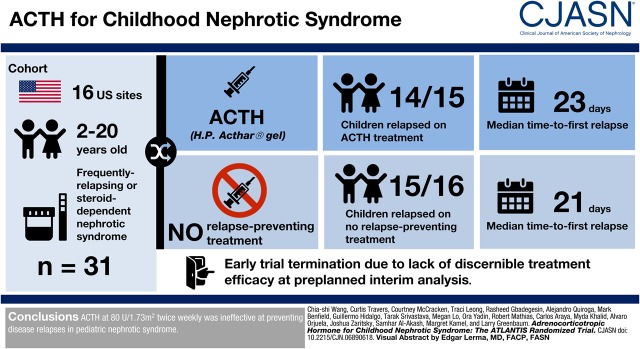
Abstract
Background and objectives
There is renewed interest in adrenocorticotropic hormone (ACTH) for the treatment of nephrotic syndrome. We evaluated the efficacy and safety of ACTH in children with frequently relapsing or steroid-dependent nephrotic syndrome in a randomized trial.
Design, setting, participants, & measurements
Participants aged 2–20 years old with frequently relapsing or steroid-dependent nephrotic syndrome were enrolled from 16 sites in the United States and randomized 1:1 to ACTH (repository corticotropin injection) or no relapse-preventing treatment. ACTH treatment regimen was 80 U/1.73 m2 administered twice weekly for 6 months, followed by 40 U/1.73 m2 administered twice weekly for 6 months. The primary outcome was disease relapse during the first 6 months. Participants in the control group were offered crossover to ACTH treatment if they relapsed within 6 months. Secondary outcomes were relapse after ACTH dose reduction and treatment side effects.
Results
The trial was stopped at a preplanned interim analysis after enrollment of 31 participants because of a lack of discernible treatment efficacy. Fourteen out of 15 (93%) participants in the ACTH arm experienced disease relapse in the first 6 months, with a median time to first relapse of 23 days (interquartile range, 9–32), compared with 15 out of 16 (94%) participants and at a median of 21 days (interquartile range, 14–51) in the control group. There was no difference in the proportion of relapsed patients (odds ratio, 0.93; 95% confidence interval, 0.05 to 16.40; P>0.99) or time to first relapse (hazard ratio, 1.03; 95% confidence interval, 0.50 to 2.15; P=0.93). Thirteen out of 16 participants in the control group crossed over to ACTH treatment. Three out of 28 participants completed 12 months of ACTH treatment; the others exited the trial because of frequent relapses or side effects. There were no disease relapses after ACTH dose reduction among the three participants. Most side effects were mild and similar to side effects of corticosteroids.
Conclusions
ACTH at 80 U/1.73 m2 administered twice weekly was ineffective at preventing disease relapses in pediatric nephrotic syndrome.
Keywords: nephrotic syndrome, clinical trial, children, Adrenocorticotropic Hormone, Childhood Nephrotic Syndrome
Visual Abstract
Introduction
Idiopathic nephrotic syndrome is one of the most common glomerular diseases and causes of ESKD in children (1,2). Although >80% respond to corticosteroids, most experience disease relapse and about half relapse frequently or become dependent on corticosteroids to maintain remission, increasing the risk of disease complications and treatment side effects (3–6). Various relapse-preventing therapies are used to maintain disease remission and decrease corticosteroid exposure, yet these agents have additional toxicity and some children continue to relapse frequently (5,7).
Adrenocorticotropic hormone (ACTH) was widely used in the 1950s as a first-line treatment of nephrotic syndrome, and was shown to induce disease remission and improve patient survival (8–12). However, by the 1960s, ACTH was replaced by oral prednisone for its ease of administration and the belief that ACTH acts via stimulation of corticosteroid production (8,13,14). More recently, several case series of patients with steroid- and multidrug-resistant nephrotic syndrome reported ACTH to be efficacious in inducing and sustaining disease remission and improving GFR, which suggests that ACTH has effects beyond steroidogenesis (13,15–18). In addition, one randomized trial demonstrated similar efficacy when comparing ACTH with methylprednisolone and a cytotoxic agent in membranous nephropathy, renewing interest in ACTH for nephrotic syndrome treatment (19). Most case series were small, retrospective, and composed of patients with heterogeneous diagnoses. Furthermore, published experience in children is limited, and clinicians rely on anecdotal evidence to guide patient treatment (13). Hence, we conducted a multisite, open-label, randomized, clinical trial to evaluate the efficacy and safety of 12 months of ACTH in children with frequently relapsing or steroid-dependent nephrotic syndrome. We hypothesized that ACTH will be efficacious in maintaining disease remission when compared with no relapse-preventing medications.
