Abstract
Background and objectives
Shorter or longer sleep duration and poor sleep quality are risk factors for numerous cardio-metabolic diseases, cardiovascular disease, and mortality in subjects with normal kidney function. The association of sleep duration and sleep quality with health outcomes in patients with CKD remains uncertain.
Design, setting, participants, & measurements
A 4-year prospective cohort study in 17 nephrology centers in Japan, the CKD Japan Cohort (CKD-JAC) Study, assessed an association of self-reported sleep duration and sleep quality, on the basis of the Pittsburgh Sleep Quality Index (PSQI) questionnaire, with incidence of ESKD in 1601 patients with eGFR 10–59 ml/min per 1.73 m2 using multivariable-adjusted Cox proportional hazards models.
Results
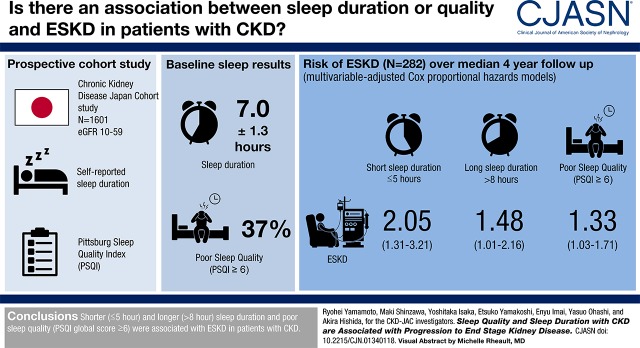
Baseline sleep duration and PSQI global score for the 1601 patients were mean±SD 7.0±1.3 hours and median 4 (interquartile range, 3–7), respectively. Poor sleep quality (PSQI global score ≥6) was common (n=588 [37%]). During a median of 4.0 (2.6–4.3) years of the follow-up period, 282 (18%) patients progressed to ESKD. After adjusting for age, sex, eGFR, urinary albumin excretion, smoking status, body mass index, history of diabetes and cardiovascular disease, systolic BP, blockade of the renin-angiotensin system, use of hypnotics, and Beck depression inventory score, both shorter (≤5 hour) and longer (>8 hour) sleep duration were associated with ESKD (adjusted hazard ratios [95% confidence intervals] for ≤5.0, 5.1–6.0, 6.1–7.0, 7.1–8.0, and ≥8.0 hours were 2.05 [1.31 to3.21], 0.98 [0.67 to 1.44], 1.00 [reference], 1.22 [0.89 to 1.66], and 1.48 [1.01 to 2.16]), suggesting a U-shaped relationship between sleep duration and ESKD. PSQI global score ≥6 was also associated with incidence of ESKD (adjusted hazard ratios [95% confidence intervals] for PSQI global score ≤5 and ≥6 were 1.00 [reference] and 1.33 [1.03 to 1.71]).
Conclusions
Shorter (≤5 hour) and longer (>8 hour) sleep duration and poor sleep quality (PSQI global score ≥6) were associated with ESKD in patients with CKD.
Keywords: Epidemiology and outcomes; chronic kidney disease; sleep hygiene; Sleep Deprivation; Hypnotics and Sedatives; Proportional Hazards Models; risk factors; Body Mass Index; glomerular filtration rate; Renin-Angiotensin System; Self Report; blood pressure; Prospective Studies; depression; Follow-Up Studies; Renal Insufficiency, Chronic; Sleep Initiation and Maintenance Disorders; diabetes mellitus; Cardiovascular Diseases; Metabolic Diseases; Smoking; Albumins
Visual Abstract
Introduction
Epidemiologic evidence demonstrates that patients with CKD, characterized by low GFR and/or proteinuria (1), are at high risk of ESKD (2), cardiovascular disease (3), and even mortality (3). The growing number of patients with CKD is a worldwide public health problem presenting an enormous economic burden (4). In Japan, the prevalence of CKD and ESKD was estimated to be approximately 13% (5) and 0.3% (6) of the adult population, respectively. The ESKD rate in Japan is one of the highest among industrialized nations (7). One potential approach to developing an effective CKD prevention program is to identify the lifestyle risk factors for incidence and progression of CKD.
One of the potential lifestyle factors for CKD is sleep. The most extensively studied sleep component is sleep duration (8). Systematic reviews identified shorter and/or longer sleep duration as associated with a wide variety of clinical outcomes, including obesity (9), metabolic syndrome (10), diabetes (11), hypertension (12), cardiovascular disease (13), stroke (14), and mortality (15). Regarding CKD, a few cohort studies identified shorter sleep duration to be associated with proteinuria (16) and GFR decline (17,18) in the general population. However, data regarding the clinical effect of sleep duration in patients with CKD are very limited (19).
Besides sleep duration, sleep quality may play a role in CKD progression. Several cohort studies suggested that self-reported sleep quality was associated with obesity (20), diabetes (21,22), cardiovascular disease (22), and mortality (23). Interventional trials to suppress deep nonrapid eye movement sleep, also known as slow-wave sleep, demonstrated adverse metabolic effects on glucose metabolism (24,25), the sympathetic nervous system (25), and adrenocortical activity (25). Although poor sleep quality is common in patients with CKD (26–28), the clinical effect of sleep quality on ESKD incidence remains to be elucidated (19).
The aims of this 4-year prospective cohort study, the CKD Japan Cohort (CKD-JAC) study, were to assess the association between self-reported sleep duration and quality and ESKD in patients with eGFR 10–59 ml/min per 1.73 m2 using the Pittsburgh Sleep Quality Index (PSQI) (29), the most widely used sleep quality questionnaire (8,30,31). The results of this study shed light on sleep as one of the key lifestyle risk factors for progression of CKD.
Materials and Methods
Participants
The CKD-JAC study (University Hospital Medical Information Network (UMIN) clinical trial number: UMIN000020038) was a prospective cohort study to determine the incidence rates of ESKD (32) and cardiovascular disease (33) and to identify their risk factors (34,35) during the 4-year follow-up period in Japanese patients with CKD. The study protocol has been described in detail elsewhere (36). Briefly, the CKD-JAC study enrolled patients aged 20–75 years with eGFR 10–59 ml/min per 1.73 m2. To calculate eGFR, a three-variable equation modified for Japanese patients was used: eGFR (ml/min per 1.73 m2)=194×age (year)−0.287×serum creatinine (mg/dl)−1.094×0.739 (if female) (37). Patients with a history of transplants or kidney replacement therapy (KRT) were not eligible for the study. All patients provided written informed consent before enrollment in this study. The study protocol was approved by the institutional review board of each participating hospital.
