better understand potential race/ethnicity-related genetic associations with IGA.
Conclusions
In summary, ours is the first study to reveal a significant association between variants in the CRHR1 gene and IGA. The AA genotype of rs28364027 in the CRHR1 gene was much more frequent in the adolescents with IGA than in those without IGA. Therefore, polymorphism in the CRHR1 gene may play an important role in IGA susceptibility in the Korean adolescent male population. These findings provide a basis for further investigation of genetic factors related to IGA. In addition, early assessment and intervention for adolescents engaging in excessive Internet gaming are needed to prevent adverse genetic consequences, including epigenetic changes resulting in cardio-metabolic health outcomes (e.g., cardiovascular disease, Type 2 diabetes, and hypertension) and psychiatric distress in adulthood [83, 84].
Acknowledgments
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and was funded by the Ministry of Education, Science and Technology (NRF-2012R1A1A4A01012884). NRF had no role in the study design; data collection, analysis, or interpretation; writing the manuscript; or the decision to submit the paper for publication.
Availability of data and materials
The datasets used and/or analyzed during the current study are available only upon reasonable request and after compliance with the policies and procedures of the Basic Science Research Program through the National Research Foundation of Korea and the Ministry of Education, Science and Technology (NRF) for data sharing.
Abbreviations
- 5HTTLPR
Serotonin transporter gene
- ACTH
Adrenocorticotropic hormone
- BMI
Body mass index
- bp
base pair
- CHRNA4
Nicotinic acetylcholine receptor subunit alpha 4
- CI
Confidence interval
- COMT
Catecholamine-o-methyltransferase
- CRH
Corticotropin releasing hormone
- CRHR1
Corticotropin releasing hormone receptor type 1
- DAT1
Dopamine transporter 1
- DNA
Deoxyribonucleic acid
- DRD2
Dopamine D2 receptor
- DRD4
Dopamine receptor D4
- DSM-V
The fifth edition of the diagnostic and statistical manual of mental disorders
- EDTA
Ethylenediaminetetraacetic acid
- GxE interaction
Gene environment interaction
- HPA
Hypothalamic pituitary adrenal
- HWE
Hardy Weinberg equilibrium
- IA
Internet addiction
- IGA
Internet gaming addiction
- IGD
Internet gaming disorder
- KADO
Korean agency for digital opportunity and promotion
- NET8
Norepiniphrine8
- PCR
Polymerase chain reaction
- SNP
Single nucleotide polymorphisms
- TE
Tris ethylenediaminetetraacetic acid
- VNTR
Variable number of tandem repeats
Authors’ contributions
Conceived and designed the present study: JP, DK, NK. Acquisition of data: IDK, NK. Analyzed and interpreted of the data: JP, JS, DK, NK. Drafted the manuscript: JP, TLH, NK. Provided significant input on the content of the manuscript: IDK, TLH. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Yonsei University Wonju College of Medicine. We obtained written informed consent from each participant and his legal guardian. Participation was voluntary, and Participants could withdraw at any time from the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jooyeon Park, Email: jypark1185@gmail.com.
Jin-Young Sung, Email: jysunny486@hanmail.net.
Dae-Kwang Kim, Email: dkkimmd@gw.kmu.ac.kr.
In Deok Kong, Email: kong@yonsei.ac.kr.
Tonda L. Hughes, Email: thughes@uic.edu
Nahyun Kim, Phone: +82-53-580-3928, Email: drkim@kmu.ac.kr.