Materials and Methods
Participants
Eligible participants were 2–20 years of age, with a clinical diagnosis of idiopathic nephrotic syndrome made at >1 year of age (edema, urine protein-to-creatinine ratio >2 mg/mg or urine protein ≥300 mg/dl on urine test strip, and hypoalbuminemia ≤2.5 g/dl) that is steroid responsive (achieve remission within 4 weeks of daily prednisone/prednisolone therapy). Patients were required to have frequently relapsing (two or more relapses within 6 months after initial therapy or four or more relapses in any 12-month period) or steroid-dependent disease (relapses during corticosteroid taper or within 2 weeks after discontinuing corticosteroids) with a disease relapse (urine protein-to-creatinine ratio >2 mg/mg or urine dipstick ≥2+ for 3 days in a row) within 4 months of screening, to indicate poor disease control on current treatment and justify enrollment into an experimental trial. Exclusion criteria were requirement of a noncorticosteroid agent to attain disease remission; prior ACTH use; cyclophosphamide or rituximab treatment within 4 months of screening; lactation, pregnancy, or refusal of birth control in patients with child-bearing potential; planned treatment with live or live-attenuated vaccines once enrolled in the study; participation in another therapeutic trial concurrently or 30 days before study randomization; active serious infection; malignancy within the last 2 years; BP >95th percentile for age/height while receiving maximal doses of three or more medications; diagnosis of diabetes mellitus or fasting glucose >200 mg/dl; organ transplantation; eGFR≤70 ml/min per 1.73 m2 via the modified bedside Schwartz equation (20); contraindications to repository corticotropin injection per product labeling; biopsy demonstrating a diagnosis other than minimal change, FSGS or a variant; or inability to consent/assent.
The study was approved by the institutional review board at each participating center and adhered with the principles of the Declaration of Helsinki. Written parental consent and minor assent were obtained in accordance with local institutional review board guidelines. An independent Data and Safety Monitoring Board (DSMB) periodically reviewed study safety and progress.
Study Design and Treatment
The A Trial of ACTH in Nephrotic Syndrome (ATLANTIS) study was an open-label, multicenter, prospective, randomized, phase III trial conducted at 16 pediatric centers within the Midwest Pediatric Nephrology Consortium (MWPNC) in the United States (Clinicaltrials.gov identifier: NCT02132195; registration date May 7, 2014). The study was advertised at biannual MWPNC meetings through study brochures distributed to the sites, and listed on websites Clinicaltrials.gov and Nephcure.org. Recruitment took place from May 2014 to November 2017, with follow-up through to March 2018. Potential participants were referred to site investigators by treating clinicians or by self-referral, then screened for eligibility before enrollment.
Participants with poorly controlled nephrotic syndrome, indicated by ongoing disease relapses on their current regimen, were randomized 1:1 to ACTH treatment or no relapse-preventing treatment. A biostatistician (T.L.) with no clinical involvement in the trial generated the random allocation sequence using a pseudorandom number generator with randomly permutated blocks stratified by treatment center. Assignments were placed in sealed, sequenced, opaque envelopes, which study coordinators opened at time of enrollment to assign participants to ACTH treatment or no relapse-preventing treatment.