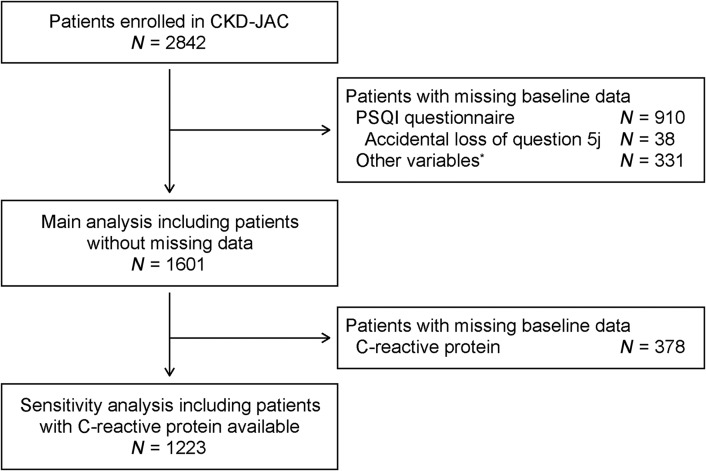
Between April of 2007 and December of 2008, 2966 patients with CKD were registered in the CKD-JAC study at 17 hospitals in Japan. After excluding patients ≥76 years of age (n=40), with eGFR≥60 or <10 ml/min per 1.73 m2 (N=13 and 69, respectively), or with a history of KRT (N=2) at their baseline visit, 2842 patients were enrolled in the CKD-JAC study. Baseline sleep duration and quality were measured using the PSQI questionnaire, comprising 18 questions (Supplemental Appendix) (29). Because completing the PSQI questionnaire was not mandatory in the CKD-JAC study, this was done primarily on a voluntary basis. Accidentally, a question pertaining to frequency of sleep disturbance (PSQI question 5j) was not included in the PSQI questionnaire in the CKD-JAC study. Depending on their answers to the missing question 5j, 38 (1%) patients had two possible PSQI global scores, whereas the rest had a single global score regardless of their answer to question 5j. After excluding the 38 (1%) patients with two possible PSQI global scores, 872 (31%) patients with missing answers to other PSQI questions, and 331 (12%) patients with missing data for other variables, this study finally included 1601 (56%) patients with CKD (Figure 1).
Figure 1.
Inclusion and exclusion process of this study. *Age, sex, smoking status, history of diabetes and cardiovascular disease, body mass index, systolic and diastolic BP, serum creatinine, eGFR, urinary albumin excretion, blockade of renin-angiotensin system, and Beck depression inventory score. CKD-JAC, CKD Japan Cohort study; PSQI, Pittsburgh Sleep Quality Index.
Measurements
The main exposures of this study were baseline sleep duration (PSQI component 3) and sleep quality (PSQI global score) during the month before the baseline visit. Poor sleep quality was defined as PSQI global score ≥6, a common cutoff value for poor sleep quality (29,30). Other baseline variables included age, sex, smoking status (non-, past, or current smoker), history of diabetes and cardiovascular disease, body mass index (body weight [kg]/height2 [m2]), systolic and diastolic BP, serum creatinine, eGFR, urinary albumin excretion, serum C-reactive protein (if available) and renin-angiotensin system (RAS) blockade, use of hypnotics (PSQI component 6), and Beck depression inventory score (38) at their baseline visit. Patients with any history of angina, myocardial infarction, congestive heart failure, stroke, and/or arteriosclerosis obliterans were regarded as those with history of cardiovascular disease. RAS blockade included use of angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists. Because the numbers of patients with hypnotic use <1 and 1–2 times/wk were small (n=41 [3%] and 34 [2%], respectively), they were classified into a single category. The Beck depression inventory is a 21-item self-administered questionnaire for depression screening (38). A score of 11 or higher is the originally defined cutoff value for depression in the general population and it is validated for use in patients with CKD (39). To assess changes in sleep duration and PSQI global score during the follow-up period, answers to the PSQI questionnaire were remeasured 2 years and 3.5 years after entry into the study.
The outcome measure of interest in this study was time to incidence of ESKD requiring KRT. Incidence of ESKD was ascertained from patient medical records by clinical research assistants and physicians in each facility. The follow-up period was defined as time from the baseline visit to (1) incidence of ESKD or (2) the last visit before March of 2013, whichever came first. Death before ESKD incidence was regarded as censored.
Statistical Analyses
Differences in baseline characteristics between the patients included in and those excluded from this study were assessed using unpaired t test, Wilcoxon rank sum test, or chi-squared test, as appropriate. The incidence rates of ESKD were compared using a Poisson regression model. After classifying baseline sleep duration into five categories (≤5.0, 5.1–6.0, 6.1–7.0, 7.1–8.0, and >8.0 hours), dose-dependent associations between baseline sleep duration and other baseline characteristics were assessed using the Cochran–Armitage trend test or the Jonckheere–Terpstera trend test, as appropriate. Baseline characteristics between patients with PSQI≤5 and ≥6 were compared using unpaired t test, Wilcoxon rank sum test, or chi-squared test, as appropriate. Correlations of sleep duration between the baseline visit and 2 and 3.5 years after study entry were assessed using Pearson’s r and those of PSQI global score were assessed using Kendall’s τ because of its tied values.
Associations of sleep duration and PSQI global score with incidence of ESKD were assessed using nested Cox proportional hazards models, whereby covariates from each prior model were retained as follows. Model 1 included age and sex. Model 2 added eGFR (ml/min per 1.73 m2) and urinary albumin excretion. Because of its skewed distribution, urinary albumin excretion was included in model 2 after logarithmic transformation (log g/g creatinine (gCr)). Model 3 added smoking status (non-, past, or current smoker), body mass index (kg/m2), history of diabetes and cardiovascular disease, systolic BP (mm Hg), and RAS blockade. Model 4 added Beck depression inventory score (≤10 versus ≥11) and use of hypnotics (none, 1–2 times per week, or ≥3 times per week). PSQI global score was not adjusted for use of hypnotics, which was included as component 6 of the PSQI global score. The proportional hazards assumption for covariates was checked using Schoenfeld residuals. Effect modifications between the baseline variables were assessed, including their interaction terms, in a fully adjusted model (model 4).