References
- 1.Weinstein A, Lejoyeux M. New developments on the neurobiological and pharmaco-genetic mechanisms underlying internet and videogame addiction. Am J Addict. 2015;24:117–125. doi: 10.1111/ajad.12110. [DOI] [PubMed] [Google Scholar]
- 2.King DL, Haagsma MC, Delfabbro PH, Gradisar M, Griffiths MD. Towards a consensus definition of pathological video-gaming: a systematic review of psychometric assessment tools. Clin Psychol Rev. 2013;33:331–342. doi: 10.1016/j.cpr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Kuss DJ. Internet gaming addiction: current perspectives. Psychol Res Behav Manag. 2013;14:125–137. doi: 10.2147/PRBM.S39476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5. Washington: American Psychiatric Association; 2013. [Google Scholar]
- 5.Griffiths MD, Davies MNO, Chappell D. Demographic factors and playing variables in online computer gaming. Cyberpsychol Behav. 2004;7:479–487. doi: 10.1089/cpb.2004.7.479. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths MD, Kuss DJ, King DL. Video game addiction: past, present, future. Curr Psychiatr Rev. 2012;8:308–318. doi: 10.2174/157340012803520414. [DOI] [Google Scholar]
- 7.Jiménez-Murcia S, Fernández-Aranda F, Granero R, Chóliz M, La Verde M, Aguglia E, et al. Video game addiction in gambling disorder: clinical, psychopathological, and personality correlates. Biomed Res Int. 2014;2014:315062. [DOI] [PMC free article] [PubMed]
- 8.Kim EJ, Kee N, Ku T, Kim SJ. The relationship between online game addiction and aggression, self-control and narcissistic personality traits. Eur Psychiatry. 2008;23:212–218. doi: 10.1016/j.eurpsy.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Rehbein F, Kleimann M, Mossle T. Prevalence and risk factors of video game dependency in adolescence: results of a German nationwide survey. Cyberpsychol Behav Soc Netw. 2010;13:269–277. doi: 10.1089/cyber.2009.0227. [DOI] [PubMed] [Google Scholar]
- 10.Spielewoy C, Gonon F, Roubert C, Fauchey V, Jaber M, Caron MG, et al. Increased rewarding properties of morphine in dopamine-transporter knockout mice. Eur J Neurosci. 2000;12:1827–1837. doi: 10.1046/j.1460-9568.2000.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinstein A, Lejoyeux M. Computer and video game addiction-a comparison between game users and nongame. Am J Drug Alcohol Abuse. 2010;36:277–283. doi: 10.3109/00952990.2010.491880. [DOI] [PubMed] [Google Scholar]
- 12.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan CS, Chiou WB. Why are adolescents addicted to online gaming? An interview study in Taiwan. Cyberpsychol Behav. 2006;9:762–766. doi: 10.1089/cpb.2006.9.762. [DOI] [PubMed] [Google Scholar]
- 14.Ray LA, Sehl M, Bujarski S, Hutchison K, Blaine S, Enoch MA. The CRHR1 gene, trauma exposure, and alcoholism risk: a test of G × E effects. Genes Brain Behav. 2013;12:361–369. doi: 10.1111/gbb.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim EH, Kim NH. Comparison of stress level and HPA axis activity of internet game addiction vs non-addiction in adolescents. J Korean Biol Nurs Sci. 2013;14:33–40. [Google Scholar]
- 16.Geisel O, Panneck P, Hellweg R, Wiedemann K, Muller CA. Hypothalamic-pituitary-adrenal axis activity in patients with pathological gambling and internet use disorder. Psychiatry Res. 2014;226:97–102. doi: 10.1016/j.psychres.2014.11.078. [DOI] [PubMed] [Google Scholar]
- 17.Kalda A, Zharkovsky A. Epigenetic mechanisms of psychostimulant-induced addiction. Int Rev Neurobiol. 2015;120:85–105. doi: 10.1016/bs.irn.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen DA, Nielsen EM, Dasari T, Spellicy CJ. Pharmacogenetics of addiction therapy. Methods Mol Biol. 2014;1175:589–624. doi: 10.1007/978-1-4939-0956-8_15. [DOI] [PubMed] [Google Scholar]
