Introduction
BK virus nephropathy is a serious complication of kidney transplantation. Although 10%–30% of kidney recipients have BK viremia, nephropathy occurs in approximately 2% (1). Nephropathy is most common early after transplant when immunosuppression is at its peak. Patients are often asymptomatic, and the diagnostic standard is biopsy (1). There are no effective viral therapies for BK, and therefore, recipients with BK nephropathy are at great risk of graft loss.
Outcomes and Risk Factors
Although uncommon, BK nephropathy represents a significant threat to allograft survival. Registry data showed that 3-year graft survival was significantly lower in recipients with BK nephropathy (79% versus 90%; P<0.001) (2). Two recent biopsy series reported frequencies of allograft loss of 15%–38%, with one half due to rejection episodes after immunosuppression reduction (3,4). Risk factors include donor seropositivity, greater mismatching, extremes of age, men, diabetes, nonblack race, treatment for rejection, lymphocyte-depleting induction, ureteral stents, and tacrolimus- or mycophenolate-based regimens (1,2,5,6).
Patient Presentation
A woman with kidney disease attributed to type 2 diabetes underwent a deceased donor kidney transplant. Because of sensitization, she received rabbit antithymocyte globulin induction (five doses at 1.5 mg/kg per dose) and maintenance immunosuppression with tacrolimus dosed to troughs of 8–10 μg/L, mycophenolate mofetil 500 mg twice daily, and prednisone 5 mg daily. Routine screening for BK viremia and donor-specific antibodies at 1 month was negative, but at 3 months, plasma BK viral load was 3.6 log copies/ml, and new class I donor-specific antibodies were detected. Laboratory values were notable for a serum creatinine of 1.9 mg/dl (baseline of 1.5 mg/dl) and a tacrolimus trough of 9 μg/L. A transplant biopsy showed focal inflammation and tubular epithelial cell nuclear positivity for simian virus-40 immunostain, confirming BK nephropathy without rejection. Interstitial fibrosis was minimal, and C4d staining was positive in only 10% of peritubular capillaries.
Is Screening Useful?
Universal, prospective screening for BK in the urine or plasma is recommended to identify early viral replication, permit intervention, and prevent progression to nephropathy or allograft loss. The optimal frequency and screening methodology remain undefined. Guidelines unanimously recommend greater screening intensity in the first year and whenever kidney dysfunction is investigated (1,5,6). We screen all kidney recipients using plasma BK PCR at 1, 3, 6, 12, and 24 months; we also screen any patients with unexplained allograft dysfunction. Plasma PCR is the preferred modality, because the correlation with nephropathy is better than with urine PCR testing. A BK viral load in the plasma of ≥10,000 copies/ml or greater than or equal to four log copies/ml is highly correlated with nephropathy and considered “presumed nephropathy,” although it can occur at lower sustained viral loads (1,5,6). Screening is hampered by lack of a universal standard assay for BK PCR; intra- and interlaboratory assay variability in quantification has been well described, especially if recipients have a less common genotype. It is recommended that testing is performed at the same laboratory to minimize assay variability and only at laboratories that operate in accordance with quality assurance programs and are certified for transplantation diagnostics (5).
Is Biopsy Indicated?
The American Society of Transplantation guidelines suggest biopsy as part of the evaluation for all kidney recipients with BK more than four log copies/ml; actual center practice varies (1). Simian virus-40 biopsy staining can be patchy, and diagnosis can be missed; additionally, the interstitial nephritis of BK can mimic rejection. We do not routinely biopsy kidney recipients with BK, even at high titers, unless there is evidence of allograft dysfunction; in that setting, our goal is to exclude rejection, calcineurin inhibitor toxicity, or recurrent native disease. However, there is utility in biopsy. Staging classifications for BK nephropathy exist; greater fibrosis and inflammation on the index biopsy correlate with worse outcomes (5). The Banff working group recently updated their classification schema; they identified three classes of BK nephropathy on the basis of the tissue replication load/level and the “ci” score (7). Allograft outcome varied by class, with 16% of those in class 1 failing by 2 years after diagnosis compared with 50% in class 3 (7). Serial biopsy studies have provided additional insights; Nankivell et al. (3) showed that interstitial fibrosis progresses more swiftly in those with higher viral loads, whereas Drachenberg et al. (4) showed that an increase in interstitial fibrosis or tubulitis scores over sequential biopsies is associated with graft loss. In both, late rejection was deleterious to allograft survival. Transplant biopsy provides valuable prognostic insights, especially serially, but biopsy-related risks must be considered.
What Are Treatment Options?