Participants randomized to ACTH treatment were started on ACTH at 80 U/1.73 m2, administered twice weekly (initial dose). All other relapse-preventing treatments, including corticosteroids and other immunosuppressive therapies, were stopped after a 2-week overlap with ACTH. Participants were treated with the initial dose of ACTH for 6 months. A disease relapse during the initial dose period was treated with prednisone/prednisolone at 2 mg/kg per day (maximum dose 60 mg daily) until remission was achieved (urine protein negative or trace for 3 days in a row), followed by 1.5 mg/kg per day every other day (maximum dose 40 mg) for 4 weeks. ACTH was continued during treatment of the relapse. If a participant developed a second relapse during the initial dose period, ACTH therapy was discontinued and the participant exited the study. Once a participant completed 6 months of initial dose ACTH, the dose was decreased to 40 U/1.73 m2, administered twice weekly (reduced dose), for an additional 6 months. A disease relapse on the reduced dose was treated by increasing ACTH back to the initial dose and with prednisone/prednisolone as described above. A participant who had two relapses after changing back to initial dose ACTH exited the study. Patients who completed 12 months of ACTH were observed for 6 months after the conclusion of ACTH therapy.
Participants randomized to no relapse-preventing therapy stopped all immunosuppressive nephrotic syndrome treatments within 2 weeks after randomization and were monitored for 6 months. A disease relapse during this period was treated with prednisone/prednisolone therapy as described above, and the patient was offered the option of ACTH treatment after remission was achieved. Patients who did not elect to cross over to ACTH exited the study. There were no changes to the methods after trial commencement.
End Points
Prespecified efficacy end point was disease relapse during the initial 6 months, comparing the ACTH arm with the control arm (primary outcome). Secondary outcomes were disease relapse after ACTH dose reduction of ACTH and treatment side effects. Exploratory outcomes included development of a second disease relapse during the initial 6 months of ACTH and relapse after completion of 12 months of ACTH.
Statistical Analyses
On the basis of a systemic review of early studies showing that 71% of patients responded to ACTH maintenance therapy (13), goal enrollment was determined to be 60 participants (30 participants in each arm) to provide 90% statistical power to detect a 40% difference in the 6-month relapse rate (70% versus 30%), with a two-sided z test, the α-spending function by Lan and DeMets (21,22), and a type 1 error rate of 5%. Two analyses at 50% and 100% enrollment (with 6 months of follow-up) were planned.
Preplanned interim results were presented to the DSMB after enrollment of 31 participants with a minimum of 6 months of follow-up. The DSMB recommended stopping trial recruitment because of the lack of discernible efficacy of ACTH compared with no relapse-preventing therapy, and the extremely low probability that continued recruitment would demonstrate treatment efficacy.