The incremental contribution of sleep duration and PSQI global score in each model were evaluated using the net reclassification improvement (NRI) approach. Continuous (category-less) NRI at a set time of 4 years comparable to the median follow-up time was estimated using the bootstrapping method (40).
As a sensitivity analysis, we assessed a potential confounding effect of serum C-reactive protein level as an inflammation marker on association of sleep duration and PSQI global score with incidence of ESKD in 1223 (76%) patients with a baseline measurement available. We made a nested Cox proportional hazards model, additionally adjusting for C-reactive protein. Because C-reactive protein serum level was 0 mg/dl (under the detection limit) in 207 (17%) patients, and its distribution was right-skewed, its serum concentration was transformed to log (C-reactive protein [mg/dl]+0.01) before inclusion in multivariable-adjusted models.
Continuous variables were expressed as mean±SD or median (interquartile range), and categorical variables were expressed as number and proportion, as appropriate. The statistical significance level was set at P<0.05. All statistical analyses were conducted using SAS Release 9.4 (SAS Institute, Cary, NC), R version 3.5.0 (www.r-project.org/; The R Foundation for Statistical Computing), and Stata, version 15 (www.stata.com; Stata Corp).
Results
Among 2842 eligible patients aged 20–75 years with eGFR 10–59 ml/min per 1.73 m2, 1601 (56%) patients with median (IQR) age 61 (54–69) years and mean±SD body mass index 23.5±3.8 kg/m2 were included in this study (Figure 1, Supplemental Table 1). Compared with the excluded patients, the included patients were significantly younger and had higher prevalence of male sex, higher diastolic BP and serum levels of C-reactive protein, and lower prevalence of nonsmokers, diabetes, cardiovascular disease, and users of hypnotics (Supplemental Table 1). Baseline sleep duration and PSQI global score were comparable between the two groups (mean±SD sleep duration, 7.0±1.3 versus 6.9±1.3 hours; PSQI global median [IQR] score, 4 [3–7] and 5 [3–7]). During a median 4.0 years of follow-up, 523 (18%) of 2842 patients developed ESKD requiring KRT. Incidence rates of ESKD in patients included in and excluded from this analysis were comparable (0.051 versus 0.059 per person-year, P=0.11).
The baseline characteristics of the 1601 patients stratified by five categories of sleep duration are listed in Table 1. Mean±SD sleep duration for 152 (9%), 300 (19%), 541 (34%), 420 (26%), and 188 (12%) patients with ≤5.0, 5.1–6.0, 6.1–7.0, 7.1–8.0, and >8.0 hours of sleep duration was 4.7±0.7, 5.9±0.2, 6.9±0.2, 7.8±0.2, and 9.2±0.7 hours, respectively. Compared with patients with longer sleep duration, those with shorter sleep duration were younger; had lower prevalence of male sex and history of cardiovascular disease, and lower levels of serum creatinine; and had higher levels of body mass index, diastolic BP, eGFR, Beck depression inventory score, and PSQI global score (Ptrend<0.05) (Table 1). Baseline median (IQR) PSQI global score in 1601 patients with CKD was 4 (3–7). More than one-third of patients with CKD (N=588 [37%]) had poor sleep quality defined as PSQI global score ≥6. Compared with patients with PSQI global score ≤5, those with PSQI global score ≥6 had significantly lower prevalence of male sex, lower levels of urinary albumin excretion, higher Beck depression inventory scores, higher prevalence of use of hypnotics, and shorter sleep duration (P<0.05) (Table 2). Baseline sleep duration and PSQI global score were moderately correlated with those at 2 and 3.5 years after the study entry (sleep duration [hour], Pearson’s r 0.65 and 0.62, respectively; PSQI global score, Kendall’s τ 0.53 and 0.52, respectively), suggesting that their baseline values reflected their values during the follow-up period.
Table 1.
Baseline sleep duration and clinical characteristics of 1601 patients
| Characteristic | Sleep Duration (h) | ||||
|---|---|---|---|---|---|
| ≤5.0 | 5.1–6.0 | 6.1–7.0 | 7.1–8.0 | >8.0 | |
| Number | 152 | 300 | 541 | 420 | 188 |
| Sleep duration, h | 4.7±0.7 | 5.9±0.2 | 6.9±0.2 | 7.8±0.2 | 9.2±0.7 |
| Age, yr | 60 (52–66) | 57 (47–66) | 60 (51–68) | 63 (57–70) | 68 (61–72) |
| Men, N (%) | 96 (63) | 179 (60) | 341 (63) | 291 (69) | 124 (66) |
| Smoking status, N (%) | |||||
| Nonsmokers | 79 (52) | 174 (58) | 301 (56) | 197 (47) | 102 (54) |
| Past smokers | 39 (26) | 72 (24) | 158 (29) | 158 (38) | 54 (29) |