- 19.Patriquin MA, Bauer IE, Soares JC, Graham DP, Nielsen DA. Addiction pharmacogenetics: a systematic review of the genetic variation of the dopaminergic system. Psychiatr Genet. 2015;25:181–193. doi: 10.1097/YPG.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuss DJ, Griffths MD. Internet and gaming addiction: a systematic literature review of neuroimaging studies. Brain Sci. 2012;2:347–374. doi: 10.3390/brainsci2030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han DH, Lee YS, Yang KC, Ki EY, Lyoo IK, Renshaw PF. Dopamine genes and reward dependence in adolescents with excessive internet video game play. J Addict Med. 2007;1:133–138. doi: 10.1097/ADM.0b013e31811f465f. [DOI] [PubMed] [Google Scholar]
- 22.Faqundo AB, Femandez-Aranda F, de la Torre R, Verdejo-Garcia A, Granero R, Penelo E, et al. Dopamine DRD2/ANKK1 Taq1A and DAT1 VNTR polymorphisms are associated with a cognitive flexibility profile in pathological gamblers. J Psychopharmacol. 2014;28:1170–1177. doi: 10.1177/0269881114551079. [DOI] [PubMed] [Google Scholar]
- 23.Kim EY, Lee YS, Han DH, Suh DS, Kee BS. Temperament and genetic polymorphism in Korean male adolescents with internet addiction tendency. J Korean Neuropsychiatr Assoc. 2006;45:468–475. [Google Scholar]
- 24.Lee YS, Han D, Yang KC, Daniels MA, Na C, Kee BS, et al. Depression like characteristics of 5HTTLPR polymorphism and temperament in excessive internet users. J Affect Disord. 2008;109:165–169. doi: 10.1016/j.jad.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Montag C, Kirsch P, Sauer C, Markett S, Reuter M. The role of the CHRNA4 gene in internet addiction: a case-control study. J Addict Med. 2012;6:191–195. doi: 10.1097/ADM.0b013e31825ba7e7. [DOI] [PubMed] [Google Scholar]
- 26.Koob GF, Zorrilla EP. Neurobiological mechanisms of addiction: focus on corticotropin-releasing factor. Curr Opin Investig Drugs. 2010;11:63–71. [PMC free article] [PubMed] [Google Scholar]
- 27.Muller MB, Wurst W. Getting closer to affective disorders: the role of CRH receptor systems. Trends Mol Med. 2004;10:409–415. doi: 10.1016/j.molmed.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, Laucht M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63:146–151. doi: 10.1016/j.biopsych.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Chen AC, Manz N, Tang Y, Rangaswamy Y, Almasy L, Kuperman S, et al. Single nucleotide polymorphisms in corticotropin releasing hormone receptor 1 gene (CRHR1) are associated with quantitative trait of event-related potential and alcohol dependence. Alcohol Clin Exp Res. 2010;34:988–996. doi: 10.1111/j.1530-0277.2010.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim NH, Hughes TL, Park CG, Quinn L, Kong ID. Altered autonomic functions and distressed personality traits in male adolescents with internet gaming addiction. Cyberpsychol Behav Soc Netw. 2016;19:667–673. doi: 10.1089/cyber.2016.0282. [DOI] [PubMed] [Google Scholar]
- 31.Kim NH, Hughes TL, Park CG, Quinn L, Kong ID. Resting-state peripheral catecholamine and anxiety level in Korean male adolescents with internet game addiction. Cyberpsychol Behav Soc Netw. 2016;19:202–208. doi: 10.1089/cyber.2015.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faul F, Erdfelder E, Lang AG, Buchner A. G* power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Meth. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 33.Kimura M, Miyakawa T, Matsushita S, So M, Higuchi S. Gender differences in the effects of ADH1B and ALDH2 polymorphisms on alcoholism. Alcohol Clin Exp Res. 2011;35:1923–1927. doi: 10.1111/j.1530-0277.2011.01543.x. [DOI] [PubMed] [Google Scholar]
- 34.Chang FM, Kidd JR, Livak KJ, Pakstis AJ, Kidd KK. The world-wide distribution of allele frequencies at the human dopamine D4 receptor locus. Hum Genet. 1996;98:91–101. doi: 10.1007/s004390050166. [DOI] [PubMed] [Google Scholar]
- 35.Korea Agency for Digital Opportunity and Promotion (KADO). A study of the development of internet game addiction scale for children and adolescents. 2006. https://www.iapc.or.kr/dia/survey/addDiaSurveyNew.do?dia_type_cd=GAYS. Accessed 10 June 2013.