Participant characteristics and outcomes were summarized by study arm, using medians and interquartile ranges (IQRs), means and SDs, and counts and percentages, where appropriate. To compare the relative odds of developing disease relapse within the first 6 months among patients randomized to ACTH versus those randomized to no relapse-preventing treatment, we calculated odds ratios with 95% confidence intervals (95% CIs). Time to first and second relapse were assessed using Kaplan–Meier curves and survival distributions were compared by treatment group, using log-rank tests. Cox proportional hazard models were used to calculate hazard ratios (HRs) when comparing time to first relapse between study arms and comparing time to first and second relapse on ACTH. All serious adverse events (SAEs) and other adverse events and their relatedness to study treatment are reported. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Participants
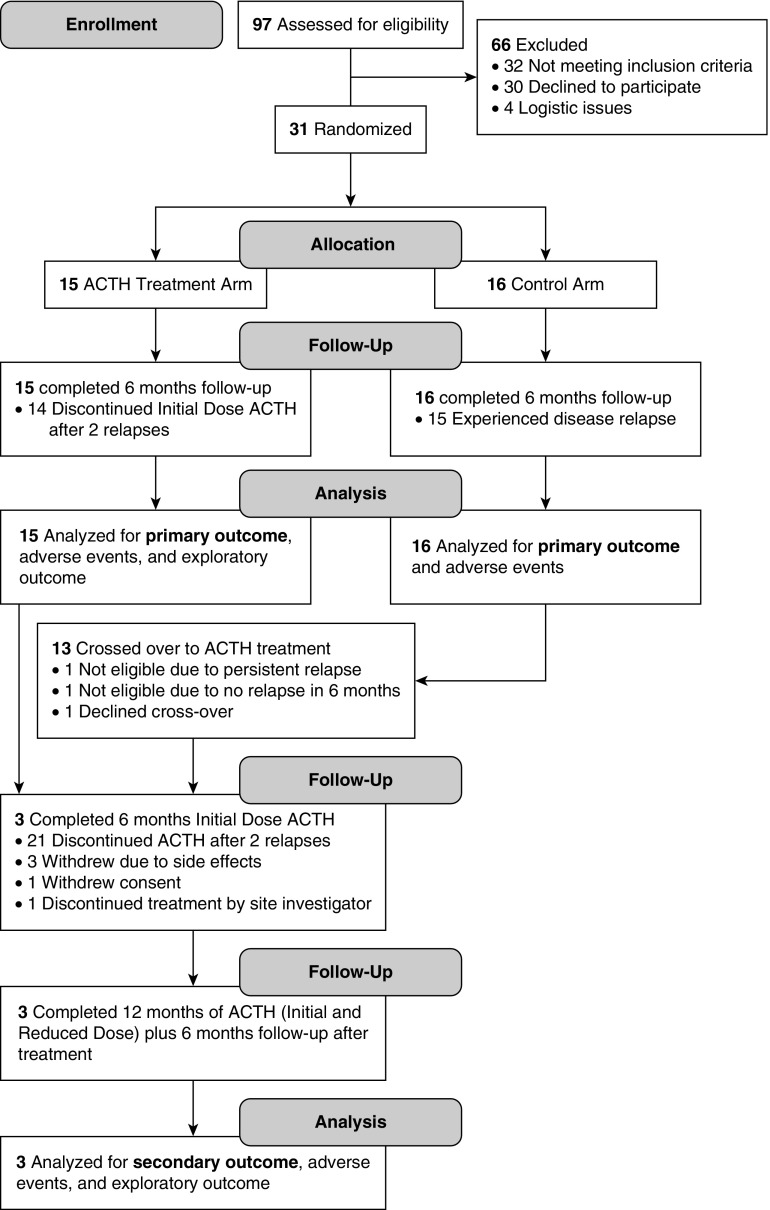
Participant enrollment, intervention allocation, and retention are summarized in Figure 1. Among 15 participants randomized to ACTH treatment, only one (7%) completed 6 months of initial dose ACTH treatment. Fourteen (93%) exited the study after experiencing two relapses on initial dose ACTH therapy, with median duration of ACTH therapy of 61 days (IQR, 36–112; range, 27–134). Among 16 participants randomized to no relapse-preventing treatment, 15 (94%) relapsed within 6 months and 13 crossed over to ACTH treatment. Of the 13 crossover participants, two (15%) completed 6 months of ACTH initial dose therapy. Reasons for study exit in the 11 crossover participants were as follows: seven (54%) had two relapses during the initial dose period, with a median duration of ACTH therapy of 49 days (IQR, 32–75; range, 32–96); two (15%) withdrew because of side effects; one (8%) stopped treatment at the discretion of the site principal investigator; and one (8%) withdrew consent. Three participants from the initially randomized and crossover groups continued on to reduced dose therapy. All three completed 6 months of reduced dose ACTH and 6 months of follow-up when not receiving any therapy to prevent relapses. Two patients had inadvertently missed a single dose of ACTH.
Figure 1.
Participant flow through the ATLANTIS trial comparing ACTH with no relapse-preventing treatment with the option of crossover.
Demographic and clinical characteristics of the participants in both study groups were similar (Table 1). The majority (24 out of 31, 77%) had been treated with immunosuppressive agents other than oral corticosteroids, but continued to experience disease relapses. Fifteen (48%) had been treated with two or more agents before the start of the trial. The most frequently used agents were tacrolimus (17 out of 31, 55%), mycophenolate (13 out of 31, 42%), and cyclophosphamide (seven out of 31, 23%).
Table 1.