| Current smokers | 34 (22) | 54 (18) | 82 (15) | 65 (15) | 32 (17) |
| Diabetes, N (%) | 55 (36) | 114 (38) | 164 (30) | 150 (36) | 75 (40) |
| Cardiovascular disease, N (%) | 27 (18) | 55 (18) | 100 (18) | 99 (24) | 49 (26) |
| Body mass index, kg/m2 | 24.4±4.2 | 23.5±4.2 | 23.4±3.7 | 23.5±3.4 | 23.0±3.7 |
| Systolic BP, mm Hg | 131±20 | 131±18 | 132±19 | 131±17 | 132±17 |
| Diastolic BP, mm Hg | 77±12 | 77±11 | 78±12 | 77±11 | 75±11 |
| Serum creatinine, mg/dl | 1.7 (1.3–2.4) | 1.7 (1.3–2.4) | 1.8 (1.4–2.5) | 1.9 (1.5–2.6) | 2.0 (1.5–2.7) |
| eGFR, ml/min per 1.73 m2 | 33 (21–40) | 32 (21–40) | 29 (20–37) | 28 (19–37) | 25 (17–34) |
| 45–59, N (%) | 18 (12) | 40 (13) | 56 (10) | 28 (7) | 12 (6) |
| 30–44, N (%) | 64 (42) | 127 (42) | 206 (38) | 168 (40) | 55 (29) |
| 15–29, N (%) | 49 (32) | 107 (36) | 224 (41) | 166 (40) | 89 (47) |
| 10–14, N (%) | 21 (14) | 26 (9) | 55 (10) | 58 (14) | 32 (17) |
| Urinary albumin excretion, g/gCr | 0.46 (0.12–1.10) | 0.44 (0.11–1.33) | 0.54 (0.15–1.24) | 0.47 (0.13–1.29) | 0.39 (0.08–1.18) |
| C-reactive protein, mg/dla | 0.10 (0.04–0.21) | 0.06 (0.04–0.19) | 0.07 (0.04–0.20) | 0.08 (0.04–0.18) | 0.10 (0.04–0.21) |
| Medications, N (%) | |||||
| RAS blockade | 128 (84) | 243 (81) | 449 (83) | 351 (84) | 153 (81) |
| Hypnotics | |||||
| None | 116 (76) | 251 (84) | 480 (89) | 357 (85) | 158 (84) |
| ≤2 times/wk | 13 (9) | 18 (6) | 23 (4) | 15 (4) | 6 (3) |
| ≥3 times/wk | 23 (15) | 31 (10) | 38 (7) | 48 (11) | 24 (13) |
| Beck depression inventory score | 11 (6–18) | 8 (5–13) | 6 (3–11) | 6 (3–11) | 7 (4–12) |
| ≥11, N (%) | 82 (54) | 112 (37) | 155 (29) | 114 (27) | 61 (32) |
| PSQI global score | 10 (8–13) | 6 (5–9) | 4 (3–6) | 3 (2–5) | 3 (2–4) |
| ≥6, N (%) | 140 (92) | 185 (62) | 164 (30) | 70 (17) | 29 (15) |
| Sleep duration | |||||
| At 2 yrb, h | 5.4±1.3 | 6.3±0.8 | 6.8±0.9 | 7.6±1.0 | 8.4±1.3 |
| At 3.5 yrc | 5.4±1.2 | 6.2±0.9 | 6.9±0.9 | 7.6±1.3 | 8.4±1.3 |
Data presented as mean±SD or median (interquartile range), unless otherwise specified. Cr, creatinine; RAS, renin angiotensin system; PSQI, Pittsburgh Sleep Quality Index.
n=113, 232, 415, 314, and 149 for patients with ≤5.0, 5.1–6.0, 6.1–7.0, 7.1–8.0, and >8.0 h baseline sleep duration, respectively.
n=101, 201, 387, 307, and 126 for patients with ≤5.0, 5.1–6.0, 6.1–7.0, 7.1–8.0, and >8.0 h baseline sleep duration, respectively.
n=64, 153, 275, 188, and 82 for patients with ≤5.0, 5.1–6.0, 6.1–7.0, 7.1–8.0, and >8.0 h baseline sleep duration, respectively.
Table 2.
Baseline PSQI global score and clinical characteristics of 1601 patients
| Characteristic | PSQI Global Score | |
|---|---|---|
| 0–5 | 6–19 | |
| Number | 1013 | 588 |
| PSQI global score | 3 (2–4) | 8 (7–10) |
| Age, yr | 61 (54–68) | 62 (53–69) |
| Men, N (%) | 681 (67) | 350 (60) |
| Smoking status, N (%) | ||
| Nonsmokers | 553 (55) | 300 (51) |
| Past smokers | 308 (30) | 173 (29) |
| Current smokers | 152 (15) | 115 (20) |
| Diabetes, N (%) | 342 (34) | 216 (37) |
| Cardiovascular disease, N (%) | 206 (20) | 124 (21) |
| Body mass index, kg/m2 | 23.5±3.6 | 23.5±4.1 |
| Systolic BP, mm Hg | 132±18 | 132±18 |
| Diastolic BP, mm Hg | 77±12 | 76±11 |
| Serum creatinine, mg/dl | 1.8 (1.4–2.5) | 1.8 (1.4–2.5) |
| eGFR, ml/min per 1.73 m2 | 29 (20–38) | 29 (20–39) |
| 45–59, N (%) | 101 (10) | 53 (9) |
| 30–44, N (%) | 389 (38) | 231 (39) |
| 15–29, N (%) | 403 (40) | 232 (39) |
| 10–14, N (%) | 120 (12) | 72 (12) |
| Urinary albumin excretion, g/gCr | 0.53 (0.15–1.30) | 0.41 (0.09–1.18) |
| C-reactive protein, mg/dla | 0.08 (0.04–0.19) | 0.09 (0.04–0.20) |
| Medications, N (%) | ||
| RAS blockade | 832 (82) | 492 (84) |
| Hypnotics | ||
| None | 975 (96) | 387 (66) |
| ≤2 times/wk | 17 (2) | 58 (10) |
| ≥3 times/wk | 21 (2) | 143 (24) |
| Beck depression inventory score | 5 (3–10) | 10 (6–17) |
| ≥11, N (%) | 236 (23) | 288 (49) |
| Sleep duration, h | 7.4±1.0 | 6.0±1.3 |
| PSQI global score at 2 yrb | 3 (2–5) | 7 (5–10) |
| ≥6, N (%) | 114 (17) | 242 (73) |
| PSQI global score at 3.5 yrc | 4 (2–5) | 8 (5–10) |
| ≥6, N (%) | 91 (21) | 157 (70) |
Data presented as mean±SD or median (interquartile range), unless otherwise specified. PSQI, Pittsburgh Sleep Quality Index; Cr, creatinine; RAS, renin angiotensin system.
n=762 and 461 for patients with 0–5 and ≥6 baseline PSQI global score, respectively.
n=654 and 233 for patients with 0–5 and ≥6 baseline PSQI global score, respectively.
n=440 and 225 for patients with 0–5 and ≥6 baseline PSQI global score, respectively.