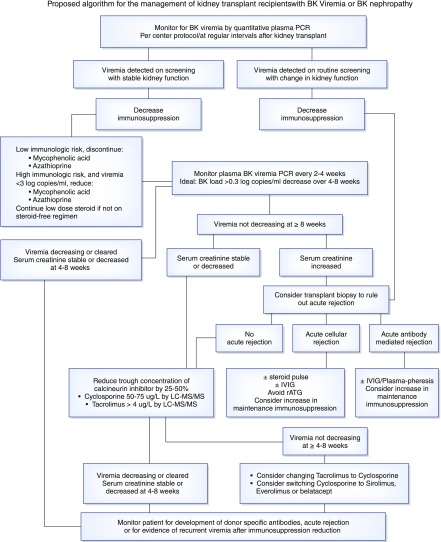
The best studied treatment for BK viremia and nephropathy is careful reduction of immunosuppression; however, there is risk rejection or development of donor-specific antibodies with immunosuppression minimization (8). No standard approach exists (Figure 1). Some centers will dose reduce the antimetabolite (azathioprine or mycophenolate) as a first step, whereas others discontinue it completely (1,5,6). There is in vitro evidence that tacrolimus inhibits anti-BK–specific T cells, which are necessary for viral clearance, and therefore, some centers reduce the calcineurin inhibitor by 25%–50% first (9). Alternatively, simultaneous dose reduction of both the antimetabolite and calcineurin inhibitor can be considered. Another option is conversion from a calcineurin inhibitor to sirolimus or everolimus. Although not specified in any of the guidelines, conversion to belatacept is another option. The patient’s level of immunologic risk, the viral load, and the degree of kidney impairment all need to be considered. Our approach is to discontinue the antimetabolite completely, except in immunologically high-risk patients with low viral loads (less than three log copies/ml), in whom dose reduction by one half is first attempted. Viremia is monitored with a goal of stabilization and ideally, a >0.3-log copies/ml decrease over 4–8 weeks. If viremia does not respond, we decrease the calcineurin inhibitor dose by 25% while still targeting tacrolimus trough levels >4 μg/L or cyclosporin trough levels 50–75 μg/L by liquid chromatography/tandem mass spectrometry. If viremia persists despite calcineurin inhibitor minimization, then conversion to another agent is considered—for patients on tacrolimus, we switch to modified cyclosporin (aiming for a trough level of 50–75 μg/L). Viral response to immunosuppression reduction and time to clearance are variable. Close monitoring of serum creatinine, BK viral loads, and calcineurin inhibitor trough levels is essential. We periodically evaluate for donor-specific antibodies. Patients with an acute or progressive rise in serum creatinine despite declining viremia should be referred for biopsy to rule out concomitant rejection. We have successfully treated patients with both BK nephropathy and acute cellular rejection with pulse steroids. Those with BK nephropathy and acute antibody-mediated rejection are treated with plasmapheresis and intravenous Ig; we avoid rabbit antithymocyte globulin. For refractory BK nephropathy, there have been reports of successful treatment with cidofovir, brincidofovir, leflunomide, or intravenous Ig; however, most of these agents have significant toxicities and are not universally accepted. There are clinical trials underway exploring the use of third party antiviral cytotoxic T cells, but this is not widely available. Despite these interventions, some kidney recipients will lose their allografts to BK nephropathy.
Figure 1.
Proposed algorithm for the management of kidney transplant recipients with BK viremia or BK nephropathy. IVIG, intravenous immunoglobulin; LC-MS/MS, liquid chromatography/tandem mass spectrometry; rATG, rabbit anti-thymocyte globulin.
Continuation of Patient Case
The mycophenolate was discontinued, and when viremia failed to decline, her tacrolimus dose was reduced (targeting trough levels of 5–7 μg/L). Four weeks later, serum creatinine decreased to 1.6 mg/dl, donor-specific antibodies were stable, and BK viral load was 3.2 log copies/ml. No further changes in immunosuppression were made due to her sensitization and proximity to transplantation. Eight weeks later, serum creatinine returned to her baseline, tacrolimus trough was 6.2 μg/L, and BK had decreased to 2.8 log copies/ml; however, she had developed new class II donor-specific antibody. One year after diagnosis, the patient remains on a tacrolimus- and prednisone-based regimen, with BK of 3.1 log copies/ml, serum creatinine of 1.4 mg/dl, tacrolimus trough of 5.2 μg/L, and stable class I and II donor-specific antibodies.
How Should Immunosuppression Be Handled after Resolution of BK Nephropathy?