ATLANTIS trial participant characteristics at enrollment
| Characteristics | ACTH 80 U/1.73 m2 Treatment Group, n=15 | No Relapse-Preventing Treatment Group, n=16 |
|---|---|---|
| Age at randomization, yr, median (IQR) | 7.0 (4.8–11.2) | 9.5 (5.2–12.0) |
| Male, n (%) | 10 (67) | 12 (75) |
| Race, n (%) | ||
| White | 13 (87) | 13 (81) |
| Black | 2 (13) | 1 (6) |
| Other | 0 (0) | 2 (12.5) |
| Hispanic ethnicity, n (%) | 7 (47) | 1 (6) |
| Age at diagnosis, yr, median (IQR) | 2.8 (2.4–4.3) | 2.8 (2.6–3.8) |
| Duration of disease at baseline, yr, median (IQR) | 3.0 (2.5–6.3) | 7.3 (1.8–9.4) |
| Time from last relapse to randomization, d, mean (SD) | 72 (36) | 75 (33) |
| Histopathology, n (%) | ||
| Minimal change | 12 (80) | 7 (44) |
| FSGS | 1 (7) | 1 (6) |
| Biopsy not performed | 2 (13) | 8 (50) |
| Treated hypertension, n (%) | 4 (27) | 4 (25) |
| Immunosuppressive agent exposure, n (%)a | ||
| None | 2 (13) | 5 (31) |
| Intravenous methylprednisolone | 3 (20) | 3 (19) |
| Tacrolimus | 11 (73) | 6 (38) |
| Cyclosporine | 2 (13) | 2 (13) |
| Cyclophosphamide | 3 (20) | 4 (25) |
| Mycophenolate | 4 (27) | 9 (56) |
| Rituximab | 1 (7) | 2 (13) |
| ≥2 agents | 7 (47) | 8 (50) |
| Immunosuppressive agents at time of enrollment, n (%)a | ||
| None | 1 (7) | 1 (6) |
| Prednisone/prednisolone | 3 (20) | 10 (63) |
| Tacrolimus | 7 (47) | 3 (19) |
| Cyclosporine | 0 | 2 (13) |
ACTH, adrenocorticotropic hormone; ATLANTIS, A Trial of ACTH for Nephrotic Syndrome; IQR, interquartile range.
More than one choice may apply to a participant.
Efficacy
In the ACTH treatment group, 14 out of 15 (93%) participants experienced a relapse within 6 months (primary outcome), with a median time to first relapse of 23 days (IQR, 9–32). In the no relapse-preventing treatment group, 15 out of 16 (94%) participants relapsed, with a median time to first relapse of 21 days (IQR, 14–51). There was no difference in the proportion of relapsed patients between the ACTH and the no relapse-preventing treatment arm (odds ratio, 0.93; 95% CI, 0.05 to 16.40; P>0.99) or time to first relapse arm (HR, 1.03; 95% CI, 0.50 to 2.15; P=0.93).
In the ACTH treatment arm, all 14 participants who developed one relapse had a second relapse within 6 months of starting ACTH (exploratory outcome), with a median time to second relapse of 21 days (IQR, 10–34) after cessation of corticosteroid therapy for relapse treatment. There was no difference between time to first relapse and time to second relapse (HR, 0.87; 95% CI, 0.43 to 1.74; P=0.69).
Three participants out of a total of 28 participants treated with ACTH, inclusive of those initially randomized and those who crossed over to ACTH treatment, completed 12 months of ACTH treatment. There were no disease relapses among the three participants on reduced dose ACTH (secondary outcome). One crossover patient relapsed once when on initial dose ACTH, but completed 12 months of ACTH and 6 months of follow-up without additional relapses. Two patients had no relapses during 12 months of ACTH treatment and 6 months of follow-up (exploratory outcome). Two patients were adolescents with a long disease duration.
Safety
SAEs and other adverse events reported with initial dose ACTH treatment, reduced dose ACTH treatment, and no relapse-preventing treatment are presented in Table 2 (secondary outcome). There were no deaths. Hospitalizations were the only SAEs, but none were deemed related to the study. Twenty four patients reported adverse events, primarily during initial dose ACTH treatment. The most common adverse events were increased appetite (32%), injection site irritation (25%), behavioral changes (25%), and cushingoid symptoms/striae/acne (18%). Adverse events of “weeping fluid” from skin striae and behavioral problems led two participants to withdraw from the study after 39 and 147 days of ACTH use, respectively.