- 36.Cho H, Kwon M, Choi JH, Lee SK, Choi JS, Choi SW, et al. Development of the internet addiction scale based on the internet gaming disorder criteria suggested in DSM-5. Addict Behav. 2014;39:1361–1366. doi: 10.1016/j.addbeh.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 37.Adamson MD, Kennedy J, Petronis A, Dean M, Virkkunen M, Linnoila M, et al. DRD4 dopamine receptor genotype and CSF monoamine metabolites in Finnish alcoholics and controls. Am J Med Genet. 1995;60:199–205. doi: 10.1002/ajmg.1320600306. [DOI] [PubMed] [Google Scholar]
- 38.Kazantseva AV, Gaysina DA, Malykh SB, Khusnutdinova EK. Role of dopamine transporter gene (DAT1) polymorphisms in personality traits variation. Genetika. 2009;45:1110–1117. [PubMed] [Google Scholar]
- 39.Chen CK, Hu X, Lin SK, Sham PC, Loh e-W, Li T, et al. Association analysis of dopamine D2-like receptor genes and methamphetamine abuse. Psychiatr Genet. 2004;14:223–226. doi: 10.1097/00041444-200412000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Skowronek MH, Laucht M, Hohm E, Becker K, Schmidt MH. Interaction between the dopamine D4 receptor and the serotonin transporter promoter polymorphisms in alcohol and tobacco use among 15-year-olds. Neurogenetics. 2006;7:239–246. doi: 10.1007/s10048-006-0050-4. [DOI] [PubMed] [Google Scholar]
- 41.Marllard TT, Doorley J, Esposito-Smythers CL, McGeary JE. Dopamine D4 receptor VNTR polymorphism associated with greater risk for substance abuse among adolescents with disruptive behavior disorders: preliminary results. Am J Addict. 2016;25:55–61. doi: 10.1111/ajad.12320. [DOI] [PubMed] [Google Scholar]
- 42.Laucht M, Becker K, Blomeyer D, Schmidt MH. Novelty seeking involved in mediating the association between the dopamine D4 receptor gene exon III polymorphism and 42 heavy drinking in male adolescents: results from a high-risk community sample. Biol Psychiatry. 2007;61:87–92. doi: 10.1016/j.biopsych.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 43.Filbey FM, Ray L, Smolen A, Claus ED, Audette A, Hutchison KE. Differential neural response to alcohol priming and alcohol taste cues is associated with DRD4 VNTR and OPRM1 genotypes. Alcohol Clin Exp Res. 2008;32:1113–1123. doi: 10.1111/j.1530-0277.2008.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laucht M, Becker K, Frank J, Schmidt MH, Esser G, Treutlein J, et al. Genetic variation in dopamine pathways differentially associated with smoking progression in adolescence. J Am Acad Child Adolesc Psychiatry. 2008;47:673–681. doi: 10.1097/CHI.0b013e31816bff77. [DOI] [PubMed] [Google Scholar]
- 45.Chang FM, Ko HC, Lu RB, Pakstis AJ, Kidd KK. The dopamine D4 receptor gene (DRD4) is not associated with alcoholism in three Taiwanese populations: six polymorphisms tested separately and as haplotypes. Biol Psychiatry. 1997;41:394–405. doi: 10.1016/S0006-3223(96)00248-X. [DOI] [PubMed] [Google Scholar]
- 46.Li T, Zhu ZH, Liu X, Hu X, Zhao J, Sham PC, et al. Association analysis of polymorphisms in the DRD4 gene and heroin abuse in Chinese subjects. Am J Med Genet. 2000;96:616–621. doi: 10.1002/1096-8628(20001009)96:5<616::AID-AJMG6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 47.Tsai SJ, Cheng CY, Shu LR, Yang CY, Pan CW, Liou YJ, et al. No association for D2 and D4 dopamine receptor polymorphisms and methamphetamine abuse in Chinese males. Psychiatr Genet. 2002;12:29–33. doi: 10.1097/00041444-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Lichter JB, Barr CL, Kennedy JL, Van Tol HH, Kidd KK, Livak KJ. A hypervariable segment in the human dopamine receptor D4 (DRD4) gene. Hum Mol Genet. 1993;2:767–773. doi: 10.1093/hmg/2.6.767. [DOI] [PubMed] [Google Scholar]
- 49.Miller G, Madras B. Polymorphisms in the 3′-untranslated region of human and monkey dopamine transporter genes affect reporter gene expression. Mol Psychiatry. 2002;7:44–55. doi: 10.1038/sj.mp.4000921. [DOI] [PubMed] [Google Scholar]
- 50.Gerra G, Garofano L, Pellegrini C, Bosari S, Zaimovic A, Moi G, et al. Allelic association of a dopamine transporter gene polymorphism with antisocial behaviour in heroin-dependent patients. Addict Biol. 2005;10:275–281. doi: 10.1080/13556210500223769. [DOI] [PubMed] [Google Scholar]