Long-term immunosuppression management of patients with cleared viremia or resolved nephropathy is individualized on the basis of patient characteristics and the level of immunologic risk. Nearly one half of patients with BK nephropathy lose their allografts from rejection rather than uncontrolled viral infection (4,5). For recipients who are low risk (age >65 years, first transplant or unsensitized), we do not restart the antimetabolite, maintaining them on a calcineurin inhibitor and prednisone alone and targeting our standard post-transplant trough levels. For recipients who are higher immunologic risk (sensitized, retransplanted, or donor-specific antibody positive), we will attempt to reintroduce the antimetabolite after three consecutive negative viral loads. We monitor viremia on a monthly basis after the antimetabolite has been restarted. In recipients with recurrent viremia, the antimetabolite is permanently stopped. For recipients with donor-specific antibody, we try to restart the antimetabolite and target calcineurin inhibitor trough levels at the upper end of our usual range while monitoring donor-specific antibody every 6–12 months; if they develop recurrent viremia, the antimetabolite is discontinued permanently.
Can Patients with Graft Loss Due to BK Nephropathy Be Retransplanted?
Retransplantation is not contraindicated after BK nephropathy. Transplant nephrectomy is not required; however, BK viral load should be undetectable to minimize recurrence (1,5,6,10). An analysis of registry data identified 823 kidney recipients who experienced graft loss due to BK nephropathy, and 15% were retransplanted (10). Retransplants received similar induction and maintenance immunosuppression as first-time recipients; 1- and 3-year graft survival rates in retransplanted recipients were 99% and 94%, respectively, with only one graft loss due to recurrent BK nephropathy.
Conclusions
BK nephropathy, although uncommon, represents a threat to allograft survival. Our understanding of the risk factors has improved, and prospective screening strategies exist, but we have not eradicated this disease. Lack of effective viral therapy remains a key limitation. Judicious immunosuppression reduction with awareness of the accompanying risks remains the best therapeutic option. Future research efforts should be focused on standardization of monitoring assays and development of safe, non-nephrotoxic, and effective prophylaxis and treatment.
Disclosures
None.
Acknowledgments
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Hirsch HH, Randhawa P; AST Infectious Diseases Community of Practice: BK polyomavirus in solid organ transplantation. Am J Transplant 13[Suppl 4]: 179–188, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Schold JD, Rehman S, Kayle LK, Magliocca J, Srinivas TR, Meier-Kriesche HU: Treatment for BK virus: Incidence, risk factors and outcomes for kidney transplant recipients in the United States. Transpl Int 22: 626–634, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Nankivell BJ, Renthawa J, Sharma RN, Kable K, O’Connell PJ, Chapman JR: BK virus nephropathy: Histological evolution by sequential pathology. Am J Transplant 17: 2065–2077, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Drachenberg CB, Papadimitriou JC, Chaudhry MR, Ugarte R, Mavanur M, Thomas B, Cangro C, Costa N, Ramos E, Weir MR, Haririan A: Histological evolution of BK virus-associated nephropathy: Importance of integrating clinical and pathological findings. Am J Transplant 17: 2078–2091, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Hirsch HH, Babel N, Comoli P, Friman V, Ginevri F, Jardine A, Lautenschlager I, Legendre C, Midtvedt K, Muñoz P, Randhawa P, Rinaldo CH, Wieszek A; ESCMID Study Group of Infection in Compromised Hosts: European perspective on human polyomavirus infection, replication and disease in solid organ transplantation. Clin Microbiol Infect 20[Suppl 7]: 74–88, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Hirsch HH, Brennan DC, Drachenberg CB, Ginevri F, Gordon J, Limaye AP, Mihatsch MJ, Nickeleit V, Ramos E, Randhawa P, Shapiro R, Steiger J, Suthanthiran M, Trofe J: Polyomavirus-associated nephropathy in renal transplantation: Interdisciplinary analyses and recommendations. Transplantation 79: 1277–1286, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Nickeleit V, Singh HK, Randhawa P, Drachenberg CB, Bhatnagar R, Bracamonte E, Chang A, Chon WJ, Dadhania D, Davis VG, Hopfer H, Mihatsch MJ, Papadimitriou JC, Schaub S, Stokes MB, Tungekar MF, Seshan SV; Banff Working Group on Polyomavirus Nephropathy: The Banff working group classification of definitive polyomavirus nephropathy: Morphologic definitions and clinical correlations. J Am Soc Nephrol 29: 680–693, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawinski D, Forde KA, Trofe-Clark J, Patel P, Olivera B, Goral S, Bloom RD: Persistent BK viremia does not increase intermediate-term graft loss but is associated with de novo donor-specific antibodies. J Am Soc Nephrol 26: 966–975, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egli A, Köhli S, Dickenmann M, Hirsch HH: Inhibition of polyomavirus BK-specific T-Cell responses by immunosuppressive drugs. Transplantation 88: 1161–1168, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Dharnidharka VR, Cherikh WS, Neff R, Cheng Y, Abbott KC: Retransplantation after BK virus nephropathy in prior kidney transplant: An OPTN database analysis. Am J Transplant 10: 1312–1315, 2010 [DOI] [PubMed] [Google Scholar]