Table 2.
Serious adverse events and other adverse events by treatment
| Event | ACTH 80U/1.73 m2, n=28a | ACTH 40U/1.73 m2, n=3 | No Relapse-Preventing Treatment, n=16 |
|---|---|---|---|
| Hospitalization, n (%) | 5 (18) | 0 | 2 (13) |
| AKI | 0 | 0 | 1 (6) |
| Edema | 2 (7) | 0 | 0 |
| Edema with GI complaints | 2 (7) | 0 | 0 |
| Edema with respiratory complaints | 1 (4) | 0 | 1 (6%) |
| Hypertension | 1 (4) | 0 | 0 |
| Other adverse events, n (%) | |||
| Infections | 1 (4) | 1 (33) | 1 (6) |
| Behavioral changes | 7 (25) | 0 | 0 |
| Sleep disturbances | 5 (18) | 0 | 1 (6) |
| Elevated BP | 1 (4) | 0 | 0 |
| Cushingoid symptoms/striae/acne | 5 (18) | 0 | 2 (13) |
| Hyperglycemia, >200 mg/dl | 0 | 0 | 0 |
| Injection site irritation | 7 (25) | 0 | N/A |
| Skin hyperpigmentation | 0 | 0 | 0 |
| Increased appetite | 9 (32) | 0 | 2 (13) |
ACTH, adrenocorticotropic hormone; GI, gastrointestinal; N/A, not applicable.
ACTH treatment participants include both those initially randomized to ACTH treatment and those who crossed over to ACTH treatment.
Discussion
The ATLANTIS trial was the first randomized trial of ACTH in childhood nephrotic syndrome. The trial was stopped early because of the lack of discernible efficacy. ACTH monotherapy at 80 U/1.73 m2, administered twice weekly, given during disease remission failed to maintain disease remission, with similar relapse rate and time to relapse when compared with no relapse-preventing treatment.
The negative trial findings contrast with recent case series that have suggested that ACTH is effective in reducing proteinuria in adults with nephrotic syndrome (18,23–26). Our trial differs from adult experiences in several important ways. First, most adult reports examined patient outcomes after >6 months of ACTH and described changes in the degree of proteinuria and eGFR compared with before treatment. Our main outcome of interest was the ability of ACTH to prevent disease relapses, assessed within the first 6 months of treatment. It has been suggested that higher cumulative doses of ACTH result in greater proteinuria reduction (23), and thus our decision to stop the study drug within 6 months of treatment if patients experienced two or more relapses may have affected the ability for the drug to establish its effects through longer treatment with higher cumulative doses. However, time to relapse was similar for the first relapse (23 days) and the second relapse (21 days) despite longer duration of ACTH treatment. This does not support a cumulative dose effect in preventing relapses in our patient population.
Our cohort has different diseases than the adults reported, which generally comprised patients with idiopathic membranous nephropathy or those with treatment-resistant disease (18,23–26). It is perhaps not surprising that a medication that reduces proteinuria and preserves eGFR in treatment-resistant disease may not be effective in preventing relapses in corticosteroid-responsive childhood nephrotic syndrome. Finally, most of the adult studies are retrospective case series and await confirmation of efficacy in randomized, clinical trials.
There is very limited published experience of ACTH in children with nephrotic syndrome. Reports of ACTH use were published in the 1950s in treatment-naïve pediatric patients as a single agent before oral prednisone became available. ACTH formulations were different in the 1950s compared with the highly purified, long-acting repository corticotropin injection used in this study. It is not possible to quantitatively compare the dosing used in the 1950s with the ACTH formulation available today; no pharmacokinetic data exist in children (13). In our study, we chose 80 U/1.73 m2 administered twice weekly on the basis of adult experiences (17), but this dosing had not been verified in children with nephrotic syndrome for the treatment goal of relapse prevention. Pediatric studies from the 1950s using ACTH as long-term, maintenance therapy generally consisted of weekly to three times weekly injections after initial daily treatment that usually lasted 1–4 weeks, with sustained proteinuria response in 71% of the patients (13). In contrast to the early literature, our patients were not treatment-naïve: all were selected for having frequent relapses or steroid dependency. The majority of our patients (77%) had been treated with immunosuppressants in addition to corticosteroids but continued to have disease relapses; nearly half had been treated with two or more agents. This group of patients likely had much more difficult to treat nephrotic syndrome compared with patients included in the reports from the 1950s.