- 51.Van der Zwaluw CS, Engels RC, Buitelaar J, Verkes RJ, Franke B, Scholte RH. Polymorphisms in the dopamine transporter gene (SLC6A3/DAT1) and alcohol dependence in humans: a systematic review. Pharmacogenomics. 2009;10:853–866. doi: 10.2217/pgs.09.24. [DOI] [PubMed] [Google Scholar]
- 52.Galeeva AR, Gareeva AE. Iur'ev EB, Khusnutdinova EK. VNTR polymorphisms of the serotonin transporter and dopamine transporter genes in male opiate addicts. Mol Biol (Mosk) 2002;36:593–598. doi: 10.1023/A:1019883806620. [DOI] [PubMed] [Google Scholar]
- 53.Choi TY, Kim HN, Han DH, Min KJ, Lee YS, Na C. Relationship between alcohol withdrawal symptoms and dopaminergic gene polymorphisms (DRD2, DAT, COMT) in alcohol dependence patients. Korean J Biol Psychiatry. 2006;13:178–190. [Google Scholar]
- 54.Lee YS, Kim SY, Han DH, Min KJ, Na C. Early treatment response of bupropion SR in smoking cessation according to genetic polymorphism and temperamental characteristics. Korean J Psychopharmacol. 2006;17:219–228. [Google Scholar]
- 55.Garvey MJ, RJr N, Cook B, Blum N. Preliminary confirmation of the proposed link between reward-dependence traits and norepinephrine. Psychiatry Res. 1996;65:61–64. doi: 10.1016/0165-1781(96)02954-X. [DOI] [PubMed] [Google Scholar]
- 56.Parish CL, Nunan J, Finkelstein DI, McNamara FN, Wong JY, Waddington JL, et al. Mice lacking the alpha4 nicotinic receptor subunit fail to modulate dopaminergic neuronal arbors and possess impaired dopamine transporter function. Mol Pharmacol. 2005;68:1376–1386. doi: 10.1124/mol.104.004820. [DOI] [PubMed] [Google Scholar]
- 57.Feng Y, Niu T, Xing H, Xu X, Chen C, Peng S, et al. A common haplotype of the nicotine acetylcholine receptor alpha 4 subunit gene is associated with vulnerability to nicotine addiction in men. Am J Hum Genet. 2004;75:112–121. doi: 10.1086/422194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lazary J, Dome P, Csala I, Kovacs G, Faludi G, Kaunisto M, et al. Massive withdrawal symptoms and affective vulnerability are associated with variants of the CHRNA4 gene in a subgroup of smokers. PLoS One. 2014;9:e87141. doi: 10.1371/journal.pone.0087141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chu CJ, Yang YC, Wei JX, Zhang L. Association of nicotinic acetylcholine receptor subunit alpha-4 polymorphisms with smoking behaviors in Chinese male smokers. Chin Med J. 2011;124:1634–1638. [PubMed] [Google Scholar]
- 60.Barlett CP, Rodeheffer C. Effects of realism on extended violent and nonviolent video game play on aggressive thoughts, feelings, and physiological arousal. Aggress Behav. 2009;35:213–224. doi: 10.1002/ab.20279. [DOI] [PubMed] [Google Scholar]
- 61.Coyne SM, Dyer WJ, Densley R, Money NM, Day RD, Harper JM. Physiological indicators of pathologic video game use in adolescence. J Adolesc Health. 2015;56:307–313. doi: 10.1016/j.jadohealth.2014.10.271. [DOI] [PubMed] [Google Scholar]
- 62.Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, et al. Effect of childhood trauma on adult depression and neuroendocrine function: sex-specific moderation by CRH receptor 1 gene. Front Behav Neurosci. 2009;6:3–41. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Labermaier C, Kohl C, Hartmann J, Devigny C, Altmann A, Weber P, et al. A polymorphism in the Crhr1 gene determines stress vulnerability in male mice. Endocrinology. 2014;155:2500–2510. doi: 10.1210/en.2013-1986. [DOI] [PubMed] [Google Scholar]
- 64.Glaser YG, Zubieta JK, Hsu DT, Villafuerte S, Mickey BJ, Trucco EM, et al. Indirect effect of corticotropin-releasing hormone receptor 1 gene variation on negative emotionality and alcohol use via right ventrolateral prefrontal cortex. J Neurosci. 2015;34:4099–4107. doi: 10.1523/JNEUROSCI.3672-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Molnader A, Vengeliene V, Heilig M, Wurst W, Deussing JM, Spanagel R. Brain-specific inactivation of the CRHR1 gene inhibits post-dependent and stress-induced alcohol intake, but does not affect relapse like drinking. Neuropsychopharmacology. 2012;37:1047–1056. doi: 10.1038/npp.2011.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, et al. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- 67.Kim CM, Kim SG, Kim JH, Kim HK, Kim MK, Ryu JH, et al. Association study of corticotropin-releasing hormone receptor (CRHR1) gene polymorphism in Korean alcohol dependent patients. J Korean Soc Biol Ther Psychiatry. 2011;17:239–249. [Google Scholar]