During a median of 4.0 (interquartile range, 2.6–4.3) years of the observational period, 282 (18%) of 1601 patients progressed to ESKD. Incidence rates of ESKD for patients with ≤5.0, 5.1–6.0, 6.1–7.0, 7.1–8.0, and ≥8.0 hours of sleep duration were 0.057, 0.041, 0.043, 0.058, and 0.073 per person-year, respectively (Table 3). In model 1, which was adjusted for age and sex, sleep duration ≥8.0 hours was significantly associated with ESKD. Its significant association was still persistent even after multivariable adjustments (models 2–4). In contrast, ≤5.0 hours of sleep duration was associated with ESKD only after adjusting for eGFR and urinary albumin excretion. NRI analyses showed that the addition of sleep duration significantly improved prediction for all models. For sleep disorders, 239 (15%) patients received hypnotics during 1 month before the baseline visit. Use of hypnotics did not affect the association between sleep duration and incidence of ESKD, or was their use associated with incidence of ESKD (adjusted hazard ratio [95% confidence interval] for use of hypnotics in model 4: none, 1.00 [reference]; 1–2 times per week, 0.72 [0.29 to 1.78]; ≥3 times per week, 1.05 [0.71 to 1.56]).
Table 3.
Sleep duration and incidence of ESKD in 1601 patients with CKD
| Sleep Duration (h) | NRI (95% CI) of Sleep Duration | |||||
|---|---|---|---|---|---|---|
| ≤5.0 | 5.1–6.0 | 6.1–7.0 | 7.1–8.0 | ≥8.0 | ||
| Number | 152 | 300 | 541 | 420 | 188 | |
| Incidence of ESKD, N (%) | 29 (19) | 43 (14) | 82 (15) | 83 (20) | 45 (24) | |
| Incidence rate (per person-year) | 0.057 | 0.041 | 0.043 | 0.058 | 0.073 | |
| Adjusted hazard ratio (95% CI) | ||||||
| Model 1 | 1.29 (0.85 to 1.97) | 0.98 (0.68 to 1.42) | 1.00 (reference) | 1.31 (0.96 to 1.78) | 1.70 (1.17 to 2.47)a | 0.10 (0.02 to 0.18)a |
| Model 2 | 2.21 (1.44 to 3.39)a | 0.98 (0.67 to 1.43) | 1.00 (reference) | 1.20 (0.88 to 1.63) | 1.54 (1.06 to 2.24)a | 0.15 (0.02 to 0.28)a |
| Model 3 | 2.27 (1.48 to 3.50)a | 1.01 (0.70 to 1.48) | 1.00 (reference) | 1.24 (0.91 to 1.69) | 1.56 (1.07 to 2.27)a | 0.15 (0.05 to 0.26)a |
| Model 4b,c | 2.05 (1.31 to 3.21)a | 0.98 (0.67 to 1.44) | 1.00 (reference) | 1.22 (0.89 to 1.66) | 1.48 (1.01 to 2.16)a | 0.12 (0.02 to 0.26)a |
Model 1 adjusted for age (yr) and sex. Model 2 adjusted for the covariates in model 1, eGFR (ml/min per 1.73 m2), and urinary albumin excretion (log g/gCr). Model 3 adjusted for the covariates in model 2, smoking status (non-, past, or current smoker), body mass index (kg/m2), history of diabetes mellitus and cardiovascular disease, systolic BP (mm Hg), and blockade of renin-angiotensin system. Model 4 adjusted for the covariates in model 3 and Beck depression inventory score (≤10 and >10) and use of hypnotics (none, 1–2 times, or ≥3 times per wk). NRI, net reclassification index; 95% CI, 95% confidence interval; Cr, creatinine.
P<0.05.
P for interaction of sleep duration with age, sex, history of diabetes and cardiovascular disease, and use of hypnotics=0.79, 0.47, 0.67, 0.49, and 0.78, respectively.
Multivariable-adjusted hazard ratio (95% CI) for none, 1–2 times, and ≥3 times per week use of hypnotics: 1.00 (reference), 0.72 (0.29 to 1.78), and 1.05 (0.71 to 1.56), respectively.
Regarding PSQI global score, incidence of ESKD was 0.049 and 0.055 per person-year in patients with PSQI≤5 and ≥6, respectively (Table 4). Incidence of ESKD was comparable between patients with PSQI global score ≤5 and ≥6 after adjusting for age and sex. As with sleep duration, PSQI global score was significantly associated with incidence of ESKD after additionally adjusting for eGFR and urinary albumin excretion. Even in a fully adjusted model (model 4), PSQI global score was significantly associated with incidence of ESKD. Although PSQI global score was identified as a significant predictor of incidence of ESKD, NRI analyses revealed no significant improvement of prediction by adding PSQI global score.
Table 4.
PSQI global score and incidence of ESKD in 1601 patients with CKD
| PSQI Global Score | NRI (95% CI) of PSQI (≤5 versus ≥6) | ||
|---|---|---|---|
| ≤5 | ≥6 | ||
| Number | 1013 | 588 | |
| Incidence of ESKD, N (%) | 172 (17) | 110 (19) | |
| Incidence rate (per person-year) | 0.049 | 0.055 | |
| Adjusted hazard ratio (95% CI) | |||
| Model 1 | 1.00 (reference) | 1.19 (0.93 to 1.51) | 0.02 (−0.04 to 0.09) |
| Model 2 | 1.00 (reference) | 1.39 (1.09 to 1.77)a | 0.02 (−0.34 to 0.09) |
| Model 3 | 1.00 (reference) | 1.40 (1.09 to 1.79)a | 0.03 (−0.12 to 0.11) |
| Model 4b | 1.00 (reference) | 1.33 (1.03 to 1.71)a | 0.03 (−0.21 to 0.10) |
Model 1 adjusted for age (yr) and sex. Model 2 adjusted for the covariates in model 1, eGFR (ml/min per 1.73 m2), and urinary albumin excretion (log g/gCr). Model 3 adjusted for the covariates in model 2, smoking status (non-, past, or current smoker), body mass index (kg/m2), history of diabetes mellitus and cardiovascular disease, systolic BP (mm Hg), and blockade of renin angiotensin system. Model 4 adjusted for the covariates in model 3 and Beck depression inventory (≤10 versus >10). PSQI, Pittsburgh Sleep Quality Index; NRI, net reclassification index; 95% CI, 95% confidence interval; Cr, creatinine.