ACTH was generally well tolerated in our study. Side effects were generally mild and similar to corticosteroids. Hyperglycemia, hypertension, and hypokalemia reported in adult studies were not seen in our cohort (18,23,24,26).
There is limited data on the risk of relapse in children with frequently relapsing or steroid-dependent nephrotic syndrome when receiving no relapse-preventing therapy. For our power analysis, we conservatively assumed a relapse rate of 70%, but observed a relapse rate of 94%. We only included patients who had relapsed within 4 months of screening and had not been treated with an agent with a potentially long duration of action within 4 months of randomization (e.g., rituximab or cyclophosphamide). These inclusion criteria and our observed relapse rate may be helpful for designing future studies in this patient population.
Our study is the first controlled trial of ACTH for childhood nephrotic syndrome. We conclude that ACTH monotherapy at 80 U/1.73 m2, administered twice weekly, is ineffective at preventing disease relapses in patients with frequently relapsing or steroid-dependent disease. Additional studies are needed to assess the efficacy of ACTH in steroid-resistant childhood nephrotic syndrome.
Disclosures
This study was an investigator-initiated trial supported by Mallinckrodt Pharmaceuticals, makers of the repository corticotropin injection used in this study, and formerly by Questcor Pharmaceuticals. C.M., T.L., and M.R.B. receive additional research support from Mallinckrodt Pharmaceuticals. T.S. and L.A.G. receive research support from Bristol-Myers Squibb and Retrophin, Inc. M.L. receives research support from Kaneka Corporation and Sanofi.
Acknowledgments
We wish to acknowledge the Steering Committee, study coordinators, and staff of the Midwest Pediatric Nephrology Consortium, who facilitated this study. We thank NephCure Kidney International (https://nephcure.org) for promoting study participation and the members of the Data Safety and Monitoring Board for their thoughtful evaluation of the study.
A summary of the trial results will be submitted to Clinicaltrials.gov within 1 year of study completion. Because of the small number of participants in this study of a rare condition, even with removal of all identifiers there is a significant risk of reidentification of the participants. Thus, we will not share participant data. Our study protocol, statistical analysis plan, informed consent and assent forms, and manual of procedures will be available for 5 years after publication of the manuscript to anyone who wishes access. Requests should be submitted to C.-s.W. at chia-shi.wang@emory.edu.
C.-s.W. was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number UL1TR000454.
These results were presented, in part, at the Pediatric Academic Societies Meeting, Toronto, Canada, May 5–7, 2018.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The study sponsors had no role in the design of the study, evaluation of the results, or writing of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Does What Goes Around Always Come Around?,” on pages 1788–1790.