- 68.Carskadon MA, Sharkey KM, Knopik VS, McGeary JE. Short sleep as an environmental exposure: a preliminary study associating 5-HTTLPR genotype to self-reported sleep duration and depressed mood in first-year university students. Sleep. 2012;35:791–796. doi: 10.5665/sleep.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hida A, Kitamura S, Katayose Y, Kato M, Ono H, Kadotani H, et al. Screening of clock gene polymorphisms demonstrates association of a PER3 polymorphism with morningness-eveningness preference and circadian rhythm sleep disorder. Sci Rep. 2014;9:6309. doi: 10.1038/srep06309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goel N. Genetic markers of sleep and sleepiness. Sleep Med Clin. 2017;12:289–299. doi: 10.1016/j.jsmc.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 71.Csala I, Egervari L, Dome P, Faludi G, Dome B, Lazary J. The possible role of maternal bonding style and CHRNB2 gene polymorphisms in nicotine dependence and related depressive phenotype. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015;3:84–90. doi: 10.1016/j.pnpbp.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 72.Vinkers CH, Van Gastel WA, Schubart CD, Van Eijk KR, Luykx JJ, Van Winkel R. The effect of childhood maltreatment and cannabis use on adult psychotic symptoms is modified by the COMT Val158Met polymorphism. Schizophr Res. 2013;150:303–311. doi: 10.1016/j.schres.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 73.Berger SP, Hall S, Mickalian JD, Reid MS, Crawfod CA. Haloperidol antagonism of cue elicited cocaine craving. Lancet. 1996;347:504–508. doi: 10.1016/S0140-6736(96)91139-3. [DOI] [PubMed] [Google Scholar]
- 74.Sinha R, Fuse T, Aubin L, O'Malley S. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152:140. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- 75.Meyer G, Heise DJ, Hauffa BP, Schedlowski M, Pawlak C, Stadler MA, et al. Casino gambling increases heart rate and salivary cortisol in regular gamblers. Biol Psychiatry. 2000;48:948–953. doi: 10.1016/S0006-3223(00)00888-X. [DOI] [PubMed] [Google Scholar]
- 76.Meyer G, Schwertfeger J, Exton MS, Janssen OE, Knapp W, Stadler MA, et al. Neuroendocrine response to casino gambling in problem gamblers. Psychoneuroendocrinology. 2004;29:1272–1280. doi: 10.1016/j.psyneuen.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 77.Torres OV, Pipkin JA, Ferree P, Carcoba LM, O'Dell LE. Nicotine withdrawal increases stress-associated genes in the nucleus accumbens of female rats in a hormone-dependent manner. Nicotine Tob Res. 2015;17:422–430. doi: 10.1093/ntr/ntu278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ivarsson M, Anderson M, Akerstedt T, Lindblad F. Playing a violent television game affects heart rate variability. Acta Paediatr. 2009;98:166–172. doi: 10.1111/j.1651-2227.2008.01096.x. [DOI] [PubMed] [Google Scholar]
- 79.Hebert S, Beland R, Dionne-Fournelle O, Crete M, Lupien SJ. Physiological stress response to video-game playing: the contribution of built-in music. Life Sci. 2005;76:2371–2380. doi: 10.1016/j.lfs.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 80.Maniaci G, Goudriaan AE, Cannizzaro C, van Holst RJ. Impulsivity and stress response in pathological gamblers during the trier social stress test. J Gambl Stud. 2018;34:147–160. doi: 10.1007/s10899-017-9685-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mistry CJ, Bawor M, Desai D, Marsh DC, Samaan Z. Genetics of opioid dependence: a review of the genetic contribution to opioid dependence. Curr Psychiatr Rev. 2014;10:156–167. doi: 10.2174/1573400510666140320000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caspi A, Moffitt TE. Gene–environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 83.Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol. 2009;5:401–408. doi: 10.1038/nrendo.2009.102. [DOI] [PubMed] [Google Scholar]
- 84.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available only upon reasonable request and after compliance with the policies and procedures of the Basic Science Research Program through the National Research Foundation of Korea and the Ministry of Education, Science and Technology (NRF) for data sharing.