P<0.05.
P for interaction of PSQI global score with age, sex, history of diabetes, and cardiovascular disease=0.20, 0.14, 0.71, and 0.52, respectively.
Effect modifications between the main exposures and the baseline variables with a significant difference between included and excluded patients, including age, sex, history of diabetes and cardiovascular disease, and use of hypnotics, were assessed in fully adjusted models, but no obvious effect modification was observed (footnotes of Tables 3 and 4).
A potential confounding effect due to inflammation was assessed by including the baseline serum level of C-reactive protein in multivariable-adjusted models in 1223 (76%) patients (Supplemental Tables 2 and 3). Even after adjusting for C-reactive protein, similar associations were observed in sleep duration and PSQI global score, although sleep duration ≥8.0 hours was not significantly associated.
Discussion
This cohort study, including 1601 patients with CKD, revealed that short and long sleep duration (≤5.0 and >8.0 hours) and poor sleep quality (PSQI global score ≥6) were significantly associated with higher incidence of ESKD. These results suggest that a quick assessment of self-reported sleep duration and sleep quality might be an easy and effective way to identify patients with CKD at high risk of ESKD. Advantages of this study included its prospective cohort design, the clinically relevant hard outcome of ESKD, and its large sample size (n=1601), which enabled us to perform statistically meaningful analyses to identify predictors of ESKD.
Although several cohort studies have reported that shorter sleep duration was associated with incidence of proteinuria (16) and GFR decline (17,18), mainly in subjects with normal kidney function, few studies have reported an association between sleep duration and incidence of ESKD. The Chronic Renal Insufficiency Cohort (CRIC) study, a cohort study of patients with CKD in the United States, measured objective sleep duration using wrist actigraphy in 431 patients and identified sleep fragmentation, an index of sleep quality, as associated with the incidence of ESKD during a median 5.2 years of follow-up (19). The CRIC study reported conflicting results of an association between short sleep duration and kidney function; shorter sleep duration was associated with eGFR decline, but not with incidence of ESKD. This study clarified that shorter sleep duration predicted incidence of ESKD in a much larger cohort (n=1601), confirming the association of shorter sleep duration with kidney survival. The findings of this and previous studies suggest that modification of sleep duration may lead to a better prognosis of kidney function. One potential method for modifying sleep duration may be the use of hypnotics. However, this study showed no significant association between use of hypnotics and incidence of ESKD (Table 3). A large number of cohort studies have reported users of hypnotics to be at risk of early death (41,42), suggesting that hypnotics should be prescribed with great caution in patients with CKD with short sleep duration. The clinical effects of modification of sleep duration on kidney function need to be examined in future studies.
In addition to shorter sleep duration (≤5 hours), this study identified longer sleep duration (>8.0 hours) as a significant predictor of ESKD, indicating a U-shaped association between sleep duration and incidence of ESKD (Table 3). A similar U-shaped association between sleep duration and cardiovascular and all-cause mortality was observed in many large cohort studies, identifying short (<6- or 7-hour) and long (>8- or 9-hour) sleep durations as risk factors of cardiovascular (13) and all-cause mortality (15,43,44). Interestingly, some systematic reviews have suggested that the elderly population (≥60 years) (15,43) and the Asian population (15,43,44) are vulnerable to the detrimental effects of long sleep duration. The reasons that the previous studies (17,18) did not identify longer sleep duration to be associated with GFR decline might be due to the different baseline characteristics of the participants. Further studies are essential to assess the clinical effect of long sleep duration on kidney function in patients with CKD.
Poor sleep quality, a potential predictor of mortality in patients with ESKD (45), is common in patients with CKD (26,27); however, its clinical effect has not been elucidated. This study measured sleep quality using PSQI, the most widely used screening tool for poor sleep quality (30), with advantages including sound reliability, validity, responsiveness, and interpretability (31). Several cross-sectional studies of patients with CKD have suggested that poor sleep quality (PSQI global index ≥6) might have some cardiovascular effects, such as a “nondipper” pattern of ambulatory BP monitoring (46) and left ventricular hypertrophy (47). However, few cohort studies have assessed the prognostic power of the PSQI. This study identified poor sleep quality, defined as PSQI global score ≥6, as a significant predictor of ESKD, a hard outcome for patients with CKD (Table 4). However, their causal relationship remains unknown. Because the PSQI questionnaire covers a broad range of indicators relevant to sleep quality (Supplemental Appendix) (29), associations might depend on the underlying diseases, including obstructive sleep apnea, restless leg syndrome, insomnia, and others. To evaluate the clinical effect of sleep quality on kidney survival, associations should be assessed in subgroups of the underlying diseases in greater detail.
This study had several limitations. First, the frequency of occurrence for “other” sleep disturbance (PSQI question 5j) was not included in the CKD-JAC study, accidentally. Thus, 38 patients whose PSQI global scores could not be calculated were excluded from this study. The exclusion of this very small number of patients was unlikely to have led to biased results. Second, many patients were excluded due to missing data (n=1241 [44%]), which might have led to biased results. Because the baseline variables with significant differences between the included and excluded patients had no obvious effect modifications with sleep duration and PSQI global score, exclusion of these patients with missing data probably had little effect on associations of sleep duration and PSQI global score with incidence of ESKD. Third, self-reported sleep duration might be biased, despite confirmation by previous studies that self-reported sleep duration was correlated moderately with the results of polysomnography (48,49) and actigraphy (50), suggesting that self-reported sleep duration was a clinical surrogate of sleep duration.
In conclusion, this cohort study, including 1601 patients with CKD, identified poor sleep quality (defined as PSQI global index ≥6) as a predictor of ESKD and revealed that both short (<5 hours) and long (≥8 hours) sleep duration were associated with ESKD incidence. Sleep duration and sleep quality may be easily measured and useful tools for identifying patients at high risk of ESKD.