References
- 1.Eddy AA, Symons JM: Nephrotic syndrome in childhood. Lancet 362: 629–639, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Leonard MB, Donaldson LA, Ho M, Geary DF: A prospective cohort study of incident maintenance dialysis in children: An NAPRTC study. Kidney Int 63: 744–755, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Primary nephrotic syndrome in children: Clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int 20: 765–771, 1981 [DOI] [PubMed] [Google Scholar]
- 4.The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr 98: 561–564, 1981 [DOI] [PubMed] [Google Scholar]
- 5.Greenbaum LA, Benndorf R, Smoyer WE: Childhood nephrotic syndrome--current and future therapies. Nat Rev Nephrol 8: 445–458, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Lombel RM, Hodson EM, Gipson DS; Kidney Disease: Improving Global Outcomes : Treatment of steroid-resistant nephrotic syndrome in children: New guidelines from KDIGO. Pediatr Nephrol 28: 409–414, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Pravitsitthikul N, Willis NS, Hodson EM, Craig JC: Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children. Cochrane Database Syst Rev 10: CD002290, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Riley CM: The management of the nephrotic syndrome. Bull N Y Acad Med 28: 630–639, 1952 [PMC free article] [PubMed] [Google Scholar]
- 9.Lauson HD, Forman CW, McNAMARA H, Mattar G, Barnett HL: The effect of corticotropin (ACTH) on glomerular permeability to albumin in children with the nephrotic syndrome. J Clin Invest 33: 657–664, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnett HL: Effect of ACTH in children with the nephrotic syndrome. Pediatrics 9: 341, 1952 [PubMed] [Google Scholar]
- 11.Metcoff J, Rance CP, Kelsey WM, Nakasone N, Janeway CA: Adrenocorticotrophic hormone (ACTH) therapy of the nephrotic syndrome in children. Pediatrics 10: 543–566, 1952 [PubMed] [Google Scholar]
- 12.Folli G, Pollak VE, Reid RT, Pirani CL, Kark RM: Electron-microscopic studies of reversible glomerular lesions in the adult nephrotic syndrome. Ann Intern Med 49: 775–795, 1958 [DOI] [PubMed] [Google Scholar]
- 13.Lieberman KV, Pavlova-Wolf A: Adrenocorticotropic hormone therapy for the treatment of idiopathic nephrotic syndrome in children and young adults: A systematic review of early clinical studies with contemporary relevance. J Nephrol 30: 35–44, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong R: The renaissance of corticotropin therapy in proteinuric nephropathies. Nat Rev Nephrol 8: 122–128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg AL, Arnadottir M: ACTH-induced improvement in the nephrotic syndrome in patients with a variety of diagnoses. Nephrol Dial Transplant 19: 1305–1307, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Berg AL, Nilsson-Ehle P, Arnadottir M: Beneficial effects of ACTH on the serum lipoprotein profile and glomerular function in patients with membranous nephropathy. Kidney Int 56: 1534–1543, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Bomback AS, Tumlin JA, Baranski J, Bourdeau JE, Besarab A, Appel AS, Radhakrishnan J, Appel GB: Treatment of nephrotic syndrome with adrenocorticotropic hormone (ACTH) gel. Drug Des Devel Ther 5: 147–153, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madan A, Mijovic-Das S, Stankovic A, Teehan G, Milward AS, Khastgir A: Acthar gel in the treatment of nephrotic syndrome: A multicenter retrospective case series. BMC Nephrol 17: 37, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponticelli C, Passerini P, Salvadori M, Manno C, Viola BF, Pasquali S, Mandolfo S, Messa P: A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis 47: 233–240, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan KKG, DeMets DL: Discrete sequential boundaries for clinical trials. Biometrika 70(3): 659–663, 1983. 21878186 [Google Scholar]
- 22.Lan KKG, DeMets D. Further comments on the alpha-spending function. Stat Biosci 1: 95–111, 2009. 21878186 [Google Scholar]
- 23.Hladunewich MA, Cattran D, Beck LH, Odutayo A, Sethi S, Ayalon R, Leung N, Reich H, Fervenza FC: A pilot study to determine the dose and effectiveness of adrenocorticotrophic hormone (H.P. Acthar® Gel) in nephrotic syndrome due to idiopathic membranous nephropathy. Nephrol Dial Transplant 29: 1570–1577, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bomback AS, Radhakrishnan J: Treatment of nephrotic syndrome with adrenocorticotropic hormone (ACTH). Discov Med 12: 91–96, 2011 [PubMed] [Google Scholar]
- 25.Watson MJ: Membranous glomerulopathy and treatment with Acthar®: A case study. Int J Nephrol Renovasc Dis 6: 229–232, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hogan J, Bomback AS, Mehta K, Canetta PA, Rao MK, Appel GB, Radhakrishnan J, Lafayette RA: Treatment of idiopathic FSGS with adrenocorticotropic hormone gel. Clin J Am Soc Nephrol 8: 2072–2081, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]