Disclosures
Y.I. has received speaker honoraria from Kyowa Hakko Kirin. The other authors have nothing to declare.
Supplementary Material
Acknowledgments
This study was supported by research funds with no restriction on publication from Kyowa Hakko Kirin Co., Ltd.
This study was conducted by principal investigators at the following medical centers: Japan Community Health Care Organization Sendai Hospital (Miyagi), JA Toride Medical Center (Ibaraki), Jichi Medical University (Tochigi), Tokyo Women’s Medical University Hospital (Tokyo), St. Luke’s International Hospital (Tokyo), Showa University Hospital (Tokyo), Showa University Yokohama Northern Hospital (Kanagawa), Showa University Fujigaoka Hospital (Kanagawa), Gifu Prefectural General Medical Center (Gifu), Kasugai Municipal Hospital (Aichi), Tosei General Hospital (Aichi), Osaka University Hospital (Osaka), Osaka General Medical Center (Osaka), Osaka City General Hospital (Osaka), Kurashiki Central Hospital (Okayama), Fukuoka Red Cross Hospital (Fukuoka), and Iizuka Hospital (Fukuoka).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01340118/-/DCSupplemental.
References
- 1.Sherwood M, McCullough PA: Chronic kidney disease from screening, detection, and awareness, to prevention. Lancet Glob Health 4: e288–e289, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, Jong PE, Coresh J, Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, de Jong PE, Coresh J, El-Nahas M, Eckardt KU, Kasiske BL, Wright J, Appel L, Greene T, Levin A, Djurdjev O, Wheeler DC, Landray MJ, Townend JN, Emberson J, Clark LE, Macleod A, Marks A, Ali T, Fluck N, Prescott G, Smith DH, Weinstein JR, Johnson ES, Thorp ML, Wetzels JF, Blankestijn PJ, van Zuilen AD, Menon V, Sarnak M, Beck G, Kronenberg F, Kollerits B, Froissart M, Stengel B, Metzger M, Remuzzi G, Ruggenenti P, Perna A, Heerspink HJ, Brenner B, de Zeeuw D, Rossing P, Parving HH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T; Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 79: 1331–1340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chronic Kidney Disease Prognosis Consortium: Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010. [DOI] [PMC free article] [PubMed]
- 4.Couser WG, Remuzzi G, Mendis S, Tonelli M: The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 80: 1258–1270, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Imai E, Horio M, Watanabe T, Iseki K, Yamagata K, Hara S, Ura N, Kiyohara Y, Moriyama T, Ando Y, Fujimoto S, Konta T, Yokoyama H, Makino H, Hishida A, Matsuo S: Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol 13: 621–630, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Masakane I, Nakai S, Ogata S, Kimata N, Hanafusa N, Hamano T, Wakai K, Wada A, Nitta K: An overview of regular dialysis treatment in Japan (As of 31 December 2013). Ther Apher Dial 19: 540–574, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Yamagata K, Yagisawa T, Nakai S, Nakayama M, Imai E, Hattori M, Iseki K, Akiba T: Prevalence and incidence of chronic kidney disease stage G5 in Japan. Clin Exp Nephrol 19: 54–64, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Turek NF, Ricardo AC, Lash JP: Sleep disturbances as nontraditional risk factors for development and progression of CKD: Review of the evidence. Am J Kidney Dis 60: 823–833, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y, Zhai L, Zhang D: Sleep duration and obesity among adults: A meta-analysis of prospective studies. Sleep Med 15: 1456–1462, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Xi B, He D, Zhang M, Xue J, Zhou D: Short sleep duration predicts risk of metabolic syndrome: A systematic review and meta-analysis. Sleep Med Rev 18: 293–297, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W, Rong Y, Jackson CL, Hu FB, Liu L: Sleep duration and risk of type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care 38: 529–537, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Mei H, Jiang Y-R, Sun W-Q, Song Y-J, Liu S-J, Jiang F: Relationship between duration of sleep and hypertension in adults: A meta-analysis. J Clin Sleep Med 11: 1047–1056, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X, Chen H, Li S, Pan L, Jia C: Association of sleep duration with the morbidity and mortality of coronary artery disease: A meta-analysis of prospective studies. Heart Lung Circ 24: 1180–1190, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Leng Y, Cappuccio FP, Wainwright NWJ, Surtees PG, Luben R, Brayne C, Khaw KT: Sleep duration and risk of fatal and nonfatal stroke: A prospective study and meta-analysis. Neurology 84: 1072–1079, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen X, Wu Y, Zhang D: Nighttime sleep duration, 24-hour sleep duration and risk of all-cause mortality among adults: A meta-analysis of prospective cohort studies. Sci Rep 6: 21480, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto R, Nagasawa Y, Iwatani H, Shinzawa M, Obi Y, Teranishi J, Ishigami T, Yamauchi-Takihara K, Nishida M, Rakugi H, Isaka Y, Moriyama T: Self-reported sleep duration and prediction of proteinuria: A retrospective cohort study. Am J Kidney Dis 59: 343–355, 2012 [DOI] [PubMed] [Google Scholar]
- 17.McMullan CJ, Curhan GC, Forman JP: Association of short sleep duration and rapid decline in renal function. Kidney Int 89: 1324–1330, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrov ME, Kim Y, Lauderdale DS, Lewis CE, Reis JP, Carnethon MR, Knutson KL, Glasser SP: Objective sleep, a novel risk factor for alterations in kidney function: The CARDIA study. Sleep Med 15: 1140–1146, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricardo AC, Knutson K, Chen J, Appel LJ, Bazzano L, Carmona-Powell E, Cohan J, Tamura MK, Steigerwalt S, Thornton JD, Weir M, Turek NF, Rahman M, Van Cauter E, Lash JP; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The association of sleep duration and quality with CKD progression. J Am Soc Nephrol 28: 3708–3715, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomson CA, Morrow KL, Flatt SW, Wertheim BC, Perfect MM, Ravia JJ, Sherwood NE, Karanja N, Rock CL: Relationship between sleep quality and quantity and weight loss in women participating in a weight-loss intervention trial. Obesity (Silver Spring) 20: 1419–1425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JA, Sunwoo S, Kim YS, Yu BY, Park HK, Jeon TH, Yoo BW: The effect of sleep quality on the development of type 2 diabetes in primary care patients. J Korean Med Sci 31: 240–246, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Twig G, Shina A, Afek A, Derazne E, Tzur D, Cukierman-Yaffe T, Shechter-Amir D, Gerstein HC, Tirosh A: Sleep quality and risk of diabetes and coronary artery disease among young men. Acta Diabetol 53: 261–270, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Martin JL, Fiorentino L, Jouldjian S, Mitchell M, Josephson KR, Alessi CA: Poor self-reported sleep quality predicts mortality within one year of inpatient post-acute rehabilitation among older adults. Sleep (Basel) 34: 1715–1721, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tasali E, Leproult R, Ehrmann DA, Van Cauter E: Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A 105: 1044–1049, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stamatakis KA, Punjabi NM: Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 137: 95–101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogna A, Forni Ogna V, Haba Rubio J, Tobback N, Andries D, Preisig M, Tafti M, Vollenweider P, Waeber G, Marques-Vidal P, Heinzer R: Sleep characteristics in early stages of chronic kidney disease in the HypnoLaus cohort. Sleep (Basel) 39: 945–953, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plantinga L, Lee K, Inker LA, Saran R, Yee J, Gillespie B, Rolka D, Saydah S, Powe NR; CDC CKD Surveillance Team : Association of sleep-related problems with CKD in the United States, 2005-2008. Am J Kidney Dis 58: 554–564, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Li J, Huang Z, Hou J, Sawyer AM, Wu Z, Cai J, Curhan G, Wu S, Gao X: Sleep and CKD in Chinese adults: A cross-sectional study. Clin J Am Soc Nephrol 12: 885–892, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ: The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 28: 193–213, 1989 [DOI] [PubMed] [Google Scholar]
- 30.Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A: The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev 25: 52–73, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Omachi TA: Measures of sleep in rheumatologic diseases: Epworth Sleepiness Scale (ESS), Functional Outcome of Sleep Questionnaire (FOSQ), Insomnia Severity Index (ISI), and Pittsburgh Sleep Quality Index (PSQI). Arthritis Care Res (Hoboken) 63[Suppl 11]: S287–S296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasegawa T, Sakamaki K, Koiwa F, Akizawa T, Hishida A; Clinical prediction models for progression of chronic kidney disease to end-stage kidney failure under pre-dialysis nephrology care: Results from the Chronic Kidney Disease Japan Cohort Study [published online ahead of print August 1, 2018]. Clin Exp Nephrol. doi: 10.1007/s10157-018-1621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka K, Watanabe T, Takeuchi A, Ohashi Y, Nitta K, Akizawa T, Matsuo S, Imai E, Makino H, Hishida A; CKD-JAC Investigators : Cardiovascular events and death in Japanese patients with chronic kidney disease. Kidney Int 91: 227–234, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Inaguma D, Imai E, Takeuchi A, Ohashi Y, Watanabe T, Nitta K, Akizawa T, Matsuo S, Makino H, Hishida A; Chronic Kidney Disease Japan Cohort Study Group : Risk factors for CKD progression in Japanese patients: Findings from the Chronic Kidney Disease Japan Cohort (CKD-JAC) study. Clin Exp Nephrol 21: 446–456, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nitta K, Iimuro S, Imai E, Matsuo S, Makino H, Akizawa T, Watanabe T, Ohashi Y, Hishida A: Risk factors for increased left ventricular hypertrophy in patients with chronic kidney disease: Findings from the CKD-JAC study [published online ahead of print June 27, 2018]. Clin Exp Nephrol 10.1007/s10157-018-1605-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imai E, Matsuo S, Makino H, Watanabe T, Akizawa T, Nitta K, Iimuro S, Ohashi Y, Hishida A; CKD-JAC Study Group : Chronic Kidney Disease Japan Cohort (CKD-JAC) study: Design and methods. Hypertens Res 31: 1101–1107, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A; Collaborators developing the Japanese equation for estimated GFR : Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 4: 561–571, 1961 [DOI] [PubMed] [Google Scholar]
- 39.Hedayati SS, Minhajuddin AT, Toto RD, Morris DW, Rush AJ: Validation of depression screening scales in patients with CKD. Am J Kidney Dis 54: 433–439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uno H, Tian L, Cai T, Kohane IS, Wei LJ: A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med 32: 2430–2442, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kripke DF: Mortality risk of hypnotics: Strengths and limits of evidence. Drug Saf 39: 93–107, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Parsaik AK, Mascarenhas SS, Khosh-Chashm D, Hashmi A, John V, Okusaga O, Singh B: Mortality associated with anxiolytic and hypnotic drugs-A systematic review and meta-analysis. Aust N Z J Psychiatry 50: 520–533, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA: Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep 33: 585–592, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.da Silva AA, de Mello RGB, Schaan CW, Fuchs FD, Redline S, Fuchs SC: Sleep duration and mortality in the elderly: A systematic review with meta-analysis. BMJ Open 6: e008119, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elder SJ, Pisoni RL, Akizawa T, Fissell R, Andreucci VE, Fukuhara S, Kurokawa K, Rayner HC, Furniss AL, Port FK, Saran R: Sleep quality predicts quality of life and mortality risk in haemodialysis patients: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 23: 998–1004, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Wang C, Gong W, Ye Z, Tang Y, Zhao W, Peng H, Lou T: Poor sleep quality is responsible for the nondipper pattern in hypertensive but not in normotensive chronic kidney disease patients. Nephrology (Carlton) 22: 690–698, 2017 [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Wang C, Gong W, Peng H, Tang Y, Li CC, Zhao W, Ye Z, Lou T: Association between sleep quality and cardiovascular damage in pre-dialysis patients with chronic kidney disease. BMC Nephrol 15: 131, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taheri S, Lin L, Austin D, Young T, Mignot E: Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 1: e62, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roumelioti M-E, Buysse DJ, Sanders MH, Strollo P, Newman AB, Unruh ML: Sleep-disordered breathing and excessive daytime sleepiness in chronic kidney disease and hemodialysis. Clin J Am Soc Nephrol 6: 986–994, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lockley SW, Skene DJ, Arendt J: Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res 8: 175–183, